Abstract
Introduction
Lipid profiles in women with early breast cancer receiving anastrozole with or without risedronate were examined within an international Phase III/IV study to assess for possible treatment related changes.
Methods
Postmenopausal women with hormone receptor-positive breast cancer were assigned to 1 of 3 strata by risk of fragility fracture. Patients with the highest risk for fracture received anastrozole plus risedronate (A+R). Moderate-risk patients were randomized in a double-blind manner to anastrozole and risedronate (A+R) or anastrozole and placebo (A+P). Lower-risk patients received anastrozole (A) alone.
Serial fasting blood samples were assessed for changes in lipid parameters relative to baseline after 12 months of treatment with anastrozole with or without risedronate. Samples were centrally analyzed for low density lipoprotein cholesterol (LDL-cholesterol), high density lipoprotein (HDL) cholesterol, total cholesterol (TC) and triglycerides (TG). Analysis was performed as primary analysis population for lipids (A plus A+P), lipid intention to treat population and secondary population (A+R).
Results
Of the 119 patients treated with A plus A+P, there were 66 patients eligible for inclusion in the primary analysis population. Of the 115 patients treated with secondary population (A+R) there were 65 patients eligible for lipid profiling. For LDL cholesterol, HDL cholesterol, TC and TG there were no significant changes between the baseline and 12 month assessments to suggest that any of these therapies have a negative impact on the lipid profile.
Conclusions
In this study of postmenopausal women with early breast cancer receiving adjuvant anastrozole with or without risedronate, there was no adverse effect on LDL cholesterol, HDL cholesterol, TC or TG values over the 12 month monitoring period.
Keywords: anastrozole, aromatase inhibitor, bisphosphonate, lipid, risedronate
Introduction
Third-generation aromatase inhibitors (AIs), anastrozole, letrozole and exemestane are routinely recommended as part of the adjuvant treatment for postmenopausal women with hormone receptor positive early stage breast cancer (EBC), either as initial therapy for newly diagnosed patients, or as sequenced therapy with tamoxifen [1]. Adjuvant AI use has shown a modest improvement in efficacy over tamoxifen and appears to have an acceptable toxicity profile, although data regarding the effect of AIs on lipid profiles is limited.
In the United States, heart disease is the leading cause of death [2].The majority of women diagnosed with EBC are not expected to die from their diagnosis of breast cancer [3]; this emphasizes the need to minimize toxicities from adjuvant breast cancer therapies and address long term side-effects. Loss of BMD leading to osteoporosis in some women has been established with AIs. There are data that suggests that the AIs may lead to an increased risk of cardiovascular disease, although the difference is estimated at less than 1% above that seen with tamoxifen [4]. Previous studies with adjuvant AI therapy have suggested a small increase in the risk of cardiovascular disease when compared to tamoxifen therapy [1]
The data reporting the impact of adjuvant AI therapy on lipid profile are mixed, with studies demonstrating both neutral and negative findings [1, 5, 6]. In addition to possible adverse changes in the lipid profile associated with AI therapy, there have been reports that bisphosphonates may have a beneficial effect on serum lipids [7,8]. If beneficial effects exist, they may be secondary to the nitrogen containing bisphosphonates serving as potent inhibitors of squalene and cholesterol synthesis by affecting farnesyl synthase diphosphate in the melvonate pathway [9, 10]. As osteoporosis remains a major cause of morbidity and mortality in the postmenopausal population and bisphosphonates represent commonly used therapy for management of osteoporosis [11], an understanding of changes in lipid profiles with the combination of anastrozole with or without risedronate may further improve understanding of the risk to benefit assessment of adjuvant aromatase inhibition in this subject population. It is of note that risedronate has been shown to decrease lipids in women with high cholesterol [12]. To investigate the impact of anastrozole on lipid profiles, with or without risedronate, analysis of serial serum specimens from the Phase III/IV randomized, placebo controlled clinical trial Study of Anastrozole with the Bisphosphonate RisedronateE (SABRE) was a pre-planned aspect of the Study.
Methods
Study design
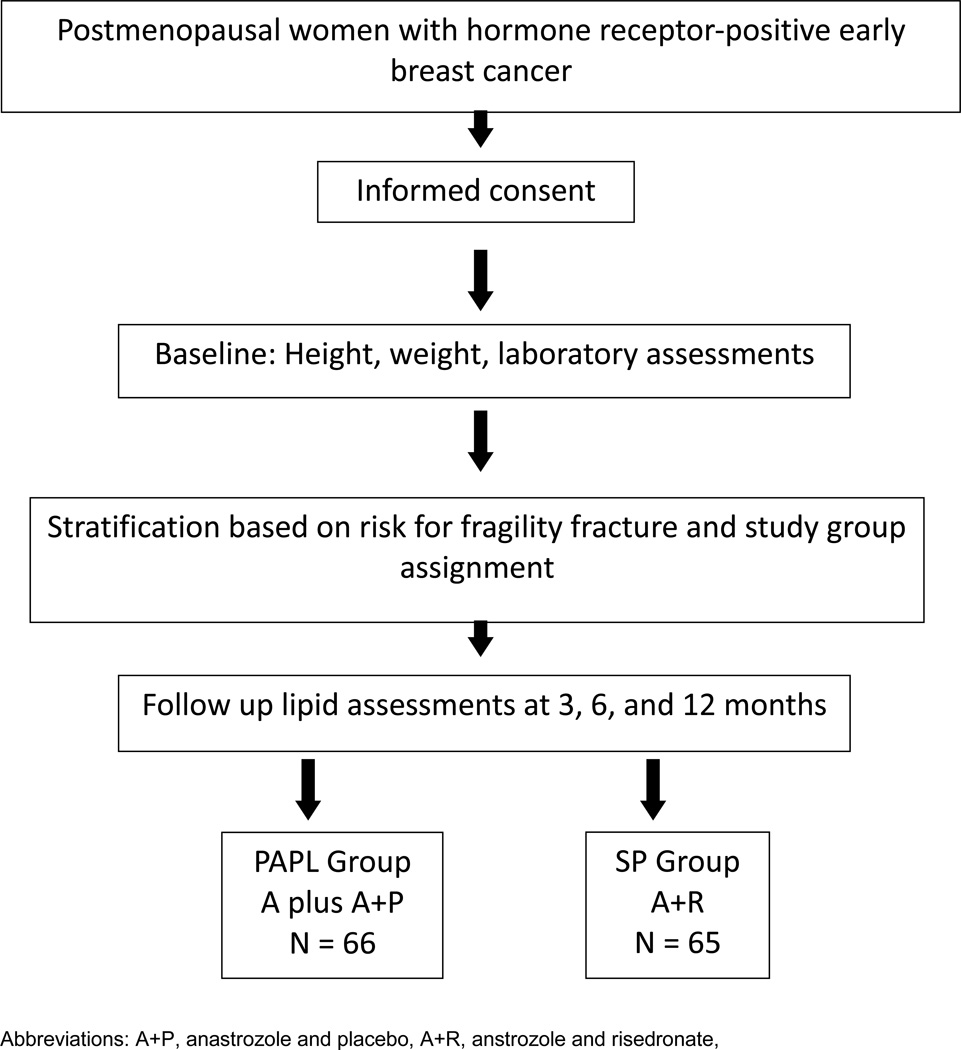
SABRE was an international, Phase III/IV study (NCT00082277) with open-label and double-blinded randomized arms, in which patients were to be treated and followed for 2 years. The bone mineral density (BMD) and biochemical bone marker data have been published separately [13]. During SABRE, blood was collected for lipid parameters during the first year of study. SABRE was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice, as required by the regulatory authorities. The study protocol was approved by the Ethics Committee of each participating site, and all enrolled patients provided written informed consent. The study schema is illustrated in Figure 1.
FIGURE 1. Study Schema.
Patients
Postmenopausal women scheduled to receive adjuvant anastrozole were eligible for inclusion in the study. Detailed information regarding inclusion and exclusion criteria of SABRE are published [13].Information specific to the lipid assessments are presented here. Patients were excluded from lipid analysis if they had elevated low density lipoprotein (LDL) cholesterol or total cholesterol (TC) at baseline, according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III) criteria, i.e. LDL cholesterol ≥ 4.2 mmol/L or TC ≥ 6.2 mmol/L [14] or if they were receiving lipid -lowering concomitant medication or there were major protocol deviations that could affect lipid metabolism.
Patients were allocated to one of three groups based on their risk of fracture (higher, moderate, or lower risk). Patients were deemed to be at a higher risk if their T-score was < -2.0 in either the lumbar spine or hip, or they had a personal history of fragility fracture. The higher-risk group was an open-label, non-comparative group, in which patients received oral anastrozole 1 mg/day with risedronate sodium 35 mg/week (A+R). Patients with a T-score ≥ -1.0 in either lumbar spine or total hip, and no history of fragility fracture, were classified as lower risk. The lower-risk group was open-label and non-comparative, with patients receiving anastrozole 1 mg/day (A). Patients with a T-score < -1.0 but ≥ -2.0 at either the lumbar spine or total hip and no history of fragility fracture could enter either the higher- or moderate-risk stratum. The decision to enter into the higher-risk stratum was based on T-score combined with clinical criteria associated with an increased risk of fracture: advanced age, early menopause (age < 45 years, low body weight (< 127 lbs/58 kg), current smoking, and history of fragility fracture in a first-degree relative. Patients stratified to the moderate-risk group were randomized in a double-blind manner to receive either anastrozole 1 mg/day plus risedronate 35 mg/week (A+R) or anastrozole 1 mg/day plus matching placebo for risedronate (A+P). Randomization was determined via a central scheme prepared by the Biostatistics group at AstraZeneca, with investigators and trial monitors unaware of each patient’s treatment assignment.
Specimen Collection
Fasting blood samples were taken at baseline, and at 3, 6, and 12 months for central analysis of lipid profiles. Samples from the same individual were stored at −70°C to −80°C and were assayed in the same batch. LDL-cholesterol (LDL), HDL-cholesterol (HDL), total cholesterol (TC), and triglycerides (TG) were measured by standard methods. A central laboratory provided investigational sites with all appropriate materials for specimen collection and sample processing, packaging and shipping to the central CLIA certified laboratory for analysis (Quintiles Central Laboratory Service, Smyrna, Georgia).
Analysis Plan
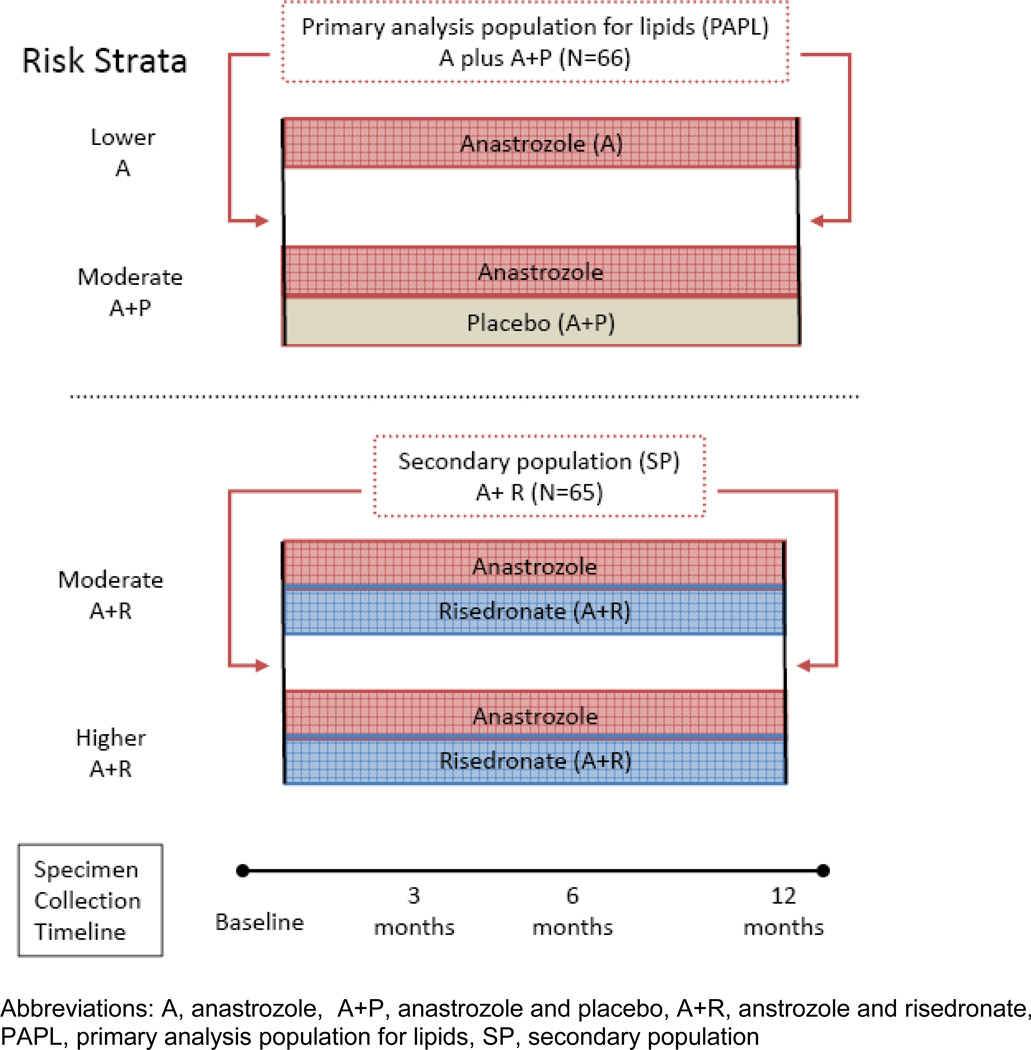
The SABRE sample size was calculated on the primary endpoint of change in lumbar spine bone mineral density at 12 months. A preplanned secondary objective of SABRE was to determine the change in LDL cholesterol relative to baseline after 12 months of treatment with anastrozole alone, and to explore other changes in lipid profiles relative to baseline at various time points after treatment with anastrozole alone, or anastrozole in combination with risedronate. The primary analysis population for lipids (PAPL) comprised those patients who received anastrozole treatment without risedronate (A plus A+P). Patients were excluded from the PAPL population if they had high LDL cholesterol or TC or were receiving lipid lowering medications at baseline, or if there were violations of the lipid phlebotomy procedure. The secondary population (SP) included patients who received anastrozole and risedronate (A+R), from the higher- and moderate-risk strata. The lipid intent-to-treat population (LITTP) was defined as all patients who received anastrozole alone or anastrozole and placebo. The LITTP population included those in the PAPL population as well as those that were excluded due to baseline lipid levels, or lipid medications. Figure 2 illustrates grouping for lipid analysis.
FIGURE 2. Study groups for lipid analysis.
Medications used to lower cholesterol include the statins and data taken from this setting suggests that a 6% reduction in LDL cholesterol with a standard deviation (SD) of around 14% is clinically important [15]. Taking 14.8% from the publication on lipid lowering drugs [15]as a conservative estimate of standard deviation, it is calculated that 67 subjects will have 90% power to detect a 6% difference from baseline using a within group paired t-test, at a two sided significance level of 5%.The analysis for LDL cholesterol at 12 months is the primary endpoint for the lipid study. The analysis of HDL cholesterol, TC and TG at 12 months are considered secondary analyses.
Since, by study design, the treatments were not assigned randomly across all the patients in the study, no formal comparison between the primary and secondary lipid populations were performed. Lipid profiles were analyzed independently of strata using paired t-test, and are presented as mean percentage changes from baseline and corresponding confidence intervals. Graphical and tabular summaries, in addition to p-values and confidence intervals (CI) describing changes from baseline, were used to assess the results. Two-sided 95% confidence intervals (CI) were produced, and all formal tests of significance were two-sided with a significance level of 5%. LDL cholesterol was classified based on ATP III criteria and a shift table showing classification at baseline and 12 months produced.
The ATP III LDL cholesterol classification defines optimal LDL cholesterol(<2.6 mmol/L or 100 mg/dL), near optimal LDL cholesterol (2.6–3.3 mmol/L or 100–129 mg/dL), borderline high LDL cholesterol (3.4–4.1 mmol/L or 130–159 mg/dL), high LDL cholesterol (4.2–4.9 mmol/L or 160–189 mg/dL), very high LDL cholesterol (4.9 mmol/L or190 mg/dL or more) [14]
Results
Two hundred and thirty-four (234) patients were enrolled in the main study. There were 38 in the higher-risk stratum treated with A+R, 154 in the moderate-risk stratum randomized to receive A+P or A+R (n = 77 each), and 42 in the lower-risk stratum treated with anastrozole alone. Baseline characteristics and demographics were similar among treatment groups, except for slight variability in weight, body mass index, and past exposure to hormone replacement therapy [13].
Of the 119 patients receiving A alone or A+P in the LITTP group, 66 were eligible for inclusion in the PAPL analysis. Forty-six patients were excluded at baseline due to high total cholesterol (≥ 6.2 mmol/L) or HDL-cholesterol (≥ 4.2 mmol/L), according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III) criteria [14] Seven patients were excluded for other reasons (received cholesterol-lowering treatment in the previous 12 months; treatment with previous hormonal therapy; and one patient with hyperparathyroidism). Similarly, of the 115 patients receiving A+R, 41 were excluded due to high baseline cholesterol levels and 9 for other reasons (received cholesterol-lowering treatment in the previous 12 months; treatment with previous hormonal therapy; concomitant treatment with corticosteroids), leaving 65 patients eligible for lipid profiling in patients treated with A+R. At the time of analysis, several additional patients were excluded due to major protocol deviations (non-fasted sample taken, cholesterol-lowering drug received while on study, or ≤ 66% of anastrozole tablets taken). Table 1 outlines the patient demographics.
TABLE 1.
Patient demographics and baseline
| Lipid populations | |||
|---|---|---|---|
| A or A+P | A+R | ||
| PAPL | LITTP | SP | |
| N=66 | N=119 | N=65 | |
| Mean | |||
| Age in years | 65.1 | 64.1 | 64.2 |
| Weight in kilograms | 71.4 | 72.7 | 74.2 |
| BMI | 27.8 | 28.0 | 28.7 |
| Percent of patients | |||
| Prior HRT | 51.5% | 43.7% | 24.6% |
| >60 months since last menstrual period |
90.9% | 84.0% | 83.1% |
Abbreviations: A, anastrozole; BMI, body mass index; HRT, hormone-replacement therapy; LITTP, lipid intention to treat population; P, placebo; PAPL, primary analysis population for lipids; R, risedronate; SP, secondary population
The mean percentage changes from baseline in lipid parameters of samples taken from eligible patients are presented in Table 2. The LDL cholesterol (primary endpoint of lipid analysis) did not demonstrate statistically significant changes from baseline to 12 months in either the PAPL (A plus A+P) or SP (A+R) study groups. The HDL cholesterol demonstrated a statistically significant increase from baseline to 12 months in the PAPL; however, there was no statistically significant change in HDL cholesterol in the SP group. At 12 months the TC and TG demonstrated no statistically significant change in either the PAPL or SP. Analysis of the ITT population supports the lack of adverse effects seen in the PAPL analysis. In the LITTP analysis anastrozole as a singlel agent and with placebo demonstrated statistically significant decreases in the LDL cholesterol and total cholesterol, as well as increases in HDL cholesterol. This finding was less pronounced in the PAPL, which consists of a smaller sample size
TABLE 2.
Mean percent change from baseline in LDL−cholesterol, HDL−cholesterol, total cholesterol and serum triglycerides at 12 months
| Group | Mean % change from baseline to 12 months (95% confidence intervals) |
p-values* | ||
|---|---|---|---|---|
| N | ||||
| LDL-cholesterol | Anastrozole (PAPL) | 54 | −2.3 (−7.64, 3.13) | 0.2859 |
| Anastrozole (LITTP) | 94 | −5.4 (−9.31, −1.54) | 0.0007 | |
| Anastrozole + risedronate (SP) |
59 | −2.9 (−7.20, 1.38) | 0.0770 | |
| HDL-cholesterol | Anastrozole (PAPL) | 54 | 6.9 (2.79, 10.91) | 0.0016 |
| Anastrozole (LITTP) | 95 | 6.8 (4.02, 9.49) | < 0.0001 | |
| Anastrozole + risedronate (SP) |
60 | 4.0 (0.21, 7.79) | 0.1070 | |
| Total cholesterol | Anastrozole (PAPL) | 54 | 0.8 (−3.08, 4.60) | 0.8647 |
| Anastrozole (LITTP) | 95 | −2.2 (−4.96, 0.67) | 0.0351 | |
| Anastrozole + risedronate (SP) |
60 | −0.4 (−3.27, 2.39) | 0.4840 | |
| Triglycerides | Anastrozole (PAPL) | 54 | −0.6 (−7.15, 5.94) | 0.9881 |
| Anastrozole (LITTP) | 95 | −0.7 (−5.84, 4.50) | 0.4133 | |
| Anastrozole + risedronate (SP) |
60 | 7.0 (−5.02, 19.09) | 0.4313 | |
Paired t−test comparing the means at baseline and 12 months are two−sided, 95% confidence intervals (CI). All formal tests of significance are two sided with a significance level of 5%.
Abbreviations: HDL, high−density lipoprotein; LITTP, lipid intent to treat population; LDL, low-density lipoprotein; PAPL, primary analysis population for lipids; SP, secondary population. Note: variation in sample size reflects sample analysis excluded due to major protocol deviations, sample collection and study drop out over the course of 12 months.
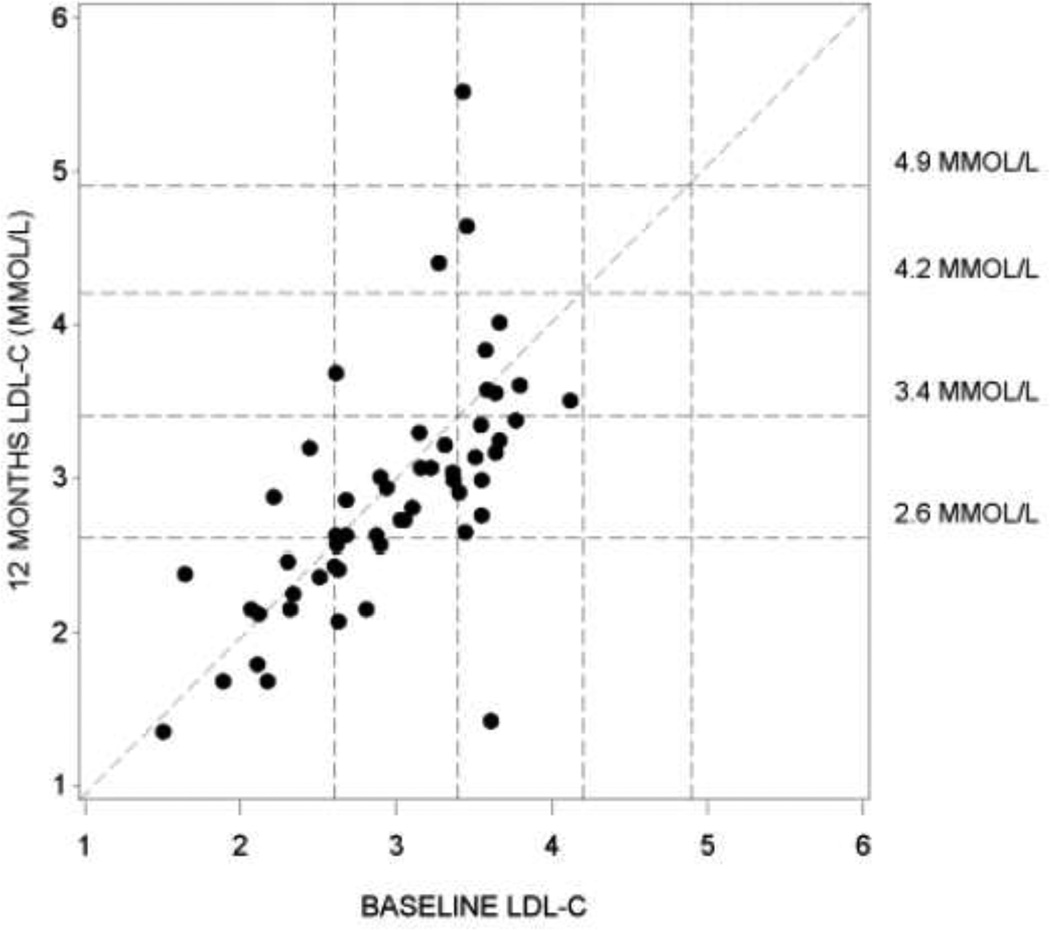
The majority of patients who had optimal or near optimal LDL cholesterol concentrations at baseline according to ATP III criteria remained in the same category at 12 months (Figure 3). In the PAPL, for patients with borderline high LDL cholesterol at baseline, there was a trend for a shift to a better ATP III category although this was not formally analyzed. The TC:HDL cholesterol ratio demonstrated a marginal decrease numerically from baseline to 12 months in the PAPL and SP populations (Table3)
FIGURE 3. Scatter plot of LDL at 12 months versus baseline in the Primary Analysis Population (PAPL).
Reference lines show LDL cholesterol classification according to the the ATPIII criteria. Optimal < 2.6 MMOL/L, Near Optimal >= 2.6 MMOL/L, Borderline High >= 3.4 to <4.2 MMOL/L, High >= 4.2 to <4.9 MMOl/L, Very High >= 4.9 MML/L (1mg/dl = 0.02586 MMOL/L)
TABLE 3.
Total Cholesterol (TC): High Density Lipoprotein Cholesterol (HDL) Ratio:
| PAPL: A plus A+P N= 66 |
SP: A+R N= 65 |
|
|---|---|---|
| Baseline | ||
| Mean (SD) | 3.30 (0.824) | 3.48 (0.895) |
| Median (Range) | 3.22 (1.7–6.0) | 3.29 (2.1–6.0) |
| 3 Months | n=53 | n=59 |
| Mean (SD) | 3.30 (0.849) | 3.38 (0.985) |
| Median (Range) | 3.27(1.8–5.7) | 3.38 (1.9–5.9) |
| 6 months | n=54 | n=59 |
| Mean (SD) | 3.20 (0.814) | 3.35 (0.927) |
| Median (Range) | 3.14 (1.9–5.4) | 3.10 (2.1–5.9) |
| 12 months | n=54 | n=60 |
| Mean (SD) | 3.11 (0.864) | 3.28 (0.849) |
| Median (Range) | 3.07 (1.8–6.0) | 3.07 (1.8–5.5) |
Abbreviations: PAPL: primary analysis population lipids, SP Secondary population, SD, standard deviation
The mean TC: HDL ration decreased from baseline to 12 months in both the PAPL and SP
Discussion
In SABRE, the serum lipid panel did not demonstrate deleterious effects in lipids or triglycerides from baseline to 12 months of follow up in postmenopausal women with hormone receptor positive EBC treated with anastrozole alone or with anastrozole and risedronate. These findings were seen not only in the PAPL, which excluded those patients with an abnormal lipid profile or who were taking antilipidemic drugs at baseline, but also in the ITT population, which included all patients receiving anastrozole alone or with placebo. Anastrozole without risedronate demonstrated a statistically significant improvement in lipid profiles within the LITTP analysis. This data do not permit insight into the potential clinical significance of these changes in lipid profiles. In the PAPL analysis a statistically significant increase was seen only in HDL cholesterol. The absence of a control group limits the interpretation of the apparent increase in HDL observed. The differences in LITTP and PAPL analysis may reflect the smaller sample size in the PAPL, or may reflect differences related to the possible concomitant use of lipid lowering medications by some of the LITTP group subjects. The bisphosphonates are often used to mitigate bone loss in patients receiving adjuvant AI therapy. There is a suggestion that the bisphosphonates may have a beneficial effect on serum cholesterol levels [16]; however, the SABRE data demonstrate that co-administration of risedronate with anastrozole in the SP does not appear to impact favorably on the lipid profile compared with anastrozole alone.
The data on AI induced changes in the lipid profiles demonstrate mixed results with studies showing either hypercholesterolemia or a neutral affect when compared to tamoxifen [5, 17]. In studies that do not use tamoxifen as a comparator, the effect of the AI on lipid profiles appears more neutral [6, 18,19], although prior therapy with adjuvant tamoxifen followed by adjuvant AI appears to have a negative impact on lipid profiles [20]. Hence, the adverse effects seen in comparison with tamoxifen, or in sequence with tamoxifen, may be due to a beneficial effect of this drug rather than a negative impact by AI therapy.
In SABRE the 12 month LDL cholesterol, TC and TG data no significant changes were seen, although an improved HDL cholesterol was seen. The clinical significance of these changes cannot be defined within the SABRE data. As can be seen in Figure 3, scatter plot demonstrating LDL cholesterol at 12 months versus baseline, there are a few outliers which suggests that there may be characteristics (genetic, environmental, or behavioral) that place an individual at a higher or lower risk of lipid alterations with the use of anastrozole. The SABRE data do not permit further investigation into these outliers.
The AIs are routinely incorporated into the adjuvant care plan of postmenopausal women with estrogen and/or progesterone receptor positive breast cancer, and like all medications are not without a risk of toxicity. There is a growing body of literature reporting on the risk of cardiovascular events in patients treated with adjuvant AIs [1]. A meta-analysis of 7 randomized controlled trials consisting of nearly 20,000 patients demonstrated a statistically significant increase in the risk of cardiovascular events in patients treated with third generation AI versus tamoxifen risk ratio (RR)1.34 (95% Confidence interval (CI) 1.09–1.63, P=0.0038) [4]. This is consistent with the meta-analysis by Amir et al which also demonstrated a higher probability of developing cardiovascular disease with an AI versus tamoxifen Odds Ratio (OR) 1.20, p =0.01 [21]. The cardiovascular risk assessment of adjuvant AIs are not uniform in all studies. In a large population based case control study using encounter and pharmacy database information from community based population of over 44,000 women over the age of 50 with breast cancer and similar matched controls Ligibel et al generated data that suggests that AIs do not associate with an increased risk of myocardial infarction or stroke [22]. The SABRE adverse events and serious adverse events at 24 months leading to study discontinuation have been reported [13] and include one patient experiencing each of the following events: cardiac failure, coronary artery insufficiency, hypertension and transient ischemic attack. Due to the small sample size, these event data are descriptive.
Conclusions
In summary, the data generated within SABRE suggest that anastrozole alone or with the addition of risedronate do not have a negative impact on LDL cholesterol, HDL cholesterol TC or TG. SABRE monitored lipids for 12 months. In order to obtain reliable data regarding the long-term effect of anastrozole on serum lipids and triglycerides studies of larger size and of greater duration are needed.
Acknowledgments
Acknowledgements for support of the clinical trial
AstraZeneca
The Alliance for Better Bone Health
Abbreviations
- A
anastrozole
- A+P
anastrozole and placebo
- A+R
anastrozole plus risedronate
- BMD
bone mineral density
- BMI
body mass index
- CI
Confidence interval
- EBC
early breast cancer
- HDL cholesterol
high density lipoprotein
- HRT
hormone-replacement therapy
- ITT
intention to treat
- LDL cholesterol
low density lipoprotein cholesterol
- LITTP
lipid intention to treat population
- NCEP ATP-III
National Cholesterol Education Program Adult Treatment Panel III
- P
placebo
- PAPL
primary analysis population for lipids
- R
risedronate
- SABRE
Study of Anastrozole with the Bisphosphonate RisedronateE
- SP
secondary population
- RR
risk ratio
- TC
total cholesterol
- TG
triglycerides
Footnotes
Competing Interests:
Employment or Leadership Position: Glen Clack, AstraZeneca
Consultant or Advisory Role: Catherine Van Poznak, Novartis, Amgen; David Barlow, AstraZeneca; Andreas Makris, AstraZeneca; Richard Eastell, AstraZeneca, Sanofi Aventis.
Stock Ownership: Glen Clack, AstraZeneca
Honoraria: Andreas Makris, AstraZeneca; Richard Eastell, AstraZeneca, Sanofi Aventis
Research Funding: Catherine Van Poznak: Novartis, Amgen; Andreas Makris, AstraZeneca; Richard Eastell, AstraZeneca, Sanofi Aventis
Expert Testimony: None
Other Remuneration: None
Author’s Contributions
CVP: Study conception and design, provision of study materials and patients, collection and assembly of the data, data analysis and interpretation, manuscript writing, final approval of manuscript.
AM: provision of study materials and patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
GC: collection and assembly of the data, data analysis and interpretation, manuscript writing, final approval of manuscript.
DHB: data analysis and interpretation, manuscript writing, final approval of manuscript.
RE: Study conception and design, collection and assembly of the data, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology. Clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clini. Oncol. 2010 Aug 10;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minino AM, Murphy SL, Xu J, Kochanek KD. [Accessed 5-9-2012];National Vital Statistics Reports. Death: Final Data for 2008 December 7. 2011. Volume 59, number 10. 2011 http://w ww.cdc,gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. [PubMed]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(Issue 9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. (2005) [DOI] [PubMed] [Google Scholar]
- 4.Cuppone F, Bria E, Verma S, Pritchard KI, Gandhi S, Carlini P, Milella M, Nisticò C, Terzoli E, Cognetti F, Giannarelli D. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer. Cancer. 2008;112:260–267. doi: 10.1002/cncr.23171. [DOI] [PubMed] [Google Scholar]
- 5.Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005;93(Suppl 1):S23–S27. doi: 10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCloskey EV, Hannon RA, Lakner G, et al. Effects of third generation aromatase inhibitors on bone health and other safety parameters: Results of an open, randomised, multi-centre study of letrozole, exemestane and anastrozole in healthy postmenopausal women. European Journal of Cancer. 2007;43:2523–2531. doi: 10.1016/j.ejca.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Montagnani A, Gonnelli C, Cepollaro MS, et al. Changes in serum HDL and LDL cholesterol in patients with Paget’s bone disease treated with pamidronate. Bone. 2003;32:15–19. doi: 10.1016/s8756-3282(02)00924-9. [DOI] [PubMed] [Google Scholar]
- 8.Adami S, Braga V, Guidi G, et al. Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. J Bone Miner Res. 2000;15:599–604. doi: 10.1359/jbmr.2000.15.3.599. [DOI] [PubMed] [Google Scholar]
- 9.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2297. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Strobach D, Lorenz RL. The bisphosphonate ibandronate stimulates reverse cholesterol transport out of monocytoid cells by enhanced ABCA1 transcription. Biochem Biophys Res Commun. 2003;307(1):23–30. doi: 10.1016/s0006-291x(03)01127-6. 18. [DOI] [PubMed] [Google Scholar]
- 11.Favus MJ. Bisphosphonates for Osteoporosis. N Engl J Med. 2010;363:2027–2035. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- 12.Tanriverdi HA, Barut A, Sarikaya S. Statins have additive effects to vertebral bone mineral density in combination with risedronate in hypercholesterolemic postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2005;120(1):63–68. doi: 10.1016/j.ejogrb.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitorinduced bone loss using risedronate: The SABRE trial. J Clin Oncol. 2010;28:967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 14.Expert panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Davidson M, Ma P, Stein EA, Gotto AM, Raza A, Chitra R, Hutchinson H. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. The American Journal of Clinical Cardiology. 2002;89:268–275. doi: 10.1016/s0002-9149(01)02226-3. [DOI] [PubMed] [Google Scholar]
- 16.Guney E, Kisakol G, Ozgen AG, et al. Effects of bisphosphonates on lipid metabolism. Neuro Endocrinol Lett. 2008;29(2):252–255. [PubMed] [Google Scholar]
- 17.Dent SF, Gaspo R, Kissner M, Pritchard KI. Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormonesensitive early breast cancer. Breast Cancer Res Treat. 2011;126:295–310. doi: 10.1007/s10549-011-1351-3. [DOI] [PubMed] [Google Scholar]
- 18.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 19.Kataja V, Hietanen P, Joensuu H, Ala-Luhtala T, Asola R, Kokko R, Korhonen T, Holli K. The effects of adjuvant anastrozole, exemestane, tamoxifen, and toremifene on serum lipids in postmenopausal women with breast cancer – A randomised study (abstract) Breast Cancer Res Treat. 2002;76(suppl 1):S156. Abstract 634. [Google Scholar]
- 20.Bell LN, Nguyen AT, Li L, Desta Z, Henry NL, Hayes DF, Wolff AC, Stearns V, Storniolo AM, Flockhart DA. Comparison of Changes in the Lipid Profile of Postmenopausal Women With Early Stage Breast Cancer Treated With Exemestane or Letrozole. J Clin Pharmacol. 2011 Dec;15 doi: 10.1177/0091270011424153. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011 Sep 7;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 22.Ligibel JA, James O'Malley A, Fisher M, Daniel GW, Winer EP, Keating NL. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat. 2012;131(2):589–597. doi: 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]