Abstract
The treatment of advanced non–small cell lung cancer (NSCLC) increasingly involves the use of molecularly targeted therapy with activity against either the tumor directly, or indirectly, through activity against host-derived mechanisms of tumor support such as angiogenesis. The most well studied signaling pathway associated with angiogenesis is the vascular endothelial growth factor (VEGF) pathway, and the only antiangiogenic agent currently approved for the treatment of NSCLC is bevacizumab, an antibody targeted against VEGF. More recently, preclinical data supporting the role of fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR) signaling in angiogenesis have been reported. The platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) pathways may also stimulate tumor growth directly through activation of downstream mitogenic signaling cascades. In addition, 1 or both of these pathways have been associated with resistance to agents targeting the epidermal growth factor receptor (EGFR) and VEGF. A number of agents that target FGF and/or PDGF signaling are now in development for the treatment of NSCLC. This review will summarize the potential molecular roles of PDGFR and FGFR in tumor growth and angiogenesis, as well as discuss the current clinical status of PDGFR and FGFR inhibitors in clinical development.
Keywords: angiogenesis, fibroblast growth factor (FGF), fibroblast growth factor receptor (FGFR), non–small cell lung cancer (NSCLC), platelet-derived growth factor (PDGF), platelet-derived growth factor receptor (PDGFR)
INTRODUCTION
In the United States, 222,520 new diagnoses and 157,300 deaths due to lung cancer (approximately 85% non-small cell lung cancer [NSCLC]) were anticipated in 2010 [1]. Of the two-thirds presenting with advanced disease [2], the 5-year survival rate is only 4% [1]. New therapeutic approaches for addressing NSCLC are urgently needed.
Many therapeutic advances will likely arise through the recognition that the term “NSCLC” indicates a molecularly heterogeneous set of diseases. The first step for therapeutic advance may be the identification of molecular targets that drive tumor proliferation directly and/or indirectly via host-derived tumor support mechanisms such as angiogenesis. This article focuses on 2 such targets, reviewing the overlapping influences of the fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR) pathways on tumor angiogenesis and on the tumor directly. The potential of these pathways to drive both de novo (primary) and/or acquired (secondary) resistance to therapies that target epidermal growth factor receptor (EGFR)- or vascular endothelial growth factor (VEGF)-related pathways is explored. Many new drugs with activity against either or both of these pathways are being developed and may eventually be “added to the mix” of relevant and effective NSCLC treatment strategies.
EGFR
Most NSCLC tumors express the EGFR protein. Almost half exhibit increased EGFR gene-copy number, but only approximately 10% of patients will possess an EGFR-activating mutation in unselected non-Asian populations [3]. Without an EGFR-activating mutation, most patients will not exhibit a dramatic response to EGFR-inhibitor monotherapy [4], either because the EGFR pathway does not drive tumor growth [5], or because other molecular co-drivers attenuate the efficacy of targeting EGFR [6]. Even among patients with EGFR-activating mutations, a small proportion will exhibit de novo resistance to reversible EGFR-tyrosine kinase inhibitor (TKI) therapy, and a majority of initial responders will likely eventually exhibit acquired resistance to reversible EGFR-TKI treatment through the selection of clones with additional resistance characteristics [7]. Although some of these acquired resistance mechanisms in EGFR-mutant cells/tumors have been identified, including c-Met amplification and the EGFR T790M mutation, the mechanism(s) remains currently unknown in at least 30% of cases [8].
OVERVIEW OF ANGIOGENESIS
Sustained angiogenesis is one of the “hallmarks of cancer” and is established in NSCLC pathogenesis [9], as tumors require a blood supply to maintain viability and metastatic potential [10]. Elevated lung tumor microvessel density correlates with metastatic potential and reduced survival [11–14]. Of the known angiogenic factors, VEGF is the best characterized and mediates angiogenesis through activation of endothelial cells, predominantly through ligand activation of VEGF receptor-2 (VEGFR-2) [15]. Endogenously produced VEGF from platelets, muscle cells, or the tumor stroma contribute to signaling [16–19]. Autocrine, paracrine, and intracrine signaling have also been described [20–23]. Because of its dominant role in angiogenesis, the VEGF/VEGFR pathway is an attractive therapeutic target. Targeting blood vessel formation with either monoclonal antibodies directed against the VEGF ligand or small-molecule TKIs directed against VEGFRs have validated VEGF pathway-directed therapy in a number of different tumors [24–27]. Bevacizumab (Avastin®, Genentech; South San Francisco, CA), a humanized VEGF-specific monoclonal antibody, initially gained approval by the Food and Drug Administration (FDA) for the treatment of metastatic colorectal cancer [28]; however, a license for NSCLC followed the results of Eastern Cooperative Oncology Group (ECOG) 4599, which showed improved median overall survival (OS; 12.3 vs 10.3 months) with the addition of bevacizumab to carboplatin/paclitaxel in the first-line treatment of advanced nonsquamous NSCLC. In ECOG 4599, 878 patients with advanced NSCLC (excluding those with squamous tumors, brain metastases, clinically significant hemoptysis, or poor performance status) were randomized to receive 6 cycles of carboplatin/paclitaxel alone or with bevacizumab, with bevacizumab continued every 3 weeks in the absence of progression or intolerance. In addition to prolonging the primary endpoint of OS, the bevacizumab arm had significant improvement in both progression-free survival (PFS; 6.2 vs 4.5 months) and response rate (RR; 35% vs 15%). Rates of hypertension, proteinuria, bleeding, neutropenia, febrile neutropenia, thrombocytopenia, hyponatremia, rash, and headache were significantly (P 0.05) higher among patients who received bevacizumab, including 15 treatment-related deaths [24]. Dowlati and colleagues evaluated correlative biomarkers in the ECOG 4599 trial via baseline plasma VEGF sampling, as well as baseline and Week 7 measurement of basic fibroblast growth factor (bFGF), soluble intercellular adhesion molecule (ICAM), and E-selectin [29]. High baseline VEGF levels were associated with an increased probability of response to bevacizumab-containing chemotherapy, but only baseline ICAM levels were both predictive of response and prognostic for survival for all patients irrespective of treatment assignment. Zhang and colleagues analyzed the sera of 133 patients enrolled in ECOG 4599 and found germline single nucleotide polymorphisms (SNPs) for VEGF G-634C, ICAM1 T469C, and WNK1-rs11064560 to be associated with improved OS (P 0.05), and SNPs for ICAM1 T469C, EGF A-61G, and CXCR2 C785T to be associated with better PFS (P 0.05)1. Prospective data are needed to further our understanding of potential prognostic and predictive markers in antiangiogenic therapy.
Factors beyond VEGF, including the angiopoietin/TIE-2 interaction, interleukins, Notch/delta-like ligand 4, PDGFs, and fibroblast growth factors (FGFs), influence angiogenesis [30–33]. These factors may drive angiogenesis directly in tumors refractory to prior VEGF/VEGFR-directed therapies or they may contribute to acquired resistance via selection pressures following VEGF/VEGFR-directed therapy. The FGF and PDGF pathways are increasingly being targeted therapeutically both alone and in combination with VEGFRs due to the spectrum of activity displayed by specific multitargeted kinase inhibitors (Figure 1).
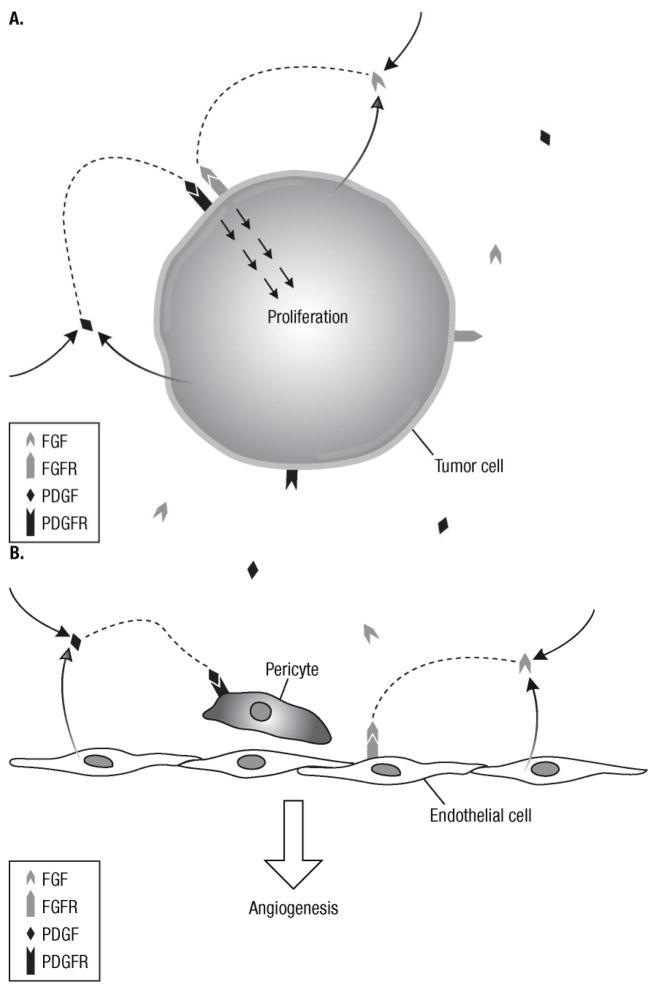
Figure 1. Schematic of the potential roles of the FGFR and PDGFR pathways in tumor proliferation and angiogenesis.
Autocrine and paracrine signaling of the FGF and PDGF pathways may contribute to tumor proliferation (A) and angiogenesis (B). (A) Activation of FGFR and PDGFR from ligands expressed by tumor cells or other tissues results in stimulation of mitogenic downstream cascades. (B) Similarly, PDGF secreted from endothelial cells may recruit pericytes necessary for angiogenesis through paracrine signaling. In addition, activation of FGFR on endothelial cells results in cellular proliferation and increased angiogenesis.
FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor.
THE FGF/FGFR PATHWAY
The mammalian FGF family plays a critical role in embryogenesis and adult tissue repair/maintenance [34] through binding FGF receptors (FGFR-1 through -4), inducing dimerization and downstream signaling [35]. Activation of FGF signaling has been reported in a number of human malignancies, including myeloproliferative disorders, lymphomas, prostate cancer, breast cancer, lung cancer, and others via activating mutations, overexpression, or gene amplification [34–39]. The ligand FGF-2 is directly associated with neovascularization [40]. Schweigerer and colleagues [41] showed that adrenal-cortex-derived capillary endothelial cells produced FGF-2, inducing proliferation of capillary endothelial cells—a mark of angiogenesis.
In addition to its role in angiogenesis, the FGF/FGFR pathway is a primary driver of tumor proliferation. Activating mutations in FGFR-2 have been described in urothelial, ovarian, gastric, and colorectal cancer cell lines [39,42]. Selective inhibition of FGFR activity caused G1 growth arrest in breast cancer cell lines [38]. Kunii and colleagues [43] identified FGFR-2 amplification as promoting cell proliferation and survival in gastric cancer cell lines, and FGFR-2 knockdown inhibited growth. Similarly, Takeda and colleagues [39] demonstrated anti-FGFR pharmacologic growth inhibition of human gastric cancer cell lines expressing FGFR-2 in vitro and in vivo.
Autocrine and paracrine signaling independent of somatic mutations have also been implicated in FGF-related tumorigenesis [44–47]. Marek and colleagues [48] noted frequent coexpression of distinct FGFs (FGF-2 and FGF-9) and FGFRs, suggesting a potential autocrine loop in NSCLC cell lines; moreover, they found cellular proliferation was inhibited by the multikinase TKI R04383596 (anti-FGFR, -PDGFR, and -VEGFR) in FGF/FGFR-expressing cell lines that lacked VEGFR and PDGFR [49]. Similar findings have been observed in NSCLC cell lines via inhibition of FGF signaling by antisense RNA, neutralizing FGF-2 antibodies, or anti-FGFR pharmacologic inhibition [48,50–52].
An emerging role of the FGFR pathway lies in its potential to mediate de novo or acquired resistance in EGFR-driven cells via upregulation of an alternative autocrine loop. Acquired resistance to EGFR TKIs in cells initially driven by EGFR-activating mutations, via the selection of alternate molecular pathway co-drivers that permit ongoing proliferation, survival, and angiogenic signaling is well documented [5,53]. Engelman and colleagues [54] described a NSCLC cell line with a known EGFR-activating mutation that was initially gefitinib-sensitive and then developed gefitinib-resistance via MET amplification; furthermore, inhibition of MET signaling restored gefitinib sensitivity.
In NSCLC, the FGF/FGFR signaling pathway may be another example of co-driver selection, but primarily through transcriptional upregulation of co-driver expression, rather than selection of hard-wired changes as with MET amplification. This pathway appears to generate an alternative autocrine loop leading to EGFR-TKI resistance in otherwise sensitive cells [48,52]. Recently, in both EGFR wild type and mutant NSCLC cell lines, FGFR-2 and FGFR-3 expression was induced at both the mRNA and protein level following gefitinib treatment [55]. Exogenous FGF-2 or FGF-7 or coculture in the presence of FGF-producing fibroblasts caused upregulation of these receptors that mediated phenotypic resistance to gefitinib in cells that were otherwise sensitive to the EGFR TKI. Pharmacologic FGFR inhibition in combination with gefitinib abrogated this effect.
The FGF/FGFR pathway is dominant among mesenchymal NSCLC histologies, typically thought of as less sensitive to EGFR TKIs [52]. The diminished sensitivity may result from a lower prevalence of EGFR mutations, or, conceivably, from the higher prevalence of FGFR signaling associated with the mesenchymal phenotype, where it may act as a primary driver or co-driver in concert with EGFR [52,56].
THE PDGF/PDGFR PATHWAY
PDGF functions in embryonal development, mesenchymal cell proliferation, connective tissue development, and wound healing [57–59]. In tumors, PDGF promotes cell proliferation, invasion, migration, and angiogenesis [57]. Four PDGF polypeptide chains (PDGF-A, -B, -C, and -D) dimerize into PDGF-AA, -BB, -CC, -DD, or -AB, and interact with receptors (PDGFR-α/β) to signal through PI3K, src, phospholipase C-γ, and Ras pathways [57,60].
The PDGF/PDGFR pathway appears to be involved in a number of distinct aspects of tumorigenesis and angiogenesis, including recruitment and activation of cancer-associated fibroblasts (CAFs) as a consequence of PDGF-CC-associated paracrine signaling [61] and the recruitment of pericytes critical to blood vessel maturation [62] and VEGF-producing stromal fibroblasts critical to both tumorigenesis and angiogenesis [63]. In fibroblastic NIH3T3 cells, Li and colleagues demonstrated PDGF-D to be involved in tumorigenesis via reorganization of the actin cytoskeleton, induction of anchorage-independent growth, and increased cell proliferation [64]. In a NSCLC model (A549 cells transfected with the PDGF-A mutant PDGF-0), inhibiting both PDGFR-α and PDGFR-β was shown to impede tumor growth by impairing periendothelial cell recruitment [65]. The underlying biologic activity of the PDGF/PDGFR pathway may also vary in NSCLC based on histologic subtype. For example, in a recent series by Tsao and colleagues [66], PDGFR-β expression by immunohistochemistry (IHC) was found to be significantly higher in rare sarcomatoid NSCLC versus non-sarcomatoid NSCLC controls, and this higher tumor cell expression was significantly associated with gene copy number gain and a higher gene copy ratio.
Direct PDGF pathway activation has also been shown in multiple tumor types. Coexpression of PDGFR and its ligands suggest a role for both autocrine and paracrine PDGF signaling [67]. Tejada and colleagues showed fibroblastic tumor infiltration to correlate with PDGF-A and -C paracrine signaling in a NSCLC cell line. Donnem and colleagues [68] analyzed NSCLC tumor specimens (stage I–IIIA) and found tumor cell coexpression by IHC of FGFR-1 and PDGF-B to be a negative prognostic factor. Similarly, they correlated IHC tumor cell expression of PDGF-A with the presence of lymph node metastasis, and coexpression of PDGF-B and VEGFR-3 with poor survival in NSCLC patients [69].
VEGF/VEGFR, FGF/FGFR, AND PDGF/PDGFR PATHWAY INTERACTIONS
In addition to their separate roles described above, the VEGF/VEGFR, FGF/FGFR, and/or PDGF/PDGFR pathways may interact, with this crosstalk believed to promote angiogenesis in malignant and non-malignant settings. For example, Casanovas and colleagues [70] used a pancreatic islet cell mouse model to demonstrate that blocking VEGFR-2 initially inhibited angiogenesis with eventual progression and that this acquired resistance could be overcome with an adenovirus-delivered soluble FGFR-2 (serving as a FGF trap), decreasing tumor burden and angiogenesis. Crawford et al [71] examined tumor-associated fibroblasts from anti–VEGF-A-sensitive and -resistant tumors in a mouse model. Anti–VEGF-A-sensitive tumors maintained growth when stimulated by tumor-associated fibroblasts obtained from anti–VEGF-A-resistant tumors, even in the presence of VEGF inhibitors. PDGF-C was upregulated in the tumor-associated fibroblasts from refractory tumors, and inhibition of PDGF-C by neutralizing antibodies blocked angiogenesis and slowed tumor growth in mice. In a preclinical series involving a mouse model of pancreatic neuroendocrine carcinoma, PDGFR inhibition led to detachment of pericytes from tumor vessels, resulting in increased sensitivity of endothelial cells to VEGFR inhibition [72].
Whereas VEGF, PDGF-BB and FGF-2 were unable to establish stable vasculature on their own in animal models of hind-limb ischemia, PDGF-BB and FGF-2 (but not VEGF plus either PDGF-BB or FGF-2) acted in a synergistic manner to induce angiogenesis and maintain functional vessels [73]. It was speculated that this interaction may have been the consequence of FGF-2-induced upregulation of PDGFR-α and PDGFR-β expression in new vasculature. Nissen and colleagues [74] later showed that FGF-2 and PDGF-BB act synergistically in both tumor angiogenesis and metastasis. Mechanistically, both FGF-2-induced upregulation of endothelial cell PDGFR expression and PDGF-BB-induced upregulation of vascular smooth muscle cell FGFR1 expression were implicated in this synergistic interaction. Consequently, while some tumors may be sensitive to only 1 or the other approach, a therapeutic advantage may be gained by targeting both pathways, offering the potential to affect both tumor and vasculature independently.
INVESTIGATIONAL TARGETED THERAPIES WITH ACTIVITY AGAINST THE FGF/FGFR AND/OR PDGF/PDGFR PATHWAYS IN CLINICAL DEVELOPMENT
Many TKIs developed as VEGFR inhibitors also inhibit PDGFR and FGFR isoforms, potentially due to homology among these receptors [75,76] (Table 1). These investigational multitargeted agents differ with respect to their specific targets, as summarized in Figure 2, and are discussed below in relation to NSCLC.
Table 1.
In vitro VEGFR, PDGFR, and FGFR kinase activity of multitargeted TKIs in clinical development for NSCLC.
| Agent | IC50, nM | Other targets | Clinical phase | ||
|---|---|---|---|---|---|
| VEGFR-1, -2, -3 | PDGFR-α, -β | FGFR-1, -2, -3 | |||
| Sorafenib [77] | NR, 90, 20a | NR, 57a | 580, NR, NR | c-kit, FLT-3, Raf | III |
| Sunitinib [78] | NR, 80, NR | NR, 2 | 2,900, NR, NR | c-kit, FLT-3, CSF-1R, RET | III |
| Cediranib [79] | 5, 1, 3 | 36, 5 | 26, NR, NR | c-kit | III |
| Motesanib [80] | 2, 3, 6 | NR, 84 | >2,800, NR, NR | c-kit, RET | III |
| Axitinib [81] | NRb | NRb | NR | c-kit | II |
| Linifanib [82] | 3, 4, 190 | NR, 66 | >12,500, NR, NR | FLT-3, CSF-1R, c-kit | II |
| Brivanib [83,84]c | 380, 25, 10 | NR, >6,000 | 148, 125, 68 | NR | II |
| BIBF 1120 [85] | 34, 21, 13 | 59, 65 | 69, 37, 108 | FLT-3, src | III |
| Pazopanib [86] | 10, 30, 47 | 71, 84 | 140, NR, 130 | c-kit | II/III |
IC50 values of sorafenib against murine VEGFR-3 and PDGFR-β.
Axitinib reportedly inhibits VEGFRs at subnanomolar concentrations and PDGFR-β at low-nanomolar concentrations [81].
IC50 values for brivanib (BMS-582664) are reported for BMS-540215, the active moiety hydrolyzed in vivo.
c-kit, stem cell factor receptor; CSF-1R, colony stimulating factor 1 receptor; FGFR, fibroblast growth factor receptor; FLT-3, fms-like tyrosine kinase 3; IC50, half maximal inhibitory concentration; NR, not reported; PDGFR, platelet-derived growth factor receptor; Raf, v-raf 1 murine leukemia viral oncogene homolog 1; RET, rearranged during transfection; VEGFR, vascular endothelial growth factor receptor.
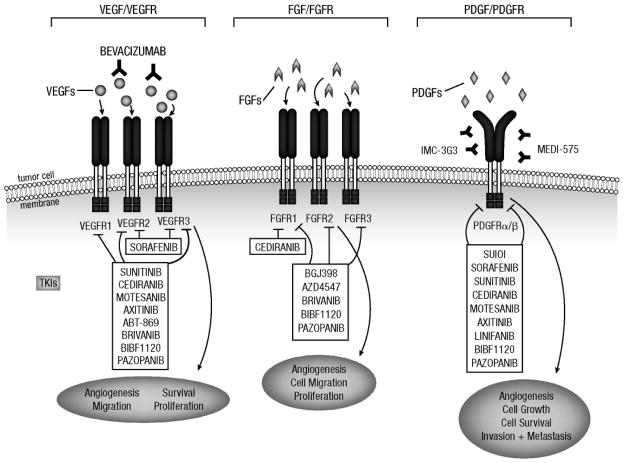
Figure 2. Molecular targets of the investigational multitargeted TKIs being studied in NSCLC and PDGFR- and FGFR-specific agents in earlier clinical development.
Illustration depicting targeted inhibition of the VEGF, PDGF, and FGF pathways by monoclonal antibodies and TKIs.
FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; TKIs, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Sorafenib
Sorafenib (Nexavar®, Bayer; Leverkusen, Germany) is a multitargeted kinase inhibitor with activity against VEGFR-2 and -3, PDGFR-β, stem cell factor receptor (c-kit), v-raf 1 murine leukemia viral oncogene homolog 1 (Raf), and fms-like tyrosine kinase 3 (FLT-3) [77,87,88]. Wilhelm et al [77] noted that sorafenib inhibited wild-type BRAF, VEGFR-2 and -3, and PDGFR-β in breast, colon, and NSCLC cell lines; additionally, human colon, lung, and breast xenograft tumor growth was inhibited. Sorafenib is FDA approved in unresectable hepatocellular carcinoma and advanced renal cell carcinoma (RCC) [89]. In a phase II study of single-agent sorafenib in refractory advanced NSCLC (Table 2), activity was reported and grade 3 or 4 toxicities included hand-foot skin reaction, hypertension, fatigue, and diarrhea [90]. An interim analysis of a phase III trial evaluating carboplatin/paclitaxel with or without sorafenib in patients with advanced NSCLC showed no clinical benefit and a higher mortality in the subset of patients with squamous histology who received sorafenib, prompting early termination [91]. The phase III NEXUS trial [92] did not show an OS benefit with the addition of sorafenib to gemcitabine/cisplatin in advanced nonsquamous NSCLC; however, PFS was significantly improved. Sorafenib is currently being evaluated in the third/fourth-line setting in the placebo-controlled MISSION trial (NCT00863746).
Table 2.
Data from published phase II trials of investigational multitargeted antiangiogenic TKIs in patients with advanced NSCLC.
| Trial description | Prior therapy/performance status | RR, % | SD, % | PFS | OS | Most common (≥25%) treatment-related AEsa (%) |
|---|---|---|---|---|---|---|
| Sorafenib 400 mg bid (N = 54); phase II [93] | 1 or 2 chemotherapy regimens ECOG PS 0–2 |
0 | 59 | 2.7 mo | 6.7 mo | Diarrhea, 40 Hand-foot skin reaction, 37 Fatigue, 27 Nausea, 25 |
| Sunitinib 37.5 mg qd (N = 47); phase II [94] | 1 or 2 chemotherapy regimens ECOG PS 0 or 1b |
2.1 | 23.4 | 11.9 wks | 37.1 wks | Diarrhea, 27.7 Fatigue, 27.7 |
| Sunitinib 50 mg qd (N = 63); phase II [95] | ≥ 1 chemotherapy regimens ECOG PS 0 or 1 |
11.1 | 28.6 | 12 wks | 23.4 wks | Fatigue/asthenia, 70c Lymphopenia, 69 Leukopenia, 68 Pain/myalgia, 60 Anemia, 58 Thrombocytopenia, 52 Nausea/vomiting, 52 Neutropenia, 49 Stomatitis/mucosal inflammation, 43 Anorexia/weight loss, 35 Dyspnea, 35 Diarrhea, 33 Dysgeusia, 27 Cough, 25 |
| Cediranib 30 mgd qd versus placebo in combination with carboplatin/paclitaxel (N = 296e); phase II/IIIf [96] | Adjuvant chemotherapy ≥1 yr prior, radiation, EGFR-targeted therapy ECOG PS 0 or 1g |
38 vs 16; P <0.001 | NR | 5.6 vs 5.0 mo; P = 0.13 | 10.5 vs 10.1 mo; P = 0.11 | Fatigue; 88 (all grades), 29 (grade ≥3)c Diarrhea; 79 (all grades), 15 (grade ≥3) Dyspnea; 75 (all grades), 10 (grade ≥3) Neutropenia; 65 (all grades), 49 (grade ≥3) Sensory neuropathy; 63 (all grades), 3 (grade ≥3) Anorexia; 61 (all grades), 6 (grade ≥3) Increased TSH; 45 (all grades), 27 (grade ≥3) Stomatitis; 41 (all grades), 6 (grade ≥3) Bleeding; 25 (all grades), 3 (grade ≥3) |
| Motesanib 125 mg qd versus motesanib 75 mg bid versus bevacizumab in combination with carboplatin/paclitaxel (N = 186); phase II [97] | Chemotherapy-naive ECOG PS ≤ 1 |
30 vs 23 vs 37 | 35 vs 50 vs 42 | 7.7 vs 5.8 vs 8.3 mo | 14.0 vs 12.8 vs 14.0 mo | Fatigue; 63 vs 52 vs 60 (all grades), 17 vs 5 vs 8 (grade 3/4)c Diarrhea; 51 vs 47 vs 28 (all grades), 19 vs 11 vs 3 (grade 3/4) Hypertension; 47 vs 27 vs 15 (all grades), 5 vs 8 vs 2 (grade 3/4) Nausea; 47 vs 47 vs 38 (all grades), 8 vs 2 vs 2 (grade 3/4) Vomiting; 47 vs 35 vs 23 (all grades), 5 vs 5 vs 3 (grade 3/4) Alopecia; 41 vs 34 vs 38 (all grades), 0 vs 0 vs 3 (grade 3/4) Constipation; 34 vs 32 vs 33 (all grades), 3 vs 2 vs 2 (grade 3/4) Dehydration; 32 vs 21 vs 10 (all grades), 17 vs 11 vs 3 (grade 3/4) Anorexia; 32 vs 24 vs 28 (all grades), 12 vs 2 vs 3 (grade 3/4) Weight decreased; 31 vs 19 vs 13 (all grades), 3 vs 0 vs 2 (grade 3/4) Peripheral neuropathy; 27 vs 26 vs 45 (all grades), 3 vs 2 vs 7 (grade 3/4) Dyspnea; 25 vs 21 vs 23 (all grades), 5 vs 0 vs 3 (grade 3/4) |
| Axitinib 5 mg bid (N = 32); phase II [98] | Chemotherapy- naive and previously treated ECOG PS 0 or 1h |
9 | 31 | 4.9 mo | 14.8 mo | Fatigue; 50 (grade 1/2), 22 (grade 3) Anorexia, 50 (grade 1/2) Diarrhea; 41 (grade 1/2), 3 (grade 3) Nausea, 34 (grade 1/2) Anemia, 31 (grade 1/2) Hoarseness, 28 (grade 1/2) |
| BIBF 1120 250 mg bid or 150 mg bid (N = 73 [ECOG PS 0–2]; n = 56 [ECOG PS 0–1]); phase II [99] | 1 or 2 chemotherapy regimens ECOG PS 0–2 |
PS 0–2, 1.4 | PS 0–2, 47.9 | PS 0–2, 6.9 wks PS 0–1, 11.6 wks |
PS 0–2, 21.9 wks PS 0–1, 37.7 wks |
Nausea; 57.5 (all grades), 6.8 (grade 3/4) Diarrhea; 47.9 (all grades), 8.2 (grade 3/4) Vomiting; 42.5 (all grades), 2.7 (grade 3/4) Anorexia, 28.8 (all grades), 1.4 (grade 3/4) |
AEs reported at all grades unless otherwise indicated.
One enrolled patient had a performance status of 2.
Only all-cause AEs were reported.
The first 45 patients received cediranib 45 mg qd.
Thirty patients were not evaluable for response.
Trial did not proceed to phase III.
Performance status was originally 0–2 but was revised per protocol amendment (in response to increased treatment-related deaths in the cediranib arm [45-mg/day cohort]).
Three enrolled patients had an unknown performance status.
AE, adverse event; bid, twice per day; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; NR, not reported; NSCLC, non–small cell lung cancer; OS, overall survival; PFS, progression-free survival; PS, performance status; qd, once per day; RR, response rate; SD, stable disease; TKI, tyrosine kinase receptor; TSH, thyroid-stimulating hormone.
Sunitinib
Sunitinib (Sutent®, Pfizer; New London, CT), a TKI with activity against PDGFR-α/β, c-kit, FLT-3, VEGFR-1 through -3, colony stimulating factor 1 receptor (CSF-1R), and rearranged during transfection (RET), is FDA approved in advanced RCC and imatinib-resistant gastrointestinal stromal tumors [100]. In phase II trials of sunitinib in patients with refractory NSCLC, RR was 2.1% with 37.5 mg/day [94] and 11.1% with 50 mg/day [95] (Table 2). The most common grade 3 or 4 toxicities observed with the 50 mg/day dosing schedule (4 weeks on and 2 off) were fatigue/asthenia, lymphopenia, pain/myalgia, dyspnea, and nausea/vomiting [95]. Scagliotti and colleagues recently published data from the SUN 1087 trial in patients with previously treated NSCLC, which showed an improvement in PFS with the combination of sunitinib plus erlotinib compared with placebo plus erlotinib (15.5 vs 8.7 weeks, respectively; P = 0.0023), but no difference in OS (9.0 vs 8.5 months)2. Ongoing studies are evaluating the role of combination therapy with sunitinib and cytotoxic chemotherapy in NSCLC.
Cediranib
Cediranib (Recentin™, AstraZeneca; Wilmington, DE), is a TKI targeting VEGFR-1 through -3, PDGFRα/β, FGFR-1, and c-kit [79]. In addition to multikinase inhibition, preclinical data from Wedge and colleagues [79] revealed significant inhibition of angiogenesis in a fibroblast/endothelial cell co-culture model, as well as inhibited growth of human tumor xenografts (colon, lung, prostate, breast, ovary). Goss and colleagues [101] reported cediranib 30 mg dosing in combination with cisplatin/gemcitabine in patients with advanced NSCLC to be tolerable, with responses in 4 of 12 evaluable patients (33.3%). However, in 2010, a phase II/III trial evaluating carboplatin/paclitaxel with or without cediranib 30 mg in advanced NSCLC was placed on hold to review imbalances in assigned causes of death [96]. The 30 mg dose was poorly tolerated due to hypertension, hand-foot syndrome, gastrointestinal toxicity, fatigue, neutropenia, and hypothyroidism; a trial of carboplatin/paclitaxel with or without 20 mg cediranib was subsequently initiated but closed early [102]. Further information is awaited.
Motesanib
Motesanib (Amgen; Thousand Oaks, CA) is a TKI targeting VEGFR-1 through -3, PDGFR, RET, and c-kit [80]. In a phase Ib study, motesanib was tolerable in conjunction with carboplatin/paclitaxel and/or panitumumab (anti-EGFR monoclonal antibody; Vectibex®, Amgen; Thousand Oaks, CA) in patients with advanced NSCLC [103]. In a randomized, open-label phase II trial of motesanib versus bevacizumab combined with paclitaxel/carboplatin, motesanib 125 mg/day continuous dosing resulted in an objective RR of 30% and a median PFS and OS of 7.7 and 14.0 months, respectively [97]. Grade ≥3 adverse events—gastrointestinal (nausea, vomiting, diarrhea) and hypertension—were more common with motesanib than with bevacizumab. The phase III MONET1 trial evaluating the combination of carboplatin, paclitaxel, and motesanib was halted after an interim safety analysis revealed unacceptable rates of hemoptysis; however, the trial had been approved to restart with accrual limited to those with nonsquamous histology (NCT00460317). Recently reported results revealed no improvement in the primary endpoint of OS with motesanib plus chemotherapy versus chemotherapy alone (13.0 vs 11.0 months) despite significant improvement in the secondary endpoints of PFS (5.6 vs 5.4 months) and RR (40% vs 26%); grade ≥3 adverse events that occurred more frequently with motesanib compared with placebo included neutropenia, diarrhea, hypertension, and cholecystitis3.
Axitinib
Axitinib (Pfizer; New London, CT) targets VEGFR-1 through -3, PDGFR-β, and c-kit [81,98]. Hu-Lowe and colleagues found that axitinib primarily inhibits VEGFR, with in vitro inhibitory effects on endothelial cell proliferation, survival, and tube formation [104]. Additionally, axitinib reduced retinal vascular VEGFR-2 phosphorylation in rats, inhibited tumor growth and angiogenesis in human xenograft tumors (colon, lung, melanoma, renal cell) in mice, and enhanced the antitumor efficacy of chemotherapy in multiple human tumor models. Schiller and colleagues [98] noted single-agent activity in patients with advanced NSCLC, with an acceptable toxicity profile (Table 2). Grade 3 adverse events included fatigue, hypertension, hyponatremia, diarrhea, and vomiting. Ongoing studies include a randomized phase II trial of axitinib in combination with cisplatin/gemcitabine (NCT00735904).
Linifanib
Linifanib (ABT-869; Abbott; Abbott Park, IL) inhibits VEGFR-1 through -3, PDGFR-β, CSF-1R, c-kit, and FLT-3 [82,105]. In addition to inhibition of VEGF and PDGF at the cellular level, Albert and colleagues showed a dose-dependent growth inhibition in human tumor xenograft models, including small cell lung, breast, and colon carcinomas [105]. In a phase I trial, stable disease for >12 weeks was seen in 16 of 29 patients with refractory solid malignancies; 2 patients with NSCLC had a partial response (PR), with toxicity rates comparable with similar drugs [106]. Dose-limiting toxicities observed included fatigue, proteinuria, and hypertension. A phase II trial is currently investigating ABT-869 (low vs high dose) in patients with advanced NSCLC (NCT00517790).
Brivanib
Brivanib (Bristol-Myers Squibb; New York, NY) inhibits VEGFR-1 through 3 and FGFR-1 through -3 [83]. Brivanib suppressed tumor growth of human hepatocellular (HCC) xenografts in mice, decreased phosphorylated VEGFR-2, microvessel density and cell proliferation, and increased apoptosis [83]. The expression of FGFR-1 and -2 in tumors was correlated with growth inhibition, suggesting a potential predictive biomarker [83]. Platero and colleagues4 correlated positive tumor FGF-2 expression by IHC (n = 24) with a trend for improved PFS (P = 0.075) and improved RR (P = 0.03) in patients treated with brivanib versus those whose tumors did not express FGF-2 (n = 19) in a phase I trial of advanced solid malignancies. In another phase I trial of brivanib in solid tumors, 1 of 4 patients enrolled had NSCLC and achieved stable disease, and no serious adverse treatment-related events were reported. Single grade 2 events including fatigue, abdominal pain, dysphagia, back pain, cognitive disorder, and cachexia were observed [107]. A randomized phase II discontinuation study evaluating the efficacy of brivanib in multiple tumor types, including lung cancer, is accruing patients (NCT00633789). Preliminary results included a 12-week stable disease rate of 24% for 42 patients with NSCLC, considered insufficient for expanding accrual to this tumor-specific cohort (as was also the case for the pancreatic, gastric, and bladder cohorts); however, early evidence of activity led to an expanded cohort of patients with soft tissue sarcoma5.
BIBF 1120
BIBF 1120 (Boehringer Ingelheim; Ingelheim, Germany) is an angiokinase inhibitor targeted against VEGFR-1 through -3, FGFR-1 through -3, PDGFR-α/β, FLT-3, and src [85]. Hilberg and colleagues [85] found that BIBF 1120 inhibited MAP kinase and Akt signaling, diminished proliferation, and induced apoptosis in endothelial cells, pericytes, and smooth muscle cells. In a phase II study of BIBF 1120 monotherapy in 73 patients (ECOG performance status 0–2) with relapsed advanced NSCLC (Table 2), the most common grade 3 or 4 toxicities were alanine aminotransferase (ALT) increase, diarrhea, and nausea [99]. Tumor stabilization (SD, complete response [CR], or PR) was achieved in 46% of all patients (ECOG 0–2) and 59% for ECOG 0–1 patients. For all patients (ECOG 0–2), median PFS was 6.9 weeks and median OS was 21.9 weeks. Median PFS and OS for ECOG 0–1 patients (n = 57) were 11.6 and 37.7 weeks, respectively. Two randomized 2-arm, placebo-controlled phase III trials of BIBF 1120 as second-line therapy in combination with either docetaxel (NCT00805194; LUME-Lung 1) or pemetrexed (NCT00806819; LUME-Lung 2) in NSCLC patients have been initiated; BIBF 1120 will be continued as maintenance therapy after cessation of combination therapy in both trials.
Pazopanib
Pazopanib (GlaxoSmithKline; London, UK) is a TKI with activity against VEGFR-1 through -3, PDGFR-α/β, FGFR-1 and -3, and c-kit [86]. In preclinical models, Kumar and colleagues found that pazopanib inhibited VEGF- and bFGF-mediated angiogenesis (mouse corneal assay); in a NSCLC mouse xenograft model, pazopanib produced an almost complete inhibition of human tumor growth [86]. Phase III data have shown efficacy of pazopanib in RCC [108]. A study of preoperative pazopanib monotherapy in 35 patients with stage I/II (94% stage I) NSCLC has completed accrual and shown a manageable safety profile [109]. Thirty (86%) patients had a post-treatment reduction in tumor volume (range, 1%–86%), 3 of whom had a PR. Grade 2 hypertension, diarrhea, and fatigue were the most common adverse events observed. Currently enrolling studies in NSCLC include pazopanib plus paclitaxel and pazopanib as third-line treatment. In an analysis of pazopanib in early-stage NSCLC, elevated baseline plasma levels of hepatocyte growth factor and IL-12 levels correlated with response, suggesting potential predictive biomarkers [110].
PDGFR-SPECIFIC THERAPIES IN CLINICAL DEVELOPMENT
In addition to trials evaluating the aforementioned multitargeted agents (Table 3), therapies specifically targeting PDGFR or FGFR are currently in development in various advanced malignancies (Table 4; Figure 2), with limited NSCLC-specific information currently available.
Table 3.
Overview of ongoing trials of investigational multitargeted antiangiogenic TKIs in NSCLC.a
| Agent (type; target) | Trial description | Identifier number (status)b |
|---|---|---|
| BIBF 1120 (TKI: VEGFR-1 through -3, FGFR-1 through -3, PDGFR-α/β, FLT-3, src) | Phase I trial of BIBF 1120 in combination with pemetrexed in Japanese patients with advanced NSCLC | NCT00979576 (Recruiting) |
| Phase I trial of BIBF 1120 in combination with docetaxel in Japanese patients with advanced NSCLC | NCT00876460 (Recruiting) | |
| Phase III trial of docetaxel in combination with BIBF 1120 or placebo as second-line therapy in patients with advanced NSCLC (LUME-Lung 1) | NCT00805194 (Active, no longer recruiting) | |
| Phase III trial of pemetrexed in combination with BIBF 1120 or placebo as second-line therapy in patients with advanced NSCLC (LUME-Lung 2) | NCT00806819 (Active, no longer recruiting) | |
| Cediranib/AZD2171 (TKI: VEGFR-1 through -3, PDGFR-α/β, FGFR-1, c-kit) | Phase I trial of cediranib in combination with chemotherapy as first-line treatment in patients with lung cancer | NCT00621361 (Active, no longer recruiting) |
| Phase II trial of gemcitabine/carboplatin with or without cediranib as first-line therapy in patients with NSCLC | NCT00326599 (Unknown) | |
| Phase II trial of cediranib and pemetrexed in patients with relapsed NSCLC | NCT00410904 (Recruiting) | |
| Phase II/III trial of paclitaxel/carboplatin with or without cediranib in patients with NSCLC | NCT00245154 (Active, no longer recruiting) | |
| Phase III trial of cediranib in combination with paclitaxel/carboplatin in patients with advanced NSCLC | NCT00795340 (Active, no longer recruiting) | |
| Pazopanib/GW786034 (TKI: VEGFR-1 through -3, PDGFR-α/β, FGFR-1 and -3, and c-kit) | Phase I trial of pazopanib and vinorelbine in patients with NSCLC and breast cancer | NCT01060514 (Recruiting) |
| Phase II trial of pazopanib as third-line therapy in patients with NSCLC | NCT01049776 (Recruiting) | |
| Phase II trial of erlotinib and pazopanib in patients with previously treated advanced NSCLC | NCT01027598 (Active, no longer recruiting) | |
| Phase II trial of pazopanib and paclitaxel in patients with advanced NSCLC | NCT00866528 (Active, no longer recruiting) | |
| Phase II/III trial of pazopanib as adjuvant treatment after surgery in patients with stage I NSCLC | NCT00775307 (Recruiting) | |
| Phase II trial of pazopanib versus pemetrexed as maintenance therapy in patients with advanced NSCLC not progressing after carboplatin or cisplatin plus pemetrexed induction | NCT01313663 (Recruiting) | |
| Phase II/III trial of pazopanib or placebo in NSCLC patients not progressing after first-line chemotherapy | NCT01208064 (Recruiting) | |
| Phase II trial of pazopanib in advanced NSCLC after progression on first-line bevacizumab- containing therapy | NCT01262820 (Recruiting) | |
| Phase II trial of pazopanib with or without pemetrexed in advanced NSCLC previously treated with bevacizumab | NCT01107652 (Recruiting) | |
| Brivanib/BMS-582664 (TKI: VEGFR-1 through -3 and FGFR-1 through -3) | Phase II trial of brivanib in patients with multiple tumor types (including advanced NSCLC) | NCT00633789 (Active, no longer recruiting) |
| Motesanib/AMG 706 (TKI: VEGFR-1 through -3, PDGFR, c-kit, RET) | Phase II trial of motesanib or bevacizumab in combination with paclitaxel/carboplatin in patients with advanced NSCLC | NCT00369070 (Active, no longer recruiting) |
| Phase III trial of motesanib or placebo in combination with paclitaxel/carboplatin in patients with advanced nonsquamous NSCLC | NCT00460317 (Active, no longer recruiting) | |
| Sorafenib (TKI: VEGFR-2 and -3, PDGFR-β, FLT-3, c-kit, Raf) | Phase I trial of sorafenib in previously untreated patients with NSCLC | NCT00533585 (Active, no longer recruiting) |
| Phase I trial of panobinostat and sorafenib in patients with renal cancer or NSCLC | NCT01005797 (Recruiting) | |
| Phase I trial of sorafenib in combination with metronomic vinorelbine in patients with advanced NSCLC | NCT00870532 (Unknown) | |
| Phase I trial of sorafenib with carboplatin, paclitaxel, and bevacizumab in patients with untreated stage IIIb NSCLC | NCT01069328 (Active, no longer recruiting) | |
| Phase II trial of sorafenib after failure of an EGFR-TKI in patients with NSCLC | NCT00922584 (Unknown) | |
| Phase II trial of sorafenib in patients with NSCLC (BATTLE) | NCT00411671 (Active, no longer recruiting) | |
| Phase II trial of sorafenib/erlotinib or erlotinib alone in patients with previously treated advanced NSCLC | NCT00600015 (Active, no longer recruiting) | |
| Phase II trial of sorafenib and erlotinib in patients with NSCLC that has not responded to chemotherapy | NCT00801385 (Unknown) | |
| Phase II trial of sorafenib/erlotinib or sorafenib alone in patients with advanced NSCLC progressing on erlotinib | NCT00609804 (Active, no longer recruiting) | |
| Phase II study of metronomic docetaxel and sorafenib as first-line therapy in patients with advanced nonsquamous NSCLC | NCT00801801 (Active, no longer recruiting) | |
| Phase II trial of erlotinib and sorafenib in chemonaive patients with locally advanced or metastatic NSCLC | NCT00722969 (Unknown) | |
| Phase II trial of sorafenib in non-smokers or former light smokers with relapsed or refractory NSCLC | NCT00754923 (Unknown) | |
| Phase II trial of sorafenib in combination with pemetrexed as second-line therapy in patients with NSCLC | NCT00454194 (Active, no longer recruiting) | |
| Phase II trial of sorafenib in patients with refractory NSCLC | NCT00064350 (Unknown) | |
| Phase III trial of sorafenib as third- or fourth-line therapy in patients with predominantly nonsquamous NSCLC | NCT00863746 (Active, no longer recruiting) | |
| Sunitinib (TKI: VEGFR-1 through -3, PDGFR-α/β, c- kit, FLT-3, CSF-1R, RET) | Phase I trial of sunitinib and rapamycin in patients with advanced NSCLC | NCT00555256 (Active, no longer recruiting) |
| Phase I trial of sunitinib and erlotinib in patients with NSCLC | NCT00581789 (Active, no longer recruiting) | |
| Phase II trial of salvage therapy with sunitinib, docetaxel, and a platinum agent in patients with NSCLC | NCT01019798 (Recruiting) | |
| Phase II trial of pemetrexed and/or sunitinib as second-line therapy in patients with NSCLC | NCT00698815 (Recruiting) | |
| Phase II trial of sunitinib in patients over 70 years of age with NSCLC | NCT00864721 (Active, no longer recruiting) | |
| Phase II trial of sunitinib/erlotinib or erlotinib alone in patients with NSCLC | NCT00265317 (Active, no longer recruiting) | |
| Phase II trial of sunitinib as maintenance therapy in patients with advanced NSCLC | NCT01210053 (Recruiting) | |
| Phase III trial of sunitinib/erlotinib or erlotinib alone in patients with NSCLC | NCT00457392 (Active, no longer recruiting) | |
| Phase III trial of sunitinib as maintenance therapy in patients with advanced NSCLC previously treated with combination chemotherapy | NCT00693992 (Recruiting) | |
| Linifanib (TKI: VEGFR-1 through -3, PDGFR-β, c-kit, and FLT-3) | Phase II trial of ABT-869 in patients with advanced NSCLC | NCT00517790 (Active, no longer recruiting) |
| Phase II trial of paclitaxel/carboplatin in combination with ABT-869 in patients with advanced or metastatic NSCLC | NCT00716534 (Recruiting) | |
| Phase I trial of ABT-869 in combination with paclitaxel/carboplatin in Japanese patients with NSCLC | NCT01225302 (Active, no longer recruiting) | |
| Axitinib/AG-013736 (TKI: VEGFR-1 through -3, PDGFR-β, c-kit) | Phase II trial of axitinib, cisplatin, and gemcitabine in patients with squamous NSCLC | NCT00735904 (Active, no longer recruiting) |
| Phase II trial of axitinib or bevacizumab in combination with carboplatin/paclitaxel in patients with advanced lung cancer | NCT00600821 (Active, no longer recruiting) |
Based on ClinicalTrials.gov accessed on March 22, 2011, with updates based on status as of November 15, 2011.
Active status indicates the trial is ongoing, but no longer recruiting patients.
c-kit, stem cell factor receptor; BAC, bronchioloalveolar carcinoma; CSF-1R, colony stimulating factor-1 receptor; EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; FLT-3, fms-like tyrosine kinase 3; FGFR, fibroblast growth factor receptor; NSCLC, non–small cell lung cancer; PDGFR, platelet-derived growth factor receptor; RET, rearranged in transformation; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
Table 4.
Overview of ongoing oncology trials of agents targeting PDGFR or FGFR signaling.a
| Agent (type; target) | Trial description | Identifier number (status)b |
|---|---|---|
| PDGFR-targeted agents | ||
| IMC-3G3 (Ab: PDGFR-α) | Phase II trial of IMC-3G3 in combination with paclitaxel/carboplatin in previously untreated patients with advanced NSCLC | NCT00918203 (Recruiting) |
| Phase II trial of liposomal doxorubicin with or without IMC-3G3 in platinum-refractory or platinum-resistant advanced ovarian cancer | NCT00913835 (Active, no longer recruiting) | |
| Phase II trial of ramucirumab or IMC-3G3 in patients with glioblastoma multiforme | NCT00895180 (Recruiting) | |
| Phase II trial of IMC-3G3 in prostate cancer | NCT01204710 (Recruiting) | |
| Phase I/II trial of IMC-3G3 in soft tissue sarcomas | NCT01185964 (Recruiting) | |
| Phase I trial of safety and pharmacokinetics of IMC-3G3 in Japanese patients with solid tumors | NCT01199822 (Active, no longer recruiting) | |
| MEDI-575 (Ab: PDGFR-α) | Phase I trial evaluating dose escalation and dose expansion of MEDI-575 in patients with advanced cancer | NCT00816400 (Active, no longer recruiting) |
| Phase I trial of MEDI-575 in patients with advanced solid malignancies | NCT01102400 (Recruiting) | |
| Phase I/II trial of MEDI-575 in combination with paclitaxel/carboplatin in previously untreated patients with advanced NSCLC | NCT01268059 (Recruiting) | |
| Phase II trial of MEDI-575 in patients with recurrent glioblastoma multiforme | NCT01268566 (Recruiting) | |
| SU101 (small molecule: PDGFR) | Phase II trial of leflunomide (SU101) in patients with relapsed anaplastic astrocytoma | NCT00003775 (Unknown) |
| FGFR-targeted agents | ||
| BGJ398 (TKI: FGFRs) | Phase I dose escalation study in patients with advanced solid malignancies | NCT01004224 (Recruiting) |
| AZD4547 (TKI: FGFR) | Phase I dose escalation study in patients with advanced tumors | NCT00979134 (Recruiting) |
| Phase I/II trial of AZD4547 in combination with exemestane in estrogen receptor-positive breast cancer | NCT01202591 (Recruiting) | |
| Phase I trial of AZD4547 in Japanese patients with advanced solid malignancies | NCT01213160 (Recruiting) | |
Based on ClinicalTrials.gov accessed on March 22, 2011, with updates based on status as of November 15, 2011.
Active status indicates the trial is ongoing, but no longer recruiting patients.
Ab, antibody; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor; TKI, tyrosine kinase inhibitor.
IMC-3G3
IMC-3G3 (ImClone Systems; New York, NY) is a fully human IgG1 monoclonal antibody with high affinity for PDGFR-α but does not cross-react with PDGFR-β. Loizos and colleagues found that IMC-3G3 inhibited signaling as well as cell proliferation through PDGFR-α, in normal, glioblastoma and leiomyosarcoma cell lines [111]. Growth inhibition was seen in glioblastoma and leiomyosarcoma human xenografts. IMC-3G3 has also been shown to inhibit proliferation in ovarian and hepatoma cell lines [112,113] and to enhance the antitumor effects of docetaxel in ovarian cancer cell lines and mouse xenograft models6. Russell and colleagues injected PC3-ML human prostate cancer cells (highly bone-metastatic) directly into the bloodstream of mice and noted that treatment with IMC-3G3 delayed progression of skeletal metastases and decreased the size of existing metastases [114]. A phase I trial evaluating IMC-3G3 in patients with advanced solid malignancies showed preliminary safety, with no dose-limiting toxicities in the first cycle; one patient had prostate-specific antigen (PSA) decrease >50%7. An ongoing phase II study (NCT00918203) is evaluating paclitaxel/carboplatin alone or in combination with IMC-3G3 as first-line treatment of advanced NSCLC8.
MEDI-575
MEDI-575 (MedImmune LLC; Gaithersburg, MD) is a fully humanized IgG2 monoclonal antibody that targets the PDGFR-α receptor without blocking PDGFR-β. Mouse tumor models showed antitumor efficacy with MEDI-575 in glioblastoma multiforme xenografts9. Another mouse xenograft model found that MEDI-575 enhanced the activity of carboplatin and paclitaxel in NSCLC, possibly related to a MEDI-575-associated reduction in phosphorylated PDGFR-α expression in tumor stroma10. In a phase I study of MEDI-575 in patients with advanced solid tumors, most treatment-related adverse events were grade 1/2 and reversible11. MEDI-575 is currently being evaluated in phase I and phase II clinical trials, including a recently initiated phase I/II study of carboplatin/paclitaxel with or without MEDI-575 in previously-untreated advanced NSCLC (NCT01268059).
SU101
SU101 (leflunomide, Arava®, Sanofi Aventis; Bridgewater, NJ) is FDA approved for the treatment of rheumatoid arthritis. In preclinical studies by Xu and colleagues, the active metabolite of SU101, A77 1776, more effectively inhibited PDGFR than EGFR and had no effect on FGFR [115]. A77 1776 demonstrated anti-proliferative activity against the C6 glioma cells both in vitro and in nude mice. In vivo, the anti-proliferative effects were independent of pyrimidine nucleotide synthesis inhibition and potentially due to tyrosine phosphorylation inhibition. In a phase I trial of 26 patients with advanced solid malignancies, SU101 was tolerable, with the most common toxicities being mild to moderate nausea, vomiting, and fever; 2 patients experienced grade 3 neutropenia [116].
FGFR-SPECIFIC THERAPIES IN CLINICAL DEVELOPMENT
BGJ398
BGJ398 (Novartis; Basel, Switzerland) is a small molecule TKI that selectively targets FGFRs (data on file, Novartis) and is currently being investigated in a phase I dose-escalation trial in patients with advanced solid malignancies (NCT01004224).
AZD4547
AZD4547 (AstraZeneca; Wilmington, DE) is a pan-FGFR inhibitor currently being evaluated in phase I trials of patients with advanced solid malignancies (NCT00979134; NCT01213160), as well as in a phase I/II breast cancer trial in combination with exemestane (NCT01202591). Preclinical data have not yet been published on this compound.
CONCLUDING REMARKS
NSCLC represents a molecularly heterogeneous set of diseases. Preclinical and clinical data suggest that the PDGFR and FGFR pathways are viable targets in NSCLC; both pathways may have a role in angiogenesis and, in some cases, stimulating tumor growth directly. Potential predictive biomarkers for these pathways, such as serum PDGF-α, -β, and tumor expression of FGF ligand or receptor, are being investigated, but none have been validated for clinical use.
Adding FGFR and/or PDGFR inhibition to the mix of antiangiogenic agents for NSCLC may have broad applicability given that these reflect host-derived angiogenic mechanisms. Mutations and gene-copy number increases in tumor cells relating to both pathways have been reported, although their clinical significance in NSCLC remains unknown. Consequently, direct antitumor activity from anti-FGFR/PDGFR therapeutic approaches is likely to be more restricted as not all NSCLC tumors will directly utilize these pathways. Recent preclinical studies have suggested targeting FGFR as a strategy for treating de novo or acquired resistance to EGFR-TKI therapy. Demonstrating the presence of FGFR pathway upregulation in the clinical setting of erlotinib/gefitinib resistance in patients with or without an activating EGFR mutation will be the next step and may suggest a population that could most benefit from dual EGFR/FGFR inhibition.
Acknowledgments
Editorial assistance and development of tables and figures were provided by Staci Heise, PhD of MedErgy, which was contracted by Boehringer Ingelheim Pharmaceuticals, Inc., for these services. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development.
Financial support
Financial support for editorial assistance was provided by Boehringer Ingelheim Pharmaceuticals, Inc. The authors received no compensation related to the development of the manuscript.
ABBREVIATIONS
- AE
adverse event
- ALT
alanine aminotransferase
- bFGF
basic fibroblast growth factor
- bid
twice per day
- CAF
cancer-associated fibroblast
- c-kit
stem cell factor receptor
- CR
complete response
- CSF-1R
colony stimulating factor 1 receptor
- ECOG
Eastern Cooperative Oncology Group
- EGFR
epidermal growth factor receptor
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FLT-3
fms-like tyrosine kinase 3
- HCC
human hepatocellular
- IC50
half maximal inhibitory concentration
- ICAM
intercellular adhesion molecule
- ICMJE
International Committee of Medical Journal Editors
- IHC
immunohistochemistry
- NR
not reported
- NSCLC
non–small cell lung cancer
- OS
overall survival
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PFS
progression-free survival
- PS
performance status
- qd
once per day
- Raf
v-raf 1 murine leukemia viral oncogene homolog 1
- RCC
renal cell carcinoma
- RET
rearranged during transfection
- RR
response rate
- SD
stable disease
- SNP
single nucleotide polymorphism
- TKI
tyrosine kinase inhibitor
- TSH
thyroid-stimulating hormone
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Zhang, W.; Dahlberg, S. E.; Yand, D.; Sandler, A. B.; Brahmer, J. R.; Schiller, J. H.; Carbone, D. P.; Johnson, D. H.; Lenz, H. Genetic variants in angiogenesis pathway associated with clinical outcome in NSCLC patients (pts) treated with bevacizumab in combination with carboplatin and paclitaxel: subset pharmacogenetic analysis of ECOG 4599. J Clin Oncol 2009, 27(suppl). Abstract 8032.
Scagliotti, G. V.; Krzakowski, M.; Szczesna, A.; Strausz, J.; Makhson, A.; Reck, M.; Tye, L.; Selaru, P.; Chao, R. C.; Govindan, R. Sunitinib (SU) in combination with erlotinib (E) for the treatment of advanced/metastatic non-small cell lung cancer (NSCLC): a phase III study. Ann Oncol 2010, 21(suppl 8), viii 3. Abstract LBA6.
Scagliotti, G.; Vynnychenko, I.; Ichinose, Y.; Park, K.; Kubota, K.; Blackhall, F. H.; Pirker, R.; Galiulin, R.; Ciuleanu, T.; Sydorenko, O.; Dediu, M.; Papai-Szekely, Z.; Martinez Banaclocha, N.; McCoy, S.; Yao, B.; Hei, Y.; Spigel, D. R. An international, randomized, placebo-controlled, double-blind phase III study (MONET1) of motesanib plus carboplatin/paclitaxel (C/P) in patients with advanced nonsquamous non-small cell lung cancer (NSCLC). J Clin Oncol 2011, 29(suppl). Abstract LBA7512.
Platero, S.; Mokliatchouk, O.; Jayson, G. C.; Jonker, D. J.; Rosen, L. S.; Luroe, S.; Kelsey, J.; Feltquate, D.; Velasquez, L.; Galbraith, S. Correlation of FGF2 tumor expression with tumor response, PFS, and changes in plasma pharmacodynamic (PD) markers following treatment with brivanib alaninate, an oral dual inhibitor of VEGFR and FGFR tyrosine kinases. J Clin Oncol 2008, 26(15S). Abstract 3506.
Ratain, M. J.; Schwartz, G. K.; Oza, A. M.; Rudin, C. M.; Kaye, S. B.; De Jonge, M. J.; Khayat, D.; Awada, A.; Sawyer, M. B.; Obel, J. C.; Medioni, J.; Evans, T.; De Greve, J.; Soetekouw, P. M.; Baurain, J.; O’Dwyer, P. J.; Hartman, C.; Poulart, V.; Walters, I. B. Brivanib (BMS-582664) in advanced solid tumors (AST): Results of a phase II randomized discontinuation trial (RDT). J Clin Oncol 2011, 29(suppl). Abstract 3079.
Matsuo, K.; Stone, R. L.; Shahzad, M.; Carroll, A. R.; Han, H-D.; Lee, S-J.; Nishimura, M.; Mora, E.; Lu, C.; Loizos, N.; Sood, A. K. Platelet-derived growth factor receptor alpha blockade significantly enhances sensitization to docetaxel in ovarian carcinoma. Proc Amer Assoc Cancer Res 2010. Abstract 1793.
Youssoufian, H.; Amato, R. J.; Sweeney, C. J.; Chiorean, E. G.; Fox, F.; Katz, T.; Rowinsky, E. K. Phase 1 study of IMC-3G3, an IgG1 monoclonal antibody targeting PDGFRα in patients with advanced solid malignancies. J Clin Oncol 2008, 26(15S). Abstract 14617.
Gerber, D. E. Randomized phase II study of human anti-platelet-derived growth factor receptor alpha (PDGFRα) monoclonal antibody (IMC-3G3) with paclitaxel/carboplatin (P/C) or P/C alone in first-line treatment of stage IIIb/IV non-small cell lung cancer (NSCLC). J Clin Oncol 2010, 28(15S). Abstract TPS296.
Steiner, P.; Wetzel, L.; Camara, M.; Schifferli, K.; Baffa, R.; LaVallee, T.; Coats, S.; Jallal, B.; Trail, P.; Chang, Y. Glioblastoma multiforme is characterized by high incidence of PDGFRalpha expression and susceptibility to the PDGFRalpha-specific antibody MEDI-575 in mouse tumor models. Eur J Cancer Suppl 2010. 8(7):27. Abstract 57.
Steiner, P.; Wetzel, L.; Schifferli, K.; Camara, M.; Baffa, R.; LaVallee, T.; Jallal, B.; Coats, S.; Trail, P.; Chang, Y. Inhibition of PDGFRalpha in tumor stroma with MEDI-575 enhances activity of carboplatin/paclitaxel and delays tumor regrowth in a NSCLC xenograft model. Eur J Cancer Suppl 2010. 8(7):39. Abstract 100.
Lechleider, R.; Becerra, C.; Liang, M.; Narwal, R.; Shi, L.; Conkling, P.; Galsky, M.; Jotte, R.; Wu, H.; Vogelzang, N. J. Phase I study of MEDI-575, a fully human monoclonal antibody targeting PDGFR-alpha in subjects with advanced solid tumors. Eur J Cancer Suppl 2010. 8(7):128. Abstract 404.
Potential conflicts of interest Drs Kono, Heasley, Doebele, and Camidge have no potential conflicts of interest to disclose.
References
- 1.American Cancer Society. Cancer Facts & Figures, 2010. American Cancer Society; Atlanta, GA: 2010. [Google Scholar]
- 2.Surveillance Epidemiology and End Results; National Cancer Institute. [Accessed April 30, 2010];SEER Stat Fact Sheets: Lung and Bronchus. 2010 http://seer.cancer.gov/statfacts/html/lungb.html.
- 3.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 6.Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11(1):397–405. [PubMed] [Google Scholar]
- 7.Metro G, Finocchiaro G, Cappuzzo F. Anti-cancer therapy with EGFR inhibitors: factors of prognostic and predictive significance. Ann Oncol. 2006;17(Suppl 2):ii42–ii45. doi: 10.1093/annonc/mdj920. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14(10):2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 11.Fontanini G, Lucchi M, Vignati S, Mussi A, Ciardiello F, De Laurentiis M, De Placido S, Basolo F, Angeletti CA, Bevilacqua G. Angiogenesis as a prognostic indicator of survival in non-small-cell lung carcinoma: a prospective study. J Natl Cancer Inst. 1997;89(12):881–886. doi: 10.1093/jnci/89.12.881. [DOI] [PubMed] [Google Scholar]
- 12.Lucchi M, Fontanini G, Mussi A, Vignati S, Ribechini A, Menconi GF, Bevilacqua G, Angeletti CA. Tumor angiogenesis and biologic markers in resected stage I NSCLC. Eur J Cardiothorac Surg. 1997;12(4):535–541. doi: 10.1016/s1010-7940(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 13.Macchiarini P, Fontanini G, Dulmet E, de MV, Chapelier AR, Cerrina J, Ladurie FL, Dartevelle PG. Angiogenesis: an indicator of metastasis in non-small cell lung cancer invading the thoracic inlet. Ann Thorac Surg. 1994;57(6):1534–1539. doi: 10.1016/0003-4975(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 14.Fontanini G, Bigini D, Vignati S, Basolo F, Mussi A, Lucchi M, Chine S, Angeletti CA, Harris AL, Bevilacqua G. Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol. 1995;177(1):57–63. doi: 10.1002/path.1711770110. [DOI] [PubMed] [Google Scholar]
- 15.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Kut C, Mac GF, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(7):978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 18.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281(2):951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 19.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2(10):727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 20.Kessler T, Fehrmann F, Bieker R, Berdel WE, Mesters RM. Vascular endothelial growth factor and its receptor as drug targets in hematological malignancies. Curr Drug Targets. 2007;8(2):257–268. doi: 10.2174/138945007779940089. [DOI] [PubMed] [Google Scholar]
- 21.Dong X, Han ZC, Yang R. Angiogenesis and antiangiogenic therapy in hematologic malignancies. Crit Rev Oncol Hematol. 2007;62(2):105–118. doi: 10.1016/j.critrevonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Dallas NA, Fan F, Gray MJ, Van BG, Lim SJ, Xia L, Ellis LM. Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev. 2007;26(3–4):433–441. doi: 10.1007/s10555-007-9070-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4(6):e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 25.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21(9):1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 29.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab-an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14(5):1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 30.Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 31.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 32.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Grose R, Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev. 2005;16(2):179–186. doi: 10.1016/j.cytogfr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrens C, Lin HY, Lee JJ, Raso MG, Hong WK, Wistuba II, Lotan R. Immunohistochemical expression of basic fibroblast growth factor and fibroblast growth factor receptors 1 and 2 in the pathogenesis of lung cancer. Clin Cancer Res. 2008;14(19):6014–6022. doi: 10.1158/1078-0432.CCR-08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koziczak M, Holbro T, Hynes NE. Blocking of FGFR signaling inhibits breast cancer cell proliferation through downregulation of D-type cyclins. Oncogene. 2004;23(20):3501–3508. doi: 10.1038/sj.onc.1207331. [DOI] [PubMed] [Google Scholar]
- 39.Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, Yamada Y, Tamura T, Fukuoka K, Kimura H, Saijo N, Nishio K. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res. 2007;13(10):3051–3057. doi: 10.1158/1078-0432.CCR-06-2743. [DOI] [PubMed] [Google Scholar]
- 40.Rusnati M, Presta M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr Pharm Des. 2007;13(20):2025–2044. doi: 10.2174/138161207781039689. [DOI] [PubMed] [Google Scholar]
- 41.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 42.Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA, Gabriel S, Wong KK, Sellers WR, Meyerson M, Greulich H. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008;105(25):8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di BA, Elbi C, Lutterbach B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68(7):2340–2348. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 44.Payson RA, Wu J, Liu Y, Chiu IM. The human FGF-8 gene localizes on chromosome 10q24 and is subjected to induction by androgen in breast cancer cells. Oncogene. 1996;13(1):47–53. [PubMed] [Google Scholar]
- 45.van Leeuwen F, Nusse R. Oncogene activation and oncogene cooperation in MMTV-induced mouse mammary cancer. Semin Cancer Biol. 1995;6(3):127–133. doi: 10.1006/scbi.1995.0018. [DOI] [PubMed] [Google Scholar]
- 46.Kwabi-Addo B, Ropiquet F, Giri D, Ittmann M. Alternative splicing of fibroblast growth factor receptors in human prostate cancer. Prostate. 2001;46(2):163–172. doi: 10.1002/1097-0045(20010201)46:2<163::aid-pros1020>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 47.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11(4):709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 48.Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE, McDermott LA, Coldren CD, Nemenoff RA, Merrick DT, Helfrich BA, Bunn PA, Jr, Heasley LE. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75(1):196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDermott LA, Simcox M, Higgins B, Nevins T, Kolinsky K, Smith M, Yang H, Li JK, Chen Y, Ke J, Mallalieu N, Egan T, Kolis S, Railkar A, Gerber L, Luk KC. RO4383596, an orally active KDR, FGFR, and PDGFR inhibitor: synthesis and biological evaluation. Bioorg Med Chem. 2005;13(16):4835–4841. doi: 10.1016/j.bmc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn H, Kopff C, Konrad J, Riedel A, Gessner C, Wirtz H. Influence of basic fibroblast growth factor on the proliferation of non-small cell lung cancer cell lines. Lung Cancer. 2004;44(2):167–174. doi: 10.1016/j.lungcan.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Fischer H, Taylor N, Allerstorfer S, Grusch M, Sonvilla G, Holzmann K, Setinek U, Elbling L, Cantonati H, Grasl-Kraupp B, Gauglhofer C, Marian B, Micksche M, Berger W. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7(10):3408–3419. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25(8):843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- 53.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 55.Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, Helfrich BA, Doebele RC, Heasley LE. Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One. 2010;5(11):e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8(4):580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 59.Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3(5):439–443. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 60.Kazlauskas A, Durden DL, Cooper JA. Functions of the major tyrosine phosphorylation site of the PDGF receptor beta subunit. Cell Regul. 1991;2(6):413–425. doi: 10.1091/mbc.2.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69(1):369–378. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain RK, Booth MF. What brings pericytes to tumor vessels? J Clin Invest. 2003;112(8):1134–1136. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, Fuh G, Gerber HP, Ferrara N. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23(14):2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Fredriksson L, Li X, Eriksson U. PDGF-D is a potent transforming and angiogenic growth factor. Oncogene. 2003;22(10):1501–1510. doi: 10.1038/sj.onc.1206223. [DOI] [PubMed] [Google Scholar]
- 65.Reinmuth N, Liersch R, Raedel M, Fehrmann F, Fehrmann N, Bayer M, Schwoeppe C, Kessler T, Berdel W, Thomas M, Mesters RM. Combined anti-PDGFRalpha and PDGFRbeta targeting in non-small cell lung cancer. Int J Cancer. 2009;124(7):1535–1544. doi: 10.1002/ijc.24109. [DOI] [PubMed] [Google Scholar]
- 66.Tsao AS, Wei W, Kuhn E, Spencer L, Solis LM, Suraokar M, Lee JJ, Hong WK, Wistuba II. Immunohistochemical overexpression of platelet-derived growth factor receptor-beta (PDGFR-beta) is associated with PDGFRB gene copy number gain in sarcomatoid non-small-cell lung cancer. Clin Lung Cancer. 2011 doi: 10.1016/j.cllc.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tejada ML, Yu L, Dong J, Jung K, Meng G, Peale FV, Frantz GD, Hall L, Liang X, Gerber HP, Ferrara N. Tumor-driven paracrine platelet-derived growth factor receptor alpha signaling is a key determinant of stromal cell recruitment in a model of human lung carcinoma. Clin Cancer Res. 2006;12(9):2676–2688. doi: 10.1158/1078-0432.CCR-05-1770. [DOI] [PubMed] [Google Scholar]
- 68.Donnem T, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM. Prognostic impact of fibroblast growth factor 2 in non-small cell lung cancer: coexpression with VEGFR-3 and PDGF-B predicts poor survival. J Thorac Oncol. 2009;4(5):578–585. doi: 10.1097/JTO.0b013e31819f2e38. [DOI] [PubMed] [Google Scholar]
- 69.Donnem T, Al-Saad S, Al-Shibli K, Busund LT, Bremnes RM. Co-expression of PDGF-B and VEGFR-3 strongly correlates with lymph node metastasis and poor survival in non-small-cell lung cancer. Ann Oncol. 2010;21(2):223–231. doi: 10.1093/annonc/mdp296. [DOI] [PubMed] [Google Scholar]
- 70.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23(5):939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 73.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9(5):604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 74.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Brakenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117(10):2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 76.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6(10):569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 77.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 78.Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J, Fukuda JY, Chu JY, Nematalla A, Wang X, Chen H, Sistla A, Luu TC, Tang F, Wei J, Tang C. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2003;46(7):1116–1119. doi: 10.1021/jm0204183. [DOI] [PubMed] [Google Scholar]
- 79.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jurgensmeier JM, Ogilvie DJ. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65(10):4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 80.Polverino A, Coxon A, Starnes C, Diaz Z, DeMelfi T, Wang L, Bready J, Estrada J, Cattley R, Kaufman S, Chen D, Gan Y, Kumar G, Meyer J, Neervannan S, Alva G, Talvenheimo J, Montestruque S, Tasker A, Patel V, Radinsky R, Kendall R. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 2006;66(17):8715–8721. doi: 10.1158/0008-5472.CAN-05-4665. [DOI] [PubMed] [Google Scholar]
- 81.Rugo HS, Herbst RS, Liu G, Park JW, Kies MS, Steinfeldt HM, Pithavala YK, Reich SD, Freddo JL, Wilding G. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23(24):5474–5483. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 82.Shankar DB, Li J, Tapang P, Owen MJ, Pease LJ, Dai Y, Wei RQ, Albert DH, Bouska JJ, Osterling DJ, Guo J, Marcotte PA, Johnson EF, Soni N, Hartandi K, Michaelides MR, Davidsen SK, Priceman SJ, Chang JC, Rhodes K, Shah N, Moore TB, Sakamoto KM, Glaser KB. ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phosphorylation and signaling in acute myeloid leukemia. Blood. 2007;109(8):3400–3408. doi: 10.1182/blood-2006-06-029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, Thng CH, Ong HS, Chung A, Chow P, Pollock P, Byron S, Tran E. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14(19):6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 84.Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, Lombardo LJ, Borzilleri RM, Zheng X, Wu LI, Barrish JC, Kim SH, Leavitt K, Mathur A, Leith L, Chao S, Wautlet B, Mortillo S, Jeyaseelan R, Sr, Kukral D, Hunt JT, Kamath A, Fura A, Vyas V, Marathe P, D’Arienzo C, Derbin G, Fargnoli J. Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem. 2006;49(7):2143–2146. doi: 10.1021/jm051106d. [DOI] [PubMed] [Google Scholar]
- 85.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 86.Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, Gilmer TM, Truesdale AT, Epperly AH, Boloor A, Stafford JA, Luttrell DK, Cheung M. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6(7):2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 87.Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother. 2010;11(11):1943–1955. doi: 10.1517/14656566.2010.496453. [DOI] [PubMed] [Google Scholar]
- 88.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7(10):3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.NEXAVAR (sorafenib) tablets, oral [package insert] Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2009. [Google Scholar]
- 90.Blumenschein GR, Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O’Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(26):4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 91.Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, brao Miziara JE, Balint B, de Marinis F, Keller A, Aren O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, DiMatteo S, Hanna N. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(11):1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 92.Gatzemeier U, Eisen T, Santoro A, Paz-Ares L, Bennouna J, Liao M, Strauss UP, Montegriffo E, Ong TJ, Biesma B. Sorafenib (S) gemcitabine/cisplatin (GC) vs GC alone in the first-line treatment of advanced non-small cell lung cancer (NSCLC): Phase III NSCLC research experience utilizing sorafenib (NEXUS) trial. Ann Oncol. 2010;21(Suppl 8):viii–7. [Google Scholar]
- 93.Blumenschein GR, Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O’Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(26):4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 94.Novello S, Scagliotti GV, Rosell R, Socinski MA, Brahmer J, Atkins J, Pallares C, Burgess R, Tye L, Selaru P, Wang E, Chao R, Govindan R. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer. 2009;101(9):1543–1548. doi: 10.1038/sj.bjc.6605346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Socinski MA, Novello S, Brahmer JR, Rosell R, Sanchez JM, Belani CP, Govindan R, Atkins JN, Gillenwater HH, Pallares C, Tye L, Selaru P, Chao RC, Scagliotti GV. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(4):650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC Clinical Trials Group BR24 study. J Clin Oncol. 2010;28(1):49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 97.Blumenschein GR, Jr, Kabbinavar F, Menon H, Mok TS, Stephenson J, Beck JT, Lakshmaiah K, Reckamp K, Hei YJ, Kracht K, Sun YN, Sikorski R, Schwartzberg L. A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdq731. [DOI] [PubMed] [Google Scholar]
- 98.Schiller JH, Larson T, Ou SH, Limentani S, Sandler A, Vokes E, Kim S, Liau K, Bycott P, Olszanski AJ, von Pawel J. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27(23):3836–3841. doi: 10.1200/JCO.2008.20.8355. [DOI] [PubMed] [Google Scholar]
- 99.Reck M, Kaiser R, Eschbach C, Stefanic M, Love J, Gatzemeier U, Stopfer P, von Pawel J. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol. 2011;22(6):1374–1381. doi: 10.1093/annonc/mdq618. [DOI] [PubMed] [Google Scholar]
- 100.SUTENT® (sunitinib malate) capsules, oral [package insert] New York, NY: Pfizer Inc; 2011. [Google Scholar]
- 101.Goss G, Shepherd FA, Laurie S, Gauthier I, Leighl N, Chen E, Feld R, Powers J, Seymour L. A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2009;45(5):782–788. doi: 10.1016/j.ejca.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 102.NCIC Clinical Trials Group. [Accessed August 11, 2011];Site Committee Open/Closed/Planned/On Hold/Withdrawn Studies. http://www.ctg.queensu.ca/public/Clinical_Trials/ph3_trial_accrual_closed.htm.
- 103.Blumenschein GR, Jr, Reckamp K, Stephenson GJ, O’Rourke T, Gladish G, McGreivy J, Sun YN, Ye Y, Parson M, Sandler A. Phase 1b study of motesanib, an oral angiogenesis inhibitor, in combination with carboplatin/paclitaxel and/or panitumumab for the treatment of advanced non-small cell lung cancer. Clin Cancer Res. 2010;16(1):279–290. doi: 10.1158/1078-0432.CCR-09-1675. [DOI] [PubMed] [Google Scholar]
- 104.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O’Connor P, Shalinsky DR, Bender SL. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14(22):7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 105.Albert DH, Tapang P, Magoc TJ, Pease LJ, Reuter DR, Wei RQ, Li J, Guo J, Bousquet PF, Ghoreishi-Haack NS, Wang B, Bukofzer GT, Wang YC, Stavropoulos JA, Hartandi K, Niquette AL, Soni N, Johnson EF, McCall JO, Bouska JJ, Luo Y, Donawho CK, Dai Y, Marcotte PA, Glaser KB, Michaelides MR, Davidsen SK. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006;5(4):995–1006. doi: 10.1158/1535-7163.MCT-05-0410. [DOI] [PubMed] [Google Scholar]
- 106.Wong CI, Koh TS, Soo R, Hartono S, Thng CH, McKeegan E, Yong WP, Chen CS, Lee SC, Wong J, Lim R, Sukri N, Lim SE, Ong AB, Steinberg J, Gupta N, Pradhan R, Humerickhouse R, Goh BC. Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies. J Clin Oncol. 2009;27(28):4718–4726. doi: 10.1200/JCO.2008.21.7125. [DOI] [PubMed] [Google Scholar]
- 107.Mekhail T, Masson E, Fischer B, Gong J, Iyer R, Gan J, Pursley J, Patricia D, Williams D, Ganapathi R. Metabolism, excretion, and pharmacokinetics of oral brivanib in patients with advanced or metastatic solid tumors. Drug Metab Dispos. 2010;38(11):1962–1966. doi: 10.1124/dmd.110.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 109.Altorki N, Lane ME, Bauer T, Lee PC, Guarino MJ, Pass H, Felip E, Peylan-Ramu N, Gurpide A, Grannis FW, Mitchell JD, Tachdjian S, Swann RS, Huff A, Roychowdhury DF, Reeves A, Ottesen LH, Yankelevitz DF. Phase II proof-of-concept study of pazopanib monotherapy in treatment-naive patients with stage I/II resectable non-small-cell lung cancer. J Clin Oncol. 2010;28(19):3131–3137. doi: 10.1200/JCO.2009.23.9749. [DOI] [PubMed] [Google Scholar]
- 110.Nikolinakos PG, Altorki N, Yankelevitz D, Tran HT, Yan S, Rajagopalan D, Bordogna W, Ottesen LH, Heymach JV. Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res. 2010;70(6):2171–2179. doi: 10.1158/0008-5472.CAN-09-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loizos N, Xu Y, Huber J, Liu M, Lu D, Finnerty B, Rolser R, Malikzay A, Persaud A, Corcoran E, Deevi DS, Balderes P, Bassi R, Jimenez X, Joynes CJ, Mangalampalli VR, Steiner P, Tonra JR, Wu Y, Pereira DS, Zhu Z, Ludwig DL, Hicklin DJ, Bohlen P, Witte L, Kussie P. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4(3):369–379. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 112.Matei D, Emerson RE, Lai YC, Baldridge LA, Rao J, Yiannoutsos C, Donner DD. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene. 2006;25(14):2060–2069. doi: 10.1038/sj.onc.1209232. [DOI] [PubMed] [Google Scholar]
- 113.Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6(7):1932–1941. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 114.Russell MR, Liu Q, Fatatis A. Targeting the {alpha} receptor for platelet-derived growth factor as a primary or combination therapy in a preclinical model of prostate cancer skeletal metastasis. Clin Cancer Res. 2010;16(20):5002–5010. doi: 10.1158/1078-0432.CCR-10-1863. [DOI] [PubMed] [Google Scholar]
- 115.Xu X, Shen J, Mall JW, Myers JA, Huang W, Blinder L, Saclarides TJ, Williams JW, Chong AS. In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: mechanisms of action. Biochem Pharmacol. 1999;58(9):1405–1413. doi: 10.1016/s0006-2952(99)00228-2. [DOI] [PubMed] [Google Scholar]
- 116.Eckhardt SG, Rizzo J, Sweeney KR, Cropp G, Baker SD, Kraynak MA, Kuhn JG, Villalona-Calero MA, Hammond L, Weiss G, Thurman A, Smith L, Drengler R, Eckardt JR, Moczygemba J, Hannah AL, Von Hoff DD, Rowinsky EK. Phase I and pharmacologic study of the tyrosine kinase inhibitor SU101 in patients with advanced solid tumors. J Clin Oncol. 1999;17(4):1095–1104. doi: 10.1200/JCO.1999.17.4.1095. [DOI] [PubMed] [Google Scholar]