SUMMARY
The cellular mechanisms that regulate the maintenance of adult tissue stem cells are still largely unknown. We show here that the p53 family member, TAp63, is essential for maintenance of epidermal and dermal precursors and that, in its absence, these precursors senesce and skin ages prematurely. Specifically, we have developed a TAp63 conditional knockout mouse and used it to ablate TAp63 in the germline (TAp63−/−) or in K14-expressing cells in the basal layer of the epidermis (TAp63fl/fl;K14cre+). TAp63−/− mice age prematurely and develop blisters, skin ulcerations, senescence of hair follicle-associated dermal and epidermal cells, and decreased hair morphogenesis. These phenotypes are likely due to loss of TAp63 in dermal and epidermal precursors since both cell types show defective proliferation, early senescence, and genomic instability. These data indicate that TAp63 serves to maintain adult skin stem cells by regulating cellular senescence and genomic stability, thereby preventing premature tissue aging.
INTRODUCTION
While the cellular mechanisms that regulate mammalian aging are still largely undefined, recent studies have suggested that aging can occur as a consequence of the functional depletion of somatic stem cells required for maintenance of adult tissues (Sharpless and DePinho, 2007). Also, regenerative failure of tissues and premature aging arise in mice lacking genes critical for genomic stability and maintenance (Hasty et al., 2003). In this regard, the tumor suppressor p53, and its related family members, p63 and p73, have all been linked to premature aging (Maier et al., 2004). Mice expressing a truncated mutant p53 exhibit phenotypes associated with premature aging (Tyner et al., 2002), by a mechanism that is still under debate. Mice heterozygous for p63 show premature aging (Flores et al., 2005; Keyes et al., 2005). However, in spite of the fact that p53 regulates cellular senescence and genomic stability and the importance of these cellular functions to ensure adult stem cell longevity, the relationship between the two is still largely an open question.
p63 is critical for skin, limb, and nervous system development (Celli et al., 1999; Jacobs et al., 2005; Mills et al., 1999; Yang et al., 1999). Multiple roles have been identified for p63 in the proliferation, differentiation, and maintenance of epidermal cells (Koster et al., 2007; Senoo et al., 2007). However, the analysis of p63 function has been complicated by the presence of multiple isoforms of p63 with various expression patterns in multiple tissues (Flores, 2007). The full-length TA isoforms contain a transactivation domain and structurally resemble p53, while the ΔN isoforms lack this domain and can act as dominant-negative family members. The existence of these isoforms has complicated the interpretation of the role of p63 in multiple processes including its role as a tumor suppressor (Flores, 2007; Flores et al., 2005). With regard to the epidermis, the functions of the TA and ΔN isoforms of p63 have been examined by overexpression or knock down in early differentiating keratinocytes (Candi et al., 2006; Koster et al., 2007). These studies, together with the high relative expression of ΔNp63 in the epidermis suggest that the ΔNp63 and not TAp63 isoforms are critical for epidermal development and maintenance. More recently, a TAp63−/− mouse was created showing that this isoform is critical for protection of the female germline (Suh et al., 2006). However, these approaches have not demonstrated a specific role for TAp63 in the epidermis, nor have they addressed a potential role for p63 in the mesenchymal compartment of the skin or other epithelial tissues that are affected in the p63−/− mice.
To understand the role of TAp63 in skin development, we generated a conditional knock out allele of TAp63 in the mouse, and used transgenic mice expressing the cre recombinase in the germline (Zp3-cre and protamine-cre) (Lewandoski et al., 1997; O'Gorman et al., 1997) or in the epidermis (K14cre) (Jonkers et al., 2001) to target genetic ablation of TAp63. We found that mice generated with germline deletion of TAp63 (TAp63−/−) develop blisters, ulcerated wounds, decreased wound healing, and accelerated aging. Surprisingly, our data indicate that these phenotypes were due to the loss of TAp63 in a dermal precursor population that resides within a hair follicle niche (Fernandes et al., 2004; Toma et al., 2001; J. Biernaskie, L. Pevny, and F.D. Miller, unpublished observations). The loss of TAp63 in these dermal precursors (termed SKPs for skin-derived precursors) led to hyperproliferation, senescence, and genomic instability in culture, and premature senescence and reduced hair follicle morphogenesis in vivo. In addition to these dermal deficits, cultured TAp63−/− epidermal cells displayed decreased colony formation, increased senescence, and genomic instability. Together, these findings indicate that TAp63 serves to maintain adult tissue stem cells thereby preventing premature aging, and to provide direct evidence that TAp63 has an important role in regulating cellular senescence and genomic stability.
EXPERIMENTAL PROCEDURES
Generation of TAp63 conditional knockout mice
The cre-loxP strategy was used to generate the TAp63 conditional knockout allele (TAp63fl). Genomic p63 DNA from intron 1 to 3 was amplified from BAC clone DNA (BAC RP23-186N8, Children’s Hospital Oakland Research Institute). LoxP sites flanking exon 2 and neomycin (neo) gene flanked by frt sites inserted in intron 2 were cloned into pL253 (Liu et al., 2003). ES cells were analyzed by Southern blot analysis. Chimeras resulting from ES cell clones injected into C57BL/6 blastocysts were mated with C57BL/6 albino females, and genotyped as described below. Mice with germline transmission of the targeted allele (conditional, floxed allele) were crossed with Zp3-Cre (C57BL/6) (Lewandoski et al., 1997) or Protamine-Cre (C57BL/6 × 129S6) (O'Gorman et al., 1997) transgenic mice. All procedures were approved by the IACUC at U.T. M.D. Anderson Cancer Center and by the Hospital for Sick Children Care Committee and were within the guidelines of the Canadian Council of Animal Care.
Blister Analysis
Thirty wild-type and TAp63−/− mice housed individually were observed throughout life for macroscopic phenotypic changes. Mice were sacrificed at 1, 2, and 4 months for blister characterization. Immunocytochemistry was performed using anti-collagen IV (1:80, Abcam) and anti-K14 (1:2000, Dennis Roop) followed by Texas Red-conjugated goat anti-rabbit and FITC conjugated goat anti-guinea pig secondary antibodies (1:1000, Jackson ImmunoResearch).
Wound healing assay
Mice were anesthetized and two 5 mm diameter full thickness punch wounds were made on each side of the dorsum using Acu-Punch (Acuderm Inc.). Mice were housed individually and wounds were measured at days 0, 1, 2, 4, and 6. At day 6, mice were euthanized and one half of the epithelium was fixed in 10% formalin and the other frozen in OCT medium (Tissue Tek). 1.2 mg/kg buprenorphine was injected subcutaneously for pain management.
Primary keratinocyte isolation and colony formation
Cells were isolated from skin of P1 mice or embryos at day 18.5 (E18.5) by treatment with DispaseII (Roche). The separated epidermis was minced and incubated in 0.25% trypsin/EDTA (GIBCO) for 20 minutes. Cells were plated on collagen-coated flasks (50 µg/ml) collagen type I (BD Bioscience) in defined K-SFM medium (GIBCO). 5×103 cells were plated on 60mm dishes containing mitomycin c (Roche)-treated J2 3T3 feeder cells in F media as described (Barrandon and Green, 1987). Colonies cultured for 8, 12, or 20 days were fixed in 10% formalin, and stained with 2% rhodamine B (Sigma) or with the following antibodies: anti-cytokeratin 5 (Abcam) (1:1000), anti-cytokeratin 10 (Chemicon)(1:1000), and anti-gamma-H2AX-FITC (Upstate Biotechnology)(1:100). Secondary antibodies used were FITC- or Texas Red-conjugated (Jackson ImmunoResearch)(1:500). Some dishes were treated with 10 mM BrdU for 15 hours and immunostained for BrdU (Becton Dickinson). Apoptotic cells were detected with the FragEL kit (Calbiochem).
SKP Isolation and culture
Dorsal and whisker pad skin were removed from E18 embryos, young (1 month), or aged (12 months) mice as described previously (Biernaskie et al., 2006). To transfect SKPs, 25,000 cells were plated on coated (1% Poly-D Lysine and 1% Laminin) chamber slides and transfected with pCMV-p57Kip2 or pCMV-EGFP expression vectors using the Fugene reagent. 48hrs after transfection, cells were fixed in 4% paraformaldehyde and analyzed for expression of p57Kip2 and Ki67 by immunocytochemistry
Sox2-EGFP sorted cells
Dorsal skin samples from adult (n=3) Sox2-EGFP mice (Ellis et al., 2004) were enzymatically digested. Viable cells were gated. Sox2-EGFP-negative, Sox2-EGFP-positive, or total live cells were collected by flow cytometry using a MoFlo (Dako) cell sorter and analyzed by RT-PCR.
Cytogenetics
Primary keratinocytes treated with colcemid (0.02 mg/ml) and fixed in 75% methanol / 25% acetic acid were dropped onto slides and stained with 5.0% Giemsa in PBS (pH 6.8). Images were processed with MetaMorph Premier (Molecular Devices) using a Nikon Eclipse E400 microscope.
Statistics
All data are represented as mean ± SEM. Data were analyzed using one-way ANOVA test or student’s t-test for comparison between two groups. A p-value of 0.05 was considered significant. All experiments were done at least in triplicate.
RESULTS
Generation of a TAp63 conditional knockout mouse
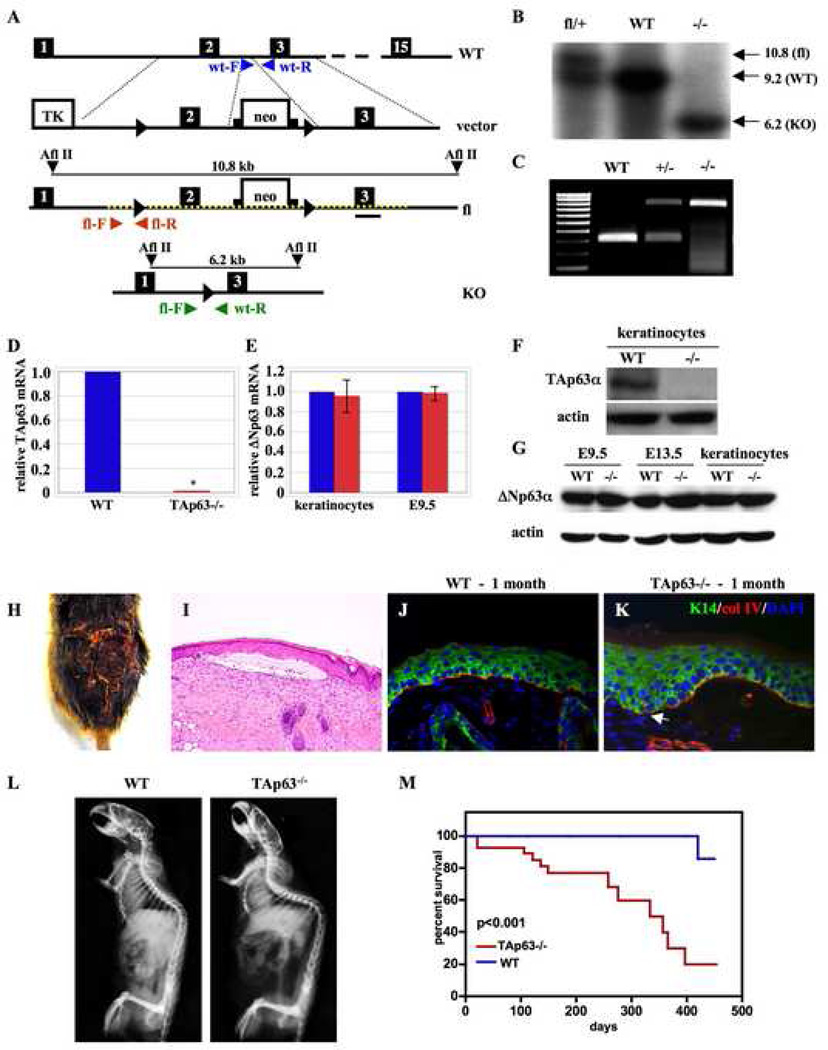
We generated a TAp63 conditional knock out mouse (TAp63fl) using the cre-loxP system (Fig. 1) allowing for tissue specific deletion of the TAp63 isoforms and retention of the ΔNp63 isoforms. LoxP sites were inserted in to the p63 gene flanking exon 2, which contains the translational start site of the TAp63 isoforms, to generate a floxed allele (TAp63fl) (Fig. 1A). Properly targeted embryonic stem (ES) cells were injected into donor blastocysts and subsequently into pseudopregnant females. Resulting male chimeras were intercrossed with albino C57/B6 females for germline transmission scored by PCR and Southern blot analysis (Fig. 1B & C).
Figure 1. TAp63−/− mice develop blisters and exhibit accelerated aging phenotypes.
(A) Generation of the TAp63 conditional knockout mice. The TAp63 targeting vector was generated by inserting loxP sites (triangles) flanking exon 2 and a neomycin (neo) cassette flanked by frt sites (squares) in intron 2. Primers used for genotyping by PCR of wild-type (blue) and TAp63 conditional knockout (fl) (red) alleles are shown. The targeted region of the floxed allele is depicted by a dashed yellow line. (B) Southern blot analysis of genomic DNA from TAp63fl/+, WT, and TAp63−/− mice. (C) PCR of DNA from wild-type (WT), TAp63+/− (+/−) and TAp63−/− (−/−) mice. (D, E) Quantitative RT-PCR of (D) TAp63 mRNA from wild-type (WT) and TAp63−/− keratinocytes or of (E) ΔNp63 mRNA from wild-type (blue) and TAp63−/− (red) keratinocytes and day 9.5 (E9.5) embryos. GAPDH was used as an internal control. (F, G) Western blot for TAp63 (F) in wild-type (WT) and TAp63−/− passage 2 keratinocytes or for ΔNp63 (G) in wild-type (WT) and TAp63−/− E9.5 and E13.5 embryos and keratinocytes. Actin was used as an internal control. (H) Dorsum of an 8-month TAp63−/− mouse with an ulcerated wound. (I) H&E cross section of skin from a 1 month TAp63−/− mouse with a blister. Arrows indicate the split at the epidermal-dermal junction with entrapped serum. (J, K) Immunocytochemistry for keratin 14 (green) and collagen IV (red) of skin from 1 month wild-type (J) and TAp63−/− mice (K). DAPI was used as a counterstain. Magnification 200X. White arrow indicates the beginning of the dermal/epidermal separation. N=6 mice per group. (L) X-ray of 8 month wild-type (WT) and TAp63−/− mice. (M) Kaplan-Meier survival curve for wild-type (WT) and TAp63−/− mice. Median survival for TAp63−/− and WT mice is 333 and 712 days, respectively, n=29 mice per group, p<0.001.
Generation of a TAp63−/− mouse
To further understand the role of TAp63 in skin development, a TAp63−/− mouse was generated by intercrossing the TAp63fl mouse (TAp63fl/fl) with germline-specific cre transgenic mice (Zp3-cre and protamine-cre)(Lewandoski et al., 1997; O'Gorman et al., 1997). We detected embryonic lethality (35%) on an enriched C57B/6 background (~95%) but not upon subsequent backcrossing to the 129S6 strain. Quantitative RT-PCR performed on keratinocytes isolated from TAp63−/− newborn mice confirmed absence of TAp63 mRNA (p<0.0001) (Fig. 1D) or protein expression (Fig. 1F) with maintenance of wild-type levels of ΔNp63 mRNA (Fig. 1E) and protein (Fig. 1G) throughout embryonic development.
TAp63−/− mice develop blisters, ulcerated wounds, and accelerated signs of aging
The TAp63−/− mice that survived to birth exhibited small skin blisters in early adulthood and signs of accelerated aging at later stages, including ulcerated wounds, and premature death (Fig. 1H). By one month of age, 33% of TAp63−/− mice developed blisters that ultimately led to ulcerated wounds on their dorsal and ventral sides (Figs. 1H–K). Keratin 14 and collagen IV immunostaining revealed a subepidermal split between the basement membrane and the dermis with accumulation of entrapped serum (Figs. 1I & K). While the blisters themselves are not likely a sign of premature aging, the wounds that result from these ruptured blisters progress to severe skin ulcerations that fail to heal with aging (Fig. 1H). The other 66% of the cohort developed blisters and ulcerations by 2 to 4 months. In addition, the TAp63−/− mice exhibited signs of premature aging. By X-ray analysis, we found that twenty-five percent of TAp63−/− mice (n=28) developed kyphosis by 8 months and 86% developed this condition by 10 months compared to 14% in the wild-type mice at 10 months (n=29) (Fig. 1L). Defects in other epithelial tissues were also noted within the kidney, stomach, esophagus, and bladder leading to the development of multiple cysts in these tissues. Finally, the median lifespan of the TAp63−/− mice was only 333 days compared to 712 days for wild-type mice (n=28) (p<0.001) (Fig. 1M).
One possible explanation for these premature aging phenotypes is that TAp63 is essential for maintenance of the adult precursor populations that maintain tissues. To address this possibility, we determined whether p63 was expressed in the dermis in addition to its reported expression in epidermal precursors and keratinocytes (Senoo et al., 2007). Immunocytochemical analysis of adult skin for p63 and antibodies specific for epidermal versus dermal cells demonstrated that p63 was expressed in epidermal cells of the hair follicle, where it was localized to the nucleus (Fig. 2A). However, it was also expressed in the fibronectin-positive dermal sheath (DS) cells of the hair follicle and in a subpopulation of NCAM-positive dermal papilla cells, where it was localized to the cytoplasm (Fig. 2A), as has been reported in other cell types (Kurita et al., 2005). To confirm this result, we took advantage of the finding that hair follicle DP and DS cells express a Sox2:EGFP reporter, and are the only cells to do so within adult backskin from the mouse (J. Biernaskie, L. Pevny, and F.D. Miller, unpublished observations). Following isolation of Sox2:EGFP-positive cells by cell sorting from Sox2:EGFP mice (Ellis et al., 2004), RT-PCR analysis demonstrated that TAp63 was expressed in these cells (Fig. 2B), and therefore, in follicle DP and DS cells. TAp63 was also expressed in the negative fraction, presumably in the Sox2-negative epidermal cells. Since the follicle dermal sheath and the related dermal papilla are niches for skin-derived precursors (SKPs), an adult dermal precursor population implicated in hair follicle morphogenesis and dermal maintenance (Fernandes et al., 2004; J. Biernaskie, L. Pevny, and F.D. Miller, unpublished observations), then this suggests that p63 is expressed in precursor cell populations in both the dermis and epidermis.
Figure 2. TAp63−/− mice lose hair follicles with age.
(A) Confocal images of immunocytochemistry of a hair follicle from a young, wild-type mouse for fibronectin (green), NCAM (dark blue), p63 (red), and Hoechst 33258 (blue). Hatched line denotes the dermal papilla (DP), yellow arrowhead indicates a p63-positive dermal sheath (DS) cell, arrows show a p63-positive dermal papilla cells, and white arrowheads epidermal cells with nuclear p63. Magnification 630X. (B) RT-PCR for TAp63 mRNA in Sox2:EGFP-positive (+ve) and negative (−ve) cells from adult backskin. #1, 2 and 3 are samples from 3 different mice, and the ungated sample is total skin cells from one mouse. (C) SA-β-gal staining (blue) of hair follicles in young wild-type or TAp63−/− skin (left two panels). The arrows indicate SA-β-gal-positive dermal papillae. The right two panels, (a) and (b), are micrographs of sequential sections through the same hair follicle, 40µm apart. The arrowhead indicates an SA-β-gal-positive dermal sheath cell (DS), and the arrows a positive dermal papilla (DP). (D) Quantification of SA-β-gal positive cells in the dermal papillae (DP). n = 3 animals per group. *p<0.05. (E) H&E stained skin sections of 1, 6, and 12 month wild-type (WT) and TAp63−/− mice. Arrows denote hair follicles, and double-arrows indicate regions of TAp63−/− skin depleted of hair follicles. Magnification 100X. (F) Quantification of hair follicles per millimeter (E). N = 8 and 4 mice each for wild-type (WT) and TAp63−/− at 1 month and 12 months, respectively. *p<0.05. (G) Quantification of hair follicles in wild-type (WT) and TAp63−/− mice at 1, 6–9, and 12 months. Only patches of skin with no follicles for greater than 400µm were included. N = 4 mice of each genotype at each time point. *p<0.05.
To determine whether TAp63 is essential for maintenance of these precursor populations, we stained sections of young adult skin (1 month) for senescence-associated β-galactosidase (SA-β-gal), a marker for cellular senescence. SA-β-gal staining was undetectable in sections of young wild-type skin (Fig. 2C), but was localized to the bulge region, the dermal sheath, and papilla of hair follicles in TAp63−/− mice (Suppl. Fig. 1 & Fig. 2C). Quantification demonstrated a 15-fold increase in total SA-β-gal-positive hair follicles (Suppl. Fig. 1B) and a 2-fold increase in hair follicles with SA-β-gal-positive dermal papillae in young TAp63−/− skin (Fig. 2D). Interestingly, similar SA-β-gal staining in follicle dermal cells was observed in aged wild-type skin at 12 months (Fig. 2D), suggesting that in the absence of TAp63, precursor cells senesce prematurely.
To investigate whether this premature senescence has functional consequences, we quantified hair follicles in backskin from TAp63−/− and wild-type mice at 1 to 12 months. Young mice of both genotypes and wild-type skin at all ages had a similar density of hair follicles (7–8 hair follicles/mm) that were uniformly distributed over the entirety of the backskin (Fig. 2E & F). In contrast, aged (6–12 month) TAp63−/− skin exhibited many areas where there were no hair follicles at all (Fig. 2E) interspersed with areas where the hair follicle density was similar to that of wild-type mice (Fig. 2E). We measured the percentage of skin lacking hair follicles across gaps of greater than 400 µm. This analysis demonstrated that, at 1 month, hair was evenly distributed along the backskin of both wild-type and TAp63−/− mice, with virtually no gaps (Fig. 2E–G). By 6–12 months, occasional hairless patches were noted in the wild-type mice, occurring as part of the normal aging process. In contrast, 30–40% of the TAp63−/− backskin was hairless by 6–12 months (Fig. 3G).
Figure 3. TAp63−/− mice have an impaired wound-healing response.
(A,B) Photomicrographs of wild-type (A) and TAp63−/− (B) H&E stained skin sections 6 days after wounding. Dotted lines indicate the dermal-epidermal boundary and boxes denote area of immunohistochemical analysis on serial sections shown at higher magnification in panels D, E, G, H, I, & J. (C) Bar graph indicating relative wound size 6 days after wounding in wild-type (WT), TAp63−/− (−/−), TAp63fl/fl;K14cre− (K14cre−), and TAp63fl/fl;K14cre+ (K14cre+) mice. N=6 mice per group, *p<0.05. (D,E) Immunohistochemistry for BrdU incorporation 6 days after wounding in wild-type (D) and TAp63−/− (E) mice. (F) Quantification of BrdU incorporation in epidermal cells in sections shown in panels D,E in WT, TAp63−/−, TAp63fl/fl;K14cre−, and TAp63fl/fl;K14Cre+ mice. N=6 mice per group, *p<0.001. (G–J) Immunohistochemistry for keratin 5 (G,H; brown) or TAp63 (I,J; brown) in wild-type (G,I) or TAp63−/− (H,J) skin. (K) Quantification of BrdU incorporation in dermal cells. In panels (D, E, G, H, I, J), tissue was counterstained with hematoxylin. Arrowheads denote BrdU positive dermal and epidermal cells (D, E) and TAp63-positive epidermal cells (I). N=6 mice per genotype. (L) qRT-PCR of mRNA from WT, TAp63−/−, and TAp63fl/fl;K14cre+ epidermal cells extracted from E18.5 skin. N=6 mice per genotype in triplicate. (M) Western blots for TAp63 and p53 in stressed keratinocytes (passage 2) and freshly-isolated keratinocytes (passage 0) from wild-type (WT) and TAp63−/− mice. (N) Western blot for TAp63 in WT, TAp63−/−, and TAp63fl/fl;K14cre+ stressed keratinocytes (passage 2). Actin was used as an internal control. Keratinocytes derived from 3 independent embryos for each genotype and performed in triplicate.
To determine whether this age-dependent hair follicle loss was due to immune-mediated destruction of hair follicles, skin from 27 TAp63−/− mice and 12 wild-type mice aged 1 to 12 months were examined. No perifollicular lymphocytic infiltrates composed of T-cells were apparent in these samples. Additionally, in autoimmune diseases where hair follicles are destroyed, affected areas show perifollicular scarring (fibrosis) as well as fibrous tracts that mark areas where intact follicles once stood (Ackerman, 1997). None of the samples examined showed such evidence. To further confirm that this hair follicle loss was not due to immune-mediated destruction, we immunostained sections for the T-cell marker (CD3) and for the macrophage marker (Iba-1). Immunostaining for the T cell marker, CD3, was performed on 11 TAp63−/− and 3 WT mice at early time points (less than 6 months of age) (Suppl. Fig. 2) and demonstrated no significant differences in lymphocytic infiltrates or evidence of active follicular injury. Similar results were obtained with immunostaining for the macrophage marker Iba-1 (Suppl. Fig. 3), indicating that the blistering and hair follicle loss seen in TAp63−/− mice was not due to an aberrant immune attack. These data, together with the skin ulceration phenotype observed in TAp63−/− mice, indicate that TAp63 is essential for maintenance of both epidermal and dermal stem cell populations, and that in its absence, there are patches of skin where hair follicles are absent and large skin ulcers form, due to premature aging/senescence of these precursors.
TAp63 is essential for wound healing
Adult tissue stem cells are essential not only for tissue maintenance, but also for appropriate wound-healing responses. To assess whether this skin ulceration phenotype was due to aberrant wound-healing, we performed a wound-healing assay using wild-type and TAp63−/− mice at one month (Fig. 3A & B). Five millimeter punch wounds were made on the dorsal side of wild-type and TAp63−/− mice, and six days later, wound sizes were measured. Wounds in TAp63−/− mice had not healed and were almost twice as larger as those in wild-type mice (Fig. 3A–C). Consistent with these findings, BrdU incorporation in the epidermis of TAp63−/− mice was less than half of that in wild-type mice (Fig. 3D–F). Similarly, levels of BrdU incorporation were low in the dermis of wounded TAp63−/− mice (Fig. 3K). Thus, TAp63 is necessary to ensure appropriate dermal and epidermal wound-healing responses.
Deletion of TAp63 in K14-positive cells is not sufficient to recapitulate the TAp63−/− phenotypes
To determine whether these skin phenotypes were due to the loss of TAp63 in the epidermal compartment, we first asked whether TAp63 was expressed in epidermal cells in vivo. Consistent with previous studies (Koster et al., 2004), TAp63 was not detected in the intact epidermis of wild-type mice. However, when skin was wounded and double-labeled for TAp63 and keratin 5, a marker for the basal layer of the epidermis, scattered epidermal cells within the wound site of wild-type mice expressed TAp63 (Fig. 3G–J) indicating it induction in response to stress. Indeed, wild-type passage 2 keratinocytes exhibited an induction of TAp63 while freshly isolated keratinocytes (passage 0) did not (Fig. 3M). To determine whether epidermal TAp63 is essential for appropriate wound-healing, we generated mice with a selective deletion of TAp63 in the epidermal compartment using keratin 14 (K14) cre transgenic mouse (Jonkers et al., 2001), which expresses the cre-recombinase in the basal layer of the epidermis. Consequently, dermal, but not epidermal, cells in these mice are wild-type. Quantitative RT-PCR analysis of epidermal cells derived from the intact epidermis of TAp63fl/fl;K14cre mice at E18.5 showed that TAp63 mRNA was not detectable, similar to what was seen with epidermal cells derived from TAp63−/− mice (Fig. 3L). To confirm that TAp63 was deleted in epidermal cells from TAp63fl/fl;K14cre mice, we derived keratinocytes from TAp63fl/fl;K14cre, passaged them in culture, and then performed Western blots (Fig. 3N). This analysis demonstrated that TAp63 was induced and detectable in cultured wild-type keratinocytes, as was p53 (Fig. 3M), but that no TAp63 was observed in passaged keratinocytes cultured from TAp63fl/fl;K14cre mice (Fig. 3N). These results indicate that most epidermal cells from TAp63fl/fl;K14cre mice extracted at E18.5 do not express TAp63, although it is possible that cre-mediated excision of the floxed allele is incomplete in epidermal cells, particularly given the low endogenous levels of TAp63 expression.
Interestingly, TAp63fl/fl;K14cre mice did not display any overt skin phenotypes under normal conditions suggesting that loss of TAp63 in K14-expressing epidermal cells alone was insufficient to cause the skin ulcerations seen in TAp63−/− mice. To further address this issue, we performed punch biopsies on the TAp63fl/fl;K14cre mice. Analysis of wound-healing revealed that TAp63fl/fl;K14cre and wild-type mice healed their wounds at similar rates (Fig. 3C). Likewise, the levels of BrdU incorporation in the epidermis and dermis of TAp63fl/fl;K14cre mice were similar to those in wild-type mice (Fig. 3F & K). Together, these data suggest that loss of TAp63 in most keratinocytes is not sufficient to recapitulate the skin ulceration or wound-healing deficit observed in TAp63−/− mice.
TAp63 is essential for colony formation from epidermal precursors
Since p63 has been implicated in the proliferation of epidermal stem cells (Senoo et al., 2007), we asked whether this phenotype was due to loss of TAp63. We isolated and cultured epidermal cells from newborn wild-type and TAp63−/− mice (Fig. 4A) (Barrandon and Green, 1987), analyzed colonies at 8, 12, and 20 days to observe their proliferative and self renewal capacities. Wild-type cells generated colonies containing keratin 5 (K5) positive immature keratinocytes, the majority of which contained up to 50% BrdU-positive proliferating cells at 8 and 20 days (Fig. 4A–D). In contrast, TAp63−/− epidermal cells generated small, irregular-shaped colonies of K5-positive cells, the majority of which contained no proliferating cells at 8 or 20 days (Fig. 4A–D). In spite of this perturbation in colony formation, cells within these TAp63−/− colonies differentiated and expressed keratin 10, a marker of the spinous layer of the epidermis, following treatment with high calcium (Fig. 4E). This deficit in colony formation is similar to that seen with knockdown of all p63 isoforms in epidermal precursors (Senoo et al., 2007), and indicates that TAp63 is required for epidermal precursor proliferation and/or the appropriate genesis of proliferation-competent keratinocytes.
Figure 4. TAp63 regulates colony formation from epidermal precursors.
(A) Epidermal colonies from wild-type (WT), TAp63fl/fl, TAp63−/−, TAp63−/−;p53−/− and TAp63fl/fl;K14Cre+ mice cultured for 8 or 12 days on J2 3T3 feeder layers and stained with rhodamine B. (B) Immunostaining for BrdU (green) and K5 (red) in wild-type and TAp63−/− epidermal clones. (C,D) Quantification of BrdU incorporation in colonies after 8 (C) or 20 (D) days in culture. *p<0.05. N = 3 cell lines per genotype in triplicate. (E) Immunostaining for the spinous marker, K10 (green), in wild-type (WT) and TAp63−/− colonies cultured in high calcium (1.2 mM) media for 2 days. DAPI was used as a counterstain.
We also performed similar experiments with epidermal cells isolated from TAp63fl/fl;K14cre mice (Fig. 4A–D). These cells formed colonies and proliferated like wild-type epidermal cells. These data are consistent with the lack of skin phenotypes in the TAp63fl/fl;K14cre mice.
Since the p53 family members interact extensively, we asked whether this colony formation phenotype could be rescued by coincident loss of p53. Thus, colony formation assays were performed with epidermal cells isolated from TAp63−/−;p53−/− mice (Fig. 4A). Eight days after culture, p53 deletion partially rescued the TAp63−/− phenotype; wild-type and TAp63−/−;p53−/− epidermal cells generated similar numbers of colonies with 50% or fewer proliferating cells, but only 18% of TAp63−/−;p53−/− colonies (p=0.038) had >50% BrdU positive cells compared to 28% of wild-type and 6% of TAp63−/− colonies (p=0.020) (Fig. 4C). This rescue was not, however, apparent at 20 days, when TAp63−/−;p53−/− colonies were similar to TAp63−/− colonies, and statistically different from wild-type colonies (Fig. 4D). Thus, coincident loss of p53 partially rescued the TAp63−/− colony formation phenotype at short but not long culture time points (Fig. 4A, C, & D).
TAp63 regulates self-renewal of dermal precursor cells
These data suggest that the skin phenotypes in TAp63−/− mice require the loss of TAp63 in dermal cells. We therefore asked whether TAp63 regulated SKPs, dermal precursors within a niche in the hair follicle where p63 is expressed (Fig. 2A & B). We isolated wild-type SKPs and characterized the repertoire of p53 family members expressed by RT-PCR. Consistent with the expression of p63 in hair follicle Sox2:EGFP-positive dermal cells in vivo (Fig. 2A & B), SKPs expressed detectable TAp63 mRNA (Fig. 5A). but not ΔNp63 or p73. p53 levels were similar in SKPs that were isolated from the dermis of p63−/− and wild-type embryos (Fig. 5A & B). Immunostaining for p63 confirmed that SKPs isolated from neonatal dermis or hair follicles expressed detectable p63 protein (Fig. 5C). Thus, of the p53 family members, SKPs express only TAp63 and p53, and expression of p53 family members is not dysregulated by the absence of p63.
Figure 5. TAp63 regulates self-renewal of dermal precursors via p57Kip2.
(A, B) RT-PCR in WT and p63−/− SKPs for (A) TAp63 and ΔNp63 mRNAs and (B) p53, p73, and GAPDH mRNAs. Embryonic day 18 cortical tissue (C) was used as a positive control. (C) Immunocytochemistry for p63 (green) in SKP spheres isolated from WT skin, from reconstituted hair follicles (WT follicle), or from E18 p63−/− skin. Hoechst (blue) used as a counterstain. Magnification 400X. (D) SKP spheres isolated from young WT or TAp63−/− skin, or from E18 p63−/− skin and immunostained for fibronectin, nestin, vimentin, smooth muscle actin (SMA) and versican. Magnification 400X. (E) SKP spheres isolated from neonatal WT skin or from E18 p63−/− skin, immunostained for Ki67 (red), and counterstained with Hoechst (blue). Magnification 400X. (F) Quantification of Ki67 positive cells. n = 3, *p < 0.05. (G) Quantification of cells that form a new sphere at clonal density (2500 cells/ml) in methylcellulose cultures. n = 3, *p < 0.05. (H) RT-PCR analysis for p57Kip2 and GAPDH mRNAs in E18 cortex (C), young WT and TAp63−/− SKPs and E18 p63−/− SKPs. (I) SKP spheres isolated from young WT and TAp63−/− skin, immunostained for p57Kip2 (red) and counterstained for Hoechst (blue). Magnification 400X. (J) Quantification of p57Kip2 positive cells. n = 3, *p < 0.05. (K) ChIP for p63 in young WT or E18 p63−/− SKPs and PCR for the p57Kip2 core promoter. (L) Immunostaining for p57Kip2 (red) and Ki67 (green) in TAp63−/− SKPs transfected with or without a p57Kip2 expression vector. Hoechst (blue) was used as a counterstain. Transfected p57Kip2-positive cells were negative for Ki67. Magnification 200X, n=3.
To determine whether TAp63 is necessary for maintenance of SKPs, we isolated them from 1 month TAp63−/− and wild-type skin. Immunocytochemical analysis of SKP spheres for fibronectin, nestin, vimentin, and versican, all markers that have been defined for SKPs (Biernaskie et al., 2006; Fernandes et al., 2004; Toma et al., 2001) demonstrated that there were no overt differences in expression of these SKP markers with the loss of TAp63 (Fig. 5D). However, immunostaining for the proliferation marker Ki67 demonstrated that TAp63−/− SKPs isolated from young mice proliferated approximately 4–5-fold more than wild-type counterparts (Fig. 5F). Similar results were obtained when SKPs were isolated from the rudimentary dermis of E18 p63−/− embryos (Fig. 5E, F). To determine if this increased proliferation reflected increased self-renewal, SKPs were dissociated to single cells, plated at low density in medium containing methylcellulose, and the percentage of cells that initiated a new sphere was determined. As seen in the proliferation assay, SKPs cultured from postnatal TAp63−/− and E18 p63−/− mice self-renewed 3–4 times more robustly than did wild-type SKPs (Fig. 5G). In spite of this hyperproliferation, TAp63−/− and wild-type SKPs were still able to differentiate into both neural and mesodermal cell types under previously defined conditions (Suppl. Fig. 4A) and when transplanted into the neural crest migratory stream of embryonic chicks in ovo at HH stage 18, both populations of SKPs migrated into neural crest targets (Suppl. Fig. 4B) (Fernandes et al., 2004). Thus, TAp63 regulates SKP proliferation and self-renewal, but does not overtly affect their phenotype, differentiation, or migratory capacity.
These data suggest that TAp63 normally functions to dampen the self-renewal rate of SKPs, potentially as a mechanism for ensuring that they last for the lifetime of the animal. One p63 target that decreases cellular proliferation is the cyclin-dependent kinase inhibitor p57Kip2 (Beretta et al., 2005). RT-PCR analysis demonstrated that p57Kip2 mRNA levels were reduced in TAp63−/− and p63−/− SKPs (Fig. 5H). Immunocytochemistry confirmed this result, and demonstrated that while p57Kip2 was robustly expressed in 50% of wild-type SKPs, it was only detectably expressed in 18% of TAp63−/− SKPs (Fig. 5I,J). In contrast, RT-PCR for two other cell cycle regulators, p27Kip1 and p21Cip1 demonstrated unchanged expression in the absence of p63 (Suppl. Fig. 4C). Chromatin immunoprecipitation for p63 identified a previously-defined binding site in the p57Kip2 promoter (Fig. 5K). We therefore asked if the hyperproliferation phenotype in TAp63−/− SKPs could be rescued by p57Kip2. Following transfection of TAp63−/− SKPs with either empty vector or p57Kip2, 20% of cells transfected with p57Kip2 expressed detectable levels of this protein, and none of these transfected cells coexpressed Ki67 (Fig. 5L). In contrast, approximately 45% of TAp63−/− cells transfected with the empty vector were dividing, as monitored by Ki67, and none of them expressed p57Kip2 (Fig. 5L). Thus, p57Kip2 completely rescued the hyperproliferation, indicating that TAp63 regulates SKPs self-renewal at least in part by regulating p57Kip2.
Loss of TAp63 causes dermal precursors to senesce
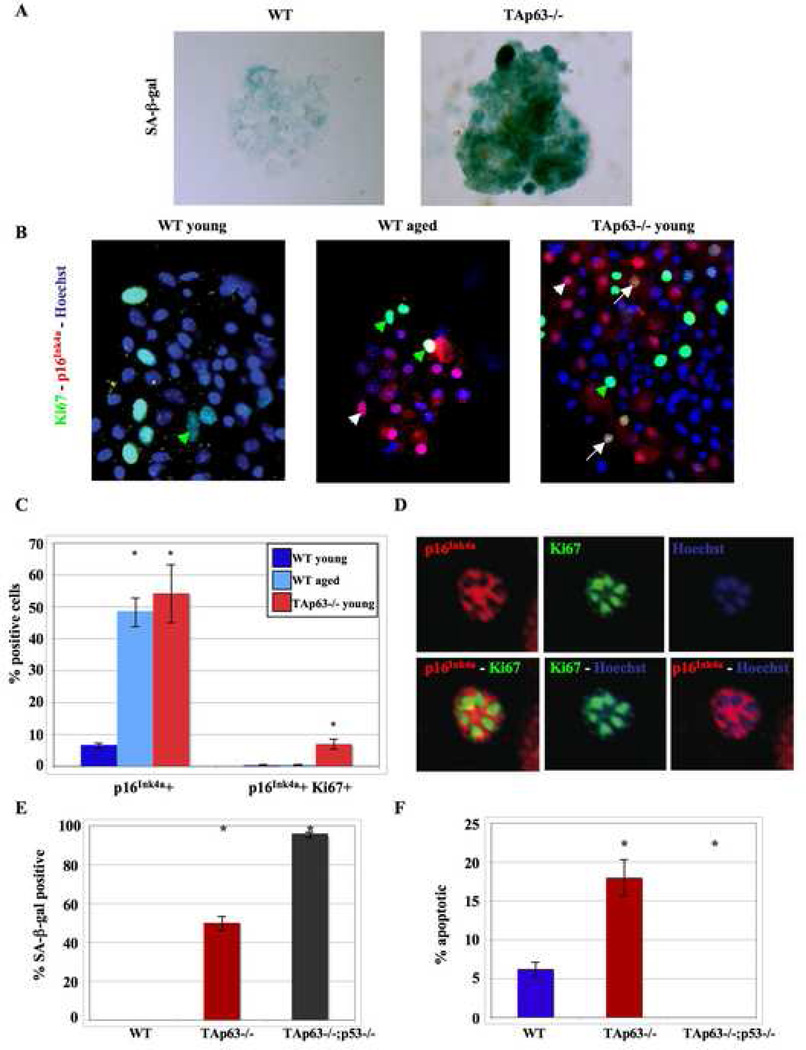
To investigate whether TAp63−/− precursors senesce, SKPs were generated from 1 month TAp63−/− and wild-type skin and analyzed for expression of SA-β-gal and p16Ink4a, a second marker for senescence that is associated with human aging (Sharpless and DePinho, 2007). SA-β-gal staining demonstrated that a very large proportion of the cells in TAp63−/− SKP spheres were SA-β-gal-positive (Fig. 6A). Similarly, immunostaining for p16Ink4a revealed that over 50% of the cells in SKP spheres were p16Ink4a-positive versus less than 10% of the wild-type SKPs (Fig. 6B & C). Interestingly, analysis of SKPs generated from aged wild-type skin demonstrated that 50–60% of the SKPs were also p16Ink4a-positive (Fig. 6B), supporting the notion that SKPs normally senesce as animals age, and that loss of TAp63 causes premature aging.
Figure 6. Loss of TAp63 causes dermal precursor cells to senesce.
(A) SKP spheres from young WT and TAp63−/− SKPs stained for SA-β-gal (blue). Magnification 400X. (B) SKP spheres from young and aged WT and young TAp63−/− skin immunostained for p16Ink4a (red) and Ki67 (green) and counterstained with Hoechst (blue). Green arrowheads indicate Ki67, white arrowheads indicate p16Ink4a, and white arrows indicate Ki67 and p16Ink4a double positive cells. Magnification 400X. (C) Quantification of p16Ink4a-positive and p16Ink4a, Ki67 double-positive cells. n = 3, *p < 0.05. (D) High magnification of a TAp63−/− SKP cell nucleus that was labeled for p16Ink4a (red) and Ki67 (green) and counterstained with Hoechst (blue). (E,F) Quantification of SA-β-gal positive (E) or apoptotic (F) keratinocytes in wild-type (WT), TAp63−/−, and TAp63−/−;p53−/− cultures. N=3 cell lines for each genotype, performed in triplicate. *p<0.0001.
Since TAp63−/− SKPs show both hyperproliferation and increased senescence, we immunostained spheres for both Ki67 and p16Ink4a. This analysis demonstrated that these two proteins were expressed in different populations of cells (Fig. 6B), consistent with the idea that as SKPs senesce, they exit the cell cycle. Interestingly, however, approximately 5% of TAp63−/− SKPs expressed both Ki67 and p16Ink4a, and in these cells, their subcellular distribution within the nucleus was mutually exclusive (Fig. 6D), indicating that these cells represent a transition state between hyperproliferation and senescence. These data are consistent with the interpretation that TAp63 serves to maintain SKPs in a largely quiescent state, and that in its absence, SKPs hyperproliferate and ultimately senesce, thereby causing premature dermal and follicle aging.
To ask whether TAp63 is a more general regulator of cellular senescence, we examined cultured epidermal cells for SA-β-gal. TAp63−/− cells had a dramatic increase in SA-β-gal relative to wild-type cells (Fig. 6E). To determine whether this cellular senescence required p53, we intercrossed the p53−/− (Jacks et al., 1994) and TAp63−/− mice. Analysis of TAp63−/−;p53−/− cells revealed that, somewhat surprisingly, the number of SA-β-gal-positive cells was further increased by the coincident loss of p53 (Fig. 6E). In contrast, apoptosis, which was increased in the absence of TAp63, was rescued by coincident deletion of p53; 0% of cultured TAp63−/−;p53−/− cells underwent apoptosis compared to 18% of TAp63−/− and 6% of wild-type cells (Fig. 6F). Consequently, the increase of senescence in the TAp63−/−;p53−/− cells may be due to the coincident decrease in apoptosis. Taken together, these data suggest that TAp63 may be a general regulator of cellular senescence, and indicate that the senescence in these cells is p53-independent.
Loss of TAp63 results in DNA damage and genomic instability
One known trigger for cellular senescence is DNA damage. In light of the wound-healing deficits and cellular senescence seen in TAp63−/− mice, we asked whether TAp63−/− skin cells might have accumulated DNA damage. Immunostaining for histone γH2AX, which is induced during DNA damage, on SKPs and epidermal cells from wild-type and TAp63−/− mice revealed that 70–80% of 1 month-old TAp63−/− SKPs expressed this DNA damage marker (Fig. 7A & B), whereas none of the age-matched controls did. Likewise, TAp63−/− epidermal cells had a marked increase in γH2AX nuclear foci. One hundred percent of TAp63−/− cells were γH2AX positive compared to less than 5% of wild-type cells (Fig. 7D). To confirm the presence of genomic instability in vivo, we immunostained young and aged skin for γH2AX. Young wild-type skin showed virtually no γH2AX-positive cells, but aged skin showed a small population of positive cells in the dermal sheath of hair follicles (Fig. 7C). In contrast, in 1 month-old TAp63−/− skin, γH2AX-positive cells were consistently observed in the dermal sheath (Fig. 7C).
Figure 7. Loss of TAp63 leads to genomic instability.
(A) SKP spheres from young and aged WT and TAp63−/− skin, immunostained for γ-H2AX (green) and counterstained for Hoechst (blue). Magnification 400X. (B) Quantification of γ-H2AX positive cells. n = 3, *p < 0.05. (C) Immunostaining for γ-H2AX (green) in aged wild-type and young TAp63−/− skin and counterstained Hoechst (blue). Arrows denote hair follicles, and arrowheads indicate positive cells within the dermal sheath. Magnification 200X. (D) Immunostaining for γ-H2AX foci (red) in wild-type and TAp63−/− keratinocytes. DAPI was used as a counterstain. (E) Metaphase spreads of wild-type and TAp63−/− primary keratinocytes (passage 0). Cytogenetic aberrations in TAp63−/− cells are indicated by colored arrows. Chromosomal fusions (black arrows), fragments (red arrows), breaks (green arrowheads), biarmed chromosomes (blue arrow), ring chromosome (blue arrowhead).
Because of the strikingly high levels of DNA damage, we assayed for chromosomal instability. More than 100 metaphase spreads from primary wild-type and TAp63−/− epidermal cells were scored for chromosomal aberrations. TAp63−/− cells had a large number of cytogenetic aberrations including chromosomal fusions, fragments, breaks, biarmed chromosomes, ring chromosomes, dicentric chromosomes, and aneuploidy (Fig. 7E), suggesting that TAp63 is a critical for maintenance of genomic stability.
DISCUSSION
Here, we show that TAp63 plays an instrumental role in the maintenance and renewal of dermal and epidermal precursors, and when this function is perturbed, it causes premature aging of both the precursors themselves and the tissues that they maintain. We demonstrated this by generating a conditional knockout allele of TAp63, preserving the ΔNp63 isoforms, and intercrossing it with germline or epidermal specific cre transgenic mice. Mice with a germline deletion of TAp63 developed blisters, ulcerated wounds, display decreased wound healing, and an accelerated aging phenotype. Surprisingly, these phenotypes were at least partially due to the loss of TAp63 in SKPs, dermal precursors that reside within a hair follicle niche. Loss of TAp63 in SKPs led to hyperproliferation, senescence, and genomic instability in culture, and premature senescence and reduced hair follicle morphogenesis in vivo. In addition to these dermal deficits, cultured TAp63−/− epidermal precursors displayed decreased colony formation, as previously seen with p63−/− epidermal precursors (Senoo et al., 2007), a phenotype that is likely at least partially due to increased senescence and genomic instability, as we show here. Together, these findings indicate that TAp63 serves to regulate and maintain the adult stem cells that are essential to prevent premature skin aging, and provide direct evidence that TAp63 has a physiologically important role in regulating cellular senescence and genomic stability.
TAp63−/− mice exhibited signs of premature aging including hair loss, impaired wound healing, kyphosis, and a median lifespan of only 48 weeks compared to 110 weeks for their wild-type littermates. Intriguingly, premature aging has also been seen in mice mutant for p53 (Tyner et al., 2002) and in p63+/− mice (Flores et al., 2005; Keyes et al., 2005). Strikingly, the life span of TAp63 knockout mice is approximately half of that seen with p63+/− mice (Flores et al., 2005; Keyes et al., 2005) suggesting that the p63+/− aging phenotype is largely attributable to loss of TAp63. It is also tempting to speculate that p53 mouse models that display premature aging may do so via TAp63. Two mouse models expressing truncated p53 mutants display premature aging (Maier et al., 2004; Tyner et al., 2002), but other mouse models with elevated p53, the super p53 and mdm2puro/Δ7–12 mice (Garcia-Cao et al., 2002; Mendrysa et al., 2006), do not. Perhaps these truncated p53 mutants bind to and inhibit TAp63, thereby causing premature aging. Precedent for such an interaction comes from mouse models of Li-Fraumeni Syndrome (Iwakuma et al., 2005; Lang et al., 2004) and from in vitro studies with point mutant p53 (Iwakuma et al., 2005).
How does TAp63 regulate aging? Our data indicate that it does so by regulating the maintenance of adult tissue stem cells. In particular, we demonstrate that in the absence of TAp63 in vivo, hair follicle dermal precursors display premature senescence, show signs of DNA damage, and lose their ability to induce hair follicle morphogenesis. Moreover, since follicle-associated precursors also contribute to dermal maintenance and wound-healing (J. Biernaskie, L. Pevny, and F.D. Miller, unpublished observations), then deficits in this same population may account for the wound healing and skin ulceration phenotypes seen in TAp63−/− mice. In addition, epidermal precursors are highly deficient in colony formation likely contributing to the skin phenotypes we document here. Moreover, we show that TAp63 is essential to keep epidermal cells from senescing and to maintain their genomic integrity. Thus, two adult skin precursor populations depend upon TAp63 for their maintenance, and we propose that the loss of TAp63 leads to functional depletion of both populations, resulting in premature skin aging. These findings provide significant support for the concept that depletion of adult stem cells may be a major cause of tissue aging (Sharpless and DePinho, 2007), and raise the possibility that functional depletion of adult stem cells is responsible for the phenotypes of humans with dysregulated TAp63 (Gu et al., 2006).
At the cellular level, our data provide a number of TAp63-dependent mechanisms that explain depletion of adult stem cell function. First, TAp63−/− SKPs showed increased proliferation and self-renewal in culture, a phenotype that correlated with decreased expression of p57Kip2, a direct transcriptional target of p63 (Beretta et al., 2005). Since adult stem cell populations are not immortal, enhanced proliferation of follicle-associated SKPs would be predicted to lead to stem cell depletion. Interestingly, re-expression of p57Kip2 in TAp63−/− SKPs rescued the hyperproliferative phenotype of TAp63−/− SKPs indicating that regulation of p57Kip2 by TAp63 is essential in the maintenance of these dermal precursors. Second, in the absence of TAp63, both SKPs and epidermal cells displayed greatly increased DNA damage and chromosomal aberrations indicating a key role for TAp63 in regulating genomic stability and stem cell maintenance. Third, in the absence of TAp63, both SKPs and epidermal cells displayed greatly increased senescence, something that was also seen in vivo. TAp63 might directly regulate cellular senescence, as does p53, or this senescence might occur in response to DNA damage or telomere shortening as a consequence of hyperproliferation. The senescence induced by TAp63 is largely p53-independent suggesting a unique senescence pathway regulated by TAp63. Regardless of the trigger, senescence would withdraw stem cells from the cell cycle, thereby effectively depleting them. All three mechanisms may be at play in the stem cell maintenance/premature aging phenotypes that are observed in TAp63−/− mice.
The phenotype of the TAp63fl/fl;K14cre mice was strikingly different from that of the TAp63−/− mice. The TAp63fl/fl;K14cre mice healed their wounds at similar rates to wild-type mice and had similar numbers of BrdU positive cells in the dermal and epidermal compartments. Additionally, epidermal cells derived from TAp63fl/fl;K14cre mice proliferated similarly to wild-type epidermal cells. There are a number of possible explanations for these data. First, the loss of TAp63 in epidermal cells may not be sufficient for the deficits that are reported here; deletion of TAp63 in both dermal and epidermal precursors might be required. Second, our results might be due to incomplete deletion of TAp63 in the TAp63fl/fl;K14cre mice. However, our data demonstrating that TAp63 mRNA is not detectable in primary embryonic epidermal cells by quantitative RT-PCR argues that recombination has occurred in most epidermal cells by E18.5. Finally, these deficits may require the loss of TAp63 in an embryonic epidermal precursor prior to the onset of K14cre expression. Our data do not distinguish between these possibilities.
The p63 deficient mouse has craniofacial abnormalities and lacks stratified epithelium resulting in death within hours after birth. Since this p63−/− mouse model lacks all isoforms of p63, it has been difficult to specifically attribute these deficits to any particular mechanism. One exception to this has been in the embryonic peripheral nervous system, where neurons only express TAp63 isoforms, and where these isoforms act as essential proapoptotic proteins during naturally-occurring cell death (Jacobs et al., 2005). The role of the TAp63 isoforms has been less clear in the skin. The ΔN isoforms, most notably ΔNp63α, are highly expressed in the basal layer of the epithelium, leading to the assumption that they are responsible for the effects of p63 on proliferation of epidermal precursors and development of the epidermis. Support for this idea comes from genetic complementation experiments where TAp63α and ΔNp63α were expressed in K5-positive cells in transgenic mice; expression of ΔNp63α showed a more impressive rescue of the epidermis than did TAp63α (Candi et al., 2006). Our data are consistent with this conclusion, since TAp63−/− mice do not display the same developmental skin phenotype as p63−/− mice. Our data using mouse models indicate that TAp63 is important for skin maintenance, primarily by acting in dermal and epidermal precursors. Importantly, the TAp63−/− mice display a phenotype similar to defects in humans with Hay-Wells syndrome where patients with mutations in p63 develop dermatitis and alopecia (McGrath et al., 2001). Thus, TAp63 and ΔNp63 might each play important roles in the skin via actions in different compartments at different developmental time points.
In addition to its critical role in stem cell maintenance, TAp63 is induced in response to stress. This is reminiscent of the induction of TAp63 in sympathetic neurons following growth factor withdrawal (Jacobs et al., 2005) and of p53 after DNA damage. Since we also observe a DNA damage response and chromosomal instability in TAp63−/− cells, then one possibility is that TAp63 is induced with p53 as an essential part of a stress response to protect the genome. Further support for this idea comes from previous reports that TAp63 plays a key role in maintaining the fidelity of the female germline (Suh et al., 2006), and that p63 can act as a tumor suppressor gene (Flores, 2007; Flores et al., 2005). Thus, TAp63 likely acts with p53 and to maintain genomic integrity, thereby protecting long-lived cells like stem cells, and potentially suppressing tumorigenesis. Interestingly, mouse models with accumulated DNA damage, such as the ATRmKO mouse exhibit age-related phenotypes (Ruzankina et al., 2007), supporting the idea that genomic instability in tissue stem cells is one way that the loss of TAp63 causes premature aging.
In summary, the findings reported here indicate that TAp63 serves to maintain the adult stem cells that are required for skin maintenance, thereby preventing premature aging. TAp63 does this by regulating precursor cell proliferation, maintaining genomic integrity, and preventing premature cellular senescence. Whether TAp63 or the related TAp73 play similar roles in other adult stem cell populations, and whether TAp63 regulates genomic integrity or cellular senescence in other normal or tumorigenic cells are key questions for the future.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants to E.R.F. from the American Cancer Society (RSG-07-082-01-MGO), March of Dimes (Basil O'Connor Scholar), Susan G. Komen Foundation (BCTR600208), Leukemia and Lymphoma Society / Hildegarde D. Becher Foundation, NCI-Cancer Center Core Grant (CA-16672)(U.T. M.D. Anderson Cancer Center) and by grants to F.D.M. from the Canadian Institutes of Health Research (MOP-64211) and HHMI. E.R.F. is a scholar of the Rita Allen Foundation and the V Foundation for Cancer Research. F.D.M. is a Canada Research Chair and an HHMI International Research Scholar. We gratefully acknowledge the work of Jan Parker-Thornburg, the Genetically Engineered Mouse Facility, and the T.C. Hsu Molecular Cytogenetics Core at M.D. Anderson (funded by NCI #CA16672). We would also like to acknowledge Wei Zhang for technical assistance, Paul Lambert and Denis Lee for J2-3T3 feeder cells, Dennis Roop for K14 antibody, Alea Mills for p63−/− mice, Elaine Fuchs for advice on wound-healing assays, David Kaplan and Chi-chung Hui for advice and input with regard to the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackerman AB. Histologic Diagnosis of Inflammatory Skin Diseases. 2nd Ed. Baltimore, MD: Williams & Wilkins; 1997. [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta C, Chiarelli A, Testoni B, Mantovani R, Guerrini L. Regulation of the cyclin-dependent kinase inhibitor p57Kip2 expression by p63. Cell Cycle. 2005;4:1625–1631. doi: 10.4161/cc.4.11.2135. [DOI] [PubMed] [Google Scholar]
- Biernaskie JA, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803–2812. doi: 10.1038/nprot.2006.422. [DOI] [PubMed] [Google Scholar]
- Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death and Differentiation. 2006;13:1037–1047. doi: 10.1038/sj.cdd.4401926. [DOI] [PubMed] [Google Scholar]
- Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, Newbury-Ecob R, Hennekam RC, Van Buggenhout G, van Haeringen A, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–153. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- Flores ER. The roles of p63 in cancer. Cell Cycle. 2007;6:300–304. doi: 10.4161/cc.6.3.3793. [DOI] [PubMed] [Google Scholar]
- Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, Yang A, McKeon F, Jacks T. Tumor predisposition in mice mutant for p63 and p73: Evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005;7:363–373. doi: 10.1016/j.ccr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. "Super p53" mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Lundqvist EN, Coates PJ, Thurfjell N, Wettersand E, Nylander K. Dysregulation of TAp63 mRNA and protein levels in psoriasis. J Invest Dermatol. 2006;126:137–141. doi: 10.1038/sj.jid.5700010. [DOI] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Iwakuma T, Lozano G, Flores ER. Li-Fraumeni syndrome: a p53 family affair. Cell Cycle. 2005;4:865–867. doi: 10.4161/cc.4.7.1800. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Govoni G, Ho D, Atwal JK, Barnabe-Heider F, Keyes WM, Mills AA, Miller FD, Kaplan DR. p63 is an essential proapoptotic protein during neural development. Neuron. 2005;48:743–756. doi: 10.1016/j.neuron.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW, Mills AA. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes & Development. 2005;19:1986–1999. doi: 10.1101/gad.342305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Dai D, Marinari B, Sano Y, Costanzo A, Karin M, Roop DR. p63 induces key target genes required for epidermal morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3255–3260. doi: 10.1073/pnas.0611376104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes & Development. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes & Development. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JA, Duijf PHG, Doetsch V, Irvine AD, de Waal R, Vanmolkot KRJ, Wessagowit V, Kelly A, Atherton DJ, Griffiths WAD, et al. Hay–Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet. 2001;10:221–229. doi: 10.1093/hmg/10.3.221. [DOI] [PubMed] [Google Scholar]
- Mendrysa SM, O'Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes & Development. 2006;20:16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.