Abstract
Context:
Laser therapy is purported to improve blood flow in soft tissues. Modulating circulation would promote healing by controlling postinjury ischemia, hypoxia, edema, and secondary tissue damage. However, no studies have quantified these responses to laser therapy.
Objective:
To determine a therapeutic dose range for laser therapy for increasing blood flow to the forearm.
Design:
Crossover study.
Setting:
Controlled laboratory setting.
Patients or Other Participants:
Ten healthy, college-aged men (age = 20.80 ± 2.16 years, height = 177.93 ± 3.38 cm, weight = 73.64 ± 9.10 kg) with no current history of injury to the upper extremity or cardiovascular conditions.
Intervention(s):
A class 4 laser device was used to treat the biceps brachii muscle. Each grid point was treated for 3 to 4 seconds, for a total of 4 minutes. Each participant received 4 doses of laser therapy: sham, 1 W, 3 W, and 6 W.
Main Outcome Measure(s):
The dependent variables were changes in blood flow, measured using venous occlusion plethysmography. We used a repeated-measures analysis of variance to analyze changes in blood flow for each dose at 2, 3, and 4 minutes and at 1, 2, 3, 4, and 5 minutes after treatment. The Huynh-Feldt test was conducted to examine differences over time.
Results:
Compared with baseline, blood flow increased over time with the 3-W treatment (F3,9 = 3.468, P < .011) at minute 4 of treatment (2.417 ± 0.342 versus 2.794 ± 0.351 mL/min per 100 mL tissue, P = .032), and at 1 minute (2.767 ± 0.358 mL/min per 100 mL tissue, P < .01) and 2 minutes (2.657 ± 0.369 mL/min per 100 mL tissue, P = .022) after treatment. The sham, 1-W, and 6-W treatment doses did not change blood flow from baseline at any time point.
Conclusions:
Laser therapy at the 3-W (360-J) dose level was an effective treatment modality to increase blood flow in the soft tissues.
Keywords: therapeutic modalities, circulation, musculo-skeletal injuries
Key Points.
Using a class 4 laser in a human clinical model, we found a protocol-response effect: a 3-W protocol at a 50% duty cycle applied to the biceps brachii muscle was the most effective for increasing blood flow to the distal forearm.
Laser therapy is an effective, noninvasive treatment modality to improve blood flow and perhaps tissue healing in the clinical setting.
The use of laser as a clinical modality has increased greatly over the past decade. Positive effects of laser therapy for the treatment of acute and chronic musculoskeletal disorders include pain control1,2 and improved tissue repair.3,4 However, the underlying mechanisms and clinical effectiveness of laser therapy remain poorly understood.
Lasers are classified by power level and their ability to produce eye injury. These power and beam characteristic ratings are established by the American National Standards Institute and the International Electrotechnical Commission. Most therapeutic lasers available for use in clinical practice are classified as 3B or 4. Class 3B lasers emit power of 5 to 500 mW, whereas class 4 lasers emit power of more than 500 mW. A few therapeutic laser manufacturers offer divergent-beam power outputs greater than 10 000 mW. Class 3B level emitting lasers are known as low-level, low-intensity, and cold lasers because they generate no significant thermal effect in the superficial tissue during irradiation. Class 4 lasers are known as high-power and hot lasers because they can produce rapid increases in superficial tissue temperatures when maximum permissible exposure limits are exceeded. Recent trends in laser therapy show a preference for class 4 lasers in patient care settings.5 Class 4 lasers can emit greater photonic energy in a shorter period of time than class 3B lasers without producing an appreciable rise in tissue temperature under normal treatment protocols.5 This higher power becomes important when treating injuries to deeper tissues such as ligaments, muscles, tendons, and cartilage.
Authors of most published clinical studies on laser therapy to treat musculoskeletal injuries have used class 3B low-power lasers. Several published reports6,7 have questioned the ability of low-power lasers to effectively transmit energy beyond the skin into deep musculoskeletal tissues. Excessive beam scattering and attenuation within the skin limit the potential bio- stimulative effects of laser in the deeper target tissues because of several factors related to dosimetry, such as subthreshold optical power, insufficient treatment durations, and varied treatment frequencies.8,9 Therefore, it is relevant and timely to study the dosimetric responses of specific infrared wavelengths of high-power class 4 lasers and their ability to modulate the physiologic effects that are conducive to healing.
Positive therapeutic effects of laser have been attributed to increased blood flow in soft tissues and, coincidentally, the limb.10–12 Maegawa et al10 showed that laser therapy induced arteriolar dilation and vastly improved blood flow in the micro-vascular bed of the rat mesentery. Moreover, Ihsan11 observed accelerated collateral circulation and increased microcirculation in rabbits exposed to laser therapy after femoral artery occlusion. Laser therapy–induced changes in blood flow and microcirculation promote healing by controlling ischemia, hypoxia, and edema after injury, thereby limiting the zone of secondary tissue damage.10,13 In addition, increased circulation in and around the injury zone creates a favorable environment for biological repair after musculoskeletal injury.11,13
An important factor associated with the therapeutic effectiveness of laser is dose.3,14,15 Achieving a therapeutic dose without understimulating or overstimulating the target tissues is often the most difficult component of clinical laser therapy practice.15 The Arndt-Schultz principle has been adopted from early toxicology studies of yeast culture to explain the optimal therapeutic dosage level of low-level laser. Optimal doses have been experimentally established in cell and tissue cultures. This therapeutic laser dosage or level of photostimulation must be attained; otherwise “no reactions or changes can occur in body tissues if the amount of energy absorbed is insufficient to stimulate the absorbing tissues.”16(379) This positive stimulatory effect of specific wavelengths of light on tissues has been called photobiomodulation. The threshold level of laser biostimulation for many human tissues is not known; however, results from cell and animal studies suggest that the therapeutic effects of laser are dose dependent and are most pronounced during the acute phase of injury. This initial therapeutic window may be within the first hours to days after soft tissue injury.14,17,18 These studies suggest that dose dependency also exists when treating humans, but except for superficial tissues and some dental applications, standard treatment doses for deep musculoskeletal tissues have yet to be firmly established in human clinical populations. Calculating an appropriate dose range for humans from studies using cell and animal models is challenging. No known transformation function can be used to calculate a comparable dose range in humans from those used in cell and animal models. Most authors have based dosage calculations on a 2-dimensional measurement (joules per square centimeter) of surface area exposure for laser. However, the actual dosage for deep tissues must take into consideration the 3-dimensional irregular Gaussian distribution of the scattered and absorbed laser beam. Without an adequate understanding of laser wavelength interactions with tissue chromophores, pigments, and tissue turbidity, this complex calculation can only be estimated. These factors dynamically influence the 3-dimensional pattern of absorption, scatter, reflectance, or transmittance of the laser beam in deep tissues. Therefore, our primary objective was to determine a therapeutic dose range using a class 4 laser to increase vasodilation and limb blood flow to the soft tissues of the forearm for treatment of the biceps brachii in humans.
METHODS
Participant Characteristics and Inclusion Criteria
Ten volunteers were recruited for participation in the study: healthy men of any racial or ethnic background (white = 8, Hispanic = 2), aged 18–35 years (age = 20.80 ± 2.16 years, height = 177.93 ± 3.38 cm, weight = 73.64 ± 9.10 kg). This age range represents a young yet mature study population readily available at our institution. Only men were recruited for this study to eliminate any influence certain phases of the menstrual cycle may have on vasoactivity.19 If a study candidate met the eligibility requirements and volunteered to participate, then he was randomly assigned to a treatment order group. Each group was given an identification code that linked the participant to the treatment order. Institutional review board approval was obtained before candidate recruitment. Written informed consent was obtained from all participants.
Exclusion Criteria
Candidates were excluded if they (1) had a recent injury (within 6 months) to the left upper extremity; (2) regularly participated in upper extremity weight training, defined as any weight (resistance) training more than once per week in the past 8 weeks; (3) took pain or prescription medication or nutritional supplements on a daily basis; (4) had any tattoos on or around the area of treatment; (5) had high blood pressure (greater than 140/90 mm Hg) or any known cardiovascular conditions (eg, coronary artery disease or peripheral arterial disease); or (6) had a history of any of the following conditions: malignant tumor in the upper extremity area, kidney or liver dysfunction, lupus or any other autoimmune disease, blood clots or phlebitis, anemia, skin allergies, severe muscle weakness, serious neurologic injury, or any disorder that might affect blood flow.
Experimental Design
A crossover, repeated-measures design was used to measure blood flow changes in the forearm before (baseline), during, and after a 4-minute laser treatment session. Participants were randomly assigned to 1 of 4 treatment order groups, and each group received the same 4 treatment doses in randomized order (Table). Each participant served as his own control (Figure 1). Laser treatments were administered during all 4 sessions, accompanied by venous occlusion plethysmography (VOP) measurements. The primary outcome measure for the experiment was percentage change in the circulation of the forearm (mL/min per 100 mL tissue). Data masking was performed to eliminate investigator, technician, and participant bias. The participant and VOP technician were blinded to the treatment order; the laser technician was blinded to the VOP measures obtained during and after treatments.
Table.
Dosing Protocol by Treatment a
| Output Power, W | Duty Cycle, % | Average Power/Treatment, W | Dose/Treatment, J |
| Sham | None | None | None |
| 1 | 50 | 0.5 | 180 |
| 3 | 50 | 1.5 | 360 |
| 6 | 50 | 3.0 | 720 |
aEach treatment lasted 4 minutes.
Figure 1.
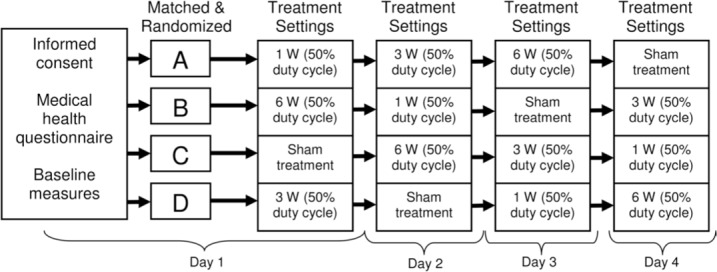
Experimental protocol.
Experimental Procedures
Participants were instructed not to consume any caffeine, alcohol, theophylline (found in tea leaves), or other substances that might affect pain perception or hydration status for 24 hours before each testing session.
All participants underwent 4 testing sessions on separate days, with 24 hours between sessions. Each participant completed all testing sessions at the same time of day to control for circadian rhythm. During the first session (day 1), participants read and signed the informed consent document; completed a brief demographic (eg, age, weight), medical health, and medical history questionnaire; and underwent random assignment to a treatment order group (Figure 1).
At each treatment session, the examiner placed an occlusion cuff on the participant's upper left arm and performed VOP (model EC-6; D.E. Hokanson, Inc, Bellevue, WA) using calibrated mercury strain gauges. A strain gauge was applied to the left forearm 5 cm below the antecubital space. The participant was instructed to lie supine for 20 minutes with the left arm passively elevated above the right atrium of the heart. This position was used to obtain stable baseline measurements of forearm blood flow (FBF). The wrist cuff was inflated to 200 mm Hg to occlude hand circulation during FBF measurements for the remainder of the study after the 20-minute baseline stabilization period. To measure FBF, the upper arm cuff was inflated to 40 mm Hg for 7 seconds every 15 seconds using a rapid cuff inflator to prevent venous outflow.20,21 Total occlusion time for the wrist cuff was 14 minutes. The FBF was measured continuously from baseline until 5 minutes after treatment.
All participants, along with the laser technician and VOP technician, wore safety eyewear (K-LaserUSA, Franklin, TN) to prevent eye damage from the laser light. The safety eyewear filtered laser light up to 1070 nm. All laser treatments were performed in a confined room; only those involved in the study were allowed in the room. In addition, a warning sign was hung on the door outside the room in which the laser treatments were performed to prevent unauthorized people from entering the room during laser applications.
Laser treatments were initiated once baseline measurements were taken and determined to be stable. A commercially available, US Food and Drug Administration–approved phototherapeutic device (K-LaserUSA) was used for all laser treatments. Laser treatments were performed using a light-touch grid pattern covering the muscle belly of the biceps brachii in order to achieve total energy absorption for the assigned treatment dose. Each grid point was treated for 3 to 4 seconds. The FBF was recorded by the plethysmograph during the laser treatment at 0 seconds, 2 minutes, and 4 minutes. Follow-up measurements were taken at 1, 2, 3, 4, and 5 minutes after treatment (Figure 2). All laser treatments were administered by the lead author (K.A.L.), who is a certified therapist for K-LaserUSA and completed a 4-hour course on laser safety and operational techniques.
Figure 2.
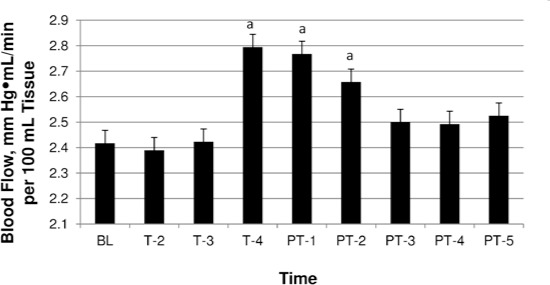
3-W dose response over time. Abbreviations: BL, baseline; T-2, mean during minute 2 of treatment; T-3, mean during minute 3 of treatment; T-4, mean during minute 4 of treatment; PT-1, mean at posttreatment minute 1; PT-2, mean at posttreatment minute 2; PT-3, mean at posttreatment minute 3; PT-4, mean at posttreatment minute 4; PT-5, mean at posttreatment minute 5.
a Significant increase in blood flow from baseline.
The FBF output signal was transmitted to the NIVP3 software program (D.E. Hokanson, Inc) loaded on a desktop computer. The FBF for each time point was calculated as the average of the first 3 plethysmographic measurements recorded during that minute.20,21 Forearm vascular resistance was calculated as the ratio of mean arterial pressure divided by FBF and expressed as mm Hg·mL/min per 100 mL tissue.
Statistical Analysis
All data analyses were performed using SPSS for Windows (version 16.0; SPSS Inc, Chicago, IL). A repeated-measures analysis of variance was used to assess changes in blood flow during laser treatments at 2, 3, and 4 minutes and 1, 2, 3, 4, and 5 minutes after treatment. We used the Huynh-Feldt test to adjust the degrees of freedom for potential violations of the sphericity assumption in the analysis of variance to produce more accurate significance (P) values. An α level of ≤.05 was the criterion for statistical significance.
RESULTS
The 3-W condition was the only treatment dose to show an increase in FBF from baseline over time (F3,9 = 3.468, P < .011). Increases in FBF occurred at minute 4 of treatment (2.417 ± 0.342 versus 2.794 ± 0.351 mL/min per 100 mL tissue, P = .032) and at 1 minute (2.417 ± 0.342 versus 2.767 ± 0.358 mL/min per 100 mL tissue, P < .01) and 2 minutes (2.417 ± 0.342 versus 2.657 ± 0.369 mL/min per 100 mL tissue, P = .022) post-treatment. At 3 minutes after treatment, FBF had returned to normal (Figure 2).
The sham, 1-W, and 6-W groups did not display any increases in FBF at any time point. However, the sham group demonstrated changes in FBF that mirrored those of the 1-W group (Figure 3), which may indicate an expectant placebo response during exposure to the sham laser treatment. Yet the 6-W group did not exhibit these changes, possibly because of overstimulation and suppression of physiologic responses (Figure 4).
Figure 3.
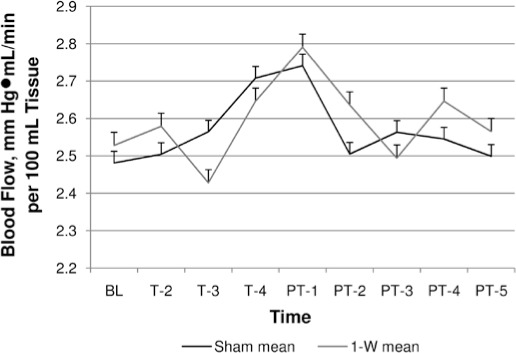
1-W versus sham dose response over time. Abbreviations: BL, baseline; T-2, mean during minute 2 of treatment; T-3, mean during minute 3 of treatment; T-4, mean during minute 4 of treatment; PT-1, mean at posttreatment minute 1; PT-2, mean at posttreatment minute 2; PT-3, mean at posttreatment minute 3; PT-4, mean at posttreatment minute 4; PT-5, mean at posttreatment minute 5.
Figure 4.
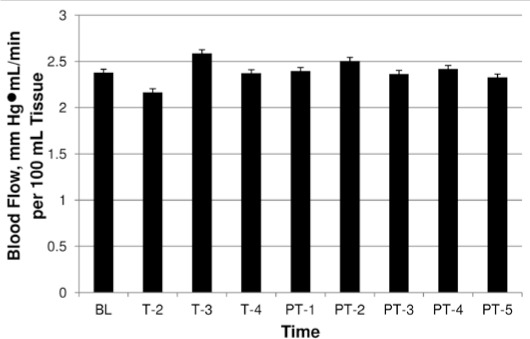
6-W dose response over time. Abbreviations: BL, baseline; T-2, mean during minute 2 of treatment; T-3, mean during minute 3 of treatment; T-4, mean during minute 4 of treatment; PT-1, mean at posttreatment minute 1; PT-2, mean at posttreatment minute 2; PT-3, mean at posttreatment minute 3; PT-4, mean at post-treatment minute 4; PT-5, mean at posttreatment minute 5.
DISCUSSION
Improved circulation is considered one of laser therapy's greatest contributions to soft tissue healing after injury.10–12 To the best of our knowledge, no authors have directly examined the effect of class 4 lasers in vivo on blood flow in the limb distal to the treatment area. Our study objective was to determine a therapeutic dose range using a class 4 laser to increase vaso-dilation and blood flow to the soft tissues in the forearm after treating the biceps brachii. We found a biphasic dose-response effect of laser therapy on FBF, with the 3-W protocol showing the greatest increase in FBF. The 3-W dosing protocol delivered 360 J of light energy over the 4-minute treatment period, whereas the 1-W dose delivered 180 J, and the 6-W dose delivered 720 J. Given this biphasic dose-response pattern, the 1-W dose was probably too weak to stimulate an increase in FBF; the 6-W dose was probably too strong and therefore suppressed any vasodilatory response.22
For the 3-W dose, increases in FBF from baseline levels were observed at minute 4 of treatment and persisted for up to 2 minutes after treatment. We did not observe any changes in blood flow for the 6-W, 1-W, or sham doses during or after laser treatment. Two factors that establish an effective treatment dose are optical output power (in watts) and energy delivery (in joules or watts over time). Optical power, or beam intensity, has a direct effect on light transmission through tissue and the depth of penetration.23 Beam intensities greater than 1 W greatly improve light transmission through soft tissues when compared with lower beam intensities (0.5 W).23 Therefore, class 4 lasers, using beam intensities greater than 1 W, may have an advantage over class 3 lasers in transmitting light to deeper tissues. In addition, using a laser with greater output power allows more light energy to be delivered to soft tissues in a shorter amount of time. Our results show that the combination of an output power of 3 W and an energy delivery of 360 J was adequate to induce a vascular response that improved FBF. The increased blow flow was transient, beginning at minute 4 of exposure and lasting 2 minutes after treatment. Short-term improvements in blood flow are helpful in therapeutic situations when cellular repair and edema control can facilitate healing as an adjunct with rehabilitative therapy. Improvements in blood flow may also aid in therapeutic exercise by delaying the onset of fatigue and improving muscle function, thereby allowing more work to be performed.24
Evidence from basic science research supports our findings. In studies in vivo, vascularized tissues exposed to laser displayed increased arteriolar dilation and increased micro-circulation.11 Nitric oxide (NO) signaling has been implicated as playing an important role in the process of light-induced changes to vascular structures. Maegawa et al10 and Chertok et al25 investigated the effect of laser therapy on rat mesenteric microcirculation in vivo. Laser therapy vastly improved vasomotor relaxation and arterial blood flow; however, these effects were completely abolished with prior inhibition of NO synthase activity.10,25 Similarly, Samoilova et al12 reported that human skin exposed to visible light showed increased micro-circulation in an NO synthase–dependent mechanism. Sources of NO in soft tissues include activated macrophages,26 vascular endothelial cells,11 and protein complexes such as hemoglobin, myoglobin, and cytochrome c oxidase.27,28 Activated macrophages and endothelial cells upregulate the synthesis of NO,11,26 whereas hemoglobin, myoglobin, and cytochrome c oxidase, once photonically stimulated, release NO via photolysis.27,29
Interestingly, the blow flow response for the sham and 1-W groups followed similar patterns of microcirculatory change during and after laser treatment. This finding may suggest a placebo response resulting from the participants' beliefs and expectation that the laser would modulate blood flow. Previous authors30 have shown that participants who believe they are receiving an active treatment display greater physiologic responses than those who believe they are not receiving an active treatment. In our case, the strength of the participants' beliefs that the laser modality would improve FBF may have caused a similar vascular response as the subthreshold 1-W dose. In addition, we did not observe a placebo response with the 6-W dose and believe this is due to overstimulation and subsequent suppression of the physiologic processes that would lead to an increase in FBF. These findings indicate the importance of blinding a patient's previous treatment outcomes as a way of controlling the placebo response.
We used VOP to analyze the responses of 4 treatment doses and the subsequent changes to FBF in real time. This technique is a sensitive and noninvasive method of measuring limb blood flow that has been used in humans for decades.20 For our study, the benefit of VOP was that it allowed us to measure FBF change in vivo noninvasively in combination with class 4 laser therapy. Although the biceps brachii muscle was our target organ for laser irradiation, FBF changes were measured as an outcome of downstream vascular responses.
Our results provide a scientific basis for establishing dosing guidelines for future studies. The ability to provide a biostimulative dose will be beneficial to future researchers studying the mechanistic effects of laser to better understand its benefits in the healing of musculoskeletal injuries. Ultimately, these new data will offer clinicians better dosing and treatment guidelines to use in health care practice settings.
Limitations and Future Studies
We did not directly monitor local tissue temperature changes during laser treatment; however, we suggest that the vascular responses were caused by photochemical changes in the tissues and were not due to thermal effects. Heating of the superficial tissue layers can be easily avoided by selecting and using a proper duty cycle and application technique. We used a 50% duty cycle and followed a grid pattern, so that each point on the skin was irradiated for a brief period of time.
Furthermore, we tested only healthy participants, as opposed to patients. Future authors should investigate the long-term effects and benefits of laser therapy in clinical populations with musculoskeletal conditions. In addition, we measured blood flow for a short time after laser treatment. It would be beneficial to measure blood flow changes for prolonged periods of time after treatment. Outcomes from such studies would provide great insight for developing appropriate clinical dosing protocols.
Information on tissue oxygenation and perfusion would also offer beneficial information. The use of a near-infrared spectrometer on the irradiated muscle tissue would allow us to measure tissue oxygenation and saturation levels of both hemoglobin and myoglobin. This information would supply additional mechanistic justification for appropriate dosing in both healthy and injured populations.
CONCLUSIONS
Our results show a dose-response effect of class 4 laser therapy in a human clinical model, similar to what has been observed in the animal model. Specifically, a 3-W dose, along with a 50% duty cycle, applied to the biceps brachii proved to be the most beneficial protocol for increasing FBF. This suggests that a properly designed laser treatment protocol with appropriate dosing guidelines is a viable therapy to increase limb blood flow. Laser therapy's ability to increase blood flow in a dose-response manner is therefore an effective, noninvasive treatment modality to improve blood flow and may promote tissue healing in the clinical setting.
REFERENCES
- 1.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martin RA. Photo-radiation in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006;24(2):158–168. doi: 10.1089/pho.2006.24.158. [DOI] [PubMed] [Google Scholar]
- 2.Laakso EL, Cabot PJ. Nociceptive scores and endorphin-containing cells reduced by low-level laser therapy (LLLT) in inflamed paws of Wistar rat. Photomed Laser Surg. 2005;23(1):32–35. doi: 10.1089/pho.2005.23.32. [DOI] [PubMed] [Google Scholar]
- 3.Bjordal JM, Couppe C, Chow RT, Tuner J, Ljunggren EA. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiother. 2003;49(2):107–116. doi: 10.1016/s0004-9514(14)60127-6. [DOI] [PubMed] [Google Scholar]
- 4.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Wood-ruff LD. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004;22(4):323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 5.Wertz RL. A higher power: therapeutic laser units feature drastically stronger intensity levels. Adv Phys Ther Rehabil Med. 2006;18(5):52. [Google Scholar]
- 6.Esnouf A, Wright PA, Moore JC, Ahmed S. Depth of penetration of an 850nm wavelength low level laser in human skin. Acupunct Electrother Res. 2007;32(1–2):81–86. doi: 10.3727/036012907815844165. [DOI] [PubMed] [Google Scholar]
- 7.Kolari PJ, Airaksinen O. Poor penetration of infra-red and helium neon low power laser light into the dermal tissue. Acupunct Electrother Res. 1993;18(1):17–21. 379. doi: 10.3727/036012993816357566. [DOI] [PubMed] [Google Scholar]
- 8.Douris P, Southard V, Ferrigi R. Effect of phototherapy on delayed onset muscle soreness. Photomed Laser Surg. 2006;24(3):377–382. doi: 10.1089/pho.2006.24.377. et al. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow PD, Hill ID, McKevitt AM, Lowe AS, Baxter D. Low intensity monochromatic infrared therapy: a preliminary study of the effects of a novel treatment unit upon experimental muscle soreness. Lasers Surg Med. 2001;28(1):33–39. doi: 10.1002/1096-9101(2001)28:1<33::AID-LSM1012>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Maegawa Y, Itoh T, Hosokawa T, Yaegashi K, Nishi M. Effects of near-infrared low-level laser irradiation on microcirculation. Lasers Surg Med. 2000;27(5):427–437. doi: 10.1002/1096-9101(2000)27:5<427::AID-LSM1004>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005;23(3):289–294. doi: 10.1089/pho.2005.23.289. [DOI] [PubMed] [Google Scholar]
- 12.Samoilova KA, Zhevago NA, Petrischev NN, Zimin AA. Role of nitric oxide in the visible light-induced rapid increase of human skin microcirculation at the local and systemic levels, II: healthy volunteers. Photomed Laser Surg. 2008;26(5):443–449. doi: 10.1089/pho.2007.2205. [DOI] [PubMed] [Google Scholar]
- 13.Leadbetter WB. Soft tissue athletic injury. In: Fu FH, Stone DA, editors. Sports Injuries: Mechanisms, Prevention, Treatment. Baltimore, MD: Lippincott Williams & Wilkins; 2001. pp. 839–888. In. eds. [Google Scholar]
- 14.Amaral AC, Parizotto NA, Salvini TF. Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci. 2001;16(1):44–51. doi: 10.1007/pl00011336. [DOI] [PubMed] [Google Scholar]
- 15.Bjordal JM, Baxter GD. Ineffective dose and lack of laser output testing in laser shoulder and neck studies. Photomed Laser Surg. 2006;24(4):533–534. [PubMed] [Google Scholar]
- 16.Starkey C. Therapeutic Modalities. 3rd ed. Philadelphia, PA: FA Davis; 2004. [Google Scholar]
- 17.Aimbire F, Albertini R, Pachecom T. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24(1):33–37. doi: 10.1089/pho.2006.24.33. et al. [DOI] [PubMed] [Google Scholar]
- 18.Ezzati A, Bayat M, Taheri S, Mohsenifar Z. Low-level laser therapy with pulsed infrared laser accelerates third-degree burn healing process in rats. J Rehabil Res Dev. 2009;46(4):543–554. doi: 10.1682/jrrd.2008.09.0121. [DOI] [PubMed] [Google Scholar]
- 19.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 20.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91(6):2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52(6):631–646. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parr JJ, Larkin KA, Borsa PA. Effect of class IV laser therapy on exercise-induced muscle injury in humans. Athl Train Sports Health Care. 2010;2(6):267–276. [Google Scholar]
- 24.Leal Junior EC, Lopes-Martin RA, Vanin AA. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009;24(3):425–431. doi: 10.1007/s10103-008-0592-9. et al. [DOI] [PubMed] [Google Scholar]
- 25.Chertok VM, Kotsyuba AE, Bespalova EV. Role of nitric oxide in the reaction of arterial vessels to laser irradiation. Bull Exp Biol Med. 2008;145(6):751–754. doi: 10.1007/s10517-008-0186-3. [DOI] [PubMed] [Google Scholar]
- 26.Klebanov GI, Kreinina MV, Poltanov EA, Khristoforova TV, Vladimirov YA. Mechanism of therapeutic effect of low-intensity infrared laser radiation. Bull Exp Biol Med. 2001;131(3):239–241. doi: 10.1023/a:1017643230376. [DOI] [PubMed] [Google Scholar]
- 27.Shiva S, Gladwin MT. Shining a light on tissue NO stores: near infrared release of NO from nitrite and nitrosylated hemes. J Mol Cell Cardiol. 2009;46(1):1–3. doi: 10.1016/j.yjmcc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Mio Y, Pratt PF. Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J Mol Cell Cardiol. 2009;46(1):4–14. doi: 10.1016/j.yjmcc.2008.09.707. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504(1):46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]


