Abstract
Autism spectrum disorders (ASD) are a complex group of neurodevelopmental disorders encompassing impairments in communication, social interactions and restricted stereotypical behaviors. Although a link between altered immune responses and ASD was first recognized nearly 40 years ago, only recently has new evidence started to shed light on the complex multifaceted relationship between immune dysfunction and behavior in ASD. Neurobiological research in ASD has highlighted pathways involved in neural development, synapse plasticity, structural brain abnormalities, cognition and behavior. At the same time, several lines of evidence point to altered immune dysfunction in ASD that directly impacts some or all these neurological processes. Extensive alterations in immune function have now been described in both children and adults with ASD, including ongoing inflammation in brain specimens, elevated pro-inflammatory cytokine profiles in the CSF and blood, increased presence of brain-specific auto-antibodies and altered immune cell function. Furthermore, these dysfunctional immune responses are associated with increased impairments in behaviors characteristic of core features of ASD, in particular, deficits in social interactions and communication. This accumulating evidence suggests that immune processes play a key role in the pathophysiology of ASD. This review will discuss the current state of our knowledge of immune dysfunction in ASD, how these findings may impact on underlying neuro-immune mechanisms and implicate potential areas where the manipulation of the immune response could have an impact on behavior and immunity in ASD.
1. Introduction
Autism spectrum disorders (ASD) are a series of pervasive development disorders which include autistic disorder, Rett’s disorder, childhood disintegrative disorder, Asperger’s syndrome or pervasive developmental disorder not otherwise specified (PDD-NOS). Autism spectrum disorders are characterized by severe and pervasive impairment in several areas of development: reciprocal social interaction skills, communication skills, or the presence of stereotyped behavior, interests and activities (APA, 2000). According to the most recent estimates calculated by the U.S. Center of Disease Control, ASD affects 1 in 110 children under the age of eight (MMWR, 2009). Although current research suggests there may be no single genetic cause for ASD, there are several lines of evidence to suggest that the disorder is highly heritable. There is a concordance rate for ASD of 0–37% reported for dizygotic twins, while concordance rates of 44–91% are reported for monozygotic twins (Bailey et al., 1995; Constantino and Todd, 2000; Kates et al., 2004; Steffenburg et al., 1989), suggesting that genetic composition may contribute to increased risk of developing ASD. In addition to the heritability observed in twin-pairs, the risk of developing ASD in non-twin siblings is increased 25-fold in comparison to the general population (Jorde et al., 1991). While the heritability of ASD suggests a genetic component in the disorders etiology, the genes involved vary greatly among individuals and family clusters.
Whole-genome linkage studies, gene association studies, copy number variation screening and SNP analyses have uncovered a large number of ASD candidate genes (Abrahams and Geschwind, 2008). Associations with ASD have been demonstrated for genes involved in a diverse range of functions including RELN (Skaar et al., 2005), SHANK3 (Moessner et al., 2007), NLGN3, NLGN4X (Jamain et al., 2003), MET (Campbell et al., 2006), GABRB3 (Buxbaum et al., 2002), OXTR (Wu et al., 2005), and SLC6A4 (Wu et al., 2005). Furthermore, in several syndromic disorders with single gene mutations, including Rett’s syndrome (MeCP2) (Nagarajan et al., 2008), Fragile X (FMR1) (Belmonte and Bourgeron, 2006), Tuberous Sclerosis (either TSC1 or TSC2) (Wiznitzer, 2004), Timothy Syndrome (CACNA1C), Cowden’s Syndrome (PTEN), and Angelman’s Syndrome (UBE3A) the occurrence of ASD is higher than the general population. Among these potential candidate genes several play important roles in immune function. Proteins within the phosphoinositide-3-kinase (PI3K) pathway, including those coded by MET, PTEN, TSC1 and TSC2, have a major role in regulating interleukin (IL)-12 production from myeloid cells and are involved in shifting macrophage phenotypes from inflammatory (M1) to alternative activated (M2) subsets (Fukao et al., 2002). Additional candidate genes including the major histocompatibility complex type 2 (MHC-II) haplotypes (Lee et al., 2006; Torres et al., 2002), as well as complement 4B (C4B) (Odell et al., 2005), and macrophage inhibitory factor (MIF) (Grigorenko et al., 2008) are important in directing and controlling immune responses. Even with the recent advancements in identifying candidate genes involved in ASD, all identified genetic risk factors combined account for only 10–20% of the total ASD population (Abrahams and Geschwind, 2008). A number of these genetic risk factors can also be present in individuals without ASD, suggesting that many of these mutations may increase the risk of developing ASD, but additional risk factors are also necessary.
The absence of a known genetic cause in the majority of cases, and the incomplete penetrance of known genetic risk factors, suggests that environmental factors are linked with the causation of ASD. Growing research has highlighted maternal immune activation (MIA), especially during the first or second trimesters of pregnancy, as one potential environmental factor that increases the risk for ASD (Patterson, 2009). In 1964 a rubella epidemic in the U.S. which affected many pregnant mothers resulted in a large increase in the number of children that developed ASD (Chess et al., 1978; Swisher and Swisher, 1975). Moreover, using medical information obtained in a large Danish database, increased risk for ASD is associated with mothers that required hospitalization for a viral infection in the first trimester of pregnancy, or mothers hospitalized for a bacterial infection in the second trimester of pregnancy (Atladottir et al., 2010), suggesting that bacterial and viral infections may confer different risks depending on gestational age. Further data suggest that season of birth is important, with associations of increased rates of ASD associated with experiencing the first trimester of pregnancy during the winter months, timing which coincided with the influenza season (Zerbo et al., 2011). Increased psoriasis, asthma and allergies during pregnancy have also been suggested as risk factors for the development of ASD (Croen et al., 2005)
The potential role of a heightened or activated maternal immune response in the risk for ASD is further strengthened by epidemiological data from large population based studies that show increased rates of autoimmune disorders in the families of individuals with ASD (Atladottir et al., 2009; Croen et al., 2005). Separately or coincidentally, the presence of specific anti-fetal brain antibodies in approximately 12% of mothers of children with ASD, which are absent in mothers of children who are typically developing or mothers of children with developmental delays, suggests a potential inflammatory process occurring in mothers of children with ASD that leads to the production of antibodies directed to the developing brain (Braunschweig et al., 2008; Croen et al., 2008; Singer et al., 2009). Such fetal brain specific antibodies could alter neurodevelopment as is seen in Systemic Lupus Erythematosis (SLE) (DeGiorgio et al., 2001; Lee et al., 2009). In experiments using IgG collected from mothers of children with ASD, administration of these antibodies to pregnant rhesus macaques, induced stereotypic behavior and hyperactivity in the offspring, symptoms that share homology to ASD (Martin et al., 2008). Similarly, anti-brain protein reactive antibodies from mothers who have children with ASD mediate behavioral changes and neuro-pathology in the offspring of a mouse model in which pregnant dams are injected with these antibodies (Singer et al., 2009). These data suggest a potential pathogenic/pathological effect of anti-fetal brain antibodies in some mothers who have children that develop ASD.
In rodent models of MIA, several abnormal behavioral features are exhibited in the offspring that may have face validity to some autistic features, including decreased prepulse inhibition and latent inhibition, as well as impaired sociability (Reviewed in Patterson, 2009). These models are becoming more established and can be induced by congenital exposure to bacteria, the bacterial compound LPS, influenza virus or, the viral mimic and toll-like receptor (TLR)3 ligand polyinosinic:polycytidylic acid [poly(I:C)]. In all four versions of the model, IL-6 appears to play an essential role and exposure to IL-6 alone during gestation is sufficient to elicit some behavioral changes in the offspring (Hsiao and Patterson, 2010; Smith et al., 2007). The similarities between the behaviors seen in models of MIA and the symptoms of ASD have spurred further investigation into the physiological features of the offspring. For example, an increase in IL-6 is present up to 24 weeks postnatally in brains of offspring of dams exposed to poly(I:C) (Samuelsson et al., 2006). While elevated numbers of splenic TH17 cells have also been observed in offspring after maternal poly(I:C) exposure (Mandal et al., 2011). This evidence suggests that in the MIA model, there are prolonged inflammatory responses that persist in adult offspring and are likely maintained by alterations in the immune system of the affected offspring. These data showing altered immune responses in MIA affected offspring bears more than passing resemblance to features of dysfunctional immune activity frequently observed in children and adults with ASD.
2. Immune Activity in ASD
2.1 Neuroinflammation
A key finding in ASD research has been the observations of marked ongoing neuroinflammation in postmortem brain specimens from individuals with ASD over a wide range of ages (4–45 years of age) (Li et al., 2009; Morgan et al., 2010; Vargas et al., 2005). These findings include prominent microglia activation and, increased inflammatory cytokine and chemokine production, including interferon (IFN)-γ, IL-1β, IL-6, IL-12p40, tumor necrosis factor (TNF)-α and chemokine C-C motif ligand (CCL)-2 in the brain tissue and cerebral spinal fluid (Li et al., 2009; Morgan et al., 2010; Vargas et al., 2005).
Microglia are the resident mononuclear phagocytic cells of the CNS, and participate in immune surveillance of the CNS as well as synaptic pruning in normal neurodevelopment (Bessis et al., 2007). Microglia are also activated in the postmortem brain specimens of individuals with other neurological diseases of unknown genetic etiology such as Multiple Sclerosis, Alzheimer’s and Parkinson’s (Kim and de Vellis, 2005). Expression profiling of postmortem brain tissue revealed increased messenger RNA transcript levels of several immune system associated genes in ASD, further implicating neuroinflammatory processes in this disorder (Garbett et al., 2008). Moreover, a recent study looking at transcriptome organization patterns showed that gene co-expression networks reflect abnormalities in cortical patterning in the brain of ASD individuals (Voineagu et al., 2011). These findings were associated with changes in microglia and immune activation; suggesting a causative role for immune dysregulation in ongoing neurological dysfunction and synapse plasticity in the brains of individuals with ASD.
2.2 Systemic immune activation
As well as signs of neuroinflammation in ASD, there are multiple lines of evidence indicating that immune responses in the peripheral are also dysfunctional and are associated with increased severity of core and related symptoms of ASD (Table 1). Immune abnormalities were first described in individuals with ASD in 1977 (Stubbs and Crawford, 1977). Since this report several research groups, from around the world, have identified a variety of immune functions that are atypical in ASD; yet, not surprisingly, these findings are often as heterogeneous as the behavioral phenotypes which make up ASD.
Table 1.
Immune Dysfunction and Behaviors in ASD
| Studies in chronological order | # in Study | Age | Assessment Method | Immune Measure | Behavior Measure |
|---|---|---|---|---|---|
| (Scifo et al., 1996) | 12 | 7–15 | BSE and CARS | Ratio of CD4 to CD8 positive T-cells | Administration of naltrexone led to reduction of “autistic” symptomology and increased ratio of CD4 to CD8 cells |
| (Gupta et al., 1996) | 25 | 3–12 | No formal testing | immunoglobuli n levels | IVIG resulted in improvements in eye contact, calmer behavior, improvement in speech and echolalia |
| (Plioplys, 1998) | 10 | 4–17 | No formal testing | IVIG | IVIG treatment resulted in transient improvements in attention span and hyperactivity; improvement declined after 5 months |
| (Sandler et al., 2000) | 11 | 3–6 | CARS | GI issues | Improvements in communication and asocial behaviors after vancomycin treatment, but were lost after conclusion of treatment |
| (Shenoy et al., 2000) | 1 | 2 | No formal testing | Autoimmune liymphoprolfer ative syndrome(ALPS) | Treatment with prednisone resulted in marked improvement in social and communication skills |
| (Curran et al., 2007) | 60 | 2–18 | ABC | Fever | Fewer aberrant behaviors were recorded for subjects with fever (>100.4°F) compared with controls. Improvements were transient. |
| (Ashwood et al., 2008) | 143 | 2–5 | ADI-R, ADOS, SCQ, VABS, MSEL, ABC | Plasma levels of active TGFβ1 | Lower TGFβ1 levels were associated with lower adaptive behaviors and worse behavioral symptoms |
| (Iwata et al., 2008a) | 37 | 20–25 | ADI-R | Plasma levels of P-selectin | Lower levels of P-selectin associated with poor social development |
| (Heuer et al., 2008) | 271 | 2–5 | ADI-R, ADOS, ABC | IgG levels in plasma | Decreased IgG associated with increased aberrant behaviors |
| (Grigorenko et al., 2008) | 1059 | n.s. | ADI-R and ADOS | Genotyping of the MIF gene, and plasma levels of MIF(n=20) | Plasma MIF levels were positively correlated with worse scores on ADOS for social impairment and imaginative skills |
| (Onore et al., 2009) | 60 | 2–5 | ADOS, ADI-R, MSEL, VABS and ABC | Induced cytokine response to PHA | Negative correlation between PHA induced IL-23 production and sociability scores of the ADOS |
| (Enstrom et al., 2010) | 30 | 2–5 | ADI-R, ADOS, SCQ, VABS, MSEL, ABC | Monocyte TLR ligand stimulation | More impaired social behaviors and non-verbal communication are associated with increased production of IL-1β and IL-6 after TLR4 stimulation |
| (Ashwood et al., 2010) | 139 | 2–5 | ADI-R, ADOS, SCQ, VABS, MSEL, ABC | Induced cytokine response to PHA and LPS | Pro-inflammatory or TH1 cytokines were associated with greater impairments in core features of ASD as well as aberrant behaviors; GM-CSF and TH2 cytokines were associated with better cognitive and adaptive function |
| (Goines et al., 2010) | 466 | 2–5 | ADI-R, ADOS, SCQ, VABS, MSEL, ABC | Antibodies directed against a 45 or 62 kDa cerebellum protein | Children with antibodies directed against a 45 kDa cerebellum protein had increased, lethargy and stereotypy; children with antibodies against a 62 kDa cerebellum protein showed increased aberrant behaviors on the VABS composite standard score |
| (Kajizuka et al., 2010a) | 62 | 6–19 | ADI-R | Serum levels of PDGF | Increased serum levels of PDGF-BB homodimers positively associated with increased restricted, repetitive and stereotyped patterns of behavior and interests |
| (Ashwood et al., 2011c) | 175 | 2–5 | ADI-R, ADOS, SCQ, VABS, MSEL, ABC | Plasma Chemokines CCL2, CCL5 and eotaxin | Plasma chemokine levels associated with higher aberrant behavior scores and more impaired developmental and adaptive function |
| (Ashwood et al., 2011b) | 223 | 2–5 | ADI-R, ADOS, SCQ, VABS, MSEL, ABC | Plasma levels of cytokines IL-1β, IL-6, IL-8 and IL-12p40 | Elevated cytokine levels in plasma were associated with more impaired communication and aberrant behaviors |
BSE = Behavior Summarized Evaluation; ABC = Aberrant Behavior Checklist; CARS = Childhood autism rating scale; ADI-R = Autism Diagnostic Interview, Revised; ADOS = Autism Diagnostic Observation Schedule; SCQ = Social Communication Questionnaire; VABS = Vineland Adaptive Behavior Scale; GI = gastrointestinal; MIF = macrophage migration inhibitory factor; PDGF = platelet derived growth factor
Proteomic analysis indicates that the levels of many immune proteins in plasma/sera, such as cytokines, chemokines, complement proteins, adhesion molecules and growth factors are altered in ASD. Notably, increased plasma levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8 and IL-12p40 as well as MIF and platelet derived growth factor (PDGF), have been reported in ASD (Ashwood et al., 2011b; Grigorenko et al., 2008; Kajizuka et al., 2010). Moreover, elevated levels of these cytokines in the plasma were found to be associated with poor communication and impaired social interaction behaviors (Table 1). Plasma levels of chemokines CCL2 and CCL5 are also higher in ASD and again are associated with worsening behavioral scores (Ashwood et al., 2011c). In parallel, decreased circulating levels of the anti-inflammatory cytokine, transforming growth factor (TGF)-β, has been documented in ASD, with lower levels associated with worsening behavioral scores (Ashwood et al., 2008; Okada et al., 2007). Collectively, these data reveal a trend towards pro-inflammatory immune activity and away from regulatory measures in ASD. As many of the cytokines have profound effects on neuronal development, migration, differentiation and synapse formation (see below), a disrupted balance in the cytokine milieu may directly influence neurodevelopment, early brain development and alter behavior. To this end it is of note that the shift in cytokine balance in ASD is associated with greater impairments in key autism behavioral domains including social interaction and communication, as well as associated features such as aberrant behaviors (Table 1).
The occurrence of a differential antibody repertoire has been studied extensively in ASD. For example, decreased total levels of IgM and IgG classes of immunoglobulin have been reported, with lower levels found to correlate with more aberrant behaviors (Heuer et al., 2008). However, within this profile, atypical antibody isotype levels are frequently reported in the plasma of individuals with ASD including increases in levels of the neutralizing IgG4 antibodies (Croonenberghs et al., 2002; Enstrom et al., 2009a). Antibodies reportedly reactive to human and non-human primate brain and CNS proteins have also been described in children and adults with ASD. Using Western blotting and ELISA techniques an increased presence of antibodies can be detected in ASD that exhibit reactivity against a diverse set of targets or specificities. These targets vary between studies, many of which have not be replicated but have at one time included; antibodies against serotonin receptors (Singh et al., 1997a), myelin basic protein (Singer et al., 2006; Singh et al., 1993; Vojdani et al., 2002), heat shock proteins (Evers et al., 2002), glial filament proteins (Singh et al., 1997b), as well as various brain tissue proteins that have yet to be identified (Cabanlit et al., 2007; Silva et al., 2004; Singer et al., 2006; Todd et al., 1988). It is tempting to speculate that the increased diversity and lack of a single specific target is due to antibody generation as a result of cellular damage and the emergence/revealing of sequestered or new epitopes. A similar finding is seen in many autoimmune diseases where diverse clonal antibody generation occurs throughout the course of the disease, such as in SLE and multiple sclerosis (MS). Whether a single antibody or cell response is responsible for initiating this cascade or whether all the antibodies reactive to brain tissue hitherto observed in ASD are as a result of cell/tissue damage, requires further research. Indeed, in contrast to that observed for the maternal antibodies (discussed above) (Martin et al., 2008; Singer et al., 2009), what role auto-antibodies play in children with ASD is unknown and it has yet to be demonstrated whether any of these antibodies block receptor function, activate neuronal/glial cells, induce cellular damage or have any pathological consequence.
In SLE, N-methyl-D-aspartate receptor (NMDAR) reactive antibodies are capable of mediating negative effects on cognitive function (DeGiorgio et al., 2001). Although antibody passage through the BBB is restricted, these antibodies can be found in the CNS (Steup-Beekman et al., 2007). In SLE, these brain protein reactive antibodies are implicitly involved in cognitive impairments in individuals who carry them, and are also suspected to exert pathological effects on fetal neurodevelopment during gestation (Lee et al., 2009). It is possible that the brain protein-reactive antibodies found in the plasma of children with ASD may mediate pathology in a similarly direct fashion. Some experimental evidence has shown, that irrespective of the target epitope, antibodies from ASD subjects bind specifically to cerebellar interneurons and golgi type II cells in tissue obtained from rhesus macaque monkeys (Wills et al., 2009; Wills et al., 2011). The binding of these antibodies to specific cellular targets could lead to decreased or increased cellular activity. Moreover, the presence of these antibodies in children with ASD correlated strongly with antibody reactivity observed to a 45 kD cerebellar protein of unknown identity and was positively associated with worsening of aberrant behavior in ASD (Goines et al., 2011). Elucidating the exact target of these cells and their function on neuronal cell cultures in vitro would help to establish their role in the pathogenesis of ASD.
Cell damage/death can be induced following the binding of complement proteins to antibodies and may be a mechanism in which auto-reactive antibodies contribute to ASD pathology. Moreover, the complement proteins C1q and C3, which classically make up a portion of the immune complement cascade, are also involved in synaptic scaling. Complement mediates synaptic pruning by opsonizing synapses, effectively targeting them for removal by phagocytic microglia (Gasque et al., 2002). An increase in complement proteins, including the lytic component C1q, has been shown in sera from children with ASD compared to age, ethnicity and gender matched typically developing children (Corbett et al., 2007). Co-localization of IgG and C1q has also been reported in the mucosa of children with ASD (Torrente et al., 2002; Torrente et al., 2004). Whether the antibodies specific for CNS proteins are the same as those in the mucosa and whether they are capable of fixing complement and eliciting cellular damage in ASD is not known. In particular, the potential interaction between auto-antibodies specific for GABAergic interneurons and complement in children with ASD requires further investigation. Increased complement production in ASD may therefore modulate neuronal function in several ways either by synapse pruning or through an interaction with specific auto-reactive antibodies leading to cell death.
2.3 Adaptive cellular response
The examination of immune cell function in ASD has been hindered by problems arising from study design including the use of small sample sizes, variable diagnostic criteria, non-matching of cases and controls for gender or age and, the use of unevaluated siblings as controls. Such issues have plagued the field and led to confusion in the interpretation of the various study findings. Despite these drawbacks many studies have observed reproducible findings of altered cellular function in ASD. Among these findings atypical adaptive T cell responses are repeatedly observed from individuals with ASD (Ashwood et al., 2010). A predominance of IL-4+ IFN-γ− T cells were observed in the circulating CD4+ T cell population in individuals with ASD (Gupta et al., 1998). This bias towards a TH2 phenotype and reduced TH1 responses has been observed in a number of other studies. In corroboration with this finding, we observed increased mononuclear cell production of IL-13 and GM-CSF in response to PHA stimulus, while IFN-γ was decreased. Production of the pro-inflammatory cytokine TNF-α was also increased in response to in vitro stimulation, and is consistent with an activated TH2 immune response in humans. Moreover increased TNF-α production was associated with increased stereotypical behaviors a hallmark symptom of ASD (Ashwood et al., 2010). Analysis of intracellular cytokine production showed an increase in frequency of TNF-α+ T cells but reduced frequency of IL-10+ T cells in both peripheral and intestinal mucosal tissue in children with ASD (Ashwood et al., 2004; Ashwood and Wakefield, 2006). These studies show, once again, a shift towards a pro-inflammatory cytokine milieu and mirror the plasma cytokine data.
The activation profile of circulating T cell phenotypes was also different in ASD and CD3+ T cells display higher levels of HLA-DR, a marker of late cellular activation (Ashwood et al., 2011a). In addition, CD26 (dipeptidyl peptidase IV), a marker associated with an effector cell phenotype in human CNS disorders such as multiple sclerosis, was increased on CD8+ T cells (Ashwood et al., 2011a). Following in vitro stimulation an altered pattern of co-stimulatory and activation markers was observed, with increased expression of CD137 (4-1BB) but decreased CD134 (OX40) and CD25 (IL-2 a receptor) on T lymphocytes of children with ASD (Ashwood et al., 2010). Increased T cell activation may also be linked with decreased apoptosis leading to the survival of activated cells that would otherwise be eliminated (Ashwood et al., 2011a), a feature that has been described in chronic inflammatory conditions such as Crohn’s disease (Monteleone et al., 2006). Collectively, evidence of atypical cytokine production, altered T cell activation and potential impaired apoptotic activity suggest there is a predisposition to chronic inflammation which could negatively affect healthy cognitive development in ASD. Exciting findings from animal models suggest that neurogenesis is modulated by the interaction between T cells and CNS cells (Ziv et al., 2006; Ziv and Schwartz, 2008). Altered T cell activation in ASD may therefore directly affect the course of neurodevelopment. However, whether the T cells that are active in the periphery in ASD also interact with CNS tissue is not known.
Adhesion molecules known to control the passage of T cells across endothelial barriers play an important role in mediating T cell passage and T cell/CNS interactions. Circulating levels of soluble adhesion molecules P-Selectin, L-Selectin and PECAM-1 accurately represent levels on endothelial cells. In high functioning individuals with ASD levels of sPECAM-1, sP-Selectin and sL-selectin were decreased compared with controls (Iwata et al., 2008; Tsuchiya et al., 2007). Furthermore, lower levels of P-Selectin were associated with more impaired social skills (Iwata et al., 2008). These data suggest that modulating immune cell access to the brain in ASD may influence abnormal social interactions. In line with these findings, during fever episodes, some children with ASD show a transient improvement in behaviors that diminishes back to baseline after the child’s fever improves (Curran et al., 2007). During fever, upregulation of adhesion molecules and changes in endothelial barriers occurs as a result of pyrogenic cytokine release. In ASD it is possible that fever may evoke a transient increase in T cell-brain interactions and hence an improvement in behavior. These data are provocative and suggest that immune activation, including activation of T-lymphocyte subsets, could be important in improving behaviors in some individuals with ASD.
2.4 Innate cellular response
As well as changes in adaptive immune responses the activity of a number of other cell subsets has been described, including atypical natural killer (NK) cell activity. Reduced ability of NK cells to kill K562 target cells in ASD was first described by Warren et al. in 1987 (Warren et al., 1987) and has now been confirmed in more contemporary reports (Enstrom et al., 2009b; Vojdani et al., 2008). In addition to reduced lytic activity, changes in several factors that contribute to NK cell activity such as perforin, granzyme B and IFN-γ have been identified in ASD. NK cells appear to produce higher levels of perforin, granzyme B, and IFN-γ while under resting conditions in children with ASD (Enstrom et al., 2009b). These data suggest that in vivo, there is increased NK cell activation; however, following a strong in vitro signal, such as with target cells, NK cells from children with ASD are unable to produce more of their effector molecules thus leading to the reduced capability to lyse the targets (Enstrom et al., 2009b). As early responders of the innate immune system, NK cells help shape the initial immune response during an inflammatory event and aberrant activity of these cells are likely an important contributor to the atypical immune activity observed in individuals with ASD.
Monocytes are among the first responders during inflammation, and are robust cytokine producers, creating a cytokine milieu that profoundly influences the activity of neighboring immune cells. Monocytes also serve as precursors for a number of tissue specific myeloid lineage cells including macrophages, dendritic cells, and microglia (Djukic et al., 2006; Geissmann et al., 2010). An increased number of circulating monocytes has been reported in ASD (Sweeten et al., 2003). Furthermore, upregulation of activation markers on circulating CD14+ monocytes suggest that these cells are activated in vivo (Ashwood et al., 2011a). In research conducted in our laboratory, an atypical pattern of cytokine responses were observed following TLR agonist stimulation of isolated CD14+ monocytes from young children with ASD (Enstrom et al., 2010). Specifically, increased inflammatory cytokine production, IL-1β, TNFα and IL-6, was observed in response to the TLR2 ligand, lipoteichoic acid (LTA) and to a lesser extent the TLR4 ligand, LPS. Conversely, reduced cytokine production was observed in response to a TLR9 agonist, an unmethylated CpG repeat synthetic oligonucleotide, resembling bacterial DNA. Furthermore, increased monocyte IL-6 and IL-1β production in response to LPS stimulation was associated with more impaired social behavior in individuals with ASD (Table 1). These atypical monocyte responses are intriguing, and indicate abnormal myeloid involvement in ASD. Hyperactivation of myeloid cells in ASD is implicated in both the periphery and CNS, as increased infiltration of monocytes and perivascular macrophages are observed in brain specimens from individuals with ASD (Vargas et al., 2005). Moreover, there is a striking similarity in cytokine levels in the plasma of children with ASD that exhibit a profile representative of myeloid cell activation, i.e. the production of IL-12p40, TNFα, IL-1β and IL-6 (Ashwood et al., 2011b).
3. Potential impact of immune dysfunction in ASD on CNS activity and behavior
Although a singular pathology of ASD remains elusive, a wealth of evidence suggests that ASD symptoms may be related to immune dysfunction (Careaga et al., 2010; Enstrom et al., 2009c; Korade and Mirnics, 2011). Further detailed investigations are needed to concretely identify whether the immunological findings in ASD converge to a single immunopathology. However, in the following section we will try and identify potential mechanisms of action in which the observed immune dysfunction in ASD could impact neuronal function and behavior in ASD.
Activity of the immune system can elicit profound effects on behavior (Table 2). Several immune proteins function within the nervous system as mediators of normal neurodevelopment (Deverman and Patterson, 2009). Cytokines, such as TNF-α, IL-1β, the TGF-β family of molecules, and the gp130 ligand family mediate direct effects on neuronal activity. For example, TNF-α is produced by wide variety of cells during an inflammatory event (Gruys et al., 2005) and, in addition to its role in inflammation, can modulate neuronal cell proliferation or cell death, and play an important role in synaptic pruning (Cacci et al., 2005; Stellwagen and Malenka, 2006; Widera et al., 2006). Other neuropoeitic cytokines, such as IL-1β and IL-6, also exert varied affects on neuronal survival, proliferation, synapse formation, migration, and differentiation. IL-6 is member of the gp130 ligand family that includes IL-11, ciliary neurotrophic factor (CNTF), oncostatin M, and leukocyte inhibitory factor (LIF), that play important roles in promoting and maintaining oligodendrocyte (ODC) survival in the CNS (Deverman and Patterson, 2009). Generation of IL-6 knock-out mouse models results in viable offspring; however, they display impaired recognition memory, suggesting an essential role for IL-6 in learning (Baier et al., 2009; Hryniewicz et al., 2007). Both IL-6 and IL-1β are involved in mediating “sickness behavior,” an adaptive change in behaviors accompanying inflammation that are characterized by lethargy, depression, loss of appetite, anxiety, impaired ability to focus and social withdrawal (Harden et al., 2008). Notably, the social withdrawal of sickness behavior can be alleviated when animals are given minocycline, a broad spectrum antibiotic with anti-inflammatory qualities (Henry et al., 2008).
Table 2.
Influence of cytokines on behaviors with relevance to ASD
| Cytokine | CNS effects relevant to the symptoms of ASD |
|---|---|
| IL-1β |
|
| IL-2 |
|
| IL-4 |
|
| IL-6 |
|
| TNF-α |
|
In addition, the cytokines IL-2 and IL-4 have been shown to influence repetitive and cognitive behaviors. Treatment of mice with IL-2 results in increased “climbing behavior” that are thought to denote repetitive behaviors, a pattern of behaviors that are characteristic of ASD (Zalcman, 2002). IL-4 knock-out mice show impaired cognition, possibly as a result of loss of T-cell function in the CNS (Derecki et al., 2010a). There is some evidence in individuals with ASD that cognition is impaired; however, largely due to the deficits in verbal and non-verbal communication as well as poor social interaction in ASD, the specific nature of these cognitive issues has been hard to define and remain somewhat controversial.
Collectively these findings suggest that cytokines are both necessary for normal neurodevelopment and behavior and that any perturbation in the cytokine network can impact neurodevelopment. Observed increases in TNF-α, IL-1β and IL-6 in in blood, CSF and brain tissues from children with ASD represents the first piece of the puzzle of a disrupted neuro-immune network in ASD. Associations with greater impairment in core autistic behaviors and increased cytokine levels highlight a potential new avenue of research in which cytokine patterns could be manipulated to benefit behavioral outcome in ASD. For example, minocycline has been shown to successfully alleviate aberrant behavior symptoms in children with Fragile X, a neurodevelopmental disorder with high rates of ASD (Paribello et al., 2010).
In ASD, linkage with specific MHC molecules has been frequently reported (Lee et al., 2006; Torres et al., 2001; Torres et al., 2002; Torres et al., 2006). In addition, two large GWAS studies in schizophrenia highlight changes within the MHC region on chromosome 6 (Shi et al., 2009; Stefansson et al., 2009). These data suggest that abnormalities in the expression of MHC genes and their effects on brain development and synaptic function may be involved in the pathogenesis of complex neurodevelopmental disorders such as ASD and schizophrenia. Until relatively recently neurons were considered to be negative for the MHC I molecules; however, emerging data support the role of these molecules in CNS development (McAllister and van de Water, 2009). MHCI is expressed on virtually every cell type within the body, and can display cytosolic proteins to CD8+ T cells, alerting them to the presence of non-self proteins, for example, during a viral infection. In the CNS, MHCI plays a dual role in regulating synaptic scaling, likely by engaging with a CD3ζ or PIRB molecules (Shatz, 2009). MHCI is expressed in the CNS under non-inflammatory conditions, and correlates with times and regions of synaptic plasticity (Corriveau et al., 1998). Double knock-out of the β-2-microglobulin (β2m) subunit of the MHCI molecule, and the transporter with antigen processing (TAP) protein, virtually eliminates all allotypes of MHCI from the cell surface (Neefjes and Momburg, 1993). The elimination of functional MHCI results in atypical synaptic plasticity in animal models, further illustrating the essential role for MHCI in synapse remodeling (Goddard et al., 2007). Thus, the role of MHC in ASD may be two fold, firstly, by their ability to modulate synapse formation during development and, secondly, in their role in shaping the T cell repertoire and the specificity, diversity and conformation of antigens that are presented to T cells.
Microglial cells are uniquely positioned to robustly respond to immune signals, and influence the CNS environment, through the production of inflammatory cytokines and the generation of reactive oxygen species (ROS) within the CNS (Garden and Moller, 2006; Hanisch and Kettenmann, 2007). The presence of activated perivascular macrophages and microglia have been described in brain specimens in ASD (Morgan et al., 2010; Vargas et al., 2005; Voineagu et al., 2011); however, what the cause of this hyperactive state is still unknown. The phagocytosis of dead or dying neurons by microglia is believed to be a normal and relatively non-inflammatory function (Bessis et al., 2007). However, upon phagocytosis of antibody-bound targets, microglia produce increased inflammatory cytokines and ROS (Ulvestad et al., 1994). The potentially pro-inflammatory activity of microglia in ASD could be associated with the presence of brain-reactive antibodies in the CNS. Similarly, dysregulated complement production in ASD may have a similar effect (Corbett et al., 2007; Odell et al., 2005), as complement opsonization can mediate the phagocytosis of neurons by microglia (Gasque et al., 2002). Peripheral and or neuronal production of cytokines could also lead to an activated profile in these cells. For example, increased GM-CSF production has been detected in ASD and will drive the release of myeloid progenitor cells that can become tissue macrophages (Ashwood et al., 2010; Ashwood et al., 2011c). In turn the production of cytokines from microglia and macrophages will influence neuronal survival, proliferation, function and synaptic plasticity.
Furthermore, perhaps due to their activities as immunological sentinels of the CNS, or their role in synaptic pruning, genetic abnormalities in microglia can result in profound effects on behavior. Recently, a landmark paper described the source of repetitive grooming behavior in Hoxb8−/− mice (Greer and Capecchi, 2002). The Hoxb8 protein is expressed exclusively in bone marrow-derived microglia in the CNS, and deficiency in this protein results in reduced numbers of bone marrow derived microglia cells within the CNS. Transplantation of bone marrow containing functional Hoxb8 was sufficient to restore normal grooming behavior in these mice, providing solid evidence of the role of microglia cells in the atypical grooming behavior of Hoxb8−/− mice (Chen et al., 2010). These data also suggests that atypical microglia function plays a role in repetitive behaviors, a characteristic of several disorders including ASD. In addition, DAP12 and the triggering receptor expressed on myeloid cells 2 (TREM2) protein form a complex in myeloid cells which is reportedly exclusively expressed in microglia cells within the CNS (Daws et al., 2001). This complex is essential for the phagocytosis of apoptotic cells, and for limiting inflammatory cytokine production in the CNS (Takahashi et al., 2005). A loss of function mutation of genes encoding either TREM2 or DAP12 results in Nasu–Hakola disease, also known as polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL). PLOSL results in cysts within the bone, and early onset dementia (Paloneva et al., 2001). The disorder is characterized by severe microglia activation in brain tissue. The example of PLOSL suggests that unchecked microglia driven inflammation can contribute to cognitive degeneration, and bears some resemblance to the microglia activation observed in individuals with ASD. A number of genetic risk factors for ASD such as MET and MIF, are known to directly impact the function of myeloid cells and their activation status and if these cells are skewed towards a tolerance or pro-inflammatory inducing phenotype. Further investigation of genes that control microglia function in ASD could provide clues to the pathogenesis and potential treatment of this disorder.
Inflammatory activity of microglia may be influence by polarization towards an inflammatory (M1) or alternatively activated (M2) phenotype. Recently, in a model of T cell deficiency and cognitive impairment, administration of M2 microglia was beneficial to cognitive performance (Derecki et al., 2010b). The recent finding of altered transciptome profiles in microglia in brain specimens from individuals with ASD (Voineagu et al., 2011) also suggests that environmental factors may play a role ion the activation of these cells. TH2 cells generate IL-4, and IL-10, skewing the microenvironment and potentially polarize neighboring microglia into an alternative M2 phenotype (Fairweather and Cihakova, 2009). In ASD the data suggest that a TH2 cytokine profile may predominate (Gupta et al 1998; Ashwood et al., 2010) and may thereby skew the microglia/macrophage response. This data highlights the importance of all arms of the immune system in immune regulation within the CNS, and the bi-directional regulation between the adaptive and innate immune system required to maintain a healthy neuro-immune environment, that may be dysfunctional in ASD.
Overall, although ASD primarily affects brain function (especially affect, social functioning and cognition) evidence of extensive immune dysfunction suggest other systems are also disrupted. The findings so far point towards a disruption of many facets of the immune responses including polymorphisms in immune genes that control and regulate function of the immune cells, microglia and astroglia activation, the production of pro-inflammatory cytokines, increased presence of CNS reactive antibodies, T cell activation and innate immune activation. Research into the immune connections in ASD is still in its infancy and, at this stage, centralizing the findings so far into one unifying theory is difficult and will only be achieved through further analysis of immune dysfunction in individuals with ASD.
4. Conclusion
The collective findings of immune aberration in ASD, and the effects of immune dysfunction in normal neurodevelopment, are difficult to ignore. Despite several early challenges the evidence of immune cell dysfunction in ASD has continued to grow. In conjunction, recent basic research has provided further evidence of how the immune system can profoundly impact neurodevelopment, cognitive function, and behavior. The dysfunctional immune activity observed in ASD spans both innate and adaptive arms of the immune system, and suggest perturbations in either area may have profound effects on neurodevelopment. Cytokines that have been observed at atypical levels in ASD, including the brain tissue, CSF, circulating blood, and GI tissues, can alter neuronal survival and proliferation. Similarly, cellular dysfunction observed in ASD may contribute to atypical CNS function in a number of ways including the production of cytokines, abnormal cell lysis and generation of brain-reactive antibodies. Abnormal levels of complement proteins and linkage to specific MHC molecules, have been repeatedly observed as in ASD, and may suggest that a role for immune function in synaptic pruning/plasticity in ASD.
The underlying cause of immune abnormalities in ASD may extend from genetic to maternal immune activation, or any number of unknown causes. Data collected thus far, suggests a wide ranging effect of immune dysfunction on behavioral outcome in ASD. Due to the heterogeneous nature of ASD, it is unlikely that all immune perturbations stem from a single origin; however, this cannot discount that they share some key commonalities that have a pathological effect on behavior and which is not yet fully understood. While a panacea for such a heterogeneous disorder may never exist, evidence suggests that manipulation of the immune response could improve core features of ASD as well as associated aberrant behaviors. A firm understanding of immune dysfunction in ASD will be essential to develop such therapies. Identifying a convergent effect of immune function on neurodevelopment and behavioral symptoms should be a focus future research.
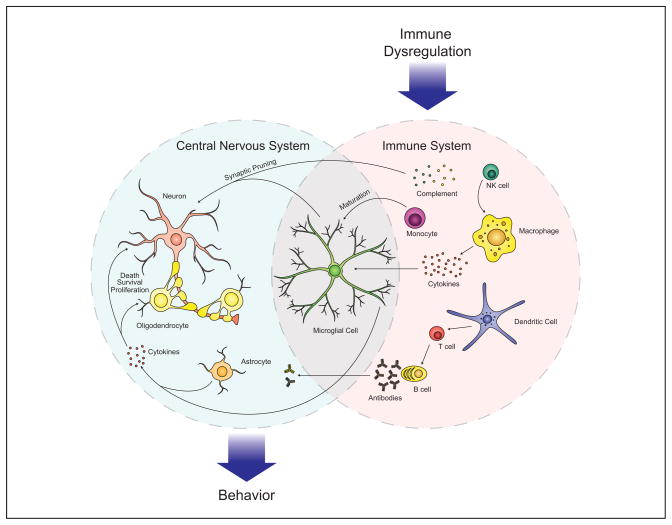
Figure.
Immune dysfunction in ASD involves a network of interactions between several cell types, from the innate and adaptive arms of the immune system. The CNS is selective but several immune factors mediate profound effects of CNS function. Increased cytokine production, such TNF-α and IL-1β inhibit neurogenesis and promote neuron death, while IL-6 may promote the growth and proliferation of neurons and oligodendrocytes. Increased levels of complement proteins can participate in synaptic scaling, opsonizing synapses and targeting them for removal by phagocytic microglia. Activated microglia may additionally mediate synaptic pruning via MHCI interactions. Collectively this immune dysfunction in ASD can exert several negative effects on behavior, including impaired cognitive function, and social withdrawal as well as aberrant behavior observed in ASD.
Research Highlight.
Immune dysfunction is increasingly observed in autism contributing to pathophysiology by impacting neurobiological process, core features and associated behaviors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc; Arlington, VA: 2000. [Google Scholar]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24:664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Corbett BA, Kantor A, Schulman H, Van de Water J, Amaral DG. In search of cellular immunophenotypes in the blood of children with autism. PLoS One. 2011a;6:e19299. doi: 10.1371/journal.pone.0019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011b;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2010;25(5):840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011c;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav Brain Res. 2009;200:192–196. doi: 10.1016/j.bbr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. J Pediatr. 1978;93:699–703. doi: 10.1016/s0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. Am J Psychiatry. 2000;157:2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Kantor AB, Schulman H, Walker WL, Lit L, Ashwood P, Rocke DM, Sharp FR. A proteomic study of serum from children with autism showing differential expression of apolipoproteins and complement proteins. Mol Psychiatry. 2007;12:292–306. doi: 10.1038/sj.mp.4001943. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64:583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, Egyed B, Deboutte D, Maes M. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120:e1386–1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- Daws MR, Lanier LL, Seaman WE, Ryan JC. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur J Immunol. 2001;31:783–791. doi: 10.1002/1521-4141(200103)31:3<783::aid-immu783>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010a;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun. 2010b doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–2403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009a;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, Hertz-Picciotto I, Van de Water JA, Sharp FR, Ashwood P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009b;23:124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Van de Water JA, Ashwood P. Autoimmunity in autism. Curr Opin Investig Drugs. 2009c;10:463–473. [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers M, Cunningham-Rundles C, Hollander E. Heat shock protein 90 antibodies in autism. Mol Psychiatry. 2002;7(Suppl 2):S26–28. doi: 10.1038/sj.mp.4001171. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gasque P, Neal JW, Singhrao SK, McGreal EP, Dean YD, Van BJ, Morgan BP. Roles of the complement system in human neurodegenerative disorders: pro-inflammatory and tissue remodeling activities. Mol Neurobiol. 2002;25:1–17. doi: 10.1385/mn:25:1:001. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, Mulder EJ, de Bildt A, Minderaa RB, Volkmar FR, Chang JT, Bucala R. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122:e438–445. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6:1045–1056. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85:106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22:838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz A, Bialuk I, Kaminski KA, Winnicka MM. Impairment of recognition memory in interleukin-6 knock-out mice. Eur J Pharmacol. 2007;577:219–220. doi: 10.1016/j.ejphar.2007.08.046. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the Maternal Immune System Induces Endocrine Changes in the Placenta via IL-6. Brain, Behavior, and Immunity. 2010 doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Tsuchiya KJ, Mikawa S, Nakamura K, Takai Y, Suda S, Sekine Y, Suzuki K, Kawai M, Sugihara G, Matsuzaki H, Hashimoto K, Tsujii M, Sugiyama T, Takei N, Mori N. Serum levels of P-selectin in men with high-functioning autism. Br J Psychiatry. 2008;193:338–339. doi: 10.1192/bjp.bp.107.043497. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde LB, Hasstedt SJ, Ritvo ER, Mason-Brothers A, Freeman BJ, Pingree C, McMahon WM, Petersen B, Jenson WR, Mo A. Complex segregation analysis of autism. Am J Hum Genet. 1991;49:932–938. [PMC free article] [PubMed] [Google Scholar]
- Kajizuka M, Miyachi T, Matsuzaki H, Iwata K, Shinmura C, Suzuki K, Suda S, Tsuchiya KJ, Matsumoto K, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Takei N, Mori N. Serum levels of platelet-derived growth factor BB homodimers are increased in male children with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:154–158. doi: 10.1016/j.pnpbp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, Reiss AL, Pearlson GD. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry. 2004;161:539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Mirnics K. Gene expression: The autism disconnect. Nature. 2011;474:294–295. doi: 10.1038/474294a. [DOI] [PubMed] [Google Scholar]
- Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, Diamond B. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Zachary AA, Leffell MS, Newschaffer CJ, Matteson KJ, Tyler JD, Zimmerman AW. HLA-DR4 in families with autism. Pediatr Neurol. 2006;35:303–307. doi: 10.1016/j.pediatrneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun. 2011;25:863–871. doi: 10.1016/j.bbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, van de Water J. Breaking boundaries in neural-immune interactions. Neuron. 2009;64:9–12. doi: 10.1016/j.neuron.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MMWR. Prevalence of Autism Spectrum Disorders --- Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill. 2009 Summ;58:1–20. [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone I, Monteleone G, Fina D, Caruso R, Petruzziello C, Calabrese E, Biancone L, Pallone F. A functional role of flip in conferring resistance of Crohn’s disease lamina propria lymphocytes to FAS-mediated apoptosis. Gastroenterology. 2006;130:389–397. doi: 10.1053/j.gastro.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Nagarajan RP, Patzel KA, Martin M, Yasui DH, Swanberg SE, Hertz-Picciotto I, Hansen RL, Van de Water J, Pessah IN, Jiang R, Robinson WP, LaSalle JM. MECP2 promoter methylation and X chromosome inactivation in autism. Autism Res. 2008;1:169–178. doi: 10.1002/aur.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes JJ, Momburg F. Cell biology of antigen presentation. Curr Opin Immunol. 1993;5:27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- Odell D, Maciulis A, Cutler A, Warren L, McMahon WM, Coon H, Stubbs G, Henley K, Torres A. Confirmation of the association of the C4B null allelle in autism. Hum Immunol. 2005;66:140–145. doi: 10.1016/j.humimm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Sugiyama T, Kawai M, Minabe Y, Takei N, Mori N. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Onore C, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen R, Van de Water J, Ashwood P. Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. J Neuroimmunol. 2009;216:126–134. doi: 10.1016/j.jneuroim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloneva J, Autti T, Raininko R, Partanen J, Salonen O, Puranen M, Hakola P, Haltia M. CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology. 2001;56:1552–1558. doi: 10.1212/wnl.56.11.1552. [DOI] [PubMed] [Google Scholar]
- Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM, Ethell DW. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 2010;10:91. doi: 10.1186/1471-2377-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural brain research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Crowe RR, Oksenberg JR, Mirel DB, Kendler KS, Freedman R, Gejman PV. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SC, Correia C, Fesel C, Barreto M, Coutinho AM, Marques C, Miguel TS, Ataide A, Bento C, Borges L, Oliveira G, Vicente AM. Autoantibody repertoires to brain tissue in autism nuclear families. J Neuroimmunol. 2004;152:176–182. doi: 10.1016/j.jneuroim.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Singh VK, Singh EA, Warren RP. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol Psychiatry. 1997a;41:753–755. doi: 10.1016/S0006-3223(96)00522-7. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997b;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7:97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, DeLong GR, Moore JH, McCauley JL, Sutcliffe JS, Ashley-Koch AE, Cuccaro ML, Folstein SE, Gilbert JR, Pericak-Vance MA. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10:563–571. doi: 10.1038/sj.mp.4001614. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Steup-Beekman G, Steens S, van Buchem M, Huizinga T. Anti-NMDA receptor autoantibodies in patients with systemic lupus erythematosus and their first-degree relatives. Lupus. 2007;16:329–334. doi: 10.1177/0961203307078224. [DOI] [PubMed] [Google Scholar]
- Stubbs EG, Crawford ML. Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr. 1977;7:49–55. doi: 10.1007/BF01531114. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. 2003;160:1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- Swisher CN, Swisher L. Letter: Congenital rubella and autistic behavior. N Engl J Med. 1975;293:198. [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, Hickok JM, Anderson GM, Cohen DJ. Antibrain antibodies in infantile autism. Biol Psychiatry. 1988;23:644–647. doi: 10.1016/0006-3223(88)90012-1. [DOI] [PubMed] [Google Scholar]
- Torrente F, Anthony A, Heuschkel RB, Thomson MA, Ashwood P, Murch SH. Focal-enhanced gastritis in regressive autism with features distinct from Crohn’s and Helicobacter pylori gastritis. Am J Gastroenterol. 2004;99:598–605. doi: 10.1111/j.1572-0241.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- Torrente F, Ashwood P, Day R, Machado N, Furlano RI, Anthony A, Davies SE, Wakefield AJ, Thomson MA, Walker-Smith JA, Murch SH. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol Psychiatry. 2002;7:375–382. 334. doi: 10.1038/sj.mp.4001077. [DOI] [PubMed] [Google Scholar]
- Torres AR, Maciulis A, Odell D. The association of MHC genes with autism. Front Biosci. 2001;6:D936–943. doi: 10.2741/torres. [DOI] [PubMed] [Google Scholar]
- Torres AR, Maciulis A, Stubbs EG, Cutler A, Odell D. The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum Immunol. 2002;63:311–316. doi: 10.1016/s0198-8859(02)00374-9. [DOI] [PubMed] [Google Scholar]
- Torres AR, Sweeten TL, Cutler A, Bedke BJ, Fillmore M, Stubbs EG, Odell D. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67:346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KJ, Hashimoto K, Iwata Y, Tsujii M, Sekine Y, Sugihara G, Matsuzaki H, Suda S, Kawai M, Nakamura K, Minabe Y, Yagi A, Iyo M, Takei N, Mori N. Decreased serum levels of platelet-endothelial adhesion molecule (PECAM-1) in subjects with high-functioning autism: a negative correlation with head circumference at birth. Biol Psychiatry. 2007;62:1056–1058. doi: 10.1016/j.biopsych.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Matre R, Nyland H, Olivier A, Antel J. Fc receptors for IgG on cultured human microglia mediate cytotoxicity and phagocytosis of antibody-coated targets. J Neuropathol Exp Neurol. 1994;53:27–36. doi: 10.1097/00005072-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojdani A, Campbell AW, Anyanwu E, Kashanian A, Bock K, Vojdani E. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J Neuroimmunol. 2002;129:168–177. doi: 10.1016/s0165-5728(02)00180-7. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K, Hirani K, Neubrander J, Woeller KN, O’Hara N, Usman A, Schneider C, Hebroni F, Berookhim J, McCandless J. Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. J Neuroimmunol. 2008;205:148–154. doi: 10.1016/j.jneuroim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Warren RP, Foster A, Margaretten NC. Reduced natural killer cell activity in autism. J Am Acad Child Adolesc Psychiatry. 1987;26:333–335. doi: 10.1097/00004583-198705000-00008. [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Rossi CC, Bennett J, Martinez-Cerdeno V, Ashwood P, Amaral DG, Van de Water J. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol Autism. 2011;2:5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiznitzer M. Autism and tuberous sclerosis. J Child Neurol. 2004;19:675–679. doi: 10.1177/08830738040190090701. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zalcman SS. Interleukin-2-induced increases in climbing behavior: inhibition by dopamine D-1 and D-2 receptor antagonists. Brain Res. 2002;944:157–164. doi: 10.1016/s0006-8993(02)02740-3. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Delwiche L, Walker C, Hertz-Picciotto I. Month of Conception and Risk of Autism. Epidemiology. 2011 doi: 10.1097/EDE.0b013e31821d0b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Schwartz M. Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun. 2008;22:167–176. doi: 10.1016/j.bbi.2007.08.006. [DOI] [PubMed] [Google Scholar]