Abstract
Objective:
To describe a case of exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion.
Background:
A healthy man (19 years old) participated in full-contact football practice followed by conditioning (2.5 hours). After practice, he entered a coach-mandated post-practice cold-water immersion and had no signs of heat illness before developing leg cramps, for which he presented to the athletic training staff. After 10 minutes of repeated stretching, massage, and replacement of electrolyte-filled fluids, he was transported to the emergency room. Laboratory tests indicated a creatine kinase (CK) level of 2545 IU/L (normal range, 45–260 IU/L), CK-myoglobin fraction of 8.5 ng/mL (normal < 6.7 ng/mL), and CK-myoglobin relative index of 30% (normal range, 25%– 30%). Myoglobin was measured at 499 ng/mL (normal = 80 ng/mL). The attending physician treated the athlete with intravenous fluids.
Differential Diagnosis:
Exercise-associated muscle cramps, dehydration, exertional rhabdomyolysis.
Treatment:
The patient was treated with rest and rehydration. One week after the incident, he began biking and swimming. Eighteen days later, the patient continued to demonstrate elevated CK levels (527 IU/L) but described no other symptoms and was allowed to return to football practice as tolerated. Two months after the incident, his CK level remained high (1900 IU/L).
Uniqueness:
The athlete demonstrated no signs of heat illness upon entering the cold-water immersion but experienced severe leg cramping after immersion, resulting in a diagnosis of exertional rhabdomyolysis. Previously described cases have not linked cold-water immersion with the pathogenesis of rhabdomyolysis.
Conclusions:
In this football player, CK levels appeared to be a poor indicator of rhabdomyolysis. Our patient demonstrated no other signs of the illness weeks after the incident, yet his elevated CK levels persisted. Cold-water immersion immediately after exercise should be monitored by the athletic training staff and may not be appropriate to prevent muscle damage, given the lack of supporting evidence.
Keywords: heat illnesses, thermoregulation, creatine kinase, myoglobin
Exertional rhabdomyolysis is a condition in which the breakdown of muscular tissues secondary to damage or overexertion causes renal failure.1–5 Rhabdomyolysis can also result from extreme crushing injuries (as in a car crash) or disease or drug toxicity, but these causes are typically not observed in athletes.1–5 Exertional rhabdomyolysis has been noted to occur with muscle activity, temperature extremes, and muscle ischemia.6 During activities that result in muscle fatigue, adenosine triphosphate (ATP) cannot supply the demand; therefore, cellular energy supplies are insufficient.6 The cellular destruction associated with rhabdomyolysis occurs much faster in extreme heat; thus, concomitant heat illnesses are likely.6 A number of reports1,2,7–13 have described muscle cramping cases in association with rhabdomyolysis in military personnel, football players, and those participating in intense physical exertion.
Regardless of the cause, the common denominator is the disruption of sarcolemma and intracellular myocyte components.3–6 The cellular destruction in rhabdomyolysis may include cell membrane breakdown, muscle cell hypoxia, ATP depletion, and electrolyte disturbances.3–6 Cell membrane destruction ultimately leads to imbalance of sodium and potassium levels, which may contribute to increased concentrations of myoglobin and creatine kinase (CK).3–6 Myoglobin and CK serve as the biochemical markers for a clinical diagnosis of rhabdomyolysis; in combination with acute renal failure, they are the best indicators that the condition is present.14 Myoglobin has a much faster elimination rate than does CK (half-life of 2 to 3 hours15), especially when patients are treated with alkaline diuresis.14 Therefore, in acute situations (within 6 to 8 hours of onset), myoglobin may be the more sensitive marker of rhabdomyolysis.15 The half-life of CK is far longer (ranging in the literature between 3615 and 7216 hours). Left untreated, the high levels of CK and myoglobin can lead to kidney failure and death.3–6 Although many cases of exertional rhabdomyolysis have been reported in the literature, this case is unique in that the CK levels remained elevated and may have falsely indicated the presence of exertional rhabdomyolysis. Although the myoglobin level was elevated (greater than 80 ng/mL), neither the physician nor the laboratory deemed the postinjury elevations to be of concern.
CASE REPORT
Personal Data and Chief Complaint
On the third day of preseason practice (August 9), a 19-year-old African American male National Collegiate Athletic Association Division I football player at a midwestern university, in good health with no personal history or family history of heat illness or conditions, presented with leg cramps. The patient had no personal or family history of diabetes. The medical history provided no indication of sickle cell testing before participation, so the patient's sickle cell trait status was unknown. He was a nonsmoker, was not ingesting any medication or supplements, and had not consumed alcohol. The patient had no signs or symptoms of heat illness before the initial complaint of cramps. Although body mass loss was recorded between prepractice and postpractice weighins, no substantive or cumulative weight loss was demonstrated in the 3 previous days of practice. The patient was in good physical shape, having participated in summer conditioning consisting of daily weight training and running drills outside. Environmental conditions for the first 3 days of practice were a temperature of 60° to 68°F (15.6° to 20.0°C) with high relative humidity (87% to 93%). During the practice in question, the air temperature rose from 74.7°F (23.7°C) at 7:00 am to 82.9°F (28.3°C) at 9:30 am. The athletes began practice in full pads and concluded the session with conditioning activities. The patient's leg cramps began after a mandatory daily postpractice 60°F (15.6°C) cold-water immersion imposed by the coach for recovery purposes, not heat illness prevention or treatment. The patient submerged his lower extremities and trunk (to just above the navel) in the cold water. He had participated in the mandatory daily cold water immersion during the 3 days of practice with no remarkable signs or symptoms. Approximately 10 minutes after the end of practice, the patient presented to the athletic trainer with cramping that started in his left quadriceps muscle and then moved to his left hamstrings, right quadriceps, and right hamstrings. The cramping continued to progress to his lower legs. The athletic training staff treated him with stretching, massage, and rehydration, with a carbohydrate-electrolyte drink containing extra electrolytes (Gatorlytes; The Gatorade Company, Chicago, IL) for approximately 10 minutes before he was transported to the emergency department. The decision to transport was made when the pain and cramping failed to subside and progressed to the patient's low and mid-back.
Physical Examination and Medical History
The team physician was on call in the emergency department and therefore present when the patient arrived. A history and physical examination were conducted. Although, no additional history information was uncovered, the patient complained of continued cramping and muscular fatigue. The physical examination indicated an alert and oriented patient who was not in cardiorespiratory distress. The cardiovascular examination was normal, and no tenderness or edema was evident. Initial laboratory tests indicated a CK level of 2545 IU/L (normal range, 0–190), CK-myoglobin (CK-MB) fraction of 8.5 ng/mL (normal < 6.7 ng/mL), and CK-MB relative index of 30% (normal range, 25%–30%). Myoglobin was measured at 499 ng/mL (normal = 0–149). Urinary pH was 7 (normal range, 4.6–8.0), urinary myoglobin was <1 mg/dL (normal = 0 mg/dL), and no myoglobinuria (dark, cola-colored urine) was present. Although other laboratory blood tests were minimally outside the normal range (Table), they were deemed unremarkable by the physician, and the patient was diagnosed with rhabdomyolysis.
Table.
Laboratory Results Over a 5-Day Period
| Biochemical Marker | Date and Time |
Normal Limitsa | ||||
| August 9, 2:34 pm | August 9, 6:55 pm | August 9, 11:30 pm | August 10, 5:05 am | August 14, 1:17 pm | ||
| Creatine kinase, IU/L | 2545 | 2352 | 2661 | 2668 | 999 | 0–190 |
| Myoglobin, ng/mL | 499 | 680 | 354 | 190 | 118 | 0–149 |
| Potassium, mEq/L | 3.2 | 4.2 | 4 | 4.5 | 4.4 | 3.3–5.3 |
| Total carbon dioxide content, mEq/L | 23 | 27.7 | 26.7 | 29.2 | 29.8 | 23.0–29.0 |
| Chloride, mEq/L | 95 | 106 | 106 | 108 | 99 | 100–112 |
| Glucose, mg/dL | 180 | 83 | 97 | 87 | 98 | 70–125 |
| Creatinine, mg/dL | 1.6 | 1.2 | 1.1 | 1 | 1.1 | 0.5–1.4 |
| Calcium, mg/dL | 10.7 | 8.7 | 9 | 8.9 | 10.5 | 8.5–10.5 |
a Provided by the laboratory for the general population.
Course of Treatment
The patient was hospitalized overnight and hydrated with normal isotonic saline with 20 mEq of potassium chloride at 200 mL/h. Early evening blood tests revealed a slightly reduced CK level but an increased myoglobin level (Table). A test for sickle cell disorder was negative. By the next day, the CK level had increased, but the myoglobin level had declined; urinary myoglobin was normal. The patient was allowed to return home on August 10 and was instructed to eat a regular diet and participate in activities as tolerated. The athletic trainer monitored the patient's symptoms daily and scheduled a follow-up appointment with the team physician on August 14.
On August 14, the patient's CK level was still elevated but had decreased greatly. Urinalysis results remained normal, and myoglobin level had returned to normal. The team physician indicated the need for continued hydration and suggested swimming and biking as tolerated. On August 17, the patient's CK level rose again and continued to fluctuate when checked on August 19 and 21 (Figure). The patient was instructed to continue a regular hydration regimen and to participate in football activities as tolerated. On August 31, the CK level was lower, and it decreased each successive week afterward. However, the myoglobin level increased acutely and then trended down to remain within normal limits by August 15.
Figure.
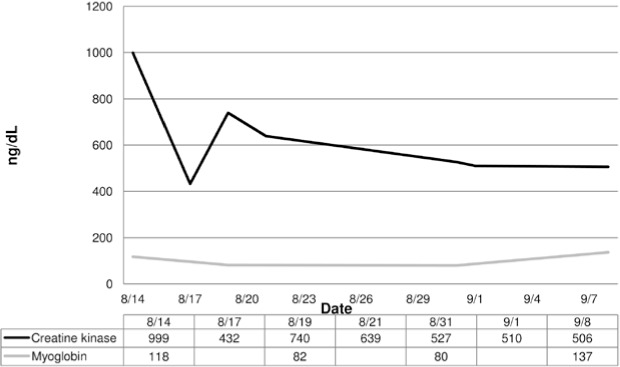
Patient's serum creatine kinase and myoglobin levels in the month after rhabdomyolysis was diagnosed. The myoglobin level was not measured on 8-17, 8-21, and 9-1. Note: We converted the creatine kinase concentrations to IU/L so that these could be shown on the same scale as the myoglobin values.
Six weeks after the incident, given the dissipation of symptoms and a normal myoglobin level, the physician released the patient for unrestricted participation. Two months after the incident, on the morning after a full practice, the patient's CK level was 1900 IU/L. Because he had no symptoms, the patient was allowed to continue monitored practice and competition. He completed the season without any complications.
DISCUSSION
Role of Creatine Kinase in Diagnosis of Exertional Rhabdomyolysis
A number of cases of exertional rhabdomyolysis have been reported,1,2,7–13 yet each case reflects a unique characteristic of the pathogenesis. Although most patients had an elevated CK level (>1000 IU/L, or more than 5 times normal),3,4 the diagnostic criteria for exertional rhabdomyolysis are inconsistent throughout the literature.6,14,15–17 In 2002, Ehlers et al18 investigated CK levels of football players during 2-a-day practices and identified elevated CK levels in all players. In 2006, authors19 of a retrospective epidemiologic study noted that almost half the secondary school students they studied (43.4%, 68 of 157) would be clinically diagnosed with exertional rhabdomyolysis after a 10-minute fitness test according to elevated CK levels. In our patient, as in many others, the CK level was significantly elevated and rhabdomyolysis was diagnosed. Unique to our case, however, was the prolonged nature of the CK elevation, lasting 18 days after the incident, even though his symptoms subsided in a few days. In fact, the only remaining indicator of rhabdomyolysis was the elevated CK level, suggesting that serum CK may not be the only factor we should use to dictate the course of care and return to activity.
Limiting the extent of the potentially life-threatening complications of rhabdomyolysis depends strongly on early diagnosis and adequate therapy,20 as in this case, when the patient received immediate attention. Return to play for this athlete was based on myoglobin levels and was therefore quicker than indicated in the position statements provided by several governing bodies (National Athletic Trainers' Association21 and American College of Sports Medicine22). Yet some independent researchers have made recommendations for return to activity that include aquatic exercise for the first 3 weeks, indoor and modified outdoor exercise for weeks 4 through 15, and a release to follow acclimatization guidelines by week 15.13 Our patient participated in sport-specific, functional conditioning exercises with limited eccentric loading to improve fitness before returning to play. Because the patient responded well to the course of treatment and because the physician used myoglobin level as an indicator of muscle damage, the patient was able to return to play within 6 weeks.
Pathophysiology
The physiologic sequelae of exertional rhabdomyolysis are unknown. However, researchers have theorized that CK leaks into the interstitial fluid from the muscle cells.20 As are most toxins, CK is absorbed by the lymphatic system.23 Although the kidneys work to filter the CK, the greater-than-normal concentration results in the accumulation of serum CK.6,23 Creatine kinase is not the only cellular constituent involved with muscle tissue breakdown; myoglobin and potassium have also been associated with the development of exertional rhabdomyolysis.3–6 Myoglobin accumulating in the kidneys can obstruct the renal tubules, leading to tubular necrosis and eventual renal failure.3–6 In addition, the blockage can limit urine flow, a common symptom in exertional rhabdomyolysis.13 The rise in potassium in the interstitial fluid may dilate arterioles, mediating a rise in muscle blood flow; however, if the skeletal muscle becomes deficient in potassium, muscle injury is likely to occur as a consequence of the ischemia.24
After muscle damage, myoglobin circulating in the blood plasma exceeds the protein-binding capacity and is filtered through kidneys and eventually excreted in the urine.6 When muscle damage is severe, as in cases of exertional rhabdomyolysis, the kidneys are unable to filter the excess myoglobin, resulting in tubular damage.5,6,25–27 Although several cellular chemicals spill into the extracellular fluid during muscle tissue breakdown, myoglobin tends to be the chief culprit in acute renal failure, and therefore its elimination is crucial.14 During alkaline diuresis, myoglobin is removed from the plasma at a faster rate than is CK, and the effects of acute renal failure can be reversed.14 The patient in our case was able to avoid renal failure, although his myoglobin and CK levels were acutely high. The drastic decline in myoglobin and lingering elevation of CK may confirm the role of myoglobin in the development of renal failure in exertional rhabdomyolysis.
Mechanism of Illness
Eccentric exercise has long been associated with the muscle breakdown leading to exertional rhabdomyolysis.28 However, the recently documented cases of rhabdomyolysis differ in onset. The condition has developed from weightlifting, sprinting, contact practices, noncontact practices, and running.1,2,7–13 Our patient participated in a full-contact practice session, followed by conditioning and then a team-mandated cold-water immersion bath. The intense nature of the exercise, in combination with the temperature extremes,1,6,7,13 may have affected the onset of rhabdomyolysis in our patient. Furthermore, the accumulated effects of 3 days of exercise may have affected the patient's CK levels. This finding is in contrast to that of Ehlers et al,18 who noted a gradual decline in serum CK after 7 and 14 days of exercise. Additional investigation is necessary to identify the effect of accumulated exercise on the onset of other biological markers for exertional rhabdomyolysis, such as myoglobin, especially in light of the acclimatization guidelines for secondary school and collegiate football players.29
Hypohydration and Rhabdomyolysis
Hypohydration has also been associated with exertional rhabdomyolysis. Patients often present with dark, reddish-brown urine (myoglobinuria)3,4 or the inability to urinate.13,30 Hypohydration often develops over the course of an exercise session but can also develop over several days of preseason practices. This phenomenon is called chronic hypohydration and can have accumulated negative effects.31 The authors2 of a recent case report suggested that dehydration was not associated with the pathogenesis of rhabdomyolysis because their patient showed no signs of myoglobinuria or inability to urinate. However, hypohydration has been reported in several other cases.1,13,32 In a large epidemiologic study19 of exertional rhabdomyolysis among high school students, myoglobinuria occurred in only 25% (17 of 68). Because our patient was initially unable to urinate, we were unable to determine his hydration status when the cramps began, but after intravenous fluid treatment, his urinalysis results were normal. The dark, cola-colored urine most commonly associated with rhabdomyolysis does not occur unless urine myoglobin levels exceed 100 mg/dL.6 Our patient did not demonstrate urinary myoglobin; in fact, his serum myoglobin levels were within the normal range of 0–149 ng/mL.33,34
Physiologic responses to decreased body water include decreased plasma volume, decreased central blood volume, increased heart rate and decreased cardiac output, and increased fatigue.35 Decreased body water and electrolyte imbalances have been theorized causes of exercise-associated muscle cramping,36 and cramping has been identified in several reported cases of exertional rhabdomyolysis.1,2,13 Cramping may be a precursor to muscle cell damage and subsequent exertional rhabdomyolysis,1,2,7,8,13,32,37 although randomized controlled trials or other robust controlled research studies should be conducted to investigate this possibility.
Uniqueness of the Case
Our patient continued to demonstrate elevated CK levels for 18 days, whereas myoglobin returned to normal limits within 5 days of the incident. The half-life of myoglobin is much shorter than that of CK, and it can be eliminated faster,14,15,38 as occurred in our patient. Creatine kinase lingers longer and, in fact, may be elevated secondary to exercise.18 When comparable data were available in other cases, the resolution of symptoms occurred in times as short as 36 hours13 to as long as 8 days.1 Once the acute symptoms resolved, no additional blood tests were performed. Therefore, drawing conclusions based on the limited data available may not be appropriate.
CONCLUSIONS
Rhabdomyolysis in athletes is an acute condition that may become life threatening because of the risk of renal failure from the elevated levels of CK and myoglobin in the blood. Although an elevated CK level is a commonly used indicator in the diagnosis of rhabdomyolysis and, more specifically, exertional rhabdomyolysis, our patient's elevated level persisted despite resolution of symptoms. In light of this case study and some of the evidence in the literature,18,34,39 the standard range of CK levels used for the general population may not be appropriate for an athletic population, particularly for athletes participating in collision sports. Data from a single patient are insufficient for drawing general conclusions, but this case study does provide the impetus for future investigations into the onset and prevention of exertional rhabdomyolysis. For example, information about the relationship between CK and myoglobin levels in both the onset and resolution of the condition is needed so that clinicians can identify the condition and determine when it is safe to return an athlete to full sport participation. Additionally, research is needed to identify precipitating factors and clinical warning signs in athletes who may be susceptible to exertional rhabdomyolysis. This case study has presented one patient's exertional rhabdomyolysis course and outcome. However, much work is needed before we truly understand this disease.
REFERENCES
- 1.Moeckel-Cole SA, Clarkson PM. Rhabdomyolysis in a collegiate football player. J Strength Cond Res. 2009;23(4):1055–1059. doi: 10.1519/JSC.0b013e3181ad316b. [DOI] [PubMed] [Google Scholar]
- 2.Anzalone ML, Green VS, Buja M, Sanchez LA, Harrykissoon RI, Eichner ER. Sickle cell trait and fatal rhabdomyolysis in football training: a case study. Med Sci Sports Exerc. 2010;42(1):3–7. doi: 10.1249/MSS.0b013e3181ae0700. [DOI] [PubMed] [Google Scholar]
- 3.Knochel JP. Catastrophic medical events with exhaustive exercise: “ white collar rhabdomyolysis.”. Kidney Int. 1990;38(4):709–719. doi: 10.1038/ki.1990.263. [DOI] [PubMed] [Google Scholar]
- 4.Knochel JP. Exertional rhabdomyolysis. N Engl J Med. 1972;287(18):927–929. doi: 10.1056/NEJM197211022871810. [DOI] [PubMed] [Google Scholar]
- 5.Vanholder RV, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11(8):1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 6.Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67(9):272–283. [PubMed] [Google Scholar]
- 7.Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 1994;159(2):160–163. [PubMed] [Google Scholar]
- 8.Kuklo TR, Tis JE, Moores LK, Schaefer RA. Fatal rhabdomyolysis with bilateral gluteal thigh and leg compartment syndrome after the Army Physical Fitness Test: a case report. Am J Sports Med. 2000;28(1):112–116. doi: 10.1177/03635465000280010401. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Kim W, Park SK, Kang ES, Kang KP, Kang SK. A case of acute renal failure, rhabdomyolysis and disseminated intravascular coagulation associated with severe exercise induced hypernatremic dehydration. Clin Nephrol. 2004;62(5):401–403. doi: 10.5414/cnp62401. [DOI] [PubMed] [Google Scholar]
- 10.Siegel SP, Verbalis JG, Clement S. Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am J Med. 2007;120(5):e1–e7. doi: 10.1016/j.amjmed.2006.10.027. et al. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson PM, Sayers SP. Etiology of exercise-induced muscle damage. Can J Appl Physiol. 1999;24(3):234–248. doi: 10.1139/h99-020. [DOI] [PubMed] [Google Scholar]
- 12.Sharma N, Winpenny H, Heyman T. Exercise-induced rhabdomyolysis: even the fit may suffer. Int J Clin Pract. 1999;53(6):476–477. [PubMed] [Google Scholar]
- 13.Cleary M, Ruiz D, Eberman L, Mitchell I, Binkley H. Dehydration, cramping, and exertional rhabdomyolysis: a case report with suggestions for recovery. J Sport Rehabil. 2007;16(3):244–259. doi: 10.1123/jsr.16.3.244. [DOI] [PubMed] [Google Scholar]
- 14.Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212–2215. doi: 10.1097/00003246-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis. An overview for clinicians. Crit Care. 2005;9(2):158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiff HB, MacSearraigh ET, Kallmeyer JC. Myoglobinuria, rhabdomyolysis and marathon running. Q J Med. 1978;47(188):463–472. [PubMed] [Google Scholar]
- 17.Feinfeld DA, Cheng JT, Beysolow TD, Briscoe AM. A prospective study of urine and serum myoglobin levels in patients with acute rhabdomyolysis. Clin Nephrol. 1992;38(4):193–195. [PubMed] [Google Scholar]
- 18.Ehlers GG, Ball TE, Liston L. Creatine kinase levels are elevated during 2-a-day practices in collegiate football players. J Athl Train. 2002;37(2):151–156. [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Chie W, Lien H. Epidemiological analysis of factors influencing an episode of exertional rhabdomyolysis in high school students. Am J Sports Med. 2006;34(3):481–486. doi: 10.1177/0363546505281243. [DOI] [PubMed] [Google Scholar]
- 20.Poels PJE, Gabreels FJM. Rhabdomyolysis: a literature review. Clin Neurol Neurosurg. 1995;95(3):175–192. doi: 10.1016/0303-8467(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 21.Binkley HM, Beckett J, Casa DJ, Kleiner DM, Plummer PE. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2002;37(3):329–343. [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. Armstrong LE, Casa DJ. American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. et al. [DOI] [PubMed] [Google Scholar]
- 23.Hortobágyi T, Denahan T. Variability in creatine kinase: methodological, exercise, and clinically related factors. Int J Sports Med. 1989;10(2):69–80. doi: 10.1055/s-2007-1024878. [DOI] [PubMed] [Google Scholar]
- 24.Knochel JP, Schlein EM. On the mechanism of rhabdomyolysis in potassium depletion. J Clin Invest. 1972;51(7):1750–1758. doi: 10.1172/JCI106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Phys. 2002;65(5):907–912. [PubMed] [Google Scholar]
- 26.Lane R, Phillips M. Rhabdomyolysis has many causes, including statins, and may be fatal. BMJ. 2003;327(7407):115–116. doi: 10.1136/bmj.327.7407.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized athletes. Medicine (Baltimore). 2005;84(6):377–385. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 28.Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc. 1983;15(1):51–56. [PubMed] [Google Scholar]
- 29.Casa DJ, Armstrong LE, Watson G. Heat acclimatization of football players during initial summer practice sessions. Med Sci Exerc Sport. 2004;36(5 suppl):S49. et al. [Google Scholar]
- 30.Greenberg J, Arneson L. Exertional rhabdomyolysis with myoglobinuria in large group of military trainees. Neurology. 1967;17(3):216–222. doi: 10.1212/wnl.17.3.216. [DOI] [PubMed] [Google Scholar]
- 31.Sawka MN. Physiological consequences of dehydration: exercise performance and thermoregulation. Med Sci Sports Exerc. 1992;24(6):657–670. [PubMed] [Google Scholar]
- 32.Msric V, Nesek Adam V, Grizelj Stojcic E, Rasic Z, Smiljanic A, Turcic I. Acute rhabdomyolysis: a case report and literature review. Acta Med Croatica. 2008;62(3):317–322. [PubMed] [Google Scholar]
- 33.Olerud JE, Homer LD, Carroll HW. Serum myoglobin levels predicted from serum enzyme values. N Engl J Med. 1976;293(10):483–485. doi: 10.1056/NEJM197509042931006. [DOI] [PubMed] [Google Scholar]
- 34.Olerud JE, Homer LD, Carrroll HW. Incidence of acute exertional rhabdomyolysis: serum myoglobin and enzyme levels as indicators of muscle injury. Arch Intern Med. 1976;136(6):692–697. doi: 10.1001/archinte.136.6.692. [DOI] [PubMed] [Google Scholar]
- 35.Murray R. Fluid needs in hot and cold environments. Int J Sport Nutr. 1995;5(suppl):S62–S73. doi: 10.1123/ijsn.5.s1.s62. [DOI] [PubMed] [Google Scholar]
- 36.Bergeron MF. Exertional heat cramps: recovery and return to play. J Sport Rehabil. 2007;16(3):190–196. doi: 10.1123/jsr.16.3.190. [DOI] [PubMed] [Google Scholar]
- 37.Dincer HE, Raza T. Compartment syndrome and fatal rhabdomyolysis in sickle cell trait. WMJ. 2005;104(6):67–71. [PubMed] [Google Scholar]
- 38.Naka T, Jones D, Baldwin I. Myoglobin clearance by super high-flux hemofiltration in a case of severe rhabdomyolysis: a case report. Crit Care. 2005;9(2):R90–R95. doi: 10.1186/cc3034. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olerud JE, Homer LD, Carroll HW. Serum myoglobin levels associated with severe exercise. Mil Med. 1981;146(4):274–276. [PubMed] [Google Scholar]


