Abstract
A number of recessive autosomal genes cause male infertility. Male mice homozygous for the blind-sterile (bs/bs) and quaking-sterile (qk/qk) gene mutations are sterile, because they either do not produce any spermatozoa or produce only a few abnormal spermatozoa. Mice lacking the cyclic AMP responsive-element modulator gene are sterile due to failure of spermiogenesis. All these mice, however, are able to produce fertile offspring when their spermatozoa or round spermatids are injected into oocytes of normal females. This implies that genetic and epigenetic elements necessary for syngamy and embryonic development are established in round spermatids and spermatozoa of these animals, even though their spermatogenic cells are destined to die (bs/bs and qk/qk) or are programmed to undergo apoptosis (cyclic AMP responsive-element modulator-null) without becoming functional spermatozoa.
In all vertebrates, with few possible exceptions, female germ cells become fertilization-competent at the metaphase of the second meiotic division (1, 2). In contrast, male germ cells become fertilization-competent only after the completion of meiosis and their transformation into motile spermatozoa. In mammals, spermatozoa in the testis are virtually immotile. They must undergo maturation in the epididymis and capacitation in the female genital tract before gaining the ability to interact with mature oocytes (2, 3). Spermatozoa become able to fuse with oocytes only after the acrosome reaction, which normally takes place immediately before passing through noncellular coats surrounding individual oocytes (4). An important question is whether all postmeiotic modifications of male germ cells are necessary for syngamy and embryonic development. Are all genes involved in postmeiotic modifications necessary for the development of new individuals?
We found that mouse oocytes fused or injected with round spermatids were able to develop into normal offspring (5, 6). Because secondary, and even late primary, spermatocytes were able to produce normal offspring after injection into oocytes (7-9), we inferred that all genomic and epigenetic elements necessary for embryonic development are established before spermatogenic cells become motile spermatozoa. In other words, all postmeiotic modifications of male germ cells are considered processes solely dedicated for the delivery of male genomes into the oocytes. However, it should be pointed out that all these experiments were performed by using male germ cells from normal fertile male mice. Thereby, a question arises: Would male germ cells that are unable to differentiate into spermatozoa because of genetic causes be able to produce normal offspring? Here, we have used three different mutant mice that display spermiogenic defects at different levels. Mice homozygous for blind-sterile (bs/bs) and quaking-sterile (qk/qk) gene mutations produce a few spermatozoa, but none are fertile (10-12). Male mice homozygous for a mutation in the cyclic AMP responsive-element modulator (CREM) gene are unable to start spermiogenesis, and therefore the most advanced spermatogenic cells in these males are round spermatids (13, 14). We have studied whether spermatozoa or spermatids from these infertile mice are able to produce normal offspring when injected into oocytes.
Materials and Methods
Animals. Females and males of B6D2F1 (B57BL/6 × DBA/2), C57BL/6, and ICR mice were purchased from the National Cancer Institute (Raleigh, NC). Male mice homozygous for the bs/bs and qk/qk mutations were purchased from The Jackson Laboratory. CREM-null mice were generated by homologous recombination and have been described (13). Phenotypes and genotypes of these mutant mice are shown in Fig. 1 and Table 1. All males and females (B6D2F1 and C57BL/6) were 3-6 and 2-3 months old, respectively, when used. Surrogate mothers (ICR) were 3-4 months old. All animals were maintained in temperature- and light-controlled rooms (14 light/10 dark, light on from 5:00 a.m.). The protocol of animal handling and treatment was reviewed and approved by the Animal Care and Use Committee of the University of Hawaii.
Fig. 1.
Sterile mutant males used in this study. (A) A homozygous bs/bs male with cataracts; bs is an autosomal recessive gene mapped to mouse chromosome 2 adjacent to the agouti gene (43). Blind bs/bs females are fertile. (B) A homologus qk/qk sterile male; qk is an autosomal recessive gene on chromosome 17. Homozygous mutation induces deficient myelination of central and peripheral nerves in both male and female (45) and causes a tremor that disappears when mice are at rest. This qk/qk male was undergoing a seizure while being photographed. qk/qk females are fertile. (C) CREM-null male (left) and female mice. CREM is a master transcription regulator of several postmeiotic genes (28, 46). Both females and males are phenotypically normal. CREM-null females are fertile, whereas CREM-null males are infertile due to a defect in spermiogenesis.
Table 1. Genotypes and phenotypes of male mice used in this study.
| Gene | Genotype | Genetic background | Phenotype | Fertility | Most advanced stage of spermatogenesis | Ref. |
|---|---|---|---|---|---|---|
| bs | bs/bs | AKR × 129 | Blind | Infertile | Deformed sperm | 10, 43 |
| bs/+ | Normal | Fertile | Normal sperm | |||
| qk | qk/qk | C57BL/6 × C3H | Tremor, seizure | Infertile | Deformed sperm | 12, 44, 45 |
| qk/+ | Normal | Fertile | Normal sperm | |||
| CREM | -/- | C57BL/6 | Normal | Infertile | Spermatid | 13 |
| +/+ | Normal | Fertile | Normal sperm |
Reagents and Media. All inorganic and organic compounds used for the preparation of media were from Sigma, unless otherwise stated. The medium for culturing sperm- or spermatid-injected oocytes was CZB medium (15) supplemented with 5.56 mM d-glucose and 5 mg/ml BSA (fraction V, Calbiochem). The medium for oocyte collection from oviducts, subsequent treatment, and injection of spermatozoa (or spermatids) was modified CZB containing 20 mM Hepes, a reduced amount of NaHCO3 (5 mM), and 0.1 mg/ml polyvinyl alcohol (PVA, cold-water soluble, 30-70 kDa) instead of BSA. This medium was called Hepes-CZB. PVA kept the wall of injection pipettes less sticky over a longer period than BSA, so that adhesion of mineral oil and cell debris was minimized. Incubation atmosphere for CZB and Hepes-CZB was 5% CO2 in air (37°C) and open air (≈25°C), respectively.
Preparation of Oocytes. Females of B6D2F1, C57BL/6, CREMnull (-/-), and normal (+/+) mice were each injected with 5 units of equine chorionic gonadotropin followed by injection of 5 units of human chorionic gonadotropin (hCG) 48 h apart. Oocytes surrounded by cumulus oophorus were collected from oviducts ≈15 h after hCG injection. The oocytes for in vitro insemination were used directly, whereas those for sperm (or spermatid) injection were freed from cumulus cells by 5- to 10-min treatment with 0.1% bovine testicular hyaluronidase (300 units/mg; ICN) in Hepes-CZB. Cumulus-free oocytes were rinsed and kept in CZB for no more than 4 h at 37°C under 5% CO2 in air before injection of spermatozoa or spermatids.
Collection and Examination of Spermatozoa and Spermatids. Spermatozoa were collected from either the caudae epididymis or testis. They were suspended in CZB medium and examined for motility at room temperature. Those with distinct motility as well as those with vibrating or undulating tails were all recorded as being alive. Viability of spermatozoa was assessed by using the sperm viability test kit (FertiLight, Molecular Probes). The nuclei of live spermatozoa with intact plasma membranes fluoresced bright green, whereas those of dead spermatozoa fluoresced bright red-orange. Round spermatids, collected from the testis (6), were also assessed for viability. Over 500 spermatozoa or spermatids were examined for each sperm sample.
In Vitro Insemination. Cauda epididymal spermatozoa of B6D2F1 or qk/qk mouse were allowed to disperse in TYH medium (16) at 37°C for 15 min. An aliquot (200 μl) of homogenous sperm suspension was incubated under mineral oil (Squibb) for 1-1.5 h at 37°C under 5% CO2 in air to allow sperm capacitation. The concentration of spermatozoa at this stage was ≈1-2 × 107/ml. Insemination was performed by adding a small volume of the sperm suspension to 200 μl of fresh TYH medium containing cumulus-enclosed oocytes that had been previously placed under mineral oil in a plastic Petri dish (30 × 10 mm; Falcon). The final sperm concentration in fertilization medium was ≈105/ml. The oocytes were examined with an inverted microscope 6-8 h after insemination. Those with two distinct pronuclei and a conspicuous second polar body were recorded as normally fertilized (17).
Intracytoplasmic Sperm Injection (ICSI) and Round Spermatid Injection (ROSI). ICSI and ROSI were carried out according to Kimura and Yanagimachi (6, 17) by using a micropipette actuated by Piezo electric power except that operations were performed at room temperature (≈25°C) rather than 17°C. For ICSI, sperm heads were injected into oocytes after separation of the head from the tail by application of a few Piezo-electric pulses to the head and tail junction (18). ICSI oocytes were transferred to CZB medium under mineral oil and cultured at 37°C in a humidified atmosphere of 5% CO2 in air. ROSI was performed according to Kimura and Yanagimachi (6) with slight modifications. Briefly, oocytes were first activated for 30-60 min with Ca2+-free CBZ medium containing 10 mM SrCl2 at 37°C, then transferred to normal CZB (37°C). Between 60 and 90 min after onset of Sr2+ treatment, when the oocytes were at late-anaphase to telophase of the second meiosis, each oocyte was injected with a round spermatid nucleus. ROSI oocytes need to be stimulated by parthenogenetic agents (for example, SrCl2, used in this study), because mouse round spermatids, unlike spermatozoa, are unable to activate oocytes (6). Oocytes can be activated immediately after ROSI, but in this study, we activated oocytes first. ROSI was performed in Hepes-CZB medium ≈1 h after onset of Sr2+ treatment, when the oocytes were at the telophase of the second meiosis.
Examination of the Development of Preimplantation Embryos and Embryo Transfer to Surrogate Mothers. ICSI and ROSI oocytes were allowed to develop in CZB medium and were examined at 24-h intervals until they reached the morula/blastocyst stage. To examine their developmental potentials, embryos at either two-cell or morula/blastocyst stages were transferred to the oviducts of ICR (albino) females that had been mated with vasectomized males of the same strain during the previous night. The day of embryo transfer was designated as day 1 of pregnancy. Because the implantation of embryos was governed by the endometrial stage of the uterus, implantation of these embryos occurred around 5 days postcoitum regardless of the developmental stage of embryos at transfer. Surrogate mothers were allowed to deliver and raise pups. Sex of infants was determined 1-2 wk after the birth by examination of external genitalia. Some males born after ICSI or ROSI were killed, and their testes were fixed and examined for histology.
Results
bs/bs Mice and Their Offspring. Homozygous mutant bs/bs mice are blind due to cataracts (Fig. 1 A). bs/bs male mice have been reported to have elongated spermatids and deformed spermatozoa (10), but the bs/bs males we used had neither elongated spermatids nor spermatozoa. The most advanced spermatogenic cells we found were round spermatids (Fig. 2A′). The discrepancy between previous reports and this study could be due to individual variations among animals or the difference in the age of animals used. Heterozygous (bs/+) males had numerous mature spermatozoa (not shown). When spermatozoa from a bs/+ male were individually injected into B6D2F1 oocytes, the majority of the oocytes were fertilized, and most of transferred embryos developed into live offspring (Table 2). Importantly, injection of round spermatids from bs/bs males was also resulted in the birth of live offspring, even though it was less efficient than injection with heterozygous (bs/+) spermatozoa (Table 2). All offspring from ROSI oocytes developed into fertile phenotypically normal adults (data not shown).
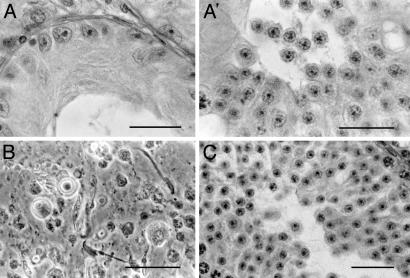
Fig. 2.
(A and A′) Sections through seminiferous tubules of the testes of a bs/bs male. (A) Some sections showed only Sertoli cells. (A′) Other tubules had spermatogenic cells. The most advanced stage of spermatogenesis was round spermatid. (B) Phase-contrast micrograph of the testicular tissue squash of a qk/qk male, showing the presence of spermatozoa among spermatogenic cells. (C) A testis section of a CREM (-/-) male showing that spermatogenesis is arrested at the earliest spermatid stage. (Bar = 20 μm.)
Table 2. Fertilization and development of B6D2F1 oocytes injected with spermatozoa of bs/bs and control (bs/+) males.
| Experimental series 1
|
Experimental series 2
|
||||||
|---|---|---|---|---|---|---|---|
| No. (%) of
|
|||||||
| Cells injected | Total no. of oocytes injected (no. exp.) | Fertilized oocytes | Blastocyst | No. of two-cell embryos transferred (no. exp.) | No. of recipients | No. (%) of live offspring | |
| bs/+ | Sperm | 27 (1) | 24 | 20 (87.5) | 10 (1) | 1 | 8 (80.0) |
| bs/bs | Spermatid | 102 (3) | 88 | 79 (89.8) | 118 (4) | 10 | 35 (29.7) |
qk/qk Mice and Their Offspring. qk/qk male mice are infertile due to defective spermiogenesis, and only a few spermatozoa were collected from the cauda epididymis. Less than 0.5% were barely motile. All had abnormal heads and/or tails. The testis contained many spermatogenic cells but few spermatozoa (Fig. 2B). None of the 131 oocytes inseminated in vitro with qk/qk spermatozoa were fertilized, whereas 82% of 133 were fertilized by heterozygous (qk/+) spermatozoa. Injection of qk/qk spermatozoa resulted in high rates of fertilization (Table 3). Two-cell embryos transferred to surrogate mothers developed into live offspring. Similarly, injection of round spermatids resulted in the birth of many live offspring (Table 3). All offspring were phenotypically normal and developed into fertile adults (data not shown).
Table 3. Fertilization and development of B6D2F1 oocytes injected with sperm and spermatids of qk/qk males.
| Experimental series 1
|
Experimental series 2
|
|||||
|---|---|---|---|---|---|---|
| Cells injected | Total no. oocytes injected (exp. no.) | No. oocytes fertilized | No. (%) of morula/blastocysts | No. of two-cell embryos transferred | No. of recipients (exp. no.) | No. of live offspring (%) |
| qk/qk sperm | 88 (4) | 75 | 39 (52.0) | 66 | 8 (4) | 11 (16.7)* |
| qk/qk spermatids | 130 (2) | 125 | 77 (61.6) | 96 | 6 (2) | 18 (18.7)† |
exp., experiment.
Eight females and three males
Ten females and eight males
CREM-Null Mice and Their Offspring. CREM-null females were fertile, whereas CREM-null males were infertile. The most advanced spermatogenic cells in the testes of CREM-null males were round spermatids (Fig. 2C). The epididymis contained only degenerated spermatids. When CREM-null spermatids were injected into oocytes, high proportions of the oocytes developed into two-cell embryos. When they were transferred to surrogate mothers, 13-25% developed into live offspring (Table 4). Four males and four females were selected randomly and mated. All proved to be fertile, with the first average litter size of 8.8 (varying between 7 and 11). Spermatogenesis in all males was normal.
Table 4. Fertilization and development of oocytes injected with spermatozoa of CREM (-/-) and CREM (+/+) males.
| Experimental series 1
|
Experimental series 2
|
||||||
|---|---|---|---|---|---|---|---|
| Spermatids | Oocytes | No. (%) oocytes injected (experiment no.) | No. (%) fertile | No. (%) of two-cell embryos | No. (%) two-cell embryos transferred | No. of recipients | No. (%) of live offspring |
| CREM (+/+) | B6D2F1 | 76 (2) | 67 | 62 (90.4) | 17 | 2 | 5 (29.4)* |
| CREM (-/-) | B6D2F1 | 129 (6) | 113 | 106 (93.8) | 60 | 4 | 8 (13.3)† |
| CREM (-/-) | CREM (+/+) C57BL/6 | 28 (2) | 26 | 11 (42.3) | 20 | 2 | 5 (25.0)‡ |
| CREM (-/-) | CREM (-/-) C57BL/6 | 25 (2) | 24 | 16 (66.7) | 16 | 2 | 3 (18.7)§ |
-/-, null; +/+, normal.
Four females and one male
Four females and four males
Two females and three males
One female and two males
Discussion
A major advance in reproductive biology during the last decade is the development of techniques that allow infertile males to gain fertility by microsurgical injection of spermatozoa and prespermatozoal cells into oocytes (19-22). We have previously shown that the majority of BALB/c spermatozoa with grossly misshapen heads are able to produce normal offspring by ICSI (23). Spermatozoa of mice carrying two complementary haplotypes (tw5/tw32) are poorly motile and totally infertile in vivo and in vitro (24), yet they are able to produce fertile offspring by ICSI (25). Some transgenic mice are infertile due to poor sperm production but are able to produce live offspring by ROSI or ICSI (26, 27). In this study, we have shown that male mice infertile due to recessive mutations (bs/bs and qk/qk) can be rescued by ICSI and/or ROSI. Round spermatids of CREM-null mice, which are programmed to undergo apoptosis (13, 28), were also able to produce fertile offspring by ROSI. According to Ogura et al. (29), the spermatozoa of ICGN mice with a hereditary nephritic syndrome are barely fertile in vivo and in vitro when animals become 9 months or older. Round spermatids of these aged mice, however, can readily produce offspring by injection into oocytes. That the nuclei of round spermatids are able to participate in normal embryonic development indicates that genomic and epigenetic elements needed for embryonic development are already established in round spermatids, even when the postmeiotic differentiation of these cells is blocked by mutations or diseases.
In infertility clinics, ICSI has been widely used as the method of choice to overcome infertility of patients with oligozoospermia, azoospermia, teratozoopermia, and asthenozoospermia. Although testicular spermatozoa or elongated spermatids are almost as effective as ejaculated spermatozoa in producing live offspring by human ICSI (30, 31), the success and failure of human ROSI have been controversial (32-35). Although it is possible that nuclei and centrosomes of human round spermatids are not quite ready for syngamy and embryonic development, the problem could be simply technical. Living human round spermatids can be distinguished from other small cells in testicular biopsies (36), but this is a difficult task for researchers with limited experience and may result in the selection of the cells that are not really round spermatids. It is also possible that activation of oocytes after ROSI have been suboptimal. According to Yazawa et al. (37), human round spermatids are able to activate oocytes but not as efficiently as spermatozoa. Additional stimulation may be necessary to fully activate human ROSI oocytes for development.
The results of this study in the mouse should be encouraging to the treatment of men who are unable to produce fertile spermatozoa. Some men are completely azoospermic or produce only a few abnormal spermatozoa. The causes of spermatogenic and spermiogenic defects are numerous and could be genetic or nongenetic. The male mice used in this study were infertile due to autosomal recessive genes, and we were able to rescue their infertility with ICSI and/or ROSI (Tables 2, 3, 4). Because we used normal fertile females as oocyte donors, all offspring we obtained were phenotypically normal and fertile. Many autosomal genes involved in human infertility are recessive (e.g., genes for cystic fibrosis, Kartagener syndrome, Usher's syndrome, Young syndrome, Fanconi anemia, persistent Mullerian duct syndrome, etc.) (38-40). As long as a male is infertile due to a recessive gene mutation and his partner has the normal alleles for that gene, the chance of their offspring being phenotypically normal and fertile is very high. If his partner carries a pair of normal alleles (+/+) for the gene affecting fertility, all of their children (+/-) will be fertile. If the partner carries heterozygous for the gene (+/-), half of the male offspring will be infertile. Usually, both the father and mother are equally responsible for male infertility, but in some cases the father is solely responsible for the infertility of his sons. Male infertility due to a microdeletion of azoopermia factors on the Y choromosome is an example (41, 42). ICSI or ROSI transmits this Y-linked infertility to his sons but not to his daughters. Autosomal dominant infertility genes (e.g., genes for myotonic dystrophy, polycystic kidney, and Noonan syndromes; refs. 38, 39) will also be transmitted to offspring by ICSI or ROSI. A fascinating scenario for the future would be the replacement of recessive or dominant infertility genes with normal alleles before or shortly after ICSI or ROSI.
Acknowledgments
We thank all members of the Yanagimachi and Sassone-Corsi laboratories for help during the course of this study. We are indebted to Drs. Norman Hecht, Edward Eddy, and Roger Gosden for invaluable advice. This study was supported by a grant from the National Institutes of Health (HD-03402), Centre Nationale de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Centre Hospitalier Universitaire Régional.
Abbreviations: ICSI, intracytoplasmic sperm injection; ROSI, round spermatid injection; CREM, cyclic AMP responsive-element modulator; bs/bs, blind-sterile; qk/qk, quaking-sterile.
References
- 1.Rothschild, L. (1956) Fertilization (Mathuen, London).
- 2.Austin, C. R. (1965) Fertilization (Prentice-Hall, Englewood Cliffs, NJ).
- 3.Bedford, J. M. (1983) Biol. Reprod. 18, 108-120. [DOI] [PubMed] [Google Scholar]
- 4.Yanagimachi, R. (1994) in The Physiology of Reproduction, eds. Knobill, E. & Neil, J. D. (Raven, New York), 2nd Ed., pp. 189-317.
- 5.Ogura, A., Matsuda, J. & Yanagimachi, R. (1994) Proc. Natl. Acad. Sci. USA 91, 7460-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura, Y. & Yanagimachi, R. (1995) Development (Cambridge, U.K.) 121, 2397-2405. [DOI] [PubMed] [Google Scholar]
- 7.Kimura, Y. & Yanagimachi R. (1995) Biol. Reprod. 53, 855-862. [DOI] [PubMed] [Google Scholar]
- 8.Kimura, Y., Tateno, H., Handel, M. A. & Yanagimachi, R. (1998) Biol. Reprod. 59, 871-877. [DOI] [PubMed] [Google Scholar]
- 9.Ogura, A., Suzuki, O., Tanemura, K., Mochida, K., Kobayashi, Y. & Matsuda, Y. (1998) Proc. Natl. Acad. Sci. USA 95, 5611-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotomayor, R, E. & Handel, M. A. (1986) Biol. Reprod. 34, 171-182. [DOI] [PubMed] [Google Scholar]
- 11.Fouquet, J. P., Valentin, A. & Kann, M. (1992) Tissue Cell 24, 655-665. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, W. I., Gall, A. M., Southard, J. L. & Sidman, R. I. (1971) Biol. Reprod. 5, 30-58. [DOI] [PubMed] [Google Scholar]
- 13.Nantel, F., Monaco, L., Foulkes, N, S., Masquiller, D., LeMeur, M., Henriksen, K., Dierich, A., Parvinen, M. & Sassone-Corsi. P. (1996) Nature 380, 159-162. [DOI] [PubMed] [Google Scholar]
- 14.Blendy, J. A., Kaestner, K. H., Weinbauer, G. H., Nieschlag, E. & Schutz, G. (1996) Nature 380, 162-165. [DOI] [PubMed] [Google Scholar]
- 15.Chatot, C. L., Torres, I. & Ziomek, C. A. (1990) Biol. Reprod. 42, 432-440. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda, Y., Yokoyama, M. & Hoshi, T. (1971) Japan J. Anim. Reprod. 16, 147-151. [Google Scholar]
- 17.Kimura, Y. & Yanagimachi, R. (1995) Biol. Reprod. 52, 709-720. [DOI] [PubMed] [Google Scholar]
- 18.Wakayama, T., Whittingham, D. G. & Yanagimachi, R. (1998) J. Reprod. Fertil. 112, 11-17. [DOI] [PubMed] [Google Scholar]
- 19.Palermo, G., Jorris, H., Devroeym P. & Van Steriteghem, A. C. (1992) Lancet 340, 17-18. [DOI] [PubMed] [Google Scholar]
- 20.Van Steirteghem, A., Nagy, Z., Joris, H., Liu, J., Staessen, C., J., Smitz, J., Winsato, A. & Devroey, P. (1993) Hum. Reprod. 8, 1061-1066. [DOI] [PubMed] [Google Scholar]
- 21.Ogura, A., Oguniki, N. & Inoue, K. (2001) Mamm. Genome 12, 803-812. [DOI] [PubMed] [Google Scholar]
- 22.Yanagimachi, R. (2001) Reprod. Fertil. Dev. 13, 3-14. [DOI] [PubMed] [Google Scholar]
- 23.Burruel, V. R., Yanagimachi, R. & Whitten, W. K. (1996) Biol. Reprod. 55, 709-714. [DOI] [PubMed] [Google Scholar]
- 24.Olds-Clarke, P. & Johnson, L. R. (1993) Dev. Biol. 155, 14-25. [DOI] [PubMed] [Google Scholar]
- 25.Kuretake, S., Maleszewski, M., Tokumasu, A., Fijimoto, H. & Yanagimachi, R. (1996) Mol. Reprod. Dev. 44, 230-233. [DOI] [PubMed] [Google Scholar]
- 26.Meng, X., Akutsu, H., Schoene, K., Reifstech, C., Fox, E. P., Olson, S., Sariola, H., Yanagimachi, R. & Baetscher, M. (2002) Biol. Reprod. 66, 726-734. [DOI] [PubMed] [Google Scholar]
- 27.Kanatsu-Shinohara, Ogura, A., Ikegawa, M., Ogunuki, N., Tashiro, K., Toyokuni, S., Honjo, T. & Shinohara, T. (2002) Proc. Natl. Acad. Sci. USA 99, 1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sassone-Corsi, P. (2002) Science 296, 2176-2178. [DOI] [PubMed] [Google Scholar]
- 29.Ogura, A., Yamamoto, Y., Suzuki, O., Takano, K., Wakayama, T., Mochida, K. & Kimura, H. (1996) Theriogenology 45, 1142-1149. [DOI] [PubMed] [Google Scholar]
- 30.Silber, S. J. & Johnson, L. (1998) Hum. Reprod. 13, 509-523. [DOI] [PubMed] [Google Scholar]
- 31.Fishel, S., Bioshop, M., Thornton, S., Hunter, A., Fleming, S. & Al-Hasson, S. (1995) Lancet 345, 1641-1642. [DOI] [PubMed] [Google Scholar]
- 32.Sousa, M., Barros, A. & Tesarik, J. (1998) Hum. Reprod. 13, 255-258. [DOI] [PubMed] [Google Scholar]
- 33.Vanderzwalmen, P. & Nijs, M. (1998) Hum. Reprod. 13, 71-84. [DOI] [PubMed] [Google Scholar]
- 34.Silber, S. J., Van Steirteghem, A., Nagy, Z., Lin, J., Tournaye, H. & Devroey, P. (1996) Fertil. Steril. 67, 408-411. [DOI] [PubMed] [Google Scholar]
- 35.Saremi, A., Esfandiari, N., Salehi, N. & Saremi, M. R. (2002) Arch. Androl. 48, 315-319. [DOI] [PubMed] [Google Scholar]
- 36.Johnson, L., Staub, C., Neacves, W. B. & Yanagimachi, R. (2001) Hum. Reprod. 16, 1575-1582. [DOI] [PubMed] [Google Scholar]
- 37.Yazawa, H., Yanagida, K., Katayose, H., Hayashi. S. & Sato, A. (2000) Hum. Reprod. 15, 2582-2590. [DOI] [PubMed] [Google Scholar]
- 38.Tuerlings, J. N., Kremer, J. & Meuleman, E. J. (1997) J. Androl. 18, 576-581. [PubMed] [Google Scholar]
- 39.Patrozio, p. & Broomfield, D. (1999) in Male Fertility and Infertility, eds Glover, T. D. & Barratt, C. L. (Cambridge Univ. Press, Cambridge, U.K.), pp. 162-176.
- 40.Wieacker, L. & Jakubiczka, S. (1997) Andrologia 29, 63-69. [DOI] [PubMed] [Google Scholar]
- 41.Vogt, P. H. (1995) Hum. Reprod. 10, Suppl. 1, 128-213 [DOI] [PubMed] [Google Scholar]
- 42.Roberts, K. P. (1998) J. Androl. 19, 255-259. [PubMed] [Google Scholar]
- 43.Varnum, D. S. (1983) J. Hered. 74, 206-207. [DOI] [PubMed] [Google Scholar]
- 44.Samorajski, T., Friende, R. L. & Reimer, P. R. (1970) J. Neuropathol. Exp. Neurol. 29, 507-523. [DOI] [PubMed] [Google Scholar]
- 45.Sidman, R. L., Dickie, M. M. & Appel, S. H. (1964) Science 144, 309-311. [DOI] [PubMed] [Google Scholar]
- 46.De Cesare, D., Fimia, G. & Sassone-Corsi, A. (2000) J. Endocrinol. Invest. 23, 592-596. [DOI] [PubMed] [Google Scholar]