Abstract
PURPOSE
Urinary tract obstruction causes hydroureteronephrosis and requires surgical intervention to prevent the development of permanent renal injury. While many studies have focused on the development of renal injuries, here we examine the molecular mechanisms that promote renal recovery following the correction of obstruction.
MATERIALS AND METHODS
A reversible murine model of ureteral obstruction was used to examine the BMP-7 and TGF-β signaling pathways during renal recovery following obstruction-induced injury. Analysis was conducted using standard molecular techniques including RT-PCR, ELISA, immunoblotting, and co-immunoprecipitation.
RESULTS
We found that the upregulation of BMP-7 following the correction of obstruction inhibits TGF-β-dependent pro-fibrotic pathways that are central to the pathogenesis of renal injury. The inhibitory effects of BMP-7 are mediated, in part, by the activation of its downstream target proteins, Smad1, Smad5, and Smad8, which suppress the activity of TGF-β-dependent Smad proteins and, in turn, inhibit the expression of TGF-β-dependent genes. Finally, the activation of the BMP-7–Smad1/5/8 pathway during renal recovery promotes the restoration of renal architecture and resolution of fibrosis in the kidney following the correction of obstruction.
CONCLUSIONS
Together, these findings demonstrate that the BMP-7–Smad1/5/8 pathway promotes the repair of the kidney following obstruction-induced injury. Accordingly, the BMP-7 pathway represents an important therapeutic target to stimulate the innate repair mechanisms of the kidney during the treatment of obstruction-induced renal injuries.
Keywords: Kidney, Ureteral Obstruction, Fibrosis, Bone Morphogenic Protein 7, Smad Proteins
INTRODUCTION
Obstructive uropathies are a leading cause of renal injury, chronic renal insufficiency, and renal failure.1–3 While current surgical approaches are frequently able to alleviate urinary tract obstructions, the possibility for irreversible renal injury remains even following treatment.2, 3 Therefore, the clinical management of obstructive uropathies would benefit from the development of therapeutic approaches that promote renal recovery following injury.
While the development of renal injuries is well understood,2, 3 renal recovery following injury is less clearly understood. Despite lacking a true regenerative ability,4 the mature kidney has an innate ability to restore renal structure and function following injury. However, this reparative ability is diminished following severe renal injury.5, 6 Understanding the processes that promote renal recovery and their molecular regulation may enable the development of therapeutic approaches to stimulate the innate repair mechanisms of the kidney during the treatment of obstruction-induced injuries.
At the molecular level, the activation of the TGF-β pathway plays a central role in the pathogenesis of renal injury by promoting apoptosis, epithelial-mesenchymal transformation, matrix protein synthesis, and other pro-fibrotic events that lead to the disruption of renal structure and function.7 Accordingly, neutralization of TGF-β inhibits the progression of obstruction-induced renal injury.8–10 Another member of the TGF-β superfamily, BMP-7, has the distinguishing property of inhibiting TGF-β-dependent biological functions.11 Both TGF-β and BMP-7 signal through receptor complexes that phosphorylate Smad transcription factors.11–13 TGF-β and BMP-7 have different functions, in part, because they promote the activating phosphorylation of distinct Smad proteins. TGF-β activates Smad2/3 whereas BMP-7 activates Smad1/5/8. These phosphorylated Smad proteins then bind the Smad4 protein and regulate the transcription of target genes in a pathway-specific manner.11–13 While the signaling pathways downstream of TGF-β and BMP-7 have been well defined, the mechanisms by which BMP-7 counters TGF-β in the kidney have not yet been clearly defined.
Importantly, treatment with exogenous BMP-7 inhibits the development of renal injuries.14–16 In this manuscript, we explore the possibility that BMP-7 may inhibit the pathogenesis of renal injury, in part, by promoting the repair of the kidney. While several studies have demonstrated that exogenous BMP-7 reverses the progression of chronic renal injury, 17, 18 chronic injury models have not been able to effectively differentiate between the effects of BMP-7 on the development of renal injury and on the repair of renal injuries since, in these models, the injurious stimuli is continuously present.
In beginning to determine the role of the BMP-7 pathway in renal recovery following injury, several important questions remain to be answered: (1) Is the BMP-7 pathway regulated during renal recovery? (2) By what mechanisms does BMP-7 counter TGF-β in the injured kidney and are these counter-regulatory mechanisms subject to regulation during renal recovery? and (3) Is pharmacologic manipulation of the BMP-7 pathway an effective therapeutic approach to stimulate the innate repair mechanisms of the kidney during the treatment of renal injuries? Here, we begin to address these questions in order to better understand the molecular mechanisms that contribute to renal recovery following obstruction-induced injury.
MATERIALS AND METHODS
Reversible Unilateral Ureteral Obstruction
8–10 week old C57BL/6J mice were obstructed by placing a vascular clamp on the proximal ureter and, when indicated, the obstruction was reversed by subsequently removing the clamp.6 Mice were treated with either PBS or 300 μg/kg of purified BMP-7 (R&D Systems), as indicated.
Masson’s Trichrome Staining
Kidneys were fixed in Histochoice (Amresco). Slides containing 5 μm tissue slices were stained using Masson’s Trichrome Reagent (Richard Allen Scientific) according to product specifications.
Type IV Collagen Immunostaining
Slides were prepared (see above), subjected to antigen retrieval by boiling in 10 mM citrate buffer (pH 6.0), and stained using rabbit anti-Collagen IV/pro-Collagen IV (Millipore) and FITC-conjugated anti-rabbit antibodies (Sigma). Three photographs/sample were uniformly taken of the region bounded by the renal capsule and the cortico-medullary junction adjacent to the papilla at 200x magnification. Interstitial/tubular volume were visually quantified by overlaying a grid on slide photographs and counting the grid points located in the interstitial/tubular regions.19
Quantification of Total Kidney Collagen Content
Kidneys were hydrolyzed, resuspended in citrate-acetate buffer (pH 6.0), and oxidized with 0.35% Chloramine T. Hydroxyproline was quantitated by measuring the conversion of 7% dimethyl-aminobenzaldehyde to a colormetric product by hydrolyzates using spectrophotometry (558 nm) and comparison to hydroxyproline standards. Collagen content was determined using the approximation that collagen contains 14% hydroxyproline.20
RT-PCR
Kidneys were pulverized in liquid nitrogen, homogenized in Trizol (Invitrogen), and RNA was isolated according to product specifications. Semiquantitative reverse transcriptase PCR was conducted using Superscript RT-PCR (Invitrogen) for 24–32 cycles with the following primers: α-Smooth Muscle Actin: 5′-ctgagcgtggctattccttc-3′; 5′-gggggccaccctataataaa-3′ Collagen α1(I): 5′-actggtacatcagcccgaac-3′; 5′-ggtggagggagtttacacga-3′ GAPDH: 5′-actccactcacggcaaattc-3′; 5′-ccttccacaatgccaaagtt-3′
ELISA
Kidneys were pulverized in liquid nitrogen and homogenized in 20 mM Tris-HCl, pH 7.5, 2M NaCl, 0.1% Tween, and 1 mM EDTA. Protein levels were determined using a BMP-7 ELISA kit (R&D Systems) according to product specifications.
Immunoblotting
Kidneys were pulverized in liquid nitrogen and lysed in 83.3 mM Tris, 150 mM NaCl, 4% SDS, and 100 mM DTT supplemented with Protease/PhosSTOP inhibitors (Roche). Immunoblotting was conducted using rabbit anti-phosho-SMAD 2/3 (Cell Signaling Technologies), rabbit anti-phosho-SMAD 1/5/8 (Santa Cruz), rabbit anti-SMAD4 (Santa Cruz), or mouse anti-GAPDH (Chemicon), and either a HRP-goat anti-rabbit or HRP-goat anti-mouse secondary antibody (Jackson Immunoresearch).
Co-Immunoprecipitation
Kidneys were pulverized in liquid nitrogen and lysed in 50mM Tris-HCl, 150mM NaCl, 1 mM EDTA, 1% Triton, 1% sodium deoxycholate, and 0.1% SDS supplemented with Complete Protease/PhosSTOP phosphatase inhibitors (Roche). Lysates were immunoprecipitated with rabbit anti-SMAD4 (Santa Cruz) conjugated to Protein G beads (Dynal). Immunoblotting was conducted (as above).
Statistical Analysis
Data are presented as mean values ± standard deviation. Statistical significance was analyzed by ANOVA with Bonferroni correction.
RESULTS
Reversible Unilateral Ureteral Obstruction (UUO) as a Model of Renal Recovery From Obstruction-Induced Injury
To better understand the mechanisms that contribute to renal recovery from obstructive uropathies, we studied a model of acute renal injury in response to unilateral ureteral obstruction (UUO).6 Importantly, in this model, the subsequent reversal of the obstruction mimics the surgical correction of obstructive uropathies in patients and serves as a model of the recovery of the kidney from acute obstruction-induced injury.
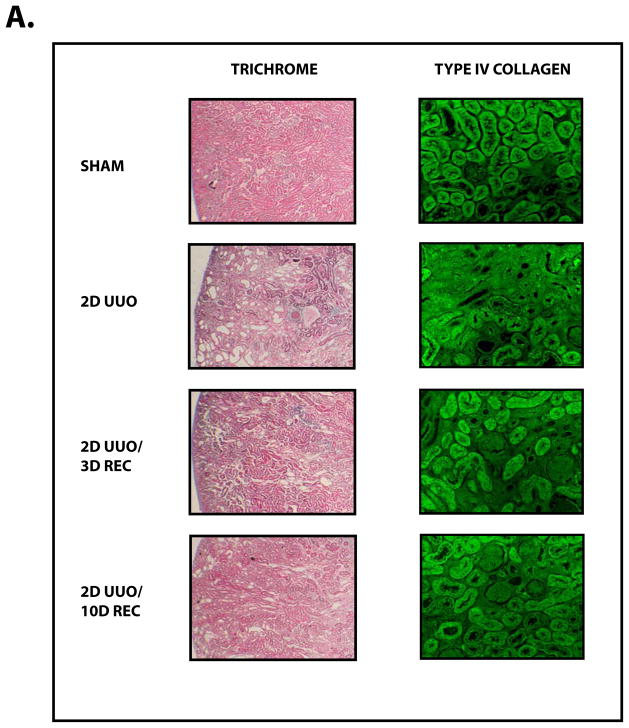
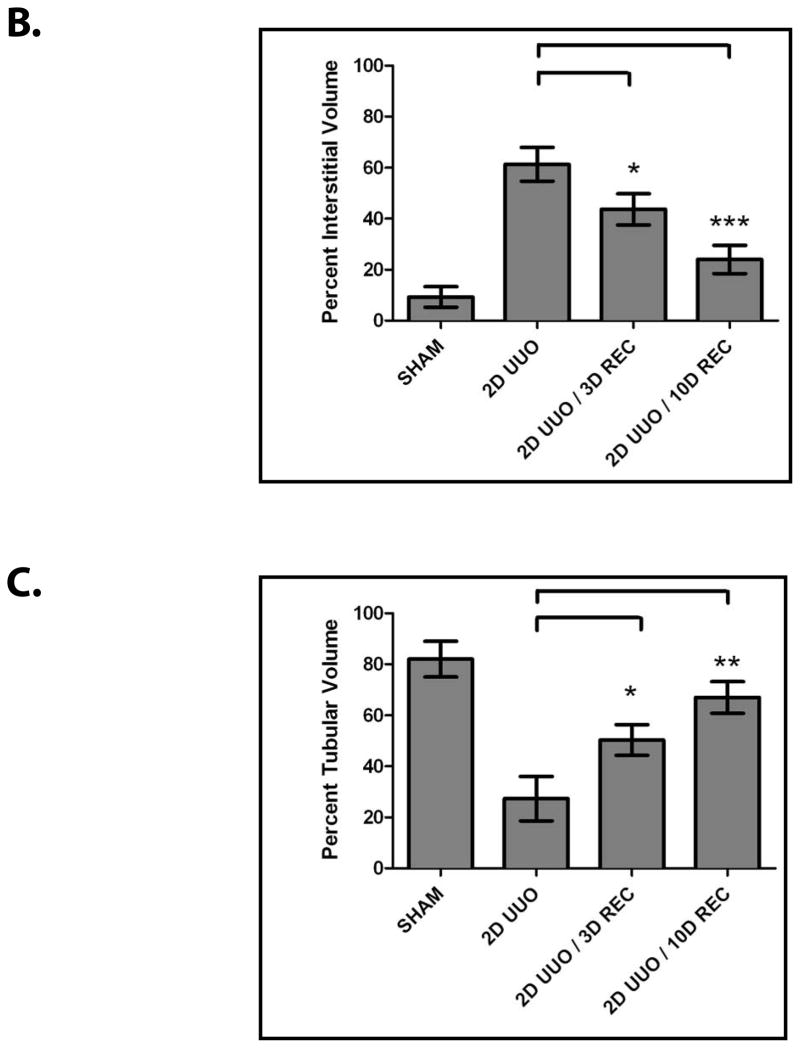
Obstructive uropathy potentially results in decreased renal function through the disruption of renal architecture and the promotion of renal fibrosis.2, 3 Indeed, these deleterious changes our effectively reproduced in our model, as UUO causes renal fibrosis (Figure 1A) characterized by interstitial expansion (Figure 1B, P<0.01), loss of tubular volume (Figure 1C, P<0.01), and accumulation of collagen (Figure 1D, P<0.005).
Figure 1. The Recovery of the Kidney Following Obstruction-induced Renal Injury.
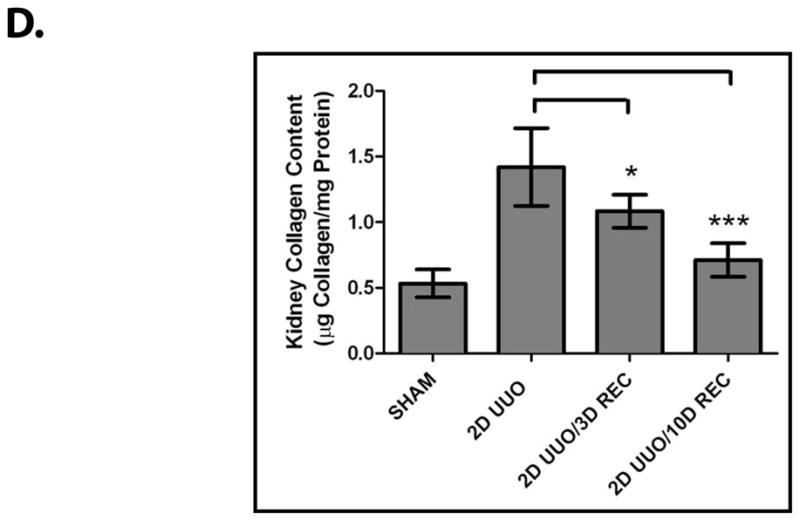
Mice (n=3) underwent either sham operation, two days of obstruction, two days of obstruction followed by reversal and three days of recovery, or two days of obstruction followed by reversal and ten days of recovery. Kidneys were analyzed by (A) Masson’s Trichrome staining (100x) (left) and Type IV Collagen immunofluorescence (200x) (right), (B) interstitial volume quantification, (C) tubular volume quantification, and (D) kidney collagen content quantification. * denotes P < 0.05; ** denotes P < 0.01; *** denotes P < 0.001.
In using the UUO model to examine renal recovery, we found that mice that undergo two days of UUO develop renal fibrosis but, following reversal of the obstruction and a recovery period, much of the renal damage subsides (Figure 1A). Reversal of the obstruction promotes a decrease in interstitial volume (Figure 1B, P<0.005), an increase in tubular volume (Figure 1C, P<0.01) and a decrease in collagen (Figure 1D, P<0.005). These findings demonstrate that the resolution of fibrosis and the restoration of renal architecture are processes that contribute to renal recovery. We next sought to examine the molecular regulation of these innate repair mechanisms.
The Activation of the BMP-7–Smad1/5/8 Pathway During Renal Recovery Inhibits TGF-β-Dependent Pro-Fibrotic Pathways in the Injured Kidney
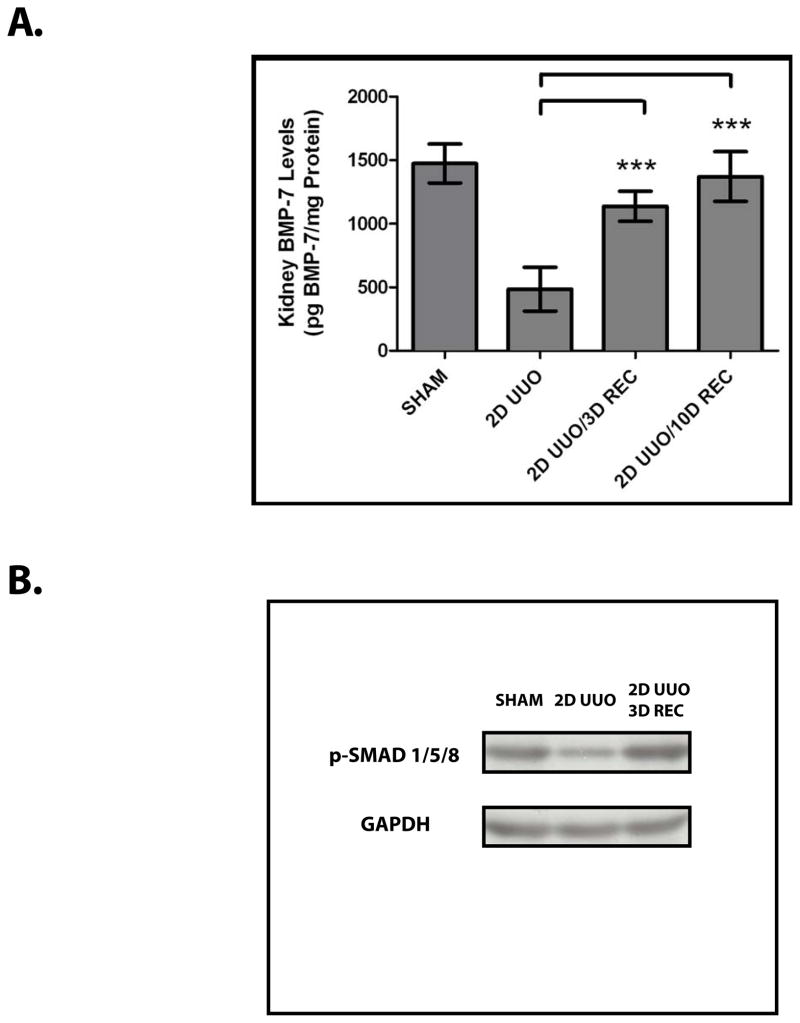
We hypothesized that BMP-7 plays a role in renal recovery following obstruction-induced injury. In examining this possibility, we found that, while BMP-7 levels are downregulated following UUO, BMP-7 levels are restored following the reversal of UUO (Figure 2A, P<0.005). Similarly, we found that the upregulation of BMP-7 is associated with the restoration of the activity of its target proteins, Smad1/5/8 (Figure 2B). Together, these findings demonstrate that the BMP-7–Smad1/5/8 pathway is activated during the repair of obstruction-induced injuries.
Figure 2. The BMP-7–Smad1/5/8 Pathway is Activated During Renal Recovery Following Obstruction-Induced Renal Injury.
Mice (n=3) underwent either sham operation, two days of obstruction, or two days of obstruction followed by reversal and three days of recovery. (A) BMP-7 ELISA. *** denotes P < 0.001. (B) Western Blot analysis for phospho-Smad 1/5/8 and GAPDH (control). Results are representative of 3 or more independent experiments.
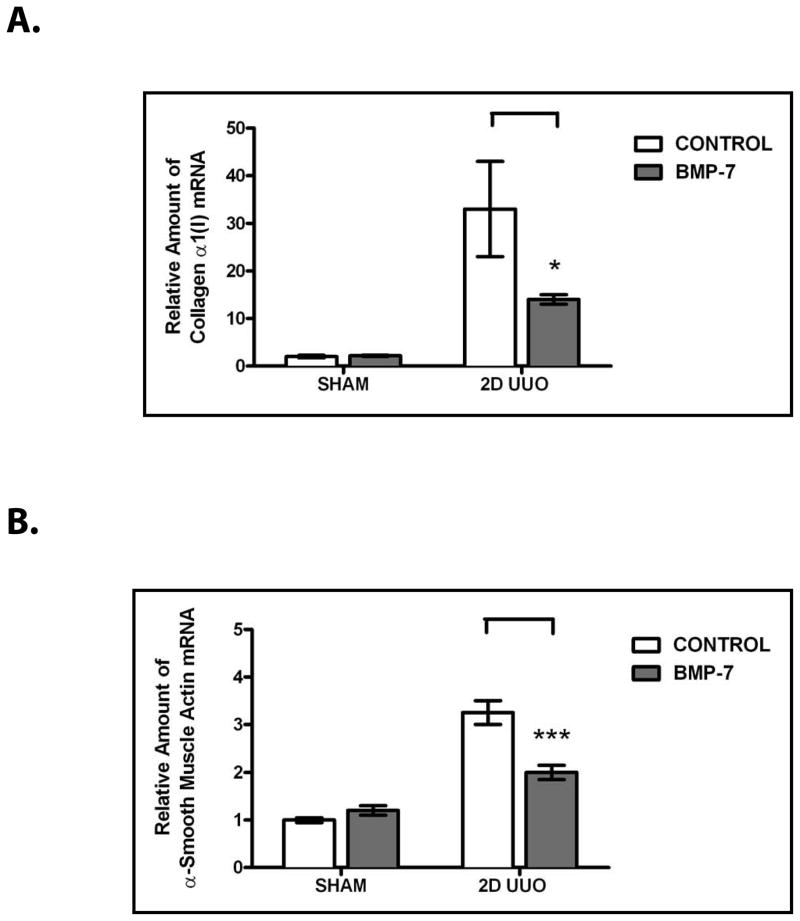
Although the BMP-7 pathway is activated during renal recovery, the possibility remained that the activation of the BMP-7 pathway is merely a consequence of renal recovery itself. To determine the functional importance of BMP-7, we examined the molecular consequences of the activation of the BMP-7 pathway in the injured kidney. We found that, treatment with exogenous BMP-7 inhibits the transcription of several TGF-β-dependent genes that are central to the pathogenesis of renal injury and that are normally increased in the injured kidney including collagen α1(I) (a gene that encodes a protein that is a significant contributor to fibrosis21) (P<0.05) and α-smooth muscle actin (a gene that encodes a protein that contributes to the differentiation of pro-fibrotic myofibroblasts22) (P<0.005) (Figure 3).
Figure 3. BMP-7 Suppresses TGF-β-dependent Pro-Fibrotic Pathways in the Injured Kidney.
Mice (n=3) underwent either sham operation or two days of obstruction and were treated either with PBS (control) or 300 μg/kg BMP-7 daily, as indicated. RT-PCR for the TGF-β-dependent target genes (A) type I collagen and (B) α-smooth muscle actin was conducted. Expression levels were normalized as a ratio to GAPDH expression and then values were compared to those obtained in sham-treated mice. * denotes P < 0.05; *** denotes P < 0.001.
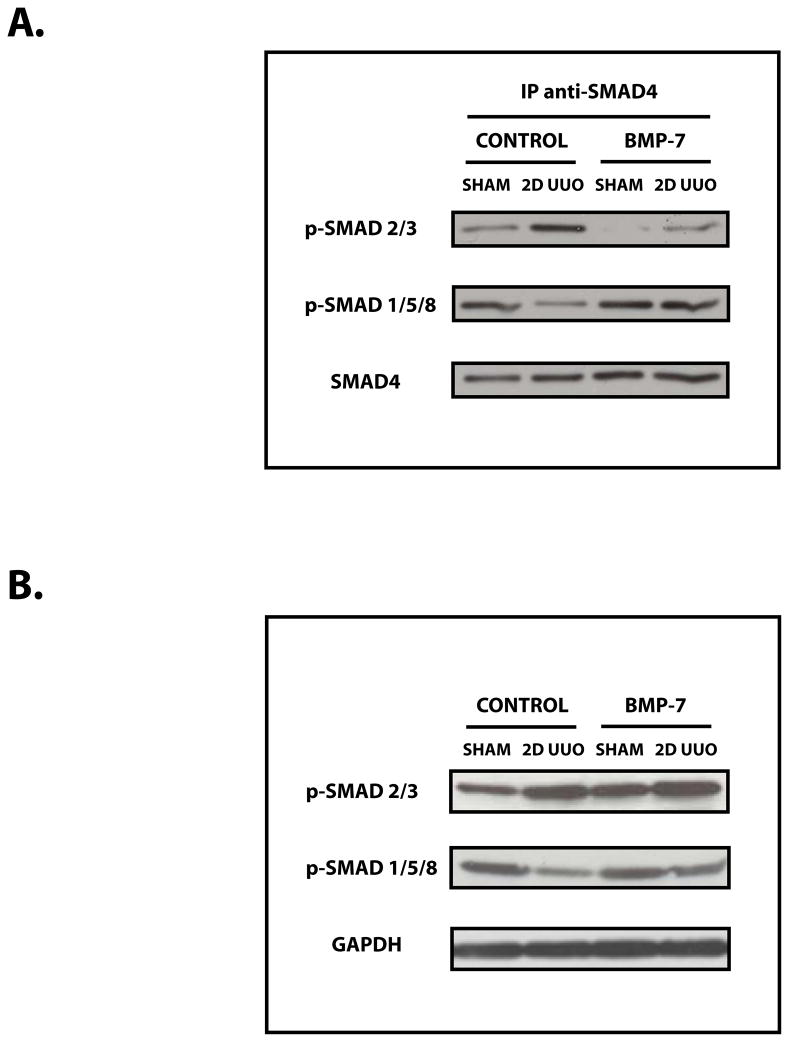
We next sought to determine the mechanisms by which BMP-7 inhibits the activity of TGF-β-dependent pathways. We found that exogenous BMP-7 promotes the formation of BMP-7-regulated Smad1/5/8–Smad4 transcription factor complexes and reduces the obstruction-induced formation of TGF-β-regulated Smad2/3–Smad4 transcription factor complexes in the injured kidney (Figure 4A) despite the continued presence of elevated levels of activated/phosphorylated Smad2/3 proteins (Figure 4B) and TGF-β (data not shown). Together, these findings demonstrate that the activation of the BMP-7–Smad 1/5/8 pathway during renal recovery inhibits the activity of TGF-β-dependent Smad proteins in the injured kidney.
Figure 4. The BMP-7–Smad1/5/8 Pathway Suppresses the Formation of TGF-β-dependent Smad4 Transcription Factor Complexes.
Mice (n=3) underwent either sham operation or two days of obstruction and were treated either with PBS (control) or 300 μg/kg BMP-7 daily, as indicated. (A) Immunoprecipitation for anti-Smad4, followed by Western Blot analysis for either phospho-Smad 2/3, phospho-Smad 1/5/8, or Smad4 (control). (B) Western Blot analysis for phospho-Smad 2/3 proteins, phospho-Smad 1/5/8 proteins, and GAPDH (control). Results are representative of 3 or more independent experiments.
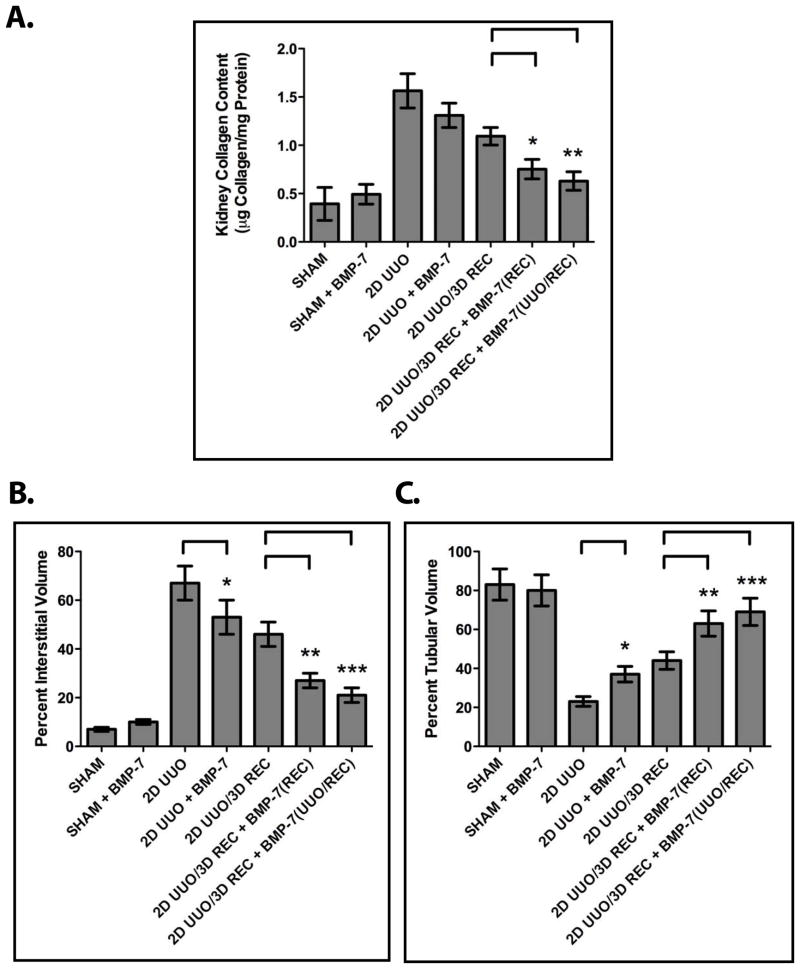
BMP-7 Promotes the Resolution of Fibrosis and the Restoration of Renal Architecture During the Repair of Obstruction-Induced Renal Injuries
Given that the upregulation of BMP-7 during renal recovery suppresses TGF-β-dependent pathways that are central to the pathogenesis of renal injury, we hypothesized that these molecular mechanisms contribute to the repair of renal injuries. Accordingly, we examined the effects of augmenting BMP-7 activity following the correction of obstruction. We found that, while mice that undergo two days of UUO followed by correction of the obstruction and a three day recovery period exhibit a 31.2% reduction in renal collagen during the recovery period, mice that are treated with exogenous BMP-7 during the recovery period exhibit a 53.1% reduction in renal collagen (Figure 5A, P<0.05). Similarly, BMP-7 treatment during recovery promotes the restoration of renal architecture by stimulating a 59.7% reduction in interstitial volume (compared to a 34.9% reduction in untreated mice) (Figure 5B, P<0.01) and a 63.0% reduction in the loss of tubular volume (compared to a 34.5% reduction in untreated mice) (Figure 5C, P<0.01). Together, these findings demonstrate that the activation of the BMP-7 pathway during renal recovery stimulates the restoration of renal architecture and the resolution of fibrosis in the kidney. Furthermore, it is notable that the renal protective effects of treatment with BMP-7 during recovery are only minimally improved by treatment with BMP-7 during both obstruction and recovery (Figures 5A–5D). Accordingly, these findings demonstrate that the renal protective effects of BMP-7 during the repair of established renal injuries may exceed even those of BMP-7 during the development of renal injuries.
Figure 5. BMP-7 Promotes the Repair of the Kidney Following Obstruction-Induced Renal Injury.
Mice (n=3) underwent either sham operation, two days of obstruction, or two days of obstruction followed by reversal and three days of recovery. Mice were treated either with PBS (control) or 300 μg/kg BMP-7 daily during obstruction, during recovery, or both during obstruction and during recovery as indicated. Kidneys were analyzed by (A) kidney collagen content quantification, (B) interstitial volume quantification, and (C) tubular volume quantification. * denotes P < 0.05; ** denotes P < 0.01; *** denotes P < 0.001.
DISCUSSION
An important long-term goal in the treatment of obstructive uropathies is the development of therapeutic approaches to stimulate the regression of fibrosis, repair of structural damage and, ultimately, recovery of renal function following obstruction-induced injury.1–3 However, little progress has been made towards achieving this goal, in part, because the processes that promote renal recovery following injury are poorly understood.5
Over the course of this study, we found that the BMP-7–Smad1/5/8 pathway is activated during the repair of the kidney following acute, reversible obstruction-induced injury. Furthermore, the activation of the BMP-7 pathway promotes the resolution of fibrosis and the restoration of renal architecture during renal recovery. While BMP-7 has previously been reported to prevent the development of renal injury,14–16 our studies demonstrate that BMP-7 has renal protective effects that extend beyond preventing the development of renal injury per se since BMP-7 is also able to stimulate the repair of established renal injuries following the correction of obstruction. Interestingly, in our model, the renal protective effects of treatment with exogenous BMP-7 during recovery are only minimally improved by treatment with exogenous BMP-7 during both obstruction and recovery. Given the absence of a synergistic effect, these findings suggest that the repair-promoting functions of BMP-7 may have played a significant role in previous studies examining the renal protective effects of BMP-7 during the development of obstruction-induced renal injury.14–16
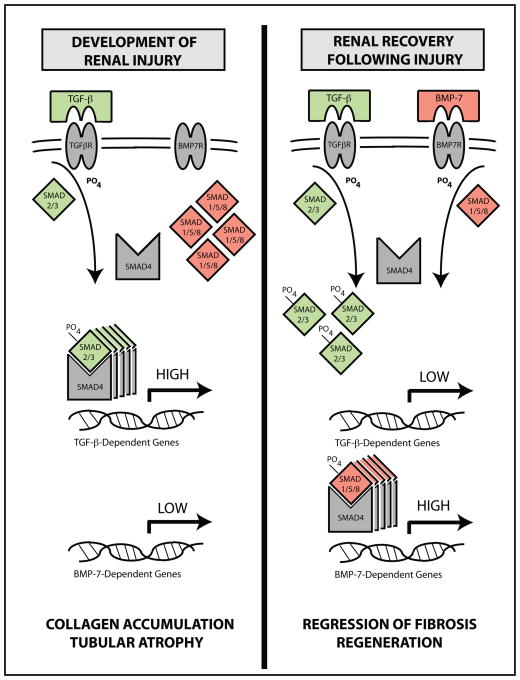
At the molecular level, the restoration of BMP-7 levels following the correction of obstruction is associated with the activation of its downstream target proteins, the Smad1/5/8 proteins. The activation of BMP-7-dependent Smad proteins opposes TGF-β-dependent pathways in the injured kidney by inhibiting the formation of TGF-β-dependent Smad2/3–Smad4 transcription factor complexes and the transcription of TGF-β-dependent genes (summarized in Figure 6). Given that TGF-β-dependent pathways are required for the pathogenesis of renal injury, 7–10 these findings suggest that the activation of BMP-7-dependent Smad proteins plays an important role in renal recovery following injury and in the renal protective effects of BMP-7.
Figure 6. A Model of BMP-7-Mediated Suppression of TGF-β-Dependent Pro-Fibrotic Pathways During the Repair of Renal Injuries.
(left) During the development of obstruction-induced renal injury, the activation of the TGF-β–Smad2/3 pathway, when unchecked by the BMP-7–Smad1/5/8 pathway, promotes the transcription of TGF-β-dependent gene products and, in turn, the disruption of renal architecture, development of renal fibrosis, and, ultimately, renal insufficiency. (right) During renal recovery following injury, BMP-7 levels are upregulated and the Smad1/5/8 pathway is activated, resulting in the redistribution of Smad4 transcription factor complexes, the suppression of activated TGF-β-dependent Smad2/3 proteins, the resolution of fibrotic lesions in the kidney, and the restoration of renal architecture.
Nonetheless, the precise molecular mechanisms downstream of BMP-7 that promote renal recovery remain to be elucidated in future studies. It is likely that at least some of the repair-promoting functions of BMP-7 may be attributed to its ability to counter TGF-β. For example, BMP-7 may oppose TGF-β-induced matrix protein synthesis, protease inhibition, and myofibroblast recruitment and differentiation.7, 23 Other repair-promoting functions of BMP-7 may be attributed to functions independent of TGF-β, such as its ability to act as a renal morphogen.14 It has been shown that BMP-7 is indispensable for kidney development during embryogenesis14, 23 and, in turn, that tissue repair and the replacement of damaged cells through regeneration involve processes analogous to embryogenesis.24 Along with identifying the downstream molecular mechanisms that contribute to BMP-7-stimulated kidney repair, important future goals will be to determine why these pathways are impaired in prolonged obstructions that lead to permanent renal injury and also to develop therapeutic approaches to reactivate these innate repair mechanisms in the kidney during the treatment of obstructive uropathies.
CONCLUSIONS
As we work towards a better understanding of renal recovery following injury, reversible models of acute renal injury will serve as invaluable tools in elucidating the innate repair mechanisms of the kidney. Here, we demonstrate that the activation of the BMP-7–Smad1/5/8 pathway promotes the resolution of fibrosis and the restoration of renal architecture during renal recovery following the reversal of obstruction. Together, these findings demonstrate that the BMP-7–Smad1/5/8 pathway plays an important role in the repair of renal injuries that result from obstructive uropathies. Accordingly, the BMP-7 pathway represents a potential target of adjuvant therapy to stimulate the innate repair mechanisms of the kidney during the treatment of obstructive uropathies.
Acknowledgments
Sources of Financial Support:
National Institutes of Health and Midwest Stone Institute
Key of Definitions for Abbreviations
- BMP-7
Bone Morphogenic Protein 7
- REC
Recovery
- SMAD
SMA and MAD-related protein
- TGF-β
Transforming Growth Factor β
- UUO
Unilateral Ureteral Obstruction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roth KS, Koo HP, Spottswood SE, et al. Obstructive uropathy: an important cause of chronic renal failure in children. Clin Pediatr (Phila) 2002;41:309. doi: 10.1177/000992280204100503. [DOI] [PubMed] [Google Scholar]
- 2.Chevalier RL. Pathogenesis of renal injury in obstructive uropathy. Curr Opin Pediatr. 2006;18:153. doi: 10.1097/01.mop.0000193287.56528.a4. [DOI] [PubMed] [Google Scholar]
- 3.Chevalier RL. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat Clin Pract Nephrol. 2006;2:157. doi: 10.1038/ncpneph0098. [DOI] [PubMed] [Google Scholar]
- 4.Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. 1995;57:263. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- 5.Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane AL, Kett MM, Samuel CS, et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 2005;16:3623. doi: 10.1681/ASN.2004090771. [DOI] [PubMed] [Google Scholar]
- 7.Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Gagliardini E, Benigni A. Role of anti-TGF-beta antibodies in the treatment of renal injury. Cytokine Growth Factor Rev. 2006;17:89. doi: 10.1016/j.cytogfr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Isaka Y, Tsujie M, Ando Y, et al. Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000;58:1885. doi: 10.1111/j.1523-1755.2000.00360.x. [DOI] [PubMed] [Google Scholar]
- 10.Miyajima A, Chen J, Lawrence C, et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 11.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 12.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 13.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Dressler GR. BMP7 signaling in renal development and disease. Trends Mol Med. 2005;11:512. doi: 10.1016/j.molmed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Zeisberg M, Bottiglio C, Kumar N, et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003;285:F1060. doi: 10.1152/ajprenal.00191.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hruska KA, Guo G, Wozniak M, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000;279:F130. doi: 10.1152/ajprenal.2000.279.1.F130. [DOI] [PubMed] [Google Scholar]
- 17.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 18.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey J, Hruska K, Guo G, et al. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002;13(Suppl 1):S14. [PubMed] [Google Scholar]
- 20.Samuel CS. Determination of collagen content, concentration, and subtypes in kidney tissue. Methods Mol Biol. 2009;466:223. doi: 10.1007/978-1-59745-352-3_16. [DOI] [PubMed] [Google Scholar]
- 21.Ritzenthaler JD, Goldstein RH, Fine A, et al. Regulation of the alpha 1(I) collagen promoter via a transforming growth factor-beta activation element. J Biol Chem. 1993;268:13625. [PubMed] [Google Scholar]
- 22.Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 23.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 24.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]