Abstract
Bacterial cell division is mediated by a multi-protein machine known as the “divisome”, which assembles at the site of cell division. Formation of the divisome starts with the polymerization of the tubulin-like protein FtsZ into a ring, the Z-ring. Z-ring formation is under tight control to ensure bacteria divide at the right time and place. Several proteins bind to the Z-ring to mediate its membrane association and persistence throughout the division process. A conserved stretch of amino acids at the C-terminus of FtsZ appears to be involved in many interactions with other proteins. Here, we describe a novel pull-down assay to look for binding partners of the FtsZ C-terminus, using a HaloTag affinity tag fused to the C-terminal 69 amino acids of B. subtilis FtsZ. Using lysates of Escherichia coli overexpressing several B. subtilis cell division proteins as prey we show that the FtsZ C-terminus specifically pulls down SepF, but not EzrA or MinC, and that the interaction depends on a conserved 16 amino acid stretch at the extreme C-terminus. In a reverse pull-down SepF binds to full-length FtsZ but not to a FtsZΔC16 truncate or FtsZ with a mutation of a conserved proline in the C-terminus. We show that the FtsZ C-terminus is required for the formation of tubules from FtsZ polymers by SepF rings. An alanine-scan of the conserved 16 amino acid stretch shows that many mutations affect SepF binding. Combined with the observation that SepF also interacts with the C-terminus of E. coli FtsZ, which is not an in vivo binding partner, we propose that the secondary and tertiary structure of the FtsZ C-terminus, rather than specific amino acids, are recognized by SepF.
Introduction
Bacterial cell division is mediated by a multi-protein machine known as the “divisome” that assembles into a ring at the site of cell division [1]. Formation of the divisome starts with the polymerization of the tubulin-like protein FtsZ into a ring, the Z-ring, just below the membrane at the cell division site. Because of its critical role in division, Z-ring formation is under tight control to ensure bacteria divide at the right time and place. Once formed, the Z-ring functions as a scaffold to which all other division proteins are recruited, and several proteins bind to the Z-ring to mediate its membrane association and persistence throughout the division process [2].
Placement of the Z-ring is mediated by two systems, that prevent cells from dividing at the poles or immediately after division is completed (Min), or from dividing through nucleoids (nucleoid occlusion). The MinC component of Min directly acts on FtsZ [3], [4], whereas nucleoid occlusion is mediated by the FtsZ-binding protein SlmA in E. coli [5], [6] and the non-homologous protein Noc in B. subtilis, which does not seem to act directly on FtsZ [7]. In B. subtilis, Z-ring formation is also controlled by the nutritional status of the cell, through the nutritional sensor UgtP that acts directly on FtsZ [8]. Another level of control is provided by ClpX. In B. subtilis ClpX destabilizes, but not degrades FtsZ polymers, whereas in E. coli ClpX degrades (preferentially polymeric) FtsZ and thus controls the amount of FtsZ available for Z-ring formation [9], [10].
The Z-ring is tethered to the membrane by FtsA. In E. coli, ZipA forms an additional membrane tethering factor [11]. A protein with a similar topology to ZipA, EzrA, acts as a destabilizer of Z-rings in B. subtilis [12]. Z-rings are further stabilized by ZapA [13], a protein that strengthens lateral associations between FtsZ filaments, and in a small subset of bacteria including E. coli, ZapC fulfills a similar function [14], [15]. Other proteins that interact directly with FtsZ have a specific function in division. FtsE functions as part of the FtsEX complex that controls hydrolysis of the cell wall in dividing E. coli [16], [17]. In B. subtilis, SepF is a ring-forming protein that binds FtsZ and plays a role in correct assembly of the cross-wall septum, presumably by bundling FtsZ polymers into ∼50 nm wide tubes that can be observed in vitro [18]. During sporulation, SpoIIE interacts with FtsZ to ensure proper formation of the asymmetric septum [19] and MciZ, a 40 amino acid protein, prevents aberrant FtsZ ring formation in the mother cell compartment [20].
Even though crystal structures of FtsZ and various interacting proteins exist, relatively little is known about the precise interactions between FtsZ and these proteins. However, it is increasingly evident that a conserved stretch of amino acids at the C-terminus of FtsZ is involved in many of these interactions. The high degree of conservation of this C-terminal peptide was identified by Ma and Margolin [21]. The C-terminal peptide consists of a highly conserved core sequence followed by a variable number of poorly conserved residues at the extreme C-terminus. This region is not resolved in the available FtsZ structures, as it is preceded by a non-conserved, flexible linker sequence of variable length. The FtsZ-binding domain of ZipA has been co-crystallized together with a peptide matching the FtsZ C-terminus, which showed an extended β-strand followed by an α-helix [22]. In addition to ZipA, evidence has been reported for the interaction of the conserved FtsZ C-terminus with MinC [23], FtsA [24], EzrA [25], ClpX [9], SepF [26] and FtsZ itself [27]. Evidence for the interaction of FtsA with the C-terminus is based on yeast two-hybrid data [24]. For MinC, mutations in the FtsZ C-terminus conserved core have been described that abolish the interaction with MinC both in vivo and in vitro [23]. Using a C-terminal truncation of FtsZ (FtsZEcΔ18) that is still capable of polymerization, it was shown that ClpX is far less active in degradation of FtsZEcΔ18 than of wt FtsZ [9]. All these studies were done in E. coli. In Mycobacterium tuberculosis, the FtsZ C-terminus mediates the interaction between FtsZ and FtsW [28]. EzrA and SepF are Gram-positive division proteins that modulate FtsZ. The effects of EzrA or SepF on FtsZ polymerization can be relieved by adding competing amounts of a synthetic peptide encoding the FtsZ C-terminus, and EzrA and SepF have no effects on a C-terminal truncation mutant of FtsZ that still polymerizes (FtsZBsΔ16) [25], [26]. The SepF study showed that SepF bundles FtsZ filaments and co-sediments with FtsZ in polymerization experiments. The FtsZBsΔ16 truncate still polymerized but no longer bundled in the presence of SepF, yet SepF still co-sedimented with FtsZBsΔ16, leading the authors to suggest that SepF also binds to a secondary site on FtsZ next to the C-terminus [26]. Recently the poorly conserved residues at the extreme C-terminus have been implicated in lateral interactions between FtsZ polymers that are potentially mediated by electrostatic forces [27].
The crystal structure of the FtsZ-binding domain of ZipA bound to a peptide matching the FtsZ C-terminus [22], and the recent crystal and NMR structures on the interaction of Thermotoga maritima FtsA (FtsATm) and a FtsZTm C-terminal peptide [29] provide more insight in how the C-terminus is bound by proteins. Bound to ZipA, the C-terminal peptide assumes the confirmation of an extended β-strand followed by an α-helix, whereas bound to FtsA the peptide is predominantly helical, but not as a single helix. Six residues in the peptide interact with ZipA, mostly through hydrophobic contacts but also through two hydrogen bonds between the backbones of ZipA and the FtsZ-peptide, formed by a conserved Asp and Leu residues (D367 and L369 in B. subtilis FtsZ) [22]. The interactions with FtsA are quite different, with three salt bridges connecting the peptide to FtsA. The salt bridges are formed by a conserved Asp and Arg residue (D370 and L376 in B. subtilis FtsZ) and the carboxyl group of the C-terminal amino acid residue [29]. Based on a comparison of both structures it has been suggested that the FtsZ C-terminus can adopt different conformations to fit different binding pockets [29].
In this study, we describe a pull-down strategy to identify interaction partners of the B. subtilis FtsZ C-terminus. Using this strategy we identify SepF as a binding partner of the C-terminus, confirming an earlier observation of Singh et al. [26]. We extend the earlier results by showing that SepF only binds to the C-terminus and by identifying residues critical for binding of SepF by an alanine-scan of the FtsZ C-terminus.
Results
SepF binds to the FtsZ C-terminus
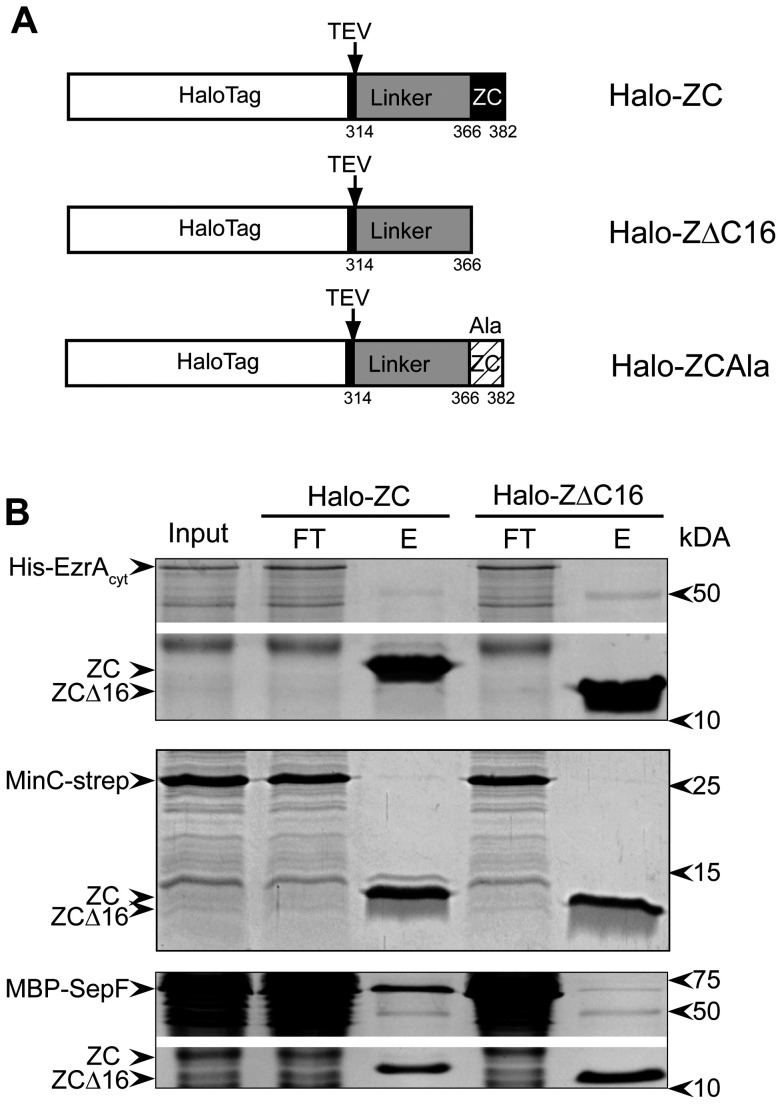
To screen for B. subtilis proteins that interact with the FtsZ C-terminus we fused the last 69 amino-acid residues of B. subtilis FtsZ to a HaloTag (Halo-ZC, Fig. 1A). The HaloTag consists of a modified dehalogenase that can covalently bind to a chloroalkane ligand coupled to sepharose beads (HaloLink resin) [30]. As a control we fused only the flexible, non-conserved linker sequence of FtsZ without the final 16 amino acid residues to a HaloTag (Halo-ZΔC16). The fusion proteins were expressed in E. coli and bound to the Halolink resin in binding buffer. Aliquots of the resin containing the covalently attached bait protein were subsequently used for pull-down experiments.
Figure 1. MBP-SepF binds to the FtsZ C-terminus.
(A) Schematic representation of the Halo-Tagged constructs used for the pull-down experiments. Halo-ZC consists of the HaloTag followed by a TEV protease cleavage site fused to the final 69 amino acids of B. subtilis FtsZ, including the 16 amino acid residues of the conserved C-terminal tail. Halo-ZΔC16 contains only the 53 amino acid residues of the FtsZ linker-domain but lacks the conserved C-terminal tail and Halo-ZAla is representative of the constructs used for the alanine scanning of the conserved C-terminal tail. (B) Pull down experiments using Halo-Zc or Halo ZΔC16 bound to resin. Pull downs were performed on lysates from E. coli cells overexpressing His-EzrAcyt (top panel), MinC-strep (middle panel) and MBP-SepF (lower panel). The input material (Input), flow-through (FT) and eluate (E) fractions of the experiments are shown and relevant proteins are indicated on the left. In the top and lower panel a fragment of the gel that did not contain the bait proteins or the protein of interest was deleted (white bar) to reduce the size of the image.
To validate the pull-down approach we analyzed the binding of three candidate interacting proteins after overexpression in E. coli. EzrA and SepF have been reported to interact with the FtsZ C-terminus in B. subtilis, and MinC interacts with the FtsZ C-terminus in E. coli [23], [25], [26]. We expressed B. subtilis MinC fused to a strep-tag (MinC-strep), the cytosolic domain of EzrA fused to a His-tag (His-EzrAcyt), and SepF fused to Maltose-Binding protein (MBP-SepF) in E. coli cells, prepared cell lysates and incubated the lysates with aliquots of resin with attached bait protein. After extensive washing, the bait protein and any interacting proteins bound to it were eluted from the resin by TEV protease cleavage. We found that MBP-SepF was retained by Halo-ZC bound to resin, and very weakly by Halo-ZΔC16 bound to resin (Fig. 1B). This confirms an earlier report that suggested that SepF binds to the FtsZ C-terminus [26]. His-EzrAcyt and MinC-strep were not retained by either construct, also not when we tested for the presence of prey protein using specific antibodies against the His- or strep-tag (not shown). Previously, we had not been able to establish a direct interaction between B. subtilis MinC and FtsZ [31].
The interaction between the FtsZ C-terminus and SepF is specific
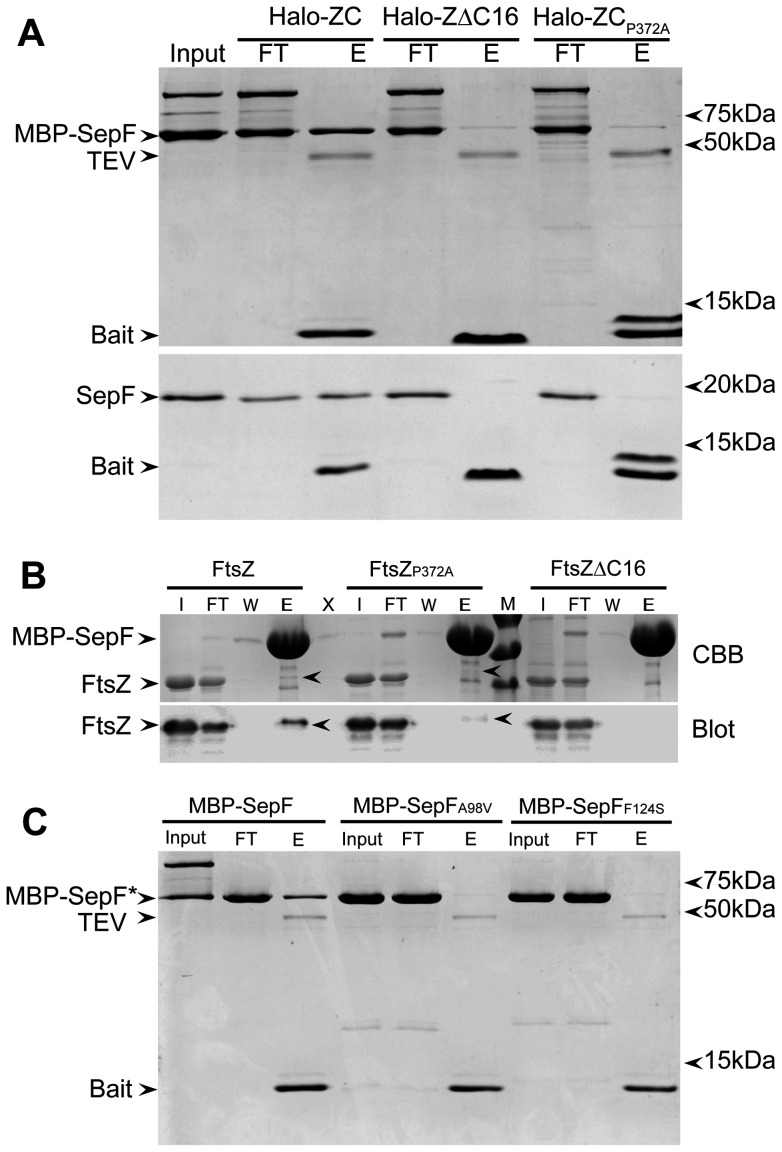
To study the interaction between SepF and the FtsZ C-terminus in more detail, and to exclude that the interaction is mediated by potential ‘bridging’ proteins present in the E. coli lysate, we repeated the pull-down experiments with purified MBP-SepF and SepF. As an extra control, we used a bait construct in which a conserved proline residue in the C-terminus was mutated into alanine, Halo-ZCP372A. Pro372 (notation for full length FtsZ) is fully conserved in the FtsZ C-terminus across species and we reasoned that a mutation of this residue would abolish the capability of the C-terminus to interact with SepF. Both pure MBP-SepF and SepF were retained when Halo-ZC was used as bait protein. When Halo-ZΔC16 or Halo-ZCP372A were used as bait a very faint band of MBP-SepF was visible in the eluate, but untagged SepF was not retained (Fig. 2A). This confirms that the interaction between SepF and the FtsZ C-terminus is specific, and that the pull-down is not caused by aspecific binding of the MBP-moiety to the C-terminus. The result also shows that the conserved proline residue indeed is critical for the function of the FtsZ C-terminus. We noticed that the ZCP372A bait protein is visible in the eluate as two bands on gel. The protein is initially expressed as full-length protein, during elution the ZCP372A bait is released from the Halo-tag by TEV cleavage. The double band pattern can be attributed to a change in the electrophoretic pattern of the mutated bait protein which is not completely resolved by the sample buffer resulting in two bands. Another, less likely explanation is that the alanine substitution gives rise to an extra TEV cleavage site. Other alanine substitutions in the C-terminus also give rise to double bands for the bait protein, and in several cases this change did not affect SepF binding (see below).
Figure 2. Specificity of the interaction between SepF and the FtsZ C-terminus.
(A) Pull down experiments using Halo-ZC, Halo ZΔC16 or Halo-ZCP372A bound to resin, with MBP-SepF (upper panel) or wild type SepF (lower panel) as prey proteins. The input material (Input), flow-through (FT) and eluate (E) fractions of the experiments are shown and relevant proteins are indicated on the left. MBP-SepF tends to form dimers as can be observed from the extra band in the input and flow-through samples. (B) Reverse pull down experiment with MBP-SepF bound to resin and purified FtsZ, FtsZP372A or FtsZΔC16 used as prey proteins. The input material (I), flow-through (FT), last wash (W) and eluate (E) fractions of the experiments are shown and relevant proteins are indicated on the left. M: molecular weight marker, bands are 70, 55 and 40 kDa. Upper panel: Coomassie stained gel (CBB), lower panel: western blot developed with anti-FtsZ antibody. (C) Pull down experiments using Halo-Zc bound to resin, with MBP-SepF, MBP-SepFA98V and MBP-SepFF124S as prey proteins. The input material (Input), flow-through (FT) and eluate (E) fractions of the experiments are shown and relevant proteins are indicated on the left.
MBP-SepF bound to amylose resin can be used to pull down FtsZ from E. coli lysates expressing B. subtilis FtsZ [18]. This allowed us to perform the reverse pull-down experiment, and we incubated MBP-SepF bound to resin with purified FtsZ, FtsZΔC16 and FtsZP372A. Importantly, in this experiment full-length FtsZ was used, so if there is a secondary binding site for SepF on FtsZ, one could expect that all three prey proteins are pulled down by MBP-SepF. We found that wild type FtsZ was bound by MBP-SepF and could be detected in the eluted fraction both by Coomassie staining and by western blotting (Fig. 2B). FtsZΔC16 and FtsZP372A were not visibly co-eluted with MBP-SepF with Coomassie, and only a very faint band was detected for co-eluted FtsZP372A. This result suggests that the FtsZ C-terminus is either the only site of interaction with SepF, or that the binding of SepF to the secondary binding site is weak.
Two mutations in SepF, SepFA98V and SepFF124S have been described that abolish SepF-FtsZ interaction [18]. We purified MBP-SepFA98V and MBP-SepFF124S and used these proteins as prey in a Halo-ZC pull-down. Neither SepF mutant was found to bind to the FtsZ C-terminus (Fig. 2C). This suggests that the mutations in SepFA98V and SepFF124S abolish binding of SepF to the FtsZ C-terminus rather than to a secondary binding site on FtsZ. It is also of note that whereas MBP-SepF runs as monomers and dimers on SDS-PAGE, the two mutants do not, confirming the observation that both mutants, most notably SepFA98V, are less prone to form large oligomeric structures [18].
Taken together, these results show that SepF interacts with the FtsZ C-terminus, that this interaction is specific, and that we cannot find evidence for a secondary interaction site on FtsZ using pull-down assays.
SepF cannot tubulate FtsZΔC16 and FtsZP372A polymers
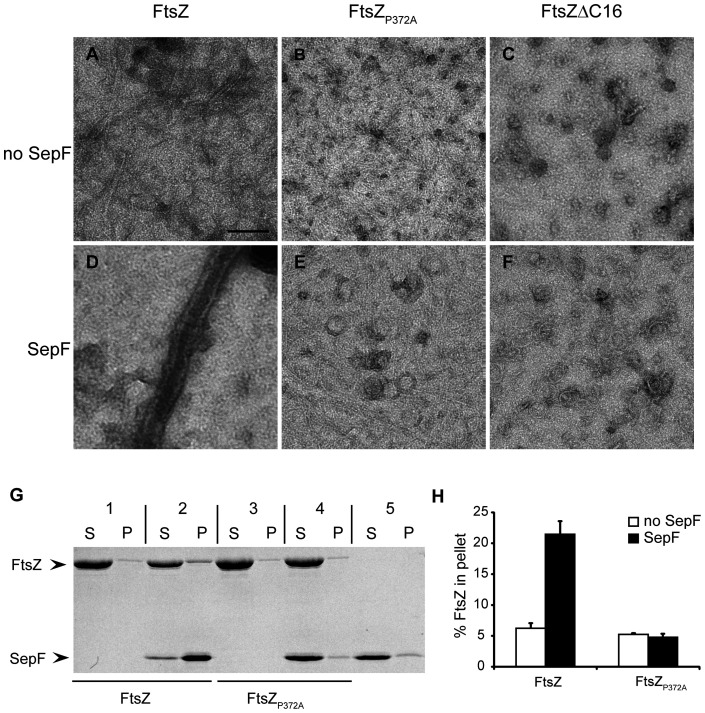
In vitro, SepF rings and FtsZ polymers combine to form large tubular structures [18]. Our pull-down experiments show that FtsZΔC16 and FtsZP372A have lost the ability to bind to SepF, but we cannot exclude that a weak residual interaction between the proteins (e.g. through a secondary interaction site on FtsZ) still allows the formation of tubules. Therefore, we mixed FtsZ, FtsZP372A and FtsZΔC16 with SepF and GTP, to induce FtsZ polymerization and possible tubule formation, and examined the samples using electron microscopy. FtsZ formed tubules with SepF (Fig. 3D), but in the case of FtsZP372A and FtsZΔC16 we could detect both FtsZ polymers and SepF rings (Fig. 3E, F), but not tubules, despite careful examination of the sample grids. We noticed that under our experimental conditions, FtsZP372A readily polymerized, but that FtsZΔC16 only formed very short polymers and aggregates (Fig. 3A–C), unlike the single stranded filaments that were reported for a FtsZΔC17 mutant [27]. We attribute this difference to the buffer system used in our study, with an increased pH and salt concentration that has previously been reported to decrease the amount of FtsZ polymerized in centrifugation and light scattering assays [18].
Figure 3. SepF cannot tubulate FtsZΔC16 and FtsZP372A polymers.
(A–F) Electron microscopy of 10 μM FtsZ (A, D), FtsZ P372A (B, E) and FtsZΔC16 (C, F) polymerized with 2 mM GTP in the absence (A–C) or presence (D–F) of 6 μM SepF. Scale bar: 100 nm. (G) Pelleting of FtsZ-SepF tubules by centrifugation. 10 μM FtsZ (1, 2) or FtsZ P372A (3,4) was polymerized with 2 mM GTP in the absence (1,3) or presence (2,4) of 12 μM SepF. As a control, SepF was used without FtsZ (5). Tubules were recovered by centrifugation and supernatant and pellet fractions were analyzed by SDS-PAGE. (H) Quantification of the amount of FtsZ pelleted in the assay shown in (G). Three independent experiments were analyzed, error bars indicate standard deviation.
As a second assay for tubule formation we used sedimentation, at a velocity whereby SepF-FtsZ tubules but not FtsZ polymers are recovered in the pellet. FtsZ and FtsZP372A were polymerized with GTP in the presence and absence of SepF. FtsZΔC16 was not used in this assay because of its tendency to aggregate. FtsZ was recovered in the pellet above background levels only when SepF was present, whereas FtsZP372A was not pelleted with SepF (Fig. 3G, H). SepF sedimentation is dependent on the presence of FtsZ, and does not occur with FtsZP372A, or when SepF is incubated alone (Fig. 3G). Combined with the pull-down experiments above this provides evidence that the FtsZ C-terminus is the only site of interaction with SepF.
The SepF binding site arises from the folding of the FtsZ C-terminus
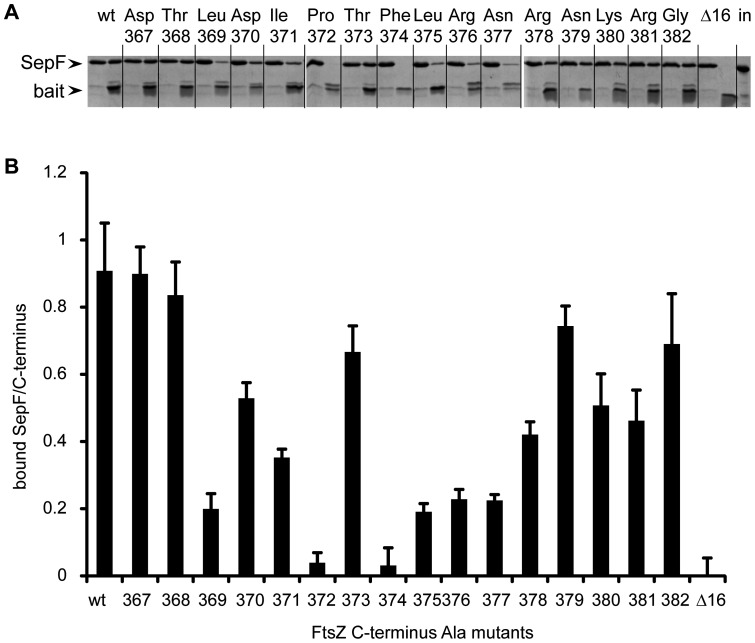
The Halo-ZC construct specifically pulls down SepF, and this interaction is lost upon deletion of the final 16 residues from the C-terminus or by mutating the conserved Pro372 to Ala. To learn which other residues in the conserved C-terminus are critical for SepF binding, we performed an alanine-scan of the C-terminus. Each of the last 16 residues of the C-terminus was mutated to Ala (Fig. 1A), resulting in a series of HaloZCAla constructs which were used to pull down purified SepF (Fig. 4A). We determined the intensity of Coomassie-stained bands from the eluted C-terminus and the co-eluted SepF, and plotted the ratio of the densities as an indication for binding, using Halo-ZC and Halo-ZΔC16 as references (Fig. 4B). The use of ratios of bound SepF to eluted bait protein means that we have corrected for possible fluctuations in the amount of the bait protein bound to and/or eluted from the beads. Changing the fully conserved Pro372 and Phe374 residues abolished SepF binding. Changing Leu369, Leu375, Arg376 and Asn377 reduced SepF binding to less than 25% of the binding observed with the wild type. Changing Asp370, Ile371, Arg378, Lys380 and Arg381 also notably affected SepF binding, whereas changing Asp367, Thr368, Thr373, Asn379 and Gly382 resulted in SepF recovery that was not noticeably different from wild type. The fact that 11 out of 16 mutations inhibited SepF binding to various extent, suggests that the recognition of the FtsZ C-terminus by SepF depends more on the folding of the C-terminus than on specific interactions between amino acids in the form of salt bridges.
Figure 4. Alanine scanning of the FtsZ C-terminus.
(A) Series of three gels from a representative pull down experiment using Halo-ZC (wt), Halo ZΔC16 (Δ16) and a series of HaloZCAla constructs (mutated residue indicated). For each pull down the flow-through (left side) and eluate fractions (right side) were loaded on the gel. The last lane is a loading control containing an equivalent amount of input SepF material. (B) Ratio of eluted SepF over eluted bait protein as determined by densitometric scanning of gels as shown in (A). Three independent experiments were analyzed, error bars indicate standard deviation.
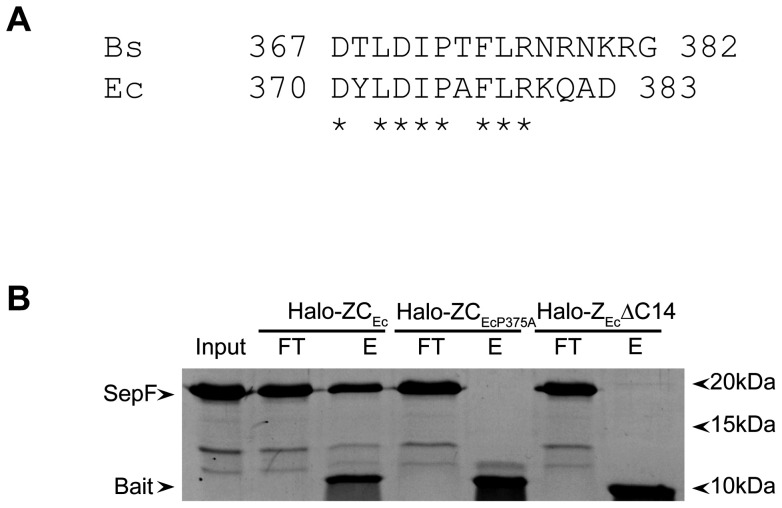
The E. coli FtsZ C-terminus has a conserved part that is very similar to that of B. subtilis FtsZ (Fig. 5A), yet as E. coli does not have SepF there is no need for FtsZEc to bind SepF. We reasoned that if the overall conformation of the C-terminus is recognized by SepF, then SepF might recognize the E. coli FtsZ C-terminus as well. We constructed Halo-ZCEc, Halo-ZEcΔC14 and Halo-ZCEcP375A similar to our B. subtilis constructs and performed a pull-down with purified SepF (Fig. 5B). SepF was bound to the Halo-ZCEc construct but not by Halo-ZEcΔC14 or Halo-ZCEcP375A. This result shows that SepF is capable of recognizing the C-terminus of a homologous FtsZ molecule, although this homologous FtsZ is not a binding partner for SepF in vivo.
Figure 5. SepF binds to the C-terminus of E. coli FtsZ.
(A) Alignment of the C-termini of B. subtilis (Bs) and E. coli (Ec) FtsZ. Identical residues are marked with an asterisk. (B) Pull down experiments using Halo-ZCEc, Halo-ZCEcP372A or Halo-ZEcΔC14 bound to resin, with wild type SepF as prey protein. The input material (Input), flow-through (FT) and eluate (E) fractions of the experiment are shown and relevant proteins are indicated on the left.
Discussion
In this paper we describe a novel pull-down assay to detect protein-protein interactions of the FtsZ C-terminus. The assay is based on the HaloTag fusion technology in which the bait protein is covalently bound to a resin allowing stringent wash steps before applying a sample to the resin [30]. Our fusion constructs consist of a modified dehalogenase fused to a part of FtsZ containing both the long flexible, non-conserved linker sequence and the conserved FtsZ C-terminus. The presence of the linker sequence has a dual function, it provides flexibility and distances the C-terminus from the dehalogenase, and it provides a control for unspecific binding through the FtsZΔC constructs. Using our assay we can specifically pull down FtsZ binding partners.
To validate our pull-down strategy we performed pull-downs with the C-terminus of B. subtilis FtsZ on lysates of E. coli cultures expressing three B. subtilis proteins that have previously been reported to bind to the FtsZ C-terminus, EzrA [25], SepF [26] and MinC. It has to be noted that evidence for MinC binding to the FtsZ C-terminus comes from the homologous E. coli proteins [23]. Although binding for each protein could be expected, we only managed to pull down SepF. Although genetic evidence suggested that MinCBs needs MinDBs to act on FtsZBs [32] our earlier work showed that MinCBs alone destabilizes bundles of FtsZBs polymers and increases the light scattering signal of non-polymerized FtsZBs in solution, indicating that FtsZBs and MinCBs interact without MinDBs [4]. However, we had not been able to detect interactions between full length MinCBs and FtsZBs through a cross-linking approach [31], whereas MinCEc cosediments with FtsZEc polymers [3] and polymerized FtsZEc can be recruited to MinCEc/MinDEc coated vesicles in a FtsZEc-C-terminus dependent manner [23]. This suggests that the interaction between B. subtilis FtsZ and MinC is not as strong as between the E. coli proteins. We do not know why we have not been able to pull down EzrA, as previously a peptide corresponding to the C-terminal 17 amino acids of FtsZ was shown to competitively inhibit EzrA binding to FtsZ and bound to EzrA with a dissociation constant of 102 μM [25]. The interaction between FtsZ and EzrA is sensitive to increasing salt concentrations [25] but our pull-down assays were performed at 150 mM NaCl, a salt concentration at which at least 50% of binding should still occur. Possibly we need a higher expression of His-EzrAcyt or to perform the pull-down assay under cross-linking conditions, or both, to detect the interaction between EzrA and the FtsZ C-terminus.
We have shown that our pull-down assay is very specific. Only SepF was pulled down from an E. coli lysate and control experiments showed that this pull-down was dependent on the presence of the C-terminus and independent of the tag attached to SepF. Furthermore, we could not detect SepF mutants that have lost the capability to bind FtsZ and we could show that FtsZ recovery in the reverse pull-down experiment depended on the presence of the FtsZ C-terminus (Fig 2). Our results strongly suggest that the FtsZ C-terminus is the only binding site for SepF. In an earlier study SepF has been found to co-precipitate with FtsZΔC16 polymers, which led the authors to suggest a potential secondary binding site for SepF on FtsZ [26]. We attribute the observed co-sedimentation of SepF with FtsZΔC16 polymers [26] to the buffer conditions used by the authors – 25 mM Pipes at pH 6.8. In an earlier report we have shown that SepF forms rings at pH 7.5 and high salt, but precipitates as aggregates at pH <7 [18]. In vitro polymerization and tubulation experiments confirmed that the formation of FtsZ-SepF tubules depends on the presence of the FtsZ C-terminus (Fig 3). SepF sedimentation is dependent on the presence of FtsZ polymers, and does not occur when we use polymers of FtsZP372A or SepF alone (Fig. 3G).
To identify residues in the FtsZ C-terminus involved in SepF binding we performed an alanine-scan of the C-terminus and performed pull-down assays. Mutation of the two residues that are totally conserved across species, Pro372 and Phe374, abolished the FtsZ-SepF interaction. In total, 11 out of the 16 mutations tested considerably affected SepF binding to the C-terminus. This suggests that the secondary and teriary structure of the FtsZ C-terminus is recognized by SepF, in a way that is more similar to how ZipA binds FtsZ, through hydrophobic contacts and hydrogen bonds to the peptide backbone, rather than to how FtsA binds FtsZ, through specific salt bridges [22], [29]. We cannot exclude that our mutations alter the structure of the C-terminus in such a way that residues are no longer in the right conformation to form salt bridges – for that structural information is required. Recently, the FtsZ C-terminus was divided into a conserved C-terminal tail (CTT) that is followed by a highly variable – both in length and composition – set of residues, the C-terminal Variable region (CTV) [27]. The CTT is thought to constitute the ‘landing pad’ for FtsZ-binding proteins whereas the CTV plays a role in promoting lateral interactions between FtsZ polymers [27]. The B. subtilis FtsZ CTV comprises residues 377–382. We noted that several residues in the CTV are likely to play a role in SepF binding, as alanine mutations of residues 377, 378, 380 and 381 led to a considerable decrease in SepF binding. Also, the salt bridge between the C-terminal residue of T. maritima FtsZ, which has a 7 residue long CTV, and FtsA [29], suggests that the CTV is part of the ‘landing pad’ for interacting proteins, next to its role in promoting lateral interactions. However, the finding that the E. coli FtsZ C-terminus, with a different and noticeably shorter CTV, also binds SepF, supports the notion that the CTT is the main binding partner for FtsZ-binding proteins. More information on the interactions between the FtsZ C-terminus and several FtsZ-binding proteins is required to elucidate this question.
In conclusion, we have developed a novel and highly specific assay to identify interaction partners of the C-terminus of FtsZ from B. subtilis and E. coli, and validated the assay with candidate binding proteins. Our results show that SepF specifically binds the C-terminus whereas the interactions of B. subtilis MinC and EzrA are too weak to be detected in our assay. We are currently using this assay to look for novel proteins interacting with the FtsZ C-termini from lysates of B. subtilis and E. coli cultures.
Materials and Methods
Plasmid construction
All plasmids are listed in Table 1. The sequence coding for the C-terminal 67 amino acid residues (317–383) from E. coli ftsZ was amplified from E. coli K12 chromosomal DNA by PCR using primers djs414 and djs415 (primers listed in Table 2). The sequence coding for the C-terminal 69 amino acid residues (314–382) from B. subtilis ftsZ was amplified from B. subtilis 168 chromosomal DNA by PCR using primers djs416 and djs417. Both PCR products were digested with PmeI and SgfI and ligated into PmeI/SgfI digested pFN18A (Promega), resulting in plasmids pDJ64 (E. coli ftsZ) and pDJ65 (B. subtilis ftsZ). pDJ65 was used as a template for site directed mutagenesis according to the Quickchange protocol (Stratagene), to introduce a stop codon at the position of D367, and to systematically change residues 367 to 382 to alanine, using primers djs426 to djs459. Similarly, pDJ64 was used as a template for site directed mutagenesis, to introduce a stop codon at the position of D370, and to change residues P375 to alanine, using primers djs461 to djs464. The sequence coding for the soluble part of B. subtilis EzrA (amino acid residues 27–562) was amplified from B. subtilis 168 chromosomal DNA by PCR using primers ek13 and ek14. The PCR product was digested with NcoI and XbaI and ligated into NcoI/XbaI digested pET302, resulting in plasmid pEK5. All constructs were verified by sequencing.
Table 1. Plasmids.
| Plasmids | Relevant characteristics | Source |
| pFN18A HaloTag® T7 Flexi® Vector | bla PT7 HaloTag-TEVsite | Promega |
| pET302 | lacZα, trc HisTag-enterokinase site | [34] |
| pDJ15 | tetR PtetA-minC-strep-tagII | [4] |
| pDJ26 | bla lacIQ PT7-his8-zapA | [4] |
| pMal-SepF | bla Ptac malE-sepF | [18] |
| pMal-SepF-A98V | bla Ptac malE-sepFA98V | [18] |
| pMal-SepF-F124S | bla Ptac malE-sepFF124S | [18] |
| pDJ64 | bla PT7 HaloTag-TEVsite-ftsZEc317-383; ZCEc | this work |
| pDJ65 | bla PT7 HaloTag-TEVsite-ftsZBs314-382; ZC | this work |
| pDJ69 | bla PT7 HaloTag-TEVsite-ftsZBs314-366; ZΔC16 | this work |
| pDJ73 | bla PT7 HaloTag-TEVsite-ftsZEc317-369; ZEcΔC14 | this work |
| pDJ74 | bla PT7 HaloTag-TEVsite-ftsZEc317-P375A-383; ZCEcP375A | this work |
| pDJ367-382 | bla PT7 HaloTag-TEVsite-ftsZBs314-382; residue in plasmid name changed to Ala | this work |
| pEK5 | lacZα, trc HisTag-enterokinase site- ezrA27-562 | this work |
Table 2. Primers.
| primer name | primer sequence 5′-3′ | aa change |
| djs414 | GTCCGCGATCGCCATGGACAAACGTCCTG | |
| djs415 | CAGCGTTTAAACTTAATCAGCTTGCTTACG | |
| djs416 | GTCCGCGATCGCCACCGGCTTTATCGAAC | |
| djs417 | GAGCGTTTAAACTTAGCCGCGTTTATTACGG | |
| djs426 | CTTCACAGCCGGCTGATTAAACGCTTGACATCCCGAC | D367Stop |
| djs427 | GTCGGGATGTCAAGCGTTTAATCAGCCGGCTGTGAAG | D367Stop |
| djs428 | GATGATACGCTTGACATCGCGACATTCTTAAGAAACC | P372A |
| djs429 | GGTTTCTTAAGAATGTCGCGATGTCAAGCGTATCATC | P372A |
| djs430 | GAAACCGTAATAAACGCGCCTAAGTTTAAACGAATTC | G382A |
| djs431 | GAATTCGTTTAAACTTAGGCGCGTTTATTACGGTTTC | G382A |
| djs432 | CTTAAGAAACCGTAATAAAGCCGGCTAAGTTTAAACG | R381A |
| djs433 | CGTTTAAACTTAGCCGGCTTTATTACGGTTTCTTAAG | R381A |
| djs434 | CATTCTTAAGAAACCGTAATGCACGCGGCTAAGTTTAAACG | K380A |
| djs435 | CGTTTAAACTTAGCCGCGTGCATTACGGTTTCTTAAGAATG | K380A |
| djs436 | CGACATTCTTAAGAAACCGTGCTAAACGCGGCTAAGTTTAAAC | N379A |
| djs437 | GTTTAAACTTAGCCGCGTTTAGCACGGTTTCTTAAGAATGTCG | N379A |
| djs438 | CCGACATTCTTAAGAAACGCTAATAAACGCGGCTAAG | R378A |
| djs439 | CTTAGCCGCGTTTATTAGCGTTTCTTAAGAATGTCGG | R378A |
| djs440 | CCCGACATTCTTAAGAGCCCGTAATAAACGCGG | N377A |
| djs441 | CCGCGTTTATTACGGGCTCTTAAGAATGTCGGG | N377A |
| djs442 | CATCCCGACATTCTTAGCAAACCGTAATAAACGCG | R376A |
| djs443 | CGCGTTTATTACGGTTTGCTAAGAATGTCGGGATG | R376A |
| djs444 | CTTGACATCCCGACATTCGCAAGAAACCGTAATAAAC | L375A |
| djs445 | GTTTATTACGGTTTCTTGCGAATGTCGGGATGTCAAG | L375A |
| djs446 | GATACGCTTGACATCCCGACAGCCTTAAGAAACCGTAATAAAC | F374A |
| djs447 | GTTTATTACGGTTTCTTAAGGCTGTCGGGATGTCAAGCGTATC | F374A |
| djs448 | CGCTTGACATCCCGGCATTCTTAAGAAACC | T373A |
| djs449 | GGTTTCTTAAGAATGCCGGGATGTCAAGCG | T373A |
| djs450 | GCTGATGATACGCTTGACGCCCCGACATTCTTAAGAAAC | I371A |
| djs451 | GTTTCTTAAGAATGTCGGGGCGTCAAGCGTATCATCAGC | I371A |
| djs452 | GGCTGATGATACGCTTGCCATCCCGACATTCTTAAG | D370A |
| djs453 | CTTAAGAATGTCGGGATGGCAAGCGTATCATCAGCC | D370A |
| djs454 | CCGGCTGATGATACGGCTGACATCCCGACATTC | L369A |
| djs455 | GAATGTCGGGATGTCAGCCGTATCATCAGCCGG | L369A |
| djs456 | CCGGCTGATGATGCGCTTGACATCC | T368A |
| djs457 | GGATGTCAAGCGCATCATCAGCCGG | T368A |
| djs458 | CAGCCGGCTGATGCTACGCTTGACATC | D367A |
| djs459 | GATGTCAAGCGTAGCATCAGCCGGCTG | D367A |
| djs461 | CAAACTGCGAAAGAGCCGTAATATCTGGATATCCCAGC | FtsZEcD370Stop |
| djs462 | GCTGGGATATCCAGATATTACGGCTCTTTCGCAGTTTG | FtsZEcD370Stop |
| djs463 | GGATTATCTGGATATCGCAGCATTCCTGCGTAAG | FtsZEcP375A |
| djs464 | CTTACGCAGGAATGCTGCGATATCCAGATAATCC | FtsZEcP375A |
| ek13 | AGACTACCATGGCCGAAATCGACCGGCTGGA | |
| ek14 | CGTTACTCTAGACTAAGCGGATATGTCAGCTTTG |
Protein purification
MBP-SepF and SepF were purified as described [18]. FtsZ was purified as described [4], FtsZΔC16 and FtsZP372A were purified using the same protocol.
Pull down experiments
Expression of HaloTagged constructs was done in E. coli BL21(DE3) carrying plasmid pBS58 which encodes an extra copy of ftsQAZ [33]. Freshly transformed cells were grown overnight at 37°C, diluted 1∶100 and grown to an OD600 ∼0.7 and induced with 1 mM IPTG. After three hours of growth cells were harvested by centrifugation and washed with PBS. Cell pellets were flash frozen in liquid nitrogen and stored at −20°C. To prepare lysates, cells were taken up in 1/10th of the original volume in buffer HPB (50 mM HEPES pH 7,5; 150 mM NaCl). Lysozyme (0,1 mg/ml) and DNAse (1 µg/ml) were added and the suspensions were incubated for 10 min on ice. Cells were disrupted using sonication (12 cycles of 5s with 5s cooling at 10 microns probe amplitude). Aliquots of 1ml were made and stored at −20°C. For a typical pull down experiment, for every construct tested a 1 ml aliquot was thawed on ice and subsequently centrifuged at 10000×g for 30 min at 4°C. DTT (to 1 mM) and Nonidet P-40 (to 0,005% v/v) were added to the supernatant. The rest of the procedure was carried out in buffer HPB+ (50 mM HEPES pH 7,5; 150 mM NaCl; 1 mM DTT; 0,005% v/v nonidet P-40). To the supernatant, 200 µl (25%) of HaloLink resin (Promega) equilibrated with HPB+ was added. The samples were incubated for 1 h at RT with gentle mixing. Subsequently, the samples were centrifuged at 1000×g for 5 min at RT. The supernatant was discarded and the resin was washed 3 times with HPB+. The resin with bound HaloTagged bait protein were subsequently incubated with 1 mL cell lysates (10× concentrated with respect to the original culture volume) of E. coli strains overexpressing potential binding partners. Alternatively, the resin with bound Halo-tagged bait protein was incubated with 1 mL of MBP-SepF, MBP-SepFA98V, MBP-SepFF124S or SepF at 15 μM diluted in HPB+. For the alanine-scanning experiments, 300 μL of 30 μM SepF was used. The samples were incubated for 1 h at RT with gentle mixing. Subsequently, the samples were centrifuged at 1000×g for 5 min at RT, and the supernatant was kept as the flow-through fraction. The resin was washed 3 times with HPB+. The HaloTagged bait protein and associated proteins were released from the resin by TEV-cleavage using 50 μL of Cleavage solution (HPB+ containing TEV-protease, Promega) for 1 h at RT with gentle mixing. The eluted material was collected by centrifugation at 1000×g for 5 min at RT and the resin was washed with an additional 50 μL of HPB+ which was collected by centrifugation and added to the eluted material. Samples were prepared for SDS-PAGE and run on 15% gels, which were stained with Coomassie Brilliant Blue.
For the FtsZ pull-downs with MBP-SepF, MBP-SepF bound to amylose-resin was prepared as described [18]. 250 μL aliquots of amylose resin with MBP-SepF were incubated with 300 μL of 30 μM FtsZ, FtsZΔC16 and FtsZP372A in buffer A (20 mM Tris–HCl pH 7.4, 200 mM KCl, 1 mM EDTA). The proteins were allowed to bind to the resin for 30 min at 4°C. Unbound protein and liquid was removed by mild centrifugation and collected as Flow-through. The resin was washed 6 times with buffer A and the last wash fraction was collected. MBP-SepF and bound FtsZ were eluted from the resin with 300 μL of 10 mM maltose in buffer A. Equal amounts of the input material, the flow-through, last wash and eluate were loaded on 12% SDS-PAGE gels, which were either stained with Coomassie Brilliant Blue, or subsequently transferred to PVDF using Western Blotting and developed using anti-FtsZ.
Electron microscopy
FtsZ, FtsZP372A, FtsZΔC16 (at 10 µM) and SepF (6 µM) were incubated in polymerization buffer (50 mM HEPES/NaOH, pH = 7,4, 300 mM KCl, 10 mM MgCl2) for 5 min. at 30°C. GTP was added to a final concentration of 2 mM to start FtsZ polymerization and the samples were incubated for 20 min. at 30°C. 2 µl of each sample was applied onto a glow-discharged 400 mesh carbon-coated copper grid, incubated for 30 s., blotted dry with filter paper and negatively stained using 5µl uranyl-acetate (2%). The grids were viewed in a Philips CM120 electron microscope equipped with a LaB6 filament operating at 120 kV. Images were recorded with a Gatan 4000 SP 4 K slow-scan CCD camera at 36,400× magnification.
Sedimentation of tubules
FtsZ or FtsZP372A (at 10µM) was mixed with SepF (12 µM) in polymerization buffer and incubated for 5 minutes at 30°C. Polymerization was started by the addition of GTP to a final concentration of 2 mM, and samples were incubated for a further 20 min. at 30°C. Control samples contained only FtsZ, FtsZP372A or SepF. Tubules were spun down for 15 min. at 24,600×g, at 25°C. Pellet and supernatant fractions were recovered and equal amounts were analyzed by SDS-PAGE and staining with Coomassie Brilliant Blue.
Acknowledgments
We would like to thank Bart van den Berg van Saparoea (VU University Amsterdam, the Netherlands), Nina van den Berghe and Zhong Yu (Promega Benelux, the Netherlands) for helpful advice regarding the use of the HaloTag system. Seline Zwarthoff (University of Groningen, the Netherlands) is acknowledged for the construction of pDJ73 and pDJ74. We thank Leendert Hamoen (University of Newcastle, UK) and Arnold Driessen (University of Groningen, the Netherlands) for helpful comments on the manuscript.
Funding Statement
This work was funded by a VIDI grant (864.09.010) from the Netherlands Organisation for Scientific Research (NWO) (www.nwo.nl) to DJS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Boer PA (2010) Advances in understanding E. coli cell fission. Curr Opin Microbiol 13: 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams DW, Errington J (2009) Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7: 642–653. [DOI] [PubMed] [Google Scholar]
- 3.Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J (1999) The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci USA 96: 14819–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheffers DJ (2008) The effect of MinC on FtsZ polymerization is pH dependent and can be counteracted by ZapA. FEBS Lett 582: 2601–2608. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt TG, de Boer PA (2005) SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol Cell 18: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonthat NK, Arold ST, Pickering BF, Van Dyke MW, Liang S, et al. (2011) Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J 30: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu LJ, Ishikawa S, Kawai Y, Oshima T, Ogasawara N, et al. (2009) Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J 28: 1940–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weart RB, Lee AH, Chien AC, Haeusser DP, Hill NS, et al. (2007) A metabolic sensor governing cell size in bacteria. Cell 130: 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camberg JL, Hoskins JR, Wickner S (2009) ClpXP protease degrades the cytoskeletal protein, FtsZ, and modulates FtsZ polymer dynamics. Proc Natl Acad Sci USA 106: 10614–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weart RB, Nakano S, Lane BE, Zuber P, Levin PA (2005) The ClpX chaperone modulates assembly of the tubulin-like protein FtsZ. Mol Microbiol 57: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hale CA, De Boer PAJ (1997) Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 88: 175–185. [DOI] [PubMed] [Google Scholar]
- 12.Levin PA, Kurtser IG, Grossman AD (1999) Identification and characterization of a negative regulator of FtsZ ring formation in Bacillus subtilis. Proc Natl Acad Sci USA 96: 9642–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueiros-Filho FJ, Losick R (2002) A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 16: 2544–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand-Heredia JM, Yu HH, De Carlo S, Lesser CF, Janakiraman A (2011) Identification and characterization of ZapC, a stabilizer of the FtsZ ring in Escherichia coli. J Bacteriol 193: 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale CA, Shiomi D, Liu B, Bernhardt TG, Margolin W, et al. (2011) Identification of Escherichia coli ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. J Bacteriol 193: 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbin BD, Wang Y, Beuria TK, Margolin W (2007) Interaction between cell division proteins FtsE and FtsZ. J Bacteriol 189: 3026–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang DC, Peters NT, Parzych KR, Uehara T, Markovski M, et al. (2011) An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc Natl Acad Sci USA 108: E1052–E1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundogdu ME, Kawai Y, Pavlendova N, Ogasawara N, Errington J, et al. (2011) Large ring polymers align FtsZ polymers for normal septum formation. EMBO J 30: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucet I, Feucht A, Yudkin MD, Errington J (2000) Direct interaction between the cell division protein FtsZ and the cell differentiation protein SpoIIE. EMBO J. 19: 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handler AA, Lim JE, Losick R (2008) Peptide inhibitor of cytokinesis during sporulation in Bacillus subtilis. Mol Microbiol 68: 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X, Margolin W (1999) Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J Bacteriol 181: 7531–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosyak L, Zhang Y, Glasfeld E, Haney S, Stahl M, et al. (2000) The bacterial cell-division protein ZipA and its interaction with an FtsZ fragment revealed by X-ray crystallography. EMBO J 19: 3179–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen B, Lutkenhaus J (2009) The conserved C-terminal tail of FtsZ is required for the septal localization and division inhibitory activity of MinC(C)/MinD. Mol Microbiol 72: 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haney SA, Glasfeld E, Hale C, Keeney D, He Z, et al. (2001) Genetic analysis of the Escherichia coli FtsZ-ZipA interaction in the yeast two-hybrid system: characterization of FtsZ residues essential for the interactions with ZipA and with FtsA. J Biol Chem 276: 11980–11987. [DOI] [PubMed] [Google Scholar]
- 25.Singh JK, Makde RD, Kumar V, Panda D (2007) A membrane protein, EzrA, regulates assembly dynamics of FtsZ by interacting with the C-terminal tail of FtsZ. Biochemistry 46: 11013–11022. [DOI] [PubMed] [Google Scholar]
- 26.Singh JK, Makde RD, Kumar V, Panda D (2008) SepF increases the assembly and bundling of FtsZ polymers and stabilizes FtsZ protofilaments by binding along its length. J Biol Chem 283: 31116–31124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buske PJ, Levin PA (2012) The extreme C-terminus of the bacterial cytoskeletal protein FtsZ plays a fundamental role in assembly independent of modulatory proteins. J Biol Chem 284: 10945–10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta P, Dasgupta A, Bhakta S, Basu J (2002) Interaction between FtsZ and FtsW of Mycobacterium tuberculosis. J Biol Chem 277(28): 24983–24987. [DOI] [PubMed] [Google Scholar]
- 29.Szwedziak P, Wang Q, Freund SM, Lowe J (2012) FtsA forms actin-like protofilaments. EMBO J 31: 2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, et al. (2008) HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3: 373–382. [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira IF, de Sousa Borges A, Kooij V, Bartosiak-Jentys J, Luirink J, et al. (2010) Characterization of ftsZ mutations that render Bacillus subtilis resistant to MinC. PLoS One 5: e12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marston AL, Errington J (1999) Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol Microbiol 33: 84–96. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Lutkenhaus J (1993) The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol 9: 435–442. [DOI] [PubMed] [Google Scholar]
- 34.van der Does C, Manting EH, Kaufmann A, Lutz M, Driessen AJ (1998) Interaction between SecA and SecYEG in micellar solution and formation of the membrane-inserted state. Biochemistry 37: 201–210. [DOI] [PubMed] [Google Scholar]