Abstract
Many species of tsetse flies are infected by a virus (GpSGHV) that causes salivary gland hypertrophy (SGH). Female Glossina pallidipes (Austen) with SGH symptoms (SGH+) have reduced fecundity and SGH+ male G. pallidipes are unable to inseminate female flies. Consequently, G. pallidipes laboratory colonies with a high prevalence of SGH have been difficult to maintain and have collapsed on several occasions. To assess the potential impact of the release of SGH+ sterile male G. pallidipes on the efficacy of an integrated control programme with a sterile insect technique (SIT) component, we examined the mating efficiency and behaviour of male G. pallidipes in field cages in relation to SGH prevalence. The results showed in a field cage setting a significantly reduced mating frequency of 19% for a male G. pallidipes population with a high prevalence of SGH (83%) compared to 38% for a male population with a low prevalence of SGH (7%). Premating period and mating duration did not vary significantly with SGH status. A high percentage (>80%) of females that had mated with SGH+ males had empty spermathecae. The remating frequency of female G. pallidipes was very low irrespective of the SGH status of the males in the first mating. These results indicate that a high prevalence of SGH+ in G. pallidipes not only affects colony stability and performance but, in view of their reduced mating propensity and competitiveness, releasing SGH+ sterile male G. pallidipes will reduce the efficiency of a sterile male release programme.
Introduction
Tsetse flies (Glossina spp.; Diptera: Glossinidae) are the cyclical vectors of two debilitating diseases in Africa, sleeping sickness in humans (human African trypanosomosis, HAT) and the cattle disease nagana (or African animal trypanosomosis, AAT) [1], [2]. Nagana, and in certain areas also sleeping sickness, has been a major obstacle to sub-Saharan African rural development and a severe constraint to agricultural production [3]. Due to the lack of effective vaccines and inexpensive drugs for HAT, and the development of resistance of the AAT parasites against available trypanocidal drugs [4], vector control remains the most efficient strategy for the sustainable management of these diseases [5].
Two attempts to establish of a colony of Glossina pallidipes (Austen) at the Insect Pest Control Laboratory (formerly Entomology Unit) of the Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture, Seibersdorf, Austria, were initially successful, but the colonies subsequently experienced a steady decline over several years and finally collapsed. Investigations revealed that up to 85% of both male and female flies showed symptoms of salivary gland hypertrophy (SGH+). This syndrome was first described in wild populations of G. pallidipes, but later detected in many tsetse species from different African countries [6]. The virus causing these symptoms was also associated with testicular degeneration and ovarian abnormalities [7]–[10] and affected the development, survival, fertility and fecundity of naturally or experimentally infected flies [6]. SGH+ G. pallidipes males were sterile and mostly unable to inseminate female flies. In G. m. morsitans and G. m. centralis, insemination by SGH+ males was also impaired [11]. In tsetse populations in nature, mother to offspring transmission, either trans-ovum or through infected milk glands, is thought to be the most likely mode of transmission of the virus [10], [12], [13], but in laboratory maintained flies horizontal transmission during membrane feeding is reported to be a significant route of virus infection [14], as each tray of blood is used to feed several successive sets of fly cages [15] leading to a build-up of virus in the blood diet.
The release of sterile males is a powerful and robust control tactic against tsetse flies but adequate competitiveness of the released sterile flies is a crucial prerequisite for success [16]. Loss in competitiveness can be compensated for by releasing higher numbers of sterile males per unit of surface, but this is not cost effective [17]. Although earlier studies report that the mating behaviour of SGH+ G. m. morsitans appeared to be normal in terms of mating duration and time to reach the jerking phase before separation [11], no information has been available on the mating propensity and competitiveness of SGH+ male G. pallidipes.
Multiple mating by wild female flies is another potential factor that may affect the efficacy of the SIT. However, it is significant only if the frequency of remating differs after a fertile or sterile mating, if females are able to discriminate between sterile and fertile males at the second mating, or if the females are able to use sperm selectively from the different matings. Information in the literature on the mating behaviour of tsetse flies in nature is scarce and the general assumption that wild female tsetse flies mate only once [18] has also received very little experimental attention.
In this paper the hypothesis that SGH in male G. pallidipes has a negative impact on their mating vigour or propensity to mating was examined. In addition, the multiple mating frequencies of female G. pallidipes were assessed in relation to SGH status of the male mates. All experiments were carried out in field cages to mimic the natural environment as closely as possible.
Materials and Methods
Tsetse flies
All experiments were carried out with flies from a G. pallidipes colony originating from pupae collected in Tororo, Uganda in 1975 and maintained since 1987 at the Insect Pest Control Laboratory, Seibersdorf, Austria. The adults were fed on warm, defibrinated, bovine blood (SVAMAN spol s.r.o., Myjava, 90701, SLOVAKIA) for 10–15 min three times per week using an in vitro membrane feeding system. The deposited larvae pupated and were incubated at 24°C until emergence [14], [19].
Screening males of G. pallidipes for salivary gland hypertrophy symptoms
Although in female G. pallidipes external signs of SGH are not reliably visible, SGH can usually be detected by the swollen and pale appearance of the abdomen in male G. pallidipes that are older than 10 days and that have been starved for two days [20]. Males of G. pallidipes were collected and fed for 10–13 days post emergence, starved for two days then chilled at 4°C for 5–10 min, prescreened visually as previously described [20] and separated into two groups of flies: one with a high prevalence and one with a low prevalence of SGH. The two groups of males were kept at 24°C and 75% RH until used in mating tests. After mating, males observed to copulate were collected and dissected to confirm the presence of salivary gland hypertrophy. The absence of hypertrophy symptoms does not mean the absence of viral infection in the flies as many individuals are infected but asymptomatic [21].
Mating and remating experiments
Mating experiments were conducted in cylindrical netted field cages, 2.9 m in diameter and 2.0 m high, placed in a laboratory rearing room at 24°C and 60–65% RH and containing a potted citrus plant to provide resting sites. All mating experiments were carried out between 9.30 am and 12.00 noon as previously described [22].
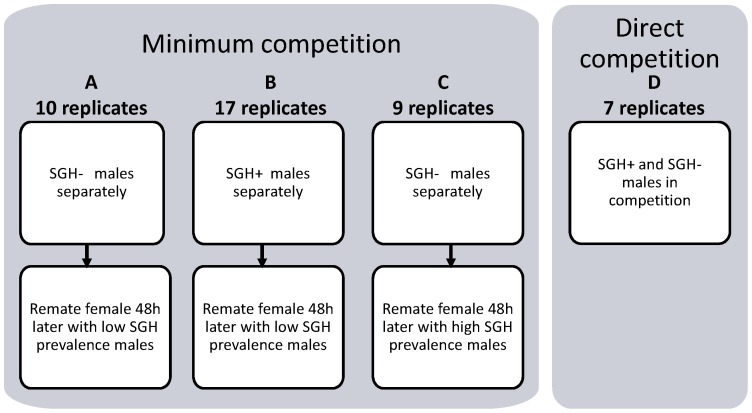
In each experiment, 7–day old virgin females were released in the field cage with 15–day old males from one of the groups described above at a ratio of 1 female: 1 male and 48 hours later given the opportunity to mate again. The salivary gland status of the males was determined by dissection after the initial mating. Only the female flies that had mated with SGH+ males from the high prevalence group and females that had mated with SGH− males from the low prevalence group were retained for remating; the remaining females (mated with SGH+ in the low prevalence group and with SGH− in the high prevalence group) were dissected to determine the spermathecal fill status. The remating experiments used a similar protocol as described above but at a ratio of 1 female: 2 males. The mating period was recorded and male dissections were carried out as described above. For the remating tests, three mating combinations were tested: (A) females that had first mated with SGH− males were given the opportunity to remate with low SGH prevalence males (B) females that had first mated with SGH+ males were given the opportunity to remate with males from the low SGH prevalence group, and (C) females that had first mated with SGH− males were given the opportunity to remate with males from the high SGH prevalence group ( Figure 1 ).
Figure 1. Schematic representation of the design of experiments A, B and C (minimum competition) and D (direct competition).
Hypertrophied males cannot be distinguished visually with complete reliability and accurate identification of individual males as being either SGH+ or SGH− required destructive dissection and observation of salivary glands. As a consequence the high prevalence (SGH+ males) group contained a number of SGH− males and the low prevalence group contained some SGH+ males. There were differences in the performance of these males in the first experiments (without competition), so in order to compare the relative competitiveness directly a second experiment with direct competition was conducted. High and low prevalence groups of 15 day-old males were released in equal numbers (30 flies of each group with one group marked with a dot of acrylic paint on the thorax) with 7 day-old virgin females to give a ratio of 1 female: 2 males in the field cage. The flies were observed for two and half hours and copulating pairs were collected in individual tubes, each tube being numbered to identify the individual male and female flies. Premating time and mating duration were recorded and after separation of the couples male flies were removed and dissected to determine salivary gland status.
The number of females that mated as a proportion of the total females in each replicate is an indication of the tendency of the flies to mate (proportion mating, PM). The relative mating index (RMI) was defined as the number of mating pairs accounted for by the salivary gland status category as a proportion of the total number of mating pairs. This measure represents the competitiveness of SGH+ males relative to the SGH− males and is equivalent to the Relative Sterility Index [22].
Pre-mating time and mating duration
The period between the flies' release in the mating cage until copulation was recorded as pre-mating time. Once genitalia were engaged and the pair was in copula, the pair was collected into individual tubes. The type of male (low or high SGH prevalence), and the starting and separation time of each successful mating were recorded to the nearest minute. The mating duration was then calculated as the difference in minutes between ending and starting times. Once copulation ended, the male was removed and dissected for salivary gland status and the female was kept in the fly holding room for the remating experiment or dissection for salivary gland status and scoring of spermathecal contents.
Insemination
The female flies were dissected in physiological saline solution under a binocular microscope and the insemination rate and spermathecal fill determined [23]. If there were any doubts when using the binocular microscope the spermathecae were removed and mounted on a microscope slide and viewed under 100× magnification. The spermathecal fill was scored to the nearest quarter for each spermatheca separately as empty (0), partially-full (0.25, 0.50 or 0.75) or full (1.0) and the quantity of sperm transferred was then computed as the sum of the two spermathecae scores.
Environmental conditions in the field cage studies
The temperature was maintained at 24±0.5°C and the relative humidity ranged from 60 to 65% during the observation periods. Light intensity (provided by high-frequency fluorescent tubes) varied from 290 to 550 lux with areas under the PVC supporting frame and tree leaves recording lower light intensity.
Data analysis
The number of matings achieved and spermathecal fill categories were tested for equality of performance by SGH+ and SGH− males using the goodness of fit statistic G [24]. Premating times and mating duration were normalized by a square root transform following Box-Cox analysis [25]. Transformed values were analyzed by ANOVA and means compared by t-test [25]. Tabulated values are presented as detransformed mean values with 95% confidence intervals.
Results
Screening of G. pallidipes males for SGH+
The prevalence of SGH+ in the G. pallidipes tsetse colony established in the Insect Pest Control Laboratory was reported previously at 4–10% [20], [21]. The results show that the male flies screened as SGH+ by external observation (high prevalence group) had 83% SGH+ prevalence when confirmed by dissection whereas the male flies screened as SGH− (low prevalence group) had 7% SGH+.
Impact of SGH+ on G. pallidipes mating behaviour
a) Impact of SGH+ on male mating efficiency
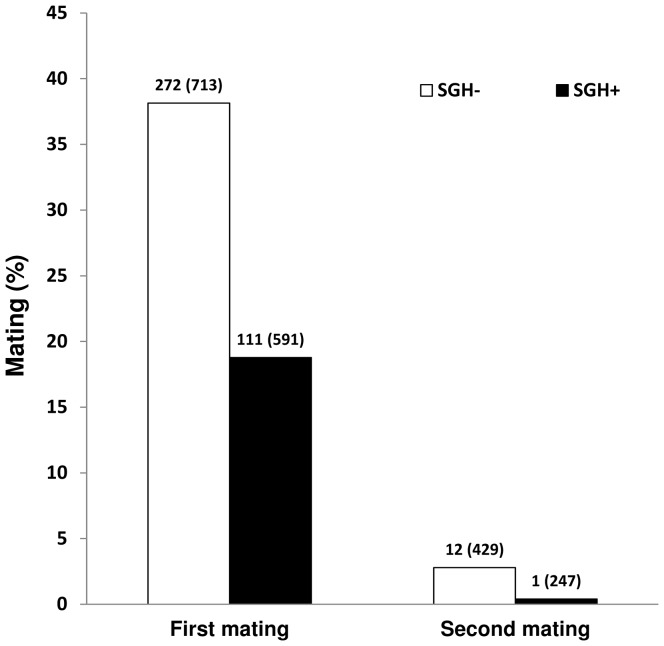
SGH+ male G. pallidipes were significantly less efficient in mating with 7 day-old females than SGH− male flies when released in a field cage either with a majority of males with SGH− (G = 10.77; d.f. = 1; P = 0.00103) or with a majority of males with SGH+ (G = 8.91; d.f. = 1; P = 0.002836) at a male: female ratio of 1∶1. Combined data of the no competition experiments indicate that only 19% of males of the high prevalence group mated, whereas 38% of the males of the low prevalence group succeeded in mating under field cage condition when they were offered the opportunity in a field cage ( Figure 2 ). Males that managed to copulate had an SGH+ prevalence of 29% whereas the males that did not copulate showed an SGH+ prevalence of 52%, a highly significant difference from the mean rate of hypertrophy in these males (45.3%, G = 42.98; d.f. = 1; P≪0.001). No difference was observed in the SGH+ prevalence between mated and non-mated females.
Figure 2. Mating percentage of SGH− and SGH+ males in the first and second matings.
Numbers above columns indicate number mating and total number of males in category.
Although the mating and remating experiments were designed to avoid competition between the two types of males, the released flies were actually a mixture of SGH+ and SGH− males (i.e. some males were classed as SGH− before the experiment started but proved to be SGH+ after dissection and vice versa, ( Table 1 ). Whilst SGH+ males constituted at least 9% of the males in the low prevalence group, only 3.6% of matings were with these SGH+ males in the initial mating and none in the remating. Conversely, SGH− males constituted 13.7% and 30% of the males in the high prevalence group in the first mating and remating respectively but constituted over 23% and 87% of the mating pairs.
Table 1. Mating frequency by SGH status in field cage mating experiments.
| SGH+ Prevalence | Reps | No of flies | Mating frequency | ||||
| Females | Males | SGH+ ♂ | No of pairs | SGH+ ♂ | SGH− ♂ | ||
| First mating | |||||||
| Low | 20 | 35.0±5.16 | 34.0±5.0 | 3.05±2.31 | 12.50±5.63 | 0.45±0.83 | 12.05±5.46** |
| High | 18 | 34.44±5.11 | 34.11±5.00 | 29.44±4.40 | 7.39±3.52 | 5.67±2.57 | 1.72±2.14** |
| Second mating (after 48 hrs) | |||||||
| A | 10 | 9.10±3.73 | 17.00±9.29 | 2.00±2.45 | 0.10±0.32 | 0.00 | 0.10±0.32 |
| B | 18 | 5.44±2.53 | 11.67±5.31 | 1.11±1.32 | 0.50±1.47 | 0.00 | 0.50±1.47 |
| C | 10 | 14.40±5.08 | 29.50±9.30 | 20.60±10.20 | 0.80±1.32 | 0.10±0.32 | 0.70±1.06 |
A: Females mated with SGH− males and remated with low SGH prevelance group, B: Females mated with SGH+ males and remated with low SGH prevelance group, and C: Females mated with SGH-males and remated with high SGH prevelance group. Data are presented as mean values ± standard deviation.
mating frequency differs between SGH+ and SGH− males in the same row at the 1% level. See Results section for full statistical analysis.
b) Impact of SGH+ on male competitiveness
In the direct competition experiment where SGH+ and SGH− males competed in almost equal numbers (a total of 192 SGH+ and 228 SGH− divided between 7 replicates), male flies with SGH+ were significantly less efficient in securing a mate (RMI of SGH+ = 0.204±0.053) in comparison to SGH− males (G = 30.6; d.f. = 6; P<0.0001). SGH+ males were substantially less competitive than SGH− males in both the low and high prevalence groups.
c) Impact of SGH+ on female mating efficiency
In general, the female flies in the no competition experiment that accepted a mate had a lower SGH+ prevalence (11.0%) than those that did not accept a mate (14.7%) although the difference was not significant (G = 1.817; d.f. = 1; P = 0.1776).
d) Impact of SGH on female remating behaviour
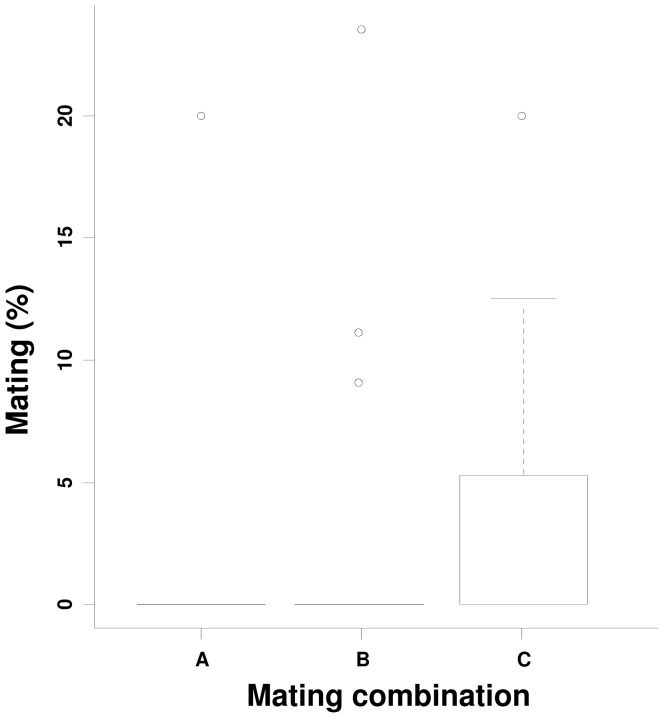
The SGH status of the male from the first mating did not significantly influence the propensity of the females to remate when offered a second mating opportunity with 7-day old males in the field cage 48 hours after the first mating (SGH+ 2.7%, SGH− 2.0%, ns). However, for the very few females that did remate, there was a significantly greater number of SGH− than SGH+ males in the second mating (G = 5.701; d.f. = 1; P = 0.0169). These data indicate a high level of remating refractoriness in female G. pallidipes independent of SGH status of the male in the first mating ( Figure 3 ).
Figure 3. Impact of first and second mating combinations on the mating percentage in the second mating of G. pallidipes.
A, Females mated with SGH− males and remated with SGH− males, B, Females mated with SGH+ males and remated with SGH− males, and C, Females mated with SGH− males and remated with SGH+ males.
Pre-mating period and mating duration
Formation of mating pairs occurred soon after the release of males until the end of the observation period. The premating period and mating duration for the three mating combinations are shown in Table 2 . As the pre-mating period and mating duration for SGH− and SGH+ males did not differ significantly in the experiments with no competition (F = 2.36; d.f. = 1,155; P = 0.126) values for all SGH− males were pooled and likewise for all SGH+ males. The pooled premating period for SGH− males was 49 and 64 minutes for first and second matings, and 64 minutes for SGH+ males.
Table 2. The mating duration and period before mating in minutes for SGH+ and SGH− male G. pallidipes in a field cage.
| SGH+ Prevalence | Salivary gland status confirmed by dissection | |||||
| Males with SGH− | Males with SGH+ | |||||
| n | Premating period | Mating duration | n | Premating period | Mating duration | |
| First mating | ||||||
| Low | 223 | 43.9–48.3–53.0a | 27.5–28.3–29.3a | 6 | 39.7–57.5–78.5a | 25.9–30.7–36.0a |
| High | 30 | 44.5–55.7–68.1a | 22.1–25.7–29.4a | 103 | 57.2–64.3–71.8a | 25.0–26.6–28.2a |
| Second mating (after 48 hrs) | ||||||
| A | 1 | 74 | 27 | 0 | ||
| B | 3 | 22.7–53.5–97.2a | 11.4–23.8–40.7a | 0 | ||
| C | 7 | 32.4–66.2–112a | 27.1–32.6–38.6a | 1 | 3 | 29 |
A: Females mated with SGH− males and remated with low SGH prevelance group, B: Females mated with SGH+ males and remated with low SGH prevelance group, and C: Females mated with SGH-males and remated with high SGH prevelance group.
Data are presented as detransformed mean values (in bold) and 95% confidence interval.
Mean values in the same column followed by the same letter do not differ at the 5% level. See Results section for full statistical analysis.
Pooled SGH− males had mean mating duration of 28 and 30 minutes in the first and second matings while SGH+ males had mean mating duration of 27 minutes. Only one SGH+ male copulated with a female mating for the second time with a premating period of 3 minutes and duration of 29 minutes. None of the differences was significant.
Insemination efficiency
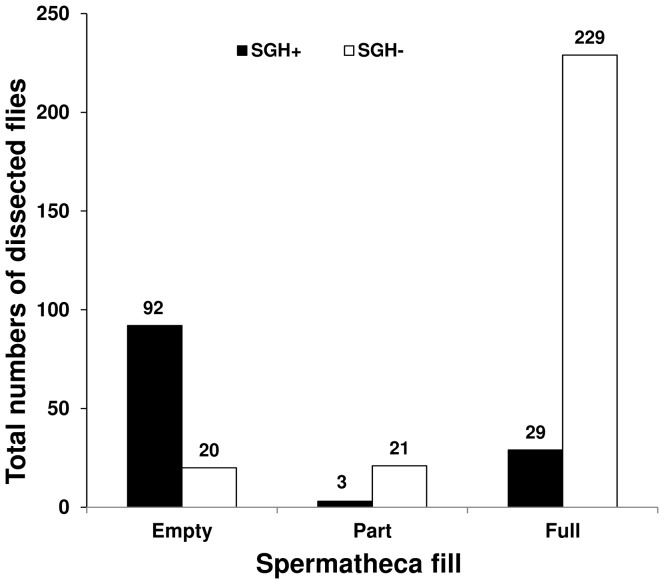
The insemination efficiency, measured by the spermathecal fill in the first mating, was affected significantly by the male's salivary gland status (G = 139.8; d.f. = 2, P≪0.0001). The percentage of females with full spermathecae was significantly lower in females mated with SGH+ males (∼10%) than those mated with SGH− males (>80%) whereas, the percentage of females with empty spermathecae was higher in the females mated with SGH+ males (>80%) than those mated with SGH− males (∼10%) ( Figure 4 ).
Figure 4. Impact of male SGH status on insemination rate (spermathecal fill) in G. pallidipes mated females.
Discussion
The SIT can only be successful if the released male insects are competitive with their wild counterparts and are able to locate virgin females to copulate. Tsetse flies, at least in G. morsitans, display a resource-based defence mating system, where the potential for mate monopolization by males is high due to the clumped distribution of the females and the resources (host animals) that are attractive to the receptive females [26]. Male mating success is largely determined by intra-sexual competition at these resources required by the females, both to intercept females and to prevent other males from gaining access to females [27]. Therefore, any reduction in male tsetse mating vigour will reduce the efficiency of the SIT component in integrated pest management programmes.
The data from our field cage studies, set-up to better mimic the natural environment as compared to small laboratory cages, indicates that the mating propensity and mating success of male G. pallidipes with SGH+ was reduced significantly as compared to SGH− male flies. Little more than half of males with SGH+ managed to secure a female mate as compared to SGH− males. These results are in agreement with previously reported data on mating in small cages in laboratory experiments [28], [29]. In addition, the results of our mating studies indicated that SGH+ males were less competitive as compared to SGH− males. This implies that releasing SGH+ G. pallidipes males would significantly decrease the effectiveness of a sterile male release programme or would increase its costs as the number of males needed would be almost double to get the same effect as compared to a programme that released SGH− males. Our observations are different from those obtained by Odindo [30] who observed no significant differences in mating performance between SGH+ and SGH− G. pallidipes. Our data also refute the speculation of Jura and Davies-Cole [11] that male flies sterilized by SGHV infection retain a competitive mating efficiency and may be useful in a sterile male release program. Our data and these of Odindo [30] and Jura and Davies-Cole [11] are most likely different because their experiments were conducted in small laboratory cages rather than field cages. The reduction in mating propensity of males with SGH+ is in agreement with the previous reported results that males of H. zea infected with the Hz-V2 virus are slower to approach healthy females and to attempt to mate compared with healthy males [31]–[33]. The reduction in mating propensity might be a result of reduction in the physical male activity i.e. flying and searching for females or negative selection by females against infected males. Odindo (1982) caught flies in the field with SGH+ that were fully engorged with blood, suggesting to him that flies with SGH+ showed normal flight and feeding behaviour under field condition [34]. However, there is no accurate data on the impact of virus infection on male flight performance. Moreover, as no data are available on the effect of virus infection on tsetse fly females' selection of males, further studies are required to understand the mechanism by which the virus infection reduces the mating propensity. Our tests were carried out with un-irradiated males, so further studies will also be needed to assess whether the addition of radiation treatment will further reduce the mating potential of SGH+ male G. pallidipes compared to SGH− males.
In addition to the lower mating propensity and success, the insemination rate was very low in females mated with SGH+ males, with more than 80% of the spermathecae empty. In comparison, more than 80% of the spermathecae of females mated with SGH− males were completely filled with sperm. These results confirm earlier data that the insemination potential of SGH+ G. pallidipes males is reduced drastically and males often were unable to inseminate females during mating, explaining the difficulties in maintaining adequate production levels in mass-reared colonies of this species [15]. The failure of males with SGH+ to inseminate females might be due to the lack of complete, functional spermatophores needed for sperm transfer as a result of short and small male accessory glands affected by the virus [9].
In a release programme, a high proportion of wild females that have mated with an SGH+ male will not have been inseminated and might have a higher inclination to remate, this time potentially with a fertile male. To test this hypothesis, the second part of the study looked at the remating potential of female G. pallidipes in relation to SGH status of their male mates. It was shown that female G. pallidipes had a very low remating frequency, irrespective of whether they had mated with a SGH− or a SGH+ male in the first mating. This accords with the results of Gillot & Langley (15) who showed in G. morsitans that inhibition of female remating was largely due to mechanical stimulation and was not dependent on the successful transfer of sperm or accessory gland factors but is in stark contrast to small cage observations of G. pallidipes [29], [35]. Jura & Davis-Cole [11] made similar observations with male G. m. morsitans, although those studies were done in small laboratory cages and not in an environment that allows mating choice. The low remating frequency of G. pallidipes females indicates again that in a sterile male release programme the first mating is very important and, hence, adequate competitiveness of the released sterile male flies is paramount.
The remating behaviour of female tsetse flies might be species-specific, as higher remating frequencies were obtained in similar remating experiments with Glossina palpalis gambiensis (GM, unpublished data). Dame and Ford [36], with chemically induced sterile sperm, demonstrated that multiple mating of female G. morsitans Westwood can occur under laboratory conditions, while males can copulate several times before depleting their sperm. Multiple mating was also reported in laboratory studies of G. palpalis palpalis Robineau-Desvoidy [37], [38] and for G. austeni [39], but refractory behaviour develops quickly in G. morsitans and it is unlikely that a female inseminated when seeking her first blood meal will permit mating to occur again when she seeks her second blood meal some days later [40]. Vreysen and Van der Vloedt [41] reported that in small laboratory cages, irradiated female G. austeni exhibited extensive multiple mating behaviour, and the ability of the irradiated flies for multiple mating decreases both with higher radiation dose (120 Gy) and treating flies later in life. For G. pallidipes, Jaenson [28] reported that it has been suggested that re-insemination of old females may take place in the field, but he mentioned that this hypothesis needs further elucidation. Recent work on G. fuscipes fuscipes has shown frequent polyandry in field samples [42].
SGH+ male G. pallidipes display a large swollen abdomen, that enables these males to be selected and separated visually [20]. A similar technique was used in our study to establish the two experimental groups with a high or low prevalence of SGH. The data indicates that the visual selection was quite efficient with 82% of males selected for the high prevalence group confirmed as being SGH+ at dissection. In the low prevalence group, only 6% of the dissected males were SGH+.
Although many tsetse species have been reported to be infected with SGHV and can display the SGH+ syndrome, only G. pallidipes has so far been shown to have a high prevalence of SGH+ in laboratory colonies, affecting colony growth and stability [6], [21]. This was epitomised by the collapse of two colonies of G. pallidipes in the Insect Pest Control Laboratory in 1987 and 2002 associated with a high prevalence of SGH syndrome The results presented in this paper clearly indicate that in addition to colony maintenance problems, releasing SGH+ G. pallidipes flies will also reduce the efficiency of a sterile male release programme and highlights again the need for an effective virus management strategy to maintain the viral load in colonies low enough that male flies can be released that are SGH−.
These studies present for the first time the impact of salivary gland hypertrophy virus infection on the mating behavior of G. pallidipes in field cages. The results support the following conclusion: (i) SGH+ males are less successful and less competitive in mating compared to SGH− males, (ii) SGH+ males did not inseminate the females when successful in mating and (iii) females mated with either SGH+ or SGH− males, with empty or full spermathecae, did not in general accept a second mating. These results also indicate the urgency of developing a virus management strategy not only to be able to effectively mass-rear G. pallidipes but to produce males with acceptable quality to be used in SIT release programmes.
Acknowledgments
We thank technical staff of the Insect Pest Control Laboratory, Seibersdorf for tsetse fly maintenance.
Funding Statement
The authors have no support or funding to report.
References
- 1.WHO (2003) Report of the Scientific Working Group meeting on African trypanosomiasis, Geneva, 4–8 June, 2001. Geneva: World Health Organization. x+169 p.
- 2. Steelman CD (1976) Effects of external and internal arthropod parasites on domestic livestock production. Annu Rev Entomol 21: 155–178. [DOI] [PubMed] [Google Scholar]
- 3.Jordan AM (1986) Trypanosomiasis control and African rural development. London: Longman. 357 p.
- 4. Aksoy S, Rio RVM (2005) Interactions among multiple genomes: Tsetse, its symbionts and trypanosomes. Insect Biochem Mol Biol 35: 691–698. [DOI] [PubMed] [Google Scholar]
- 5.Leak SGA (1998) Tsetse biology and ecology: their role in the epidemiology and control of trypanosomosis. Wallingford: CABI Publishing. xxiii+568 p.
- 6. Lietze VU, Abd-Alla AMM, Vreysen MJB, Geden CJ, Boucias DG (2010) Salivary gland hypertrophy viruses: a novel group of insect pathogenic viruses. Annu Rev Entomol 56: 63–80 doi:10.1146/annurev-ento-120709-144841. [DOI] [PubMed] [Google Scholar]
- 7. Jura WGZO, Odhiambo TR, Otieno LH, Tabu NO (1988) Gonadal lesions in virus-infected male and female tsetse, Glossina pallidipes (Diptera: Glossinidae). J Invertebr Pathol 52: 1–8. [DOI] [PubMed] [Google Scholar]
- 8. Kokwaro ED, Nyindo M, Chimtawi M (1990) Ultrastructural changes in salivary glands of tsetse, Glossina morsitans morsitans, infected with virus and rickettsia-like organisms. J Invertebr Pathol 56: 337–346. [DOI] [PubMed] [Google Scholar]
- 9. Sang RC, Jura WGZO, Otieno LH, Mwangi RW, Ogaja P (1999) The effects of a tsetse DNA virus infection on the functions of the male accessory reproductive gland in the host fly Glossina morsitans centralis (Diptera; Glossinidae). Curr Microbiol 38: 349–354. [DOI] [PubMed] [Google Scholar]
- 10. Sang RC, Jura WGZO, Otieno LH, Mwangi RW (1998) The effects of a DNA virus infection on the reproductive potential of female tsetse flies, Glossina morsitans centralis and Glossina morsitans morsitans (Diptera: Glossinidae). Mem Inst Oswaldo Cruz 93: 861–864. [DOI] [PubMed] [Google Scholar]
- 11. Jura WGZO, Davies-Cole JOA (1992) Some aspects of mating behavior of Glossina morsitans morsitans males infected with a DNA virus. Biol Control 2: 188–192. [Google Scholar]
- 12. Jura WGZO, Otieno LH, Chimtawi MMB (1989) Ultrastructural evidence for trans-ovum transmission of the DNA virus of tsetse, Glossina pallidipes (Diptera: Glossinidae). Curr Microbiol 18: 1–4. [Google Scholar]
- 13. Sang RC, Jura WGZO, Otieno LH, Ogaja P (1996) Ultrastructural changes in the milk gland of tsetse Glossina morsitans centralis (Diptera; Glissinidae) female infected by a DNA virus. J Invertebr Pathol 68: 253–259. [DOI] [PubMed] [Google Scholar]
- 14.Feldmann U (1994) Guidelines for the rearing of tsetse flies using the membrane feeding technique. In: Ochieng'-Odero JPR editor. Techniques of insect rearing for the development of integrated pest and vector management strategies. Nairobi, Kenya: ICIPE Science Press. pp. 449–471.
- 15. Abd-Alla AMM, Kariithi H, Parker AG, Robinson AS, Kiflom M, et al. (2010) Dynamics of the salivary gland hypertrophy virus in laboratory colonies of Glossina pallidipes (Diptera: Glossinidae). Virus Res 150: 103–110 doi:10.1016/j.virusres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Calkins CO, Parker AG (2005) Sterile insect quality. In: Dyck VA, Hendrichs J, Robinson AS editors. Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht: Springer. pp. 269–296.
- 17.Hendrichs JP, Vreysen MJB, Enkerlin WR, Cayol JP (2005) Strategic options in using sterile insects for area-wide integrated pest management. In: Dyck VA, Hendrichs J, Robinson AS editors. Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht, The Netherlands: Springer. pp. 563–600.
- 18.Glasgow JP (1963) The distribution and abundance of tsetse. Oxford, New York: Pergamon Press. xi+241 p.
- 19.Gooding RH, Feldmann U, Robinson AS (1997) Care and maintenance of tsetse colonies. In: Crampton JM, Beard CB, Louis C editors. The molecular biology of insect disease vectors: a methods manual. London, UK: Chapman & Hall Ltd. pp. 41–55.
- 20. Abd-Alla A, Cousserans F, Parker A, Bergoin M, Chiraz J, et al. (2009) Quantitative PCR analysis of the salivary gland hypertrophy virus (GpSGHV) in a laboratory colony of Glossina pallidipes . Virus Res 139: 48–53 doi:10.1016/j.virusres.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 21. Abd-Alla A, Bossin H, Cousserans F, Parker A, Bergoin M, et al. (2007) Development of a non-destructive PCR method for detection of the salivary gland hypertrophy virus (SGHV) in tsetse flies. J Virol Methods 139: 143–149 Available: http://dx.doi.org/10.1016/j.jviromet.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 22. Mutika GN, Opiyo E, Robinson AS (2001) Assessing mating performance of male Glossina pallidipes (Diptera: Glossinidae) using a walk-in field cage. Bull Entomol Res 91: 281–287. [DOI] [PubMed] [Google Scholar]
- 23.Pollock JN (1982) Training Manual for Tsetse Control Personnel. Vol. 1: Tsetse biology, systematics and distribution, techniques. Rome: Food and agricultural Organization of the United Nations. x+280 p.
- 24.Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. New York: Freeman and Company. xiii+887 p.
- 25.Minitab Inc. (2000) Minitab Release 13.32 Minitab Inc. State College, PA.:
- 26.Thornhill R, Alcock J (1983) The evolution of insect mating systems. Lincoln, NE: iUniverse.com. Inc. ix+547 p.
- 27. Hendrichs J, Robinson AS, Cayol JP, Enkerlin WR (2002) Medfly areawide Sterile Insect Technique programmes for prevention, suppression or eradication: the importance of mating behavior studies. Fla Entomol 85: 1–13. [Google Scholar]
- 28. Jaenson TGT (1978) Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes . Trans R Soc Trop Med Hyg 72: 234–238. [DOI] [PubMed] [Google Scholar]
- 29. Jaenson TGT (1986) Sex ratio distortion and reduced lifespan of Glossina pallidipes infected with the virus causing salivary gland hyperplasia. Entomol Exp Appl 41: 256–271. [Google Scholar]
- 30. Odindo MO (1988) Glossina pallidipes virus: its potential for use in biological control of tsetse. Insect Sci Appl 9: 399–403. [Google Scholar]
- 31. Burand JP, Tan W, Kim W, Nojima S, Roelofs W (2005) Infection with the insect virus Hz-2v alters mating behavior and pheromone production in female Helicoverpa zea moths. J Insect Sci 5 6. insectscience.org/5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burand JP, Tan W (2006) Male preference and mating behaviour of male Helicoverpa zea (Lepidoptera: Noctuidae) infected with the sexually transmitted insect virus Hz-2V. Ann Entomol Soc Am 99: 969–973. [Google Scholar]
- 33. Burand JP (2009) The sexually transmitted insect virus, Hz-2V. Virol Sin 24: 428–435 DOI: 10.1007/s12250-009-3046-z. [Google Scholar]
- 34. Odindo MO (1982) Incidence of salivary gland hypertrophy in field populations of the tsetse Glossina pallidipes on the South Kenya coast. Insect Sci Appl 3: 59–64. [Google Scholar]
- 35. Leegwater-van der Linden ME, Tiggelman EPM (1984) Multiple mating and inseminating potential of Glossina pallidipes . Entomol Exp Appl 35: 283–294. [Google Scholar]
- 36. Dame DA, Ford HR (1968) Multiple mating of Glossina morsitans Westw. and its potential effect on the sterile male technique. Bull Entomol Res 58: 213–219. [Google Scholar]
- 37. Jordan AM (1958) The mating behaviour of females of Glossina palpalis (R.-D.). Bull Entomol Res 49: 35–43. [Google Scholar]
- 38. Van Der Vloedt AMV, Taher M (1978) Effect of gamma radiation on the tsetse fly Glossina palpalis palpalis (Rob.-Desv.) (Diptera:Glossinidae) with observations on the reproductive biology. Int J Appl Radiat Isot 29: 713–716. [DOI] [PubMed] [Google Scholar]
- 39. Curtis CF (1968) Radiation sterilization and the effect of multiple mating of females in Glossina austeni . J Insect Physiol 14: 1365–1380. [Google Scholar]
- 40. Gillott C, Langley PA (1981) The control of receptivity and ovulation in the tsetse fly, Glossina morsitans . Physiol Entomol 6: 269–281. [Google Scholar]
- 41. Vreysen MJB, Van der Vloedt AMV (1992) The use of gamma irradiated Glossina austeni females as sentinel insects for entomological monitoring in tsetse control programmes. Rev Élev Méd Vét Pays Trop 45: 303–309. [Google Scholar]
- 42. Bonomi A, Bassetti F, Gabrieli P, Beadell J, Falchetto M, et al. (2011) Polyandry is a common event in wild populations of the tsetse fly Glossina fuscipes fuscipes and may impact population reduction measures. PLoS Negl Trop Dis 5: e1190 doi:10.1371/journal.pntd.0001190. [DOI] [PMC free article] [PubMed] [Google Scholar]