Abstract
Context:
Conditions such as osteoarthritis, obesity, and spinal cord injury limit the ability of patients to exercise, preventing them from experiencing many well-documented physiologic stressors. Recent evidence indicates that some of these stressors might derive from exercise-induced body temperature increases.
Objective:
To determine whether whole-body heat stress without exercise triggers cardiovascular, hormonal, and extra-cellular protein responses of exercise.
Design:
Randomized controlled trial.
Setting:
University research laboratory.
Patients or Other Participants:
Twenty-five young, healthy adults (13 men, 12 women; age = 22.1 ± 2.4 years, height = 175.2 ± 11.6 cm, mass = 69.4 ± 14.8 kg, body mass index = 22.6 ± 4.0) volunteered.
Intervention(s):
Participants sat in a heat stress chamber with heat (73°C) and without heat (26°C) stress for 30 minutes on separate days. We obtained blood samples from a subset of 13 participants (7 men, 6 women) before and after exposure to heat stress.
Main Outcome Measure(s):
Extracellular heat shock protein (HSP72) and catecholamine plasma concentration, heart rate, blood pressure, and heat perception.
Results:
After 30 minutes of heat stress, body temperature measured via rectal sensor increased by 0.8°C. Heart rate increased linearly to 131.4 ± 22.4 beats per minute (F6,24 = 186, P < .001) and systolic and diastolic blood pressure decreased by 16 mm Hg (F6,24 = 10.1, P < .001) and 5 mm Hg (F6,24 = 5.4, P < .001), respectively. Norepinephrine (F1,12 = 12.1, P = .004) and prolactin (F1,12 = 30.2, P < .001) increased in the plasma (58% and 285%, respectively) (P < .05). The HSP72 (F1,12 = 44.7, P < .001) level increased with heat stress by 48.7% ± 53.9%. No cardiovascular or blood variables showed changes during the control trials (quiet sitting in the heat chamber with no heat stress), resulting in differences between heat and control trials.
Conclusions:
We found that whole-body heat stress triggers some of the physiologic responses observed with exercise. Future studies are necessary to investigate whether carefully prescribed heat stress constitutes a method to augment or supplement exercise.
Keywords: whole-body heat stress, HSP72, catecholamine, prolactin
Key Points.
Whole-body heat stress stimulated the sympathetic nervous system and led to some of the physiologic responses that have been observed with exercise.
Timely heat stress might serve as an adjunct to training for people who cannot exercise as needed because of age, injury, or chronic disease.
Regular exercise is a powerful nonpharmacologic treatment that can prevent and reduce the incidence of various age-related chronic diseases. However, only about 27% of the adult population in the United States engages in exercise at the recommended level to prevent chronic disease.1 Because of various injuries and disabilities (sports injury, osteoarthritis, spinal cord injury, aging), some people cannot participate in regular activity for extended periods. In addition, some athletes and soldiers need to acclimate to high-heat environments to perform safely. A common element of exercise that has gained attention is increased body temperature, leading to profuse sweating and the triggering of cell chaperones and hormones. The physiologic value of safely increasing body temperature in the absence of exercise is the focus of this investigation.
When cells are exposed to thermal stress, stress proteins called heat shock proteins (HSPs) are upregulated intracellularly, and they are thought to serve as molecular chaperones to prevent protein aggregation and help transport repair proteins.2 In addition to these well-characterized intracellular functions of HSPs, researchers have suggested that extracellular HSPs enhance the immune system.3 The most inducible and abundant, and therefore most studied, is HSP72, which was reclassified recently as HSPA1A.4 Although various stressors can trigger upregulation of HSP72, thermal stress appears to be one of the most effective stressors to increase the intracellular5 and extra-cellular6 concentrations of HSP72.
In humans, accumulating evidence has shown that intense exercise can increase extracellular HSP72.7,8 Associated with intense exercise is profuse sweating in response to the elevation in core body temperature. This raises the question of whether heat stress alone in the absence of exercise similarly triggers extracellular HSP72 in humans. Investigators have reported that the elevated extracellular HSP72 level with exercise is not attributed to passive release of intracellular HSP72 from exercising muscles.8 Instead, hepatosplanchnic organs were at least partly responsible for the active release of the HSP72 into the bloodstream, possibly for systemic use,7 indicating that mechanical stress is not necessary to increase the extracellular HSP72 level. Furthermore, Fleshner et al9 showed that psychological rather than physical stress could trigger the systemic release of HSP72 in animal models.
Researchers have reported that an increase in extracellular HSP72 due to exercise was much greater than that due to passive heating.6 However, they induced passive heat stress with water immersion, in which the head and face are not heated directly.6,10 Whole-body heat stress that includes the head and face (ie, heat stress chamber) might effectively modulate cardiovascular, hormonal, and protective chaperones (extracellular HSP72). For example, cardiovascular work increases to stabilize blood pressure during heat-induced skin vasodilation.11 Hormones related to stressful stimuli (eg, catecholamines and prolactin) also should increase in the circulating blood. Prolactin, which is one of these hormones, is an indirect measure of dopaminergic-serotonergic transmitters in the brain.12 The extent to which passive heat stress triggers a cascade of responses is the basis for this study. Therefore, the primary purpose of our study was to determine whether whole-body passive heat stress triggers cardiovascular (heart rate, blood pressure), hormonal (prolactin, catecholamines), and extracellular protein (HSP72) responses that commonly are reported during exercise. We hypothesized that whole-body heat stress would reproduce many of the responses observed with exercise. If passively increasing body temperature elicits many of the exercise-induced responses as hypothesized, whole-body heat stress might produce positive health adaptations during key periods of rehabilitation. Indeed, people who cannot exercise but need to maintain their fitness status (eg, injured athletes) might be able to use this as an alternative or supplemental intervention during key periods of recovery from injury.
METHODS
Participants
Twenty-five participants (13 men, 12 women; age = 22.1 ± 2.4 years, height = 175.2 ± 11.6 cm, mass = 69.4 ± 14.8 kg, and body mass index = 22.6 ± 4.0) were recruited for the study, but blood samples were collected only from a subset of 13 randomly selected participants (7 men, 6 women). Female participants were excluded if they were menstruating at the time of the study. No participants had known neurologic or cardiovascular diseases, and none engaged regularly in heat stress (sauna, hot tub). Each person participated in 2 sessions (no heat, heat) separated by at least 1 week, and the starting time of the experiments was similar within participants. The only difference between these sessions was the temperature used in the heat stress chamber (no heat = 26°C, heat = 73°C). We counterbalanced the order of participation in the sessions so that half of the participants had the no-heat (control) session and the other half had the heat session as their first session. Participants refrained from exhaustive exercise and consumption of alcohol and caffeine for 24 hours before sessions. Participants logged their dietary intakes and were encouraged to maintain their pre-enrollment caloric input for the 2 sessions. Thus, participants varied in their individual diets but demonstrated that their diets remained consistent during the study. All participants provided written informed consent, and the study was approved by the Institutional Review Board at the University of Iowa.
Instrumentation and Experimental Setup
Heat Stress Chamber.
We induced whole-body passive heat stress using a specially instrumented, custom-designed heat stress chamber (Saunatec, Inc, Cokata, MN). It was equipped with an access port so heart rate and blood pressure could be sampled without removing the participant from the chamber. The temperature was servo controlled via a sensor (Saunatec, Inc) placed at the head level of participants, who sat within 3 in (7.62 cm) of the interior ceiling. We also recorded temperature at face level using a second sensor (model MSR12; MSR Electronics GmbH, Henggart, Switzerland) to verify the accuracy of the measurement, which was 73° ± 1°C during the heat and 26.1° ± 1°C during the control sessions. This datalogger also recorded the rectal temperature. The humidity in the heat stress chamber was recorded and was low (10% relative humidity). Participants sat upright in the same position in the same heat stress chamber for 30 minutes for both sessions. They sat comfortably in the chamber with their backs resting on the wall and placed their feet comfortably on a lower seat (approximately 65 cm from the floor). After the 30-minute heat session, they sat on this lower seat for 3 minutes while drying their bodies. During the heat session, the face-level temperature at the lower seat was approximately 30°C lower than the temperature at the upper seat. The purpose of sitting on the lower seat was to avoid a sudden temperature change upon leaving the heat chamber. During the entire data collection session, participants were allowed to talk freely with the investigators through the glass window of the chamber. Although only 1 participant at a time sat in the chamber for the data collection, the chamber was spacious enough for 4 people to sit comfortably at the same time.
Body Temperature, Heart Rate, and Blood Pressure Measurements.
We measured heart rate and blood pressure in real time using a beat-by-beat finger arterial blood pressure monitor (model Ohmeda 2003; Finapres Medical Systems B.V., Amsterdam, The Netherlands). Participants placed their upper extremities in a fixed position on a wooden platform adjacent to the small access port. We dried the middle finger before the blood pressure cuff was applied. The analog signal from the device was digitized using commercially available software (Datapac 2K2 version 3.18; RUN Technologies Co, Mission Viejo, CA) with a 100-Hz sampling rate for 7 seconds. Heart rate and blood pressure were measured immediately and every 5 minutes after participants entered the heat stress chamber and for 10 minutes after they exited it.
We monitored rectal temperature using a thermistor probe (model B10014; MSR Electronics GmbH) on a subset of 13 participants. We inserted the rectal probe 10 cm beyond the anal sphincter and used the same datalogger to sample the rectal temperature every 30 seconds.
Blood Sample.
For both trials, we collected venous blood samples from a vein in the forearm in prechilled, 6-mL tubes containing ethylenediaminetetraacetic acid before and immediately after the 30-minute session while participants were sitting quietly. We measured the hormone (norepinephrine [NE], epinephrine [EPI], prolactin [PRL]) and HSP72 concentrations in the plasma of the circulating blood. To assess the variability of plasma concentrations without heat stress, we took 2 samples separated by 30 minutes before participants entered the heat stress chamber. Using paired t tests, we found no differences in any of the hormones and HSP72 for repeated blood samples taken consecutively (Table 1). Intraclass correlation coefficients showed strong concordance between the 2 consecutive samples (Table 1). The effect of posture on plasma volume13 is believed to be negligible because an upright seated position was used for the blood draws and the heat stress intervention, and enough time (approximately 10 minutes) was given in the seated position before each blood draw.
Table 1.
Assessment of First and Second Blood Samples Taken Before Heat Stress
| Concentration | Blood Sample |
t1,12 Valuea | P Value | Intraclass Correlation Coefficient | |
| First | Second | ||||
| Norepinephrine, nmol/L | 3.12 ± 1.71 | 2.99 ± 2.0 | 0.56 | .62 | 0.94 |
| Epinephrine, pmol/L | 228.0 ± 141.2 | 239.4 ± 145.1 | 0.76 | .51 | 0.92 |
| Prolactin, pmol/L | 318.0 ± 212.6 | 342.1 ± 220.8 | 1.03 | .37 | 0.97 |
| Extracellular heat shock protein, ng/mL | 2.5 ± 1.2 | 2.3 ± 0.7 | 0.86 | .46 | 0.99 |
aIndicates that paired t tests showed no differences. All t statistics are absolute values.
Subjective Ratings of Thermosensation and Pain.
Participants rated their comfort, thermosensation,14 and pain.15,16 The comfort scale ranged from 1 (comfortable) to 4 (very uncomfortable), and the thermosensation scale ranged from 1 (cold) to 7 (hot), with 4 indicating neutral. The pain scale ranged from 0 (no pain at all) to 10 (worst pain imaginable). Participants provided a whole number associated with their subjective ratings before, immediately on entering the heat stress chamber, and every 5 minutes after they entered the chamber.
Experimental Procedure
When they arrived at the laboratory, participants were weighed without clothes, then dressed in bathing suits. We drew blood samples and recorded subjective ratings before participants entered the heat stress chamber. After the participants were seated in the chamber, we recorded heart rate, blood pressure, and subjective ratings every 5 minutes for 30 minutes. After the last measurements, participants sat on the lower seat for 3 minutes and dried their bodies before they left the chamber. After they left the heat stress chamber, we instructed them to remove their bathing suits, weighed the participants, and drew blood samples again. Heart rate and blood pressure were monitored at 5 and 10 minutes after heat stress. Participants drank as needed after all blood work was completed.
Data Reduction and Analysis
We determined systolic blood pressure (SBP) and diastolic blood pressure (DBP) by taking the maximal and minimal peaks in each cardiac cycle, respectively. Heart rate was determined by calculating the time between 2 consecutive maximal peaks.
We centrifuged the blood samples at 2300g for 10 minutes at 4°C, and the separated plasma was stored at −70°C until analyzed. We measured NE, EPI, and PRL concentrations using a radioimmunoassay technique (Skybio Ltd, Bedford, UK). All hormone analyses from a single participant were carried out in the same assay batch. We measured HSP72 using the HSP72 high-sensitivity enzyme immunometric assay (EIA) kit (model EKS 715; Assay Designs Inc, Ann Arbor, MI). All analyses were conducted in triplicate, and the samples were thawed only once in the analysis process.
Statistical Analysis
Two-way, repeated-measures analyses of variance (ANOVAs) were used to assess whether time and intervention had effects on the dependent variables. When repeated-measures ANOVAs are used, intrasubject relative change determines a difference, which is independent from between-subjects variability (standard deviation). The α level was set at .05 for all statistical analyses. The standard deviation was used to present variability. Because no sex differences were found in any dependent variable obtained (P > .05), we present only pooled data.
RESULTS
Core Body Temperature
We found a main effect of time (F6,24 = 500.5, P < .001). As expected, the rectal temperature started increasing at about 10 minutes, and the increase continued until about 10 minutes after the heat sessions. The total increase in the rectal temperature ranged from 0.71°C to 1.20°C. The average increase was 0.82°C (38.50° ± 0.27°C).
Heart Rate and Blood Pressure
We found main effects of time for heart rate (F6,24 = 186, P < .001), SBP (F6,24 = 10.1, P < .001), and DBP (F6,24 = 5.4, P < .001). Heart rate showed a near linear increase with time during the heat stress sessions. By the end of the session, heart rate increased to 131.4 ± 22.4 beats per minute. The SBP and DBP decreased with time during heat stress by 16 and 5 mm Hg, respectively (Figure 1).
Figure 1.
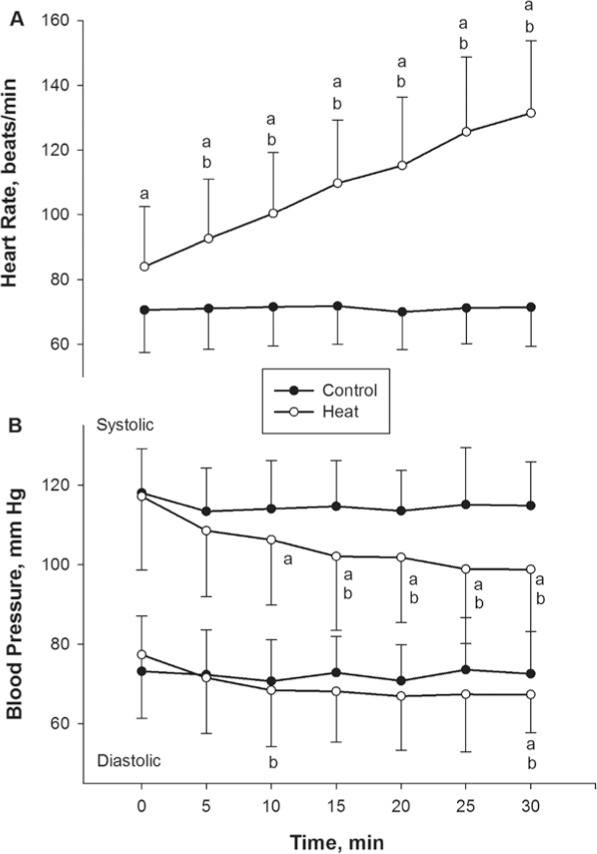
Changes in A, heart rate, and B, blood pressure, during heat and control sessions (n = 25 for both). The heart rate increased, whereas systolic blood pressure (top pair of lines) and diastolic (bottom pair of lines) blood pressure decreased during the heat session. a Indicates difference between heat and control sessions (P < .01). b Indicates difference from 0 minutes within sessions (P < .01).
Hormonal and HSP72 Changes
The coefficients of variation for the repeated analyses for the hormonal and HSP72 concentrations were 4%, 6%, 2%, and 8% for NE, EPI, PRL, and HSP72, respectively. The preheat (baseline) and postheat concentrations of the hormones and HSP72 are shown in Table 2. The NE (F1,12 = 12.1, P = .004), PRL (F1,12 = 30.2, P < .001), and HSP72 (F1,12 = 44.7, P < .001) concentrations increased from baseline levels after the heat stress. Although the heat stress increased the average EPI level, the heat-induced change in the EPI concentration was not different (F1,12 = 2.97, P = .11) because of variation across participants (range, 21% decrease to 396% increase).
Table 2.
Hormone and Extracellular Heat Shock Protein Concentrations Before and After Heat Stress
| Concentration | Before Heat Stress | After Heat Stress |
| Norepinephrine, nmol/L | 3.22 ± 1.43 | 4.54 ± 1.97a |
| Epinephrine, pmol/L | 236.4 ± 128.9 | 388.2 ± 1818.7 |
| Prolactin, pmol/L | 326.1 ± 182.6 | 1117.4 ± 513.9a |
| Extracellular heat shock protein, ng/mL | 2.7 ± 2.1 | 3.8 ± 2.2a |
aIndicates that concentration was greater after than before heat stress (P < .01).
Subjective Ratings and Weight Loss
We found a main effect of time (F1,12 = 24.2, P < .001). Participants felt that sitting in the heat stress chamber for 30 minutes at 73°C was hot and moderately uncomfortable by the end of the session, which was confirmed by the thermosensation and comfort levels reaching 6.8 ± 0.4 (7 indicated hot) and 2.6 ± 0.9 (3 indicated uncomfortable) (Figure 2). However, no one needed to leave the stress chamber before the 30-minute heat session ended. Three participants felt the heat was painful, but the highest rating observed was 2 on the scale of 0 to 10 (data not shown). No participant felt that sitting in the heat stress chamber for the control trial was stressful, which was confirmed by no change from the comfort scale of 3 throughout the trial (Figure 2). Body mass was reduced through dehydration by 0.5 ± 0.2 kg after the heat stress trial (F1,12 = 13.2, P = .01).
Figure 2.
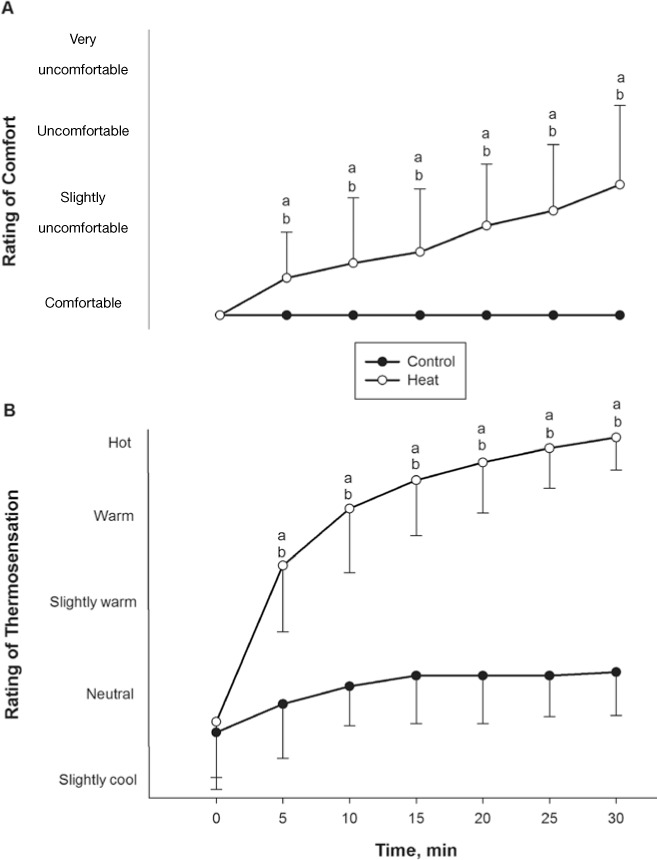
Subjective ratings for A, comfort, and B, thermal sensations, during heat and control sessions (n = 25). During heat stress, participants felt slightly uncomfortable and hot. The scales for A and B are both discrete and noncontinuous (P < .01).
a Indicates difference between heat and control sessions. b Indicates difference from baseline within sessions (P < .01).
DISCUSSION
We systematically investigated the effect of whole-body heat stress on physiologic responses that included cardiovascular, hormonal, and stress protein factors in humans. A dose of heat stress that triggered an increase in heart rate up to 60% of age-predicted maximal heart rate in young, healthy people was associated with increases in the extracellular protective chaperone HSP72 and other endocrine factors.
Cardiovascular Responses
Increased body temperature is a form of stress that stimulates the sympathetic nervous system17 and therefore induces various physiologic responses, the most obvious of which is cardiovascular response. The increase in heart rate with heat stress was consistent with previous findings.18 Researchers have shown that during heat stress, peripheral vascular resistance is reduced and blood volume shifts from the central body to the periphery to facilitate heat exchange.19 The increase in heart rate is compensatory to avoid a large drop in mean arterial blood pressure so cardiac output can be maintained even with a reduced stroke volume. The decrease we found in SBP and DBP also was consistent with findings in previous heat stress studies.19,20 The reduction in blood pressure observed with heat corresponds to about an 8-mm Hg reduction in mean arterial blood pressure estimated using the equation mean arterial pressure ≈ DBP + 1/3(SBP – DBP) for the resting condition and the equation 1/2(SBP + DBP) for the end of heat stress.21 We used different equations for the 2 conditions to account for the change in shape of arterial pulse pressure with an increase in heart rate.21 The estimated reduction we found is very close to the value reported using an intra-arterial technique (10 mm Hg).20 Vuori20 suggested that the workload of the heart is less with whole-body heat stress than with exercise despite the same increase in the heart rate. This reduced workload during heat stress occurs because blood pressure does not increase as it does with exercise,22 suggesting that appropriately dosed whole-body heat stress might offer an option besides exercise for health benefits even for people with cardiac conditions. Heart rate increased to about 130 beats per minute on average by the end of heat stress, and this corresponds to about 66% of the age-predicted maximal heart rate calculated using the equation 220 – age. A stimulus that increases heart rate up to 66% of the age-predicted maximal heart rate is considered moderate if induced by exercise.23
Endocrine Responses
We found that whole-body heat stress increased NE but not EPI concentration. Our finding is in line with findings of a previous study24 in which heat load added to exercise increased the release of NE but not EPI. Based on our findings and previous evidence, NE might be a better index than EPI to quantify the extent of whole-body heat stress. Although Kappel et al25 reported that NE and EPI increased with passive heating in humans, this discrepancy might be explained by the method (heat stress chamber versus water immersion) and the resultant rectal temperature (38.5°C versus 39.5°C) for our study.25 The important role of NE in blood pressure control (homeostatic reflexes) has been shown by an increase in NE secretion when simply changing from the recumbent to erect position.26
The PRL increase was greater (close to a 4-fold increase) than increases observed in catecholamines. The release of PRL is very sensitive to heat stress, requiring only a 0.25°C increase in rectal temperature.18 The greater than 0.8°C increase in rectal temperature in our study appeared to offer a very strong stimulus for PRL release.18 Prolonged exercise has been shown to increase the concentration of PRL in the circulating blood in an intensity-dependent manner.27 This increase in PRL is explained mainly by the exercise-induced increase in core (rectal) body temperature.28 The effect of increased core temperature on PRL release was further supported by Brisson and colleagues,29 who found no increase in PRL even after a 45-minute bicycle exercise at a workload of 65% maximal oxygen consumption in a cold environment (10°C). Prolonged exercise capacity is reduced markedly if the ambient temperature is high enough to trigger PRL release.28 In fact, the heat-induced increase of PRL with exercise or heat stress has been used as an indirect marker of serotonergic activity12 and central fatigue.28 Serotonergic and dopaminergic neurotransmitter pathways in the brain have been implicated as contributing to central fatigue during prolonged exercise.30
Extracellular HSP72 Response
Extracellular HSP72 increased after heat stress. Researchers have suggested that catecholamines (EPI in humans31 and NE in animals9) mediate the release of HSP72 in response to stress. If catecholamines influence the release of HSP72, as suggested by other investigators,31 it probably varies depending on the associated alternative stressors that might occur simultaneously. Humans probably have a genetic predisposition for HSP72 induction,31 necessitating further research on this topic.
The extent of the increase in HSP72 release in our study (49%) was comparable with that previously seen with water immersion (approximately 50%),6 although the dose of heat stress as assessed by rectal temperature was smaller (0.82°C increase in our study versus 2.3°C for water immersion).6 This difference could be caused partly by the variance between heat conduction using a rectal thermistor in water compared with whole-body heat stress in a chamber with low humidity.32 Taken together, these findings indicate that the whole-body heat stress we used is an adequate trigger for HSP72 release.33–35
Although we observed an increase in HSP72 level that was comparable with that of other passive heat stress methods (water immersion),6 the increase was much smaller than the exercise-induced increase in HSP72 level.6 The role of elevated extracellular HSP72 level in humans and the extent to which the HSP72 level must be elevated to trigger some responses to the level at which they become clinically and physiologically meaningful still is unclear. Further studies are necessary to determine whether a threshold or even a range of elevated HSP72 levels is meaningful for health.
CONCLUSIONS
Whole-body heat stress (sitting in a heat stress chamber for 30 minutes at 73°C) was well tolerated by young, non–heat-acclimated participants. However, the dose of heat (0.82°C increase in the core body temperature) was intense enough to stimulate the sympathetic nervous system and led to many of the physiologic responses observed with exercise, including increased heart rate to 66% of age-predicted maximum. Although still unclear, new evidence suggests that increasing the extracellular HSP72 level in humans might trigger a stress adaptation response that is beneficial. More studies are necessary to understand the role of extracellular HSP72 in humans with various injuries, disabilities, and ages. Exercise clearly offers the most substantiated benefits for overall health,6–8,31 but our results raise a possibility that timely heat stress might serve as an adjunct to athletic activity for people who cannot exercise to the extent they need because of age, injury, or chronic disease. Studies are under way to determine the longitudinal adaptive responses to heat stress in people with acute and chronic injury.
Acknowledgments
This work was supported in part by the Roy J. Carver Foundation, the National Institutes of Health, and the United States Department of Veterans Affairs.
REFERENCES
- 1.Stewart KJ. Physical activity and aging. Ann N Y Acad Sci. 2005;1055:193–206. doi: 10.1196/annals.1323.029. [DOI] [PubMed] [Google Scholar]
- 2.Kopecek P, Altmannova K, Weigl E. Stress proteins: nomenclature, division and functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145(2):39–47. doi: 10.5507/bp.2001.010. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Whittall T, McGowan E. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174(6):3306–3316. doi: 10.4049/jimmunol.174.6.3306. et al. [DOI] [PubMed] [Google Scholar]
- 4.Kampinga HH, Hageman J, Vos MJ. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol Cell. 1998;2(1):101–108. doi: 10.1016/s1097-2765(00)80118-5. [DOI] [PubMed] [Google Scholar]
- 6.Whitham M, Laing SJ, Jackson A, Maassen N, Walsh NP. Effect of exercise with and without a thermal clamp on the plasma heat shock protein 72 response. J Appl Physiol. 2007;103(4):1251–1256. doi: 10.1152/japplphysiol.00484.2007. [DOI] [PubMed] [Google Scholar]
- 7.Febbraio MA, Ott P, Nielsen HB. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544(pt 3):957–962. doi: 10.1113/jphysiol.2002.025148. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum HSP72 in humans. Cell Stress Chaperones. 2001;6(4):386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleshner M, Campisi J, Amiri L, Diamond DM. Cat exposure induces both intra- and extracellular HSP72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29(9):1142–1152. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Mundel T, Bunn SJ, Hooper PL, Jones DA. The effects of face cooling during hyperthermic exercise in man: evidence for an integrated thermal, neuroendocrine and behavioural response. Exp Physiol. 2007;92(1):187–195. doi: 10.1113/expphysiol.2006.034934. [DOI] [PubMed] [Google Scholar]
- 11.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Bridge MW, Weller AS, Rayson M, Jones DA. Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol. 2003;89(5):451–459. doi: 10.1007/s00421-003-0800-z. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgrim C, Pottgiesser T, Robinson N, Sottas PE, Ruecker G, Schumacher YO. Are 10 min of seating enough to guarantee stable haemoglobin and haematocrit readings for the athlete's biological passport? Int J Lab Hematol. 2010;32(5):506–511. doi: 10.1111/j.1751-553X.2009.01213.x. [DOI] [PubMed] [Google Scholar]
- 14.Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res. 1967;1(1):1–20. doi: 10.1016/0013-9351(67)90002-3. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson AM. Assessment of chronic pain, I: aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 16.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175–184. [PubMed] [Google Scholar]
- 17.Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15(5):505–507. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- 18.Mundel T, Hooper PL, Bunn SJ, Jones DA. The effects of face cooling on the prolactin response and subjective comfort during moderate passive heating in humans. Exp Physiol. 2006;91(6):1007–1014. doi: 10.1113/expphysiol.2006.034629. [DOI] [PubMed] [Google Scholar]
- 19.Luurila OJ. The sauna and the heart. J Intern Med. 1992;231(4):319–320. doi: 10.1111/j.1365-2796.1992.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 20.Vuori I. Sauna bather' s circulation. Ann Clin Res. 1988;20(4):249–256. [PubMed] [Google Scholar]
- 21.Klabunde RE. Cardiovascular Physiology Concepts. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 44–72. [Google Scholar]
- 22.Luurila OJ. Arrhythmias and other cardiovascular responses during Finnish sauna and exercise testing in healthy men and post-myocardial infarction patients. Acta Med Scand Suppl. 1980;641:1–60. [PubMed] [Google Scholar]
- 23.Gibbons RJ, Balady GJ, Beasley JW. ACC/AHA guidelines for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30(1):260–311. doi: 10.1016/s0735-1097(97)00150-2. et al. [DOI] [PubMed] [Google Scholar]
- 24.Powers SK, Howley ET, Cox R. A differential catecholamine response during prolonged exercise and passive heating. Med Sci Sports Exerc. 1982;14(6):435–439. doi: 10.1249/00005768-198206000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kappel M, Stadeager C, Tvede N, Galbo H, Pedersen BK. Effects of in vivo hyperthermia on natural killer cell activity, in vitro proliferative responses and blood mononuclear cell subpopulations. Clin Exp Immunol. 1991;84(1):175–180. doi: 10.1111/j.1365-2249.1991.tb08144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundin T. The effect of body posture on the urinary excretion of adrenaline and noradrenaline. Acta Med Scand Suppl. 1958;336:1–59. [PubMed] [Google Scholar]
- 27.Luger A, Watschinger B, Deuster P, Svoboda T, Clodi M, Chrousos GP. Plasma growth hormone and prolactin responses to graded levels of acute exercise and to a lactate infusion. Neuroendocrinology. 1992;56(1):112–117. doi: 10.1159/000126912. [DOI] [PubMed] [Google Scholar]
- 28.Pitsiladis YP, Strachan AT, Davidson I, Maughan RJ. Hyperprolactinaemia during prolonged exercise in the heat: evidence for a centrally mediated component of fatigue in trained cyclists. Exp Physiol. 2002;87(2):215–226. doi: 10.1113/eph8702342. [DOI] [PubMed] [Google Scholar]
- 29.Brisson GR, Peronnet F, Perrault H, Boisvert P, Massicotte D, Gareau R. Prolactinotrophic effect of endogenous and exogenous heat loads in human male adults. J Appl Physiol. 1991;70(3):1351–1355. doi: 10.1152/jappl.1991.70.3.1351. [DOI] [PubMed] [Google Scholar]
- 30.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29(1):45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Whitham M, Walker GJ, Bishop NC. Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol. 2006;101(4):1222–1227. doi: 10.1152/japplphysiol.00409.2006. [DOI] [PubMed] [Google Scholar]
- 32.Craig AB, Jr, Dvorak M. Thermal regulation during water immersion. J Appl Physiol. 1966;21(5):1577–1585. doi: 10.1152/jappl.1966.21.5.1577. [DOI] [PubMed] [Google Scholar]
- 33.Haak J, Kregel KC. 1962–2007: a cell stress odyssey. Novartis Found Symp. 2008;291:3–22. 137–140. doi: 10.1002/9780470754030.ch2. [DOI] [PubMed] [Google Scholar]
- 34.Whitham M, Fortes MB. Effect of blood handling on extracellular HSP72 concentration after high-intensity exercise in humans. Cell Stress Chaperones. 2006;11(4):304–308. doi: 10.1379/CSC-212.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Front Biosci. 2008;13(4):1328–1339. doi: 10.2741/2765. [DOI] [PubMed] [Google Scholar]


