The roles of BMP and FGF during the transition of proliferating lens epithelial cells to differentiated primary lens fiber cells are examined. The results show that proliferation, cell cycle exit, and early differentiation of primary lens fiber cells are regulated by counterbalancing BMP and FGF signals.
Abstract
In embryonic and adult lenses, a balance of cell proliferation, cell cycle exit, and differentiation is necessary to maintain physical function. The molecular mechanisms regulating the transition of proliferating lens epithelial cells to differentiated primary lens fiber cells are poorly characterized. To investigate this question, we used gain- and loss-of-function analyses to modulate fibroblast growth factor (FGF) and/or bone morphogenetic protein (BMP) signals in chick lens/retina explants. Here we show that FGF activity plays a key role for proliferation independent of BMP signals. Moreover, a balance of FGF and BMP signals regulates cell cycle exit and the expression of Ccdc80 (also called Equarin), which is expressed at sites where differentiation of lens fiber cells occurs. BMP activity promotes cell cycle exit and induces Equarin expression in an FGF-dependent manner. In contrast, FGF activity is required but not sufficient to induce cell cycle exit or Equarin expression. Furthermore, our results show that in the absence of BMP activity, lens cells have increased cell cycle length or are arrested in the cell cycle, which leads to decreased cell cycle exit. Taken together, these findings suggest that proliferation, cell cycle exit, and early differentiation of primary lens fiber cells are regulated by counterbalancing BMP and FGF signals.
INTRODUCTION
A balance between proliferation and differentiation is required for proper formation of various tissues and organs. During early lens morphogenesis in vertebrates, the majority of presumptive lens cells are actively proliferating. At these stages, lens development is revealed by the thickening of the nonneural ectoderm into the lens placode, in the vicinity of the optic vesicle. At the placodal stage, lens development involves up-regulation of L-Maf expression in chicks and c-Maf expression in mice (Ogino and Yasuda, 1998; Kawauchi et al., 1999). Somewhat later, the lens placode invaginates and subsequently forms the lens vesicle. By the lens vesicle stage, posterior lens cells exit from the cell cycle and differentiate into primary lens fiber cells, whereas the anteriorly situated lens epithelial cells retain a high proliferative capacity (Modak et al., 1968). The first step of primary lens fiber differentiation involves up-regulation of crystallin proteins, where δ-crystallin is the first crystallin protein up-regulated in chicks (Shinohara and Piatigorsky, 1976; Bower et al., 1983; Reza and Yasuda, 2004). During later stages of development, the proliferation rate of lens cells gradually declines, but it never ceases completely, and secondary fiber cells will be generated throughout life. Cells that exit the cell cycle and start to differentiate into secondary lens fiber cells becomes restricted to a small region above the lens equator, known as the germinative zone or equatorial region (reviewed in Lovicu and McAvoy, 2005; Robinson, 2006; West-Mays et al., 2010; Gunhaga, 2011). Thus, the lens provides a good model system in which to study proliferation, cell cycle exit, and differentiation (reviewed in Wride, 1996).
For several decades, fibroblast growth factor (FGF) signals have been known to regulate different aspects of lens development, and retina cells are one source of FGF signals known to affect lens fiber cell differentiation (reviewed in Gunhaga, 2011; Lovicu et al., 2011). In lens epithelial cell assays in both rodents and chicks, FGF signals induce differentiation of cells resembling secondary lens fiber cells (McAvoy and Chamberlain, 1989; Le and Musil, 2001). In addition, based on the same lens epithelial cell assay, low levels of FGF activity are found to induce cell proliferation, whereas sequentially higher doses promote fiber cell differentiation (McAvoy and Chamberlain, 1989). It is not surprising that the vitreous humor that bathes the lens fiber cells includes FGF proteins (Caruelle et al., 1989; Schulz et al., 1993). Consistently, modulations of FGF signaling disturb secondary lens fiber cell differentiation in vivo. Overexpression of FGFs lead to ectopic fiber differentiation in the epithelial compartment (Robinson et al., 1995; Lovicu and Overbeek, 1998), and deletion of several FGF receptors results in failure of fiber differentiation (Robinson, 2006; Zhao et al., 2008). Whether FGF activity plays a similar role during earlier stages of lens development and primary fiber cell differentiation remains to be determined.
Bone morphogenetic protein (BMP) signals have also been suggested to play important roles in lens development. At gastrula stages, lens placodal progenitors situated at the rostral neural plate border are exposed to BMP activity (Chapman et al., 2002; Faure et al., 2002). In agreement with this, in mice, chicks, and zebrafish, the generation of lens placodal cells and up-regulation of early lens markers depends on BMP signals (Furuta and Hogan, 1998; Wawersik et al., 1999; Sjodal et al., 2007; French et al., 2009; Rajagopal et al., 2009). Lens progenitors require sustained exposure of BMP signals to maintain lens character, in part by inhibiting the generation of olfactory placodal cells (Sjodal et al., 2007), and also to induce the early lens marker L-Maf (Pandit et al., 2011). After L-Maf onset, the subsequent up-regulation of δ-crystallin in primary lens fiber cells is independent of BMP signals (Pandit et al., 2011). Further studies are required to examine whether BMP and FGF interact to regulate proliferation, cell cycle exit, and/or primary lens fiber cell differentiation.
Ccdc80 is a secreted, soluble protein that contains a coiled-coil domain (also known as Equarin, DRO1, and URB), which is expressed in various cell types (Mu et al., 2003; Liu et al., 2004; Tremblay et al., 2009). In the eye region, the name Equarin has been used (Mu et al., 2003), and we will therefore use this term throughout this article. Equarin is up-regulated in the early-formed lens vesicle, just before the generation of the first primary lens fiber cells, and is later restricted to the lens equatorial region (Mu et al., 2003). The molecular regulation of Equarin and its role in lens development remain to be defined.
In this study, we examined the roles of BMP and FGF and a possible interaction between BMP and FGF signals during the early differentiation of primary lens fiber cells. To accomplish this, we performed gain- and loss-of-function experiments in chick explant assays of lens fiber cell differentiation. In addition, we identified Equarin as a molecular marker restricted to regions of primary fiber cell differentiation and examined how Equarin is regulated by BMP and FGF activity. Briefly, our results show that FGF activity is sufficient to promote proliferation independent of BMP activity. In contrast, both BMP and FGF signals are required for cell cycle exit and for Equarin expression. For these processes, BMP activity is the most important pathway; it promotes cell cycle exit and induces Equarin expression in lens cells but in an FGF-dependent manner. In summary, these results provide evidence that BMP and FGF signals interact to regulate proliferation and cell cycle exit coupled to induction of Equarin expression in lens cells.
RESULTS
Equarin expression is located in the p27-positive region of the lens
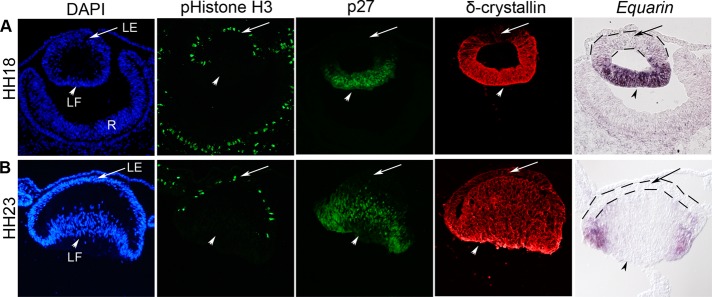
To better understand the process of cell cycle exit in the lens and lens fiber cell differentiation, we first examined whether Equarin can be used as a molecular marker for this purpose. We analyzed Equarin expression in Hamilton and Hamburger stage (HH) 18 and 23 chick embryos (Hamburger and Hamilton, 1951) and compared this with the expression of δ-crystallin, which is expressed in lens fiber cells that constitute the major part of the transparent lens (Sullivan et al., 1998), p27 and p57, two inhibitors of G1 cyclin-Cdk protein kinase activity, which enables cells to leave the cell cycle (Toyoshima and Hunter, 1994; Dyer and Cepko, 2000), and phosphorylated histone H3 (pHistone H3), which labels proliferating cells in late G2 and M phases, indicative of mitotic cells (Sholl-Franco et al., 2010).
At HH18, Equarin and p27 are expressed in most of the posterior lens compartment, which will develop into primary lens fiber cells, indicated by the expression of δ-crystallin (Figure 1A). By HH23, the expression of Equarin is restricted to the equatorial region, where lens epithelial cells leave the cell cycle and differentiate into lens fiber cells (Figure 1B). At this stage, p27 is expressed both in the equatorial region and in the primary δ-crystallin+ fiber cells (Figure 1B). In contrast, p57 is not expressed at HH18 or at HH23 in the chick lens (Supplemental Figure S1), indicating that p27 is critical for cell cycle exit in the chick lens at these stages. At both HH18 and HH23, pHistone H3+ mitotic cells are detected in the anterior lens epithelium, where no Equarin expression is detected (Figure 1, A and B). Taken together, these expression patterns suggest that Equarin is confined to cells instructed to leave the cell cycle and can be used as a marker to study cell cycle exit and early differentiation of lens fiber cells.
FIGURE 1:
Equarin is expressed in the p27-positive region of the lens at HH18 and HH23. (A) At HH18, Equarin and p27 are expressed in most of the posterior lens compartment, whereas pHistone H3+ cells are detected in the anterior lens epithelium. At this stage, δ-crystallin is expressed in the majority of cells in the lens vesicle, except the most anterior part. (B) By HH23, Equarin is expressed in the equatorial region, whereas p27 is expressed both in the equatorial region and in the young primary δ-crystallin+ fiber cells. pHistone H3+ cells are detected in the anterior lens epithelium. LE, lens epithelium; LF, lens fiber cells; R, retina.
FGF signals promote both mitosis and cell cycle exit of lens cells
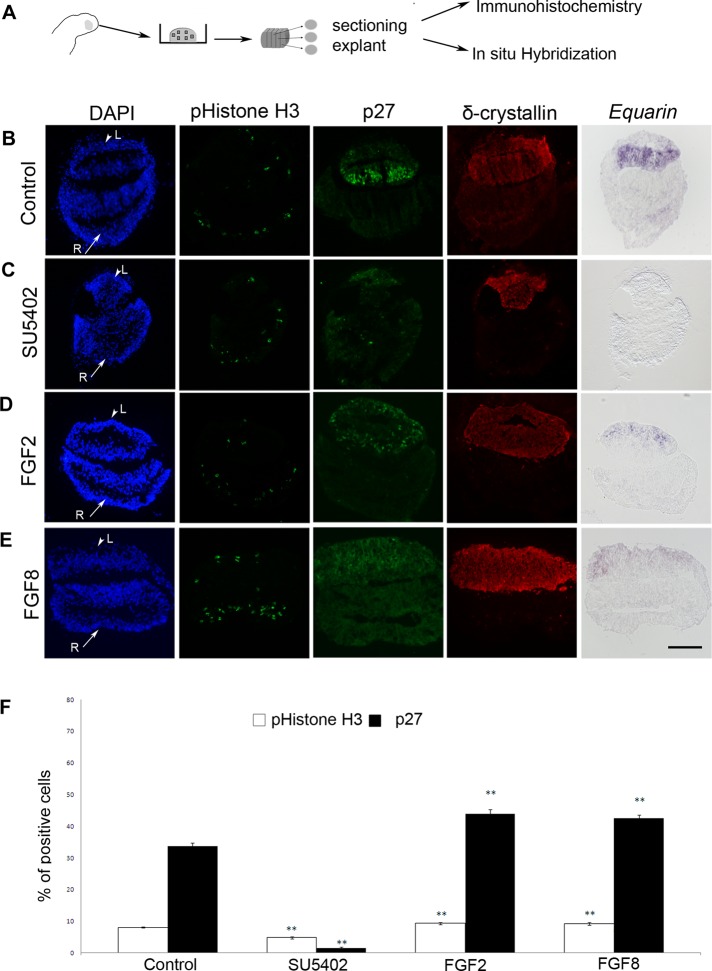
BMP and FGF signals have been shown to influence the development of the lens (reviewed in Gunhaga, 2011; Lovicu et al., 2011), and these two pathways have been suggested to interact during fiber cell differentiation (Boswell et al., 2008b). To analyze in more detail whether BMP and/or FGF signals regulate the cell cycle and early differentiation of primary lens fiber cells, we established an explant assay of lens cell differentiation in which the BMP and/or FGF pathways were modulated. HH13 prospective lens/retina (LR) chick explants were cultured for 26 h, which in intact chick embryos would correspond to approximately HH18, alone or together with factors that modulate the BMP and/or FGF pathways (Figure 2A). Then the explants were processed, serially sectioned, and analyzed by in situ hybridization and immunohistochemistry (Figure 2A). The lens region of the explants was defined by the expression of the lens-specific protein δ-crystallin (Figure 2, B–E).
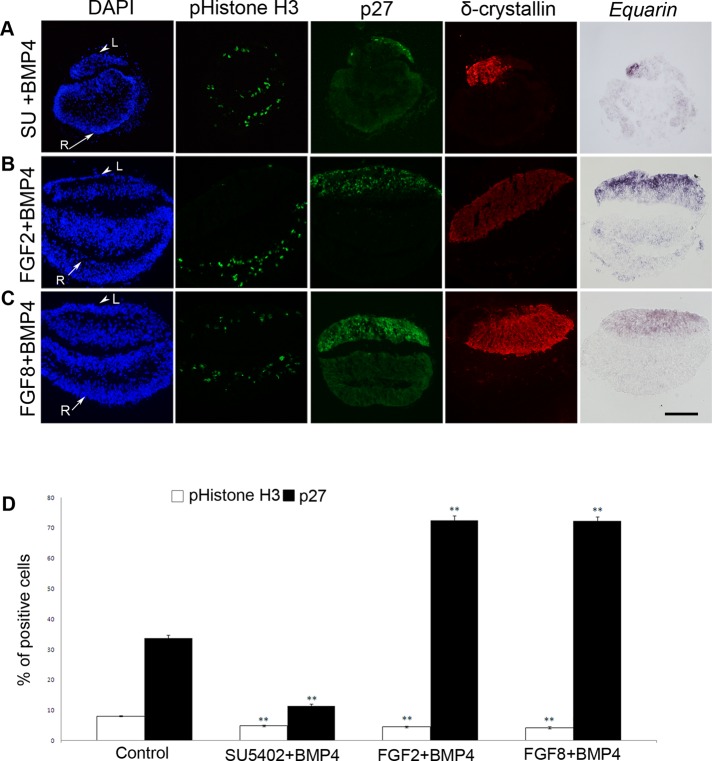
FIGURE 2:
FGF signals promote proliferation and cell cycle exit of lens cells. (A) Schematic drawing depicting the explant method. HH13 LR explants were dissected and cultured in vitro to the developmental equivalent time point of HH18. The explants were then fixed, frozen, and sectioned. (B–E) Arrowheads indicate prospective lens cells (L) positive for δ-crystallin, and arrows indicate prospective retinal cells (R). (B) LR explants generated Equarin+ and p27+ cells in a restricted domain of the lens compartment, whereas pHistone H3+ cells were detected in separate regions of the explants. (C) In LR explants cultured in the presence of SU5402 the generation of Equarin+ cells was inhibited, and p27+ and pHistone H3+ cells were reduced. (D, E) In LR explants cultured together with FGF2 (D) or FGF8 (E) the generation of p27+ and pHistone H3+ cells was increased, whereas Equarin expression was reduced. (F) Graph showing the percentage of pHistone H3+ and p27+ cells in the lens compartment. Error bars represent mean ± SEM. **p < 0.02. Scale bar, 100 μm.
LR explants cultured alone (n = 54) generated Equarin+ and p27+ cells in a restricted domain, whereas pHistone H3+ cells were detected in separate regions of the explants (Figure 2B). In contrast, in LR explants cultured in the presence of SU5402 (5μM; n = 42), an inhibitor of FGF receptor signaling (Mohammadi et al., 1997), the generation of Equarin+ cells was completely inhibited, and the numbers of both p27+ and pHistone H3+ cells were significantly reduced (Figure 2, C and F). Thus, these results suggest that FGF activity is required for mitosis, as well as for cell cycle exit and the expression of Equarin in lens cells.
Next we explored whether increased FGF activity promotes mitosis and/or cell cycle exit of lens cells, by exposing LR explants to FGF2 (50–250 ng/ml) or FGF8 (50–250 ng/ml). In the presence of 250 ng/ml FGF2 (n = 42) or FGF8 (n = 18), the numbers of both p27+ and pHistone H3+ cells were significantly increased, whereas Equarin expression was reduced compared with control LR explants (Figure 2, D–F). At lower levels of FGF2 or FGF8 (50 ng/ml; n = 12 in each group) the results were not as homogeneous; some explants exhibited the same expression patterns as control explants and some explants mimicked the results we observed using 250 ng/ml FGF2 or FGF8, whereas in other explants the generation of Equarin+, p27+, and pHistone H3+ cells was down-regulated (Supplemental Figure S2, B–E). Thus, we cannot observe that different concentrations of FGF2 or FGF8 control proliferation versus differentiation during primary lens fiber cell differentiation. Nevertheless, a threshold level of FGF activity (250 ng/ml) resulted in an increased number of mitotic cells and cells exiting the cell cycle. However, the reduced expression of Equarin during these conditions indicated that correct levels of FGF activity are crucial for the generation of Equarin+ cells. These results also suggest that other signaling pathways are important for the induction of Equarin expression.
BMP activity promotes cell cycle exit and Equarin expression in lens cells
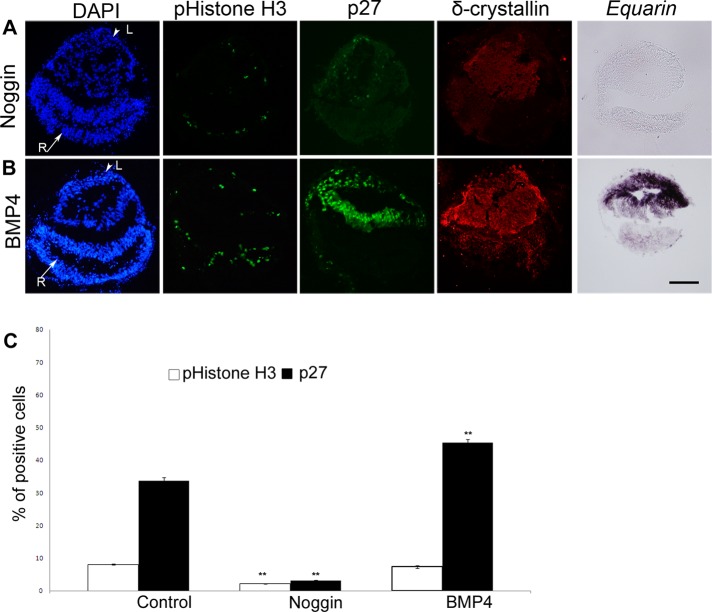
We continued to examine the role of BMP signals during lens cell proliferation and cell cycle exit of lens cells by culturing LR explants in the presence of Noggin, a known BMP inhibitor (Lamb et al., 1993). In LR explants cultured together with Noggin (n = 48), the generation of Equarin+ cells was completely inhibited and the generation of both p27+ and pHistone H3+ cells was significantly reduced (Figure 3, A and C). Thus, at HH13, BMP signals are required for mitosis, as well as for the ability of cells to leave the cell cycle and for the generation of Equarin+ cells.
FIGURE 3:
BMP activity promotes cell cycle exit and Equarin expression in lens cells. (A, B) Cultured LR explants. Arrowheads indicate prospective lens cells (L) positive for δ-crystallin, and arrows indicate prospective retinal cells (R). (A) In LR explants cultured in the presence of Noggin the generation of Equarin+ cells was inhibited, and p27+ and pHistone H3+ cells were reduced. (B) In LR explants cultured together with BMP4 the expression domain of Equarin+ cells was expanded and the generation of p27+ cells was increased, whereas the number of pHistone H3+ cells was unaffected. (C) Graph showing the percentage of pHistone H3+ and p27+ cells in the lens compartment. Error bars represent mean ± SEM. **p < 0.02. Scale bar, 100 μm.
To analyze whether at HH13 elevated levels of BMP signals promote the generation of Equarin+ cells and/or affect the cell cycle, we exposed LR explants to BMP4 (3.5 ng/ml, n = 15; 35 ng/ml, n = 30). Under these conditions, the expression domain of Equarin+ cells was expanded, and the generation of p27+ cells was significantly increased, whereas the number of pHistone H3+ cells was similar to that in the control LR explants (Figure 3, B and C, and Supplemental Figure S2, F and G). Thus, additional BMP signals in lens cells promote cell cycle exit and Equarin expression. These results provide evidence that at early stages of lens development, BMP activity is both required and sufficient for promoting cell cycle exit and for the generation of Equarin+ lens cells.
BMP and FGF signals are required for the induction of Equarin
Suppression of either BMP or FGF signals inhibits the generation of Equarin+ lens cells. To directly determine whether FGF and/or BMP signals are required for the induction of Equarin expression, we cultured LR explants alone (n = 13) or in the presence of SU5402 (n = 13) or Noggin (n = 13) for only 12 h, which in intact chick embryos would correspond approximately to the time of onset for Equarin.
After 12 h of culture, Equarin was up-regulated in control LR explants (Figure 4A), whereas in the absence of FGF or BMP signals, the induction of Equarin expression was inhibited (Figure 4, B and C). These results provide evidence that both FGF and BMP signals are required for the induction of Equarin expression in lens cells.
FIGURE 4:
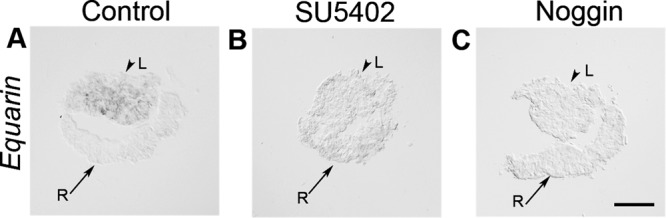
FGF and BMP signals are required for the induction of Equarin. (A–C) LR explants cultured for 12 h. (A) LR explants cultured alone generated Equarin+ cells. (B, C) In the presence of SU5402 (B) or Noggin (C) the generation of Equarin+ cells was inhibited. Scale bar, 100 μm. L, lens; R, retina.
BMP activity is critical for cell cycle exit but not for mitosis in lens cells
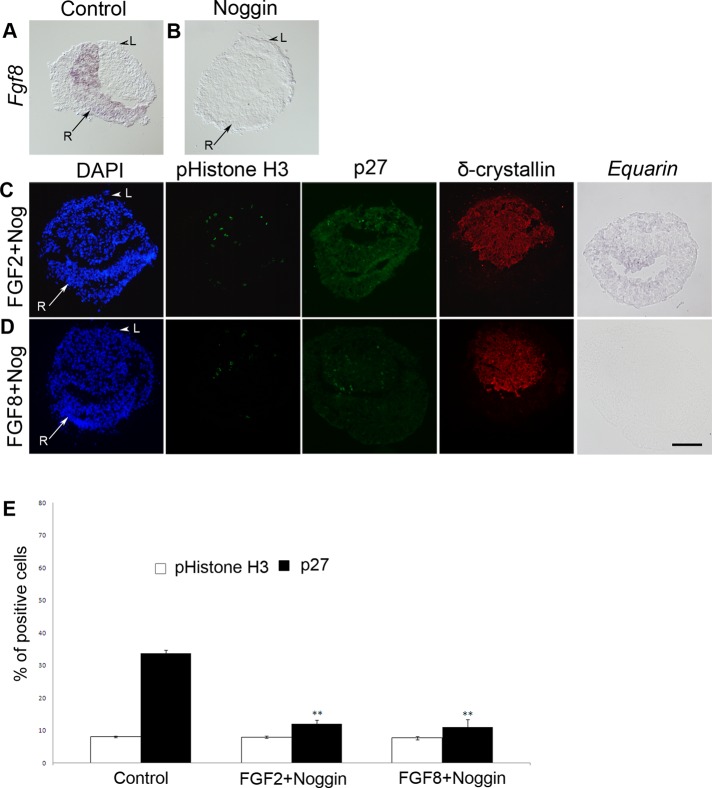
Although our results show that BMP inhibition suppresses mitosis in lens cells, elevated BMP activity does not affect the number of mitotic cells. On the other hand, our results suggest that increased FGF signals promote mitosis in the lens. To evaluate whether decreased mitosis after BMP inhibition could be a consequence of disturbed FGF signaling, we analyzed the expression of Fgf8 in our cultured LR explants. During control conditions, Fgf8 is expressed in the retinal compartment of the explants (n = 10; Figure 5A). In contrast, in LR explants cultured together with Noggin (n = 10), no Fgf8 expression is detected (Figure 5B). Thus, after BMP inhibition, Fgf8 expression is suppressed in retinal cells, indicating that the reduced proliferation of lens cells observed after BMP inhibition is caused by decreased FGF activity.
FIGURE 5:
BMP activity is critical for cell cycle exit but not for proliferation in lens cells. (A) Fgf8 is expressed in the retinal compartment of the cultured LR explants. (B) In LR explants cultured in the presence of Noggin the expression of Fgf8 is suppressed. (A–D) Arrowheads indicate prospective lens cells (L) positive for δ-crystallin, and arrows indicate prospective retinal cells (R) in cultured LR explants. (C, D) In LR explants cultured together with Noggin and FGF2 or FGF8, the generation of pHistone H3+ cells was unaffected, whereas p27+ cells were reduced and Equarin expression was inhibited. (E) Graph showing the percentage of pHistone H3+ and p27+ cells in the lens compartment. Error bars represent mean ± SEM. **p < 0.02. Scale bar, 100 μm.
To test whether exogenous FGF activity can rescue mitosis in the absence of BMP signals, we cultured LR explants in the presence of FGF2 (250 ng/ml) and Noggin (n = 30). In the presence of FGF activity and absence of BMP signals, the numbers of pHistone H3+ mitotic cells were restored to control levels (Figure 5, C and E). In addition, a few pHistone H3+ cells were detected in the δ-crystallin+ region of the explants, indicating that during these conditions mitotic cells expand outside of the lens epithelial compartment (Figure 5C). Furthermore, the number of p27+ cells was significantly reduced, and the expression of Equarin was blocked (Figure 5, C and E). Similar results were observed when using FGF8 (Figure 5, D and E). Thus, in the absence of BMP activity, FGF signals restore the levels of mitosis but are not sufficient to rescue cell cycle exit or Equarin expression in lens cells. In addition, when compared with LR explants cultured together with FGF2 or FGF8 alone (Figure 2F), the inhibition of BMP activity in LR explants cultured together with Noggin and FGF2 or FGF8 dramatically reduced the levels of cell cycle exit, as indicated by the percentage of p27+ cells (Figure 5E). Subsequently, even in the presence of exogenous FGF, BMP activity appears to be critical for proper cell cycle exit and Equarin expression in lens cells.
FGF and BMP interaction promotes cell cycle exit of lens cells
Next we analyzed whether BMP activity can rescue cell cycle exit in the absence of FGF signals, by culturing LR explants in the presence of BMP4 (35 ng/ml) in combination with SU5402 (5 μM; n = 30). Under these conditions, the number of p27+ cells was still significantly reduced compared with control LR explants, as was the number of pHistone H3+ mitotic cells (Figure 6, A and D). Compared to LR explants cultured together with the FGF inhibitor (Figure 2F), activation of the BMP pathway led to a minor increase in the levels of cell cycle exit (Figure 6D). However, in the absence of FGF signals, BMP activity was sufficient to rescue parts of the Equarin expression, although in an altered pattern compared with control explants (Figure 6A). These results suggest that even in the presence of elevated levels of BMP activity, FGF signals are required for proper cell cycle exit of lens cells, which indicates that FGF and BMP pathways act together to promote cell cycle exit of lens cells.
FIGURE 6:
FGF and BMP interaction promotes cell cycle exit of lens cells. (A–C) Arrowheads indicate prospective lens cells (L) positive for δ-crystallin, and arrows indicate prospective retinal cells (R) in cultured LR explants. (A) In LR explants cultured together with BMP4 and SU5402 the generation of pHistone H3+ and p27+ cells was reduced, and Equarin expression was reduced and had an altered pattern. (B, C) In LR explants cultured together with BMP4 and FGF2 (B) or FGF8 (C) the generation of p27+ cells and the expression of Equarin were increased, whereas pHistone H3+ cells were reduced. (D) Graph showing the percentage of pHistone H3+ and p27+ cells in the lens compartment. Error bars represent mean ± SEM. **p < 0.02. Scale bar, 100 μm.
To further assess this issue, we cultured LR explants in the presence of both FGF2 (250 ng/ml) and BMP4 (35 ng/ml; n = 30). Under these conditions, the number of p27+ cells was significantly increased in comparison to both control LR explants (Figure 6, B and D) and LR explants cultured in the presence of FGF2, FGF8, or BMP4 alone (Figures 2F and 3C). In addition, Equarin expression was extended in all compartments of the cultured lens (Figure 6B). The number of pHistone H3+ cells was reduced (Figure 6, B and D) compared with LR control explants and LR explants cultured together with BMP4, FGF2, or FGF8 alone (Figures 2F and 3C). Similar results were observed when using FGF8 (Figure 6C). Taken together, these results indicate that FGF activity is the key pathway to regulate mitosis in the developing lens, whereas an interaction between FGF and BMP signals promote cell cycle exit, expression of Equarin, and early differentiation of lens cells.
Lens cells are halted in the cell cycle in the absence of BMP activity
Our results suggest that FGF and BMP signals act in parallel to promote cell cycle exit of lens cells and that a balance of FGF and BMP signals is required for this process. To further address how these two pathways affect the progression of the cell cycle in developing lens cells, we performed bromodeoxyuridine (BrdU) experiments. BrdU is incorporated in proliferating cells in the S phase of the cell cycle and provides detection of active DNA synthesis; it can be used in addition to pHistone H3, which labels mitotic cells, and p27 to mark cell cycle exit. During the last 20 min of the 26-h culture period, BrdU was added to the LR explants cultured alone or together with different factors modulating the BMP and/or FGF pathways.
In LR explants cultured together with Noggin (n = 24), a significant increase in BrdU incorporation was detected, whereas pHistone H3+ and p27+ cells were decreased compared with control LR explants (Figure 7). These findings indicate that lens cells have an increased S phase length or are arrested in the early G2 phase, thereby explaining the decreased level of cell cycle exit. Consistently, in LR explants cultured together with BMP4 (35 ng/ml; n = 24), the level of incorporated BrdU was significantly decreased compared with control LR explants (Figure 7). At the same time, the number of pHistone H3+ cells was unaffected, whereas the generation of p27+ cells was increased. These results highlight that during early development of the lens, BMP activity promotes cell cycle exit, in part by affecting the length of the S phase for proliferating lens cells.
FIGURE 7:
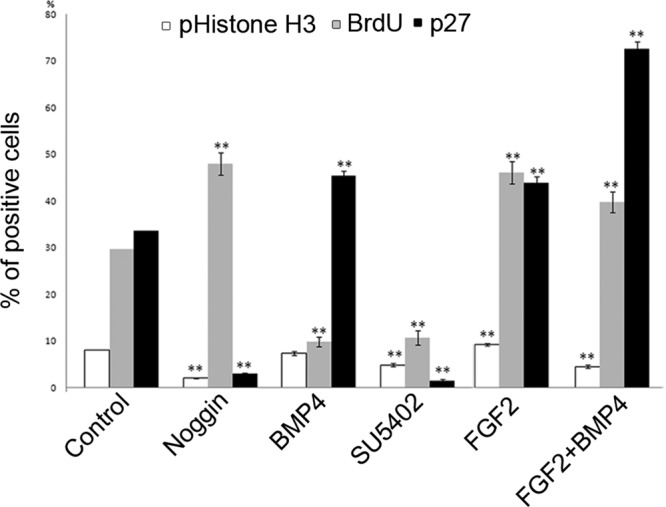
FGF signals promote and BMP activity decrease BrdU incorporation in lens cells. LR explants cultured alone or together with different factors modulating the BMP and/or FGF pathways. Graph showing the percentage of pHistone H3+, p27+, and BrdU+ cells in the lens compartment. Error bars represent mean ± SEM. **p < 0.02.
FGF signals increase BrdU incorporation in lens cells
Next we explored how FGF signals affect BrdU incorporation in cultured lens cells. In LR explants cultured together with SU5402 (5 μM; n = 24), both the levels of incorporated BrdU and the number of pHistone H3+ cells were significantly decreased compared with control LR explants (Figure 7). Consistently, in the presence of FGF2 (250 ng/ml), the numbers of BrdU+ cells and pHistone H3+ cells were significantly increased in LR explants (Figure 7), supporting our results that FGF activity plays an important role in proliferation of lens cells.
We also analyzed how cooperation of FGF and BMP signals affects the progression of the cell cycle in lens cells, by culturing LR explants together with FGF2 (250 ng/ml) and BMP4 (35 ng/ml; n = 24). Under these conditions, the number of BrdU+ cells was significantly increased compared with LR explants cultured in the presence of BMP4 alone (Figure 7), which supports and explains the high number of p27+ cells exiting the cell cycle. Thus, FGF is the most important signaling pathway to promote cell proliferation, whereas BMP and FGF activity interact during cell cycle exit in early lens cells.
DISCUSSION
The BMP and FGF signaling pathways are known to regulate developmental processes during lens formation by regulating proliferation, differentiation, and cell fate decisions. In this study, our results indicate that FGF and BMP signals play key roles in proliferation, cell cycle exit, and Equarin expression in the lens. In addition, our results provide evidence that the onset of Equarin expression in the lens is restricted to cells that are committed to leave the cell cycle and that this is regulated by both BMP and FGF activity. Taken together, these findings suggest that proliferation, cell cycle exit, and early differentiation of primary lens fiber cells are regulated by counterbalancing BMP and FGF signals.
Through comparative immunohistochemistry studies, we show that Equarin (Ccdc80) expression is restricted to the p27-positive region of the lens, indicative of cells exiting the cell cycle. In mice, p27 and p57 function redundantly to control cell cycle exit and differentiation of lens fiber cells (Zhang et al., 1998). However, our results show that p57 is not expressed in the chick lens at early lens vesicle stages, thereby indicating that p27 is critical for cell cycle exit of chick lens cells at these stages. Moreover, our results suggest that decreased or increased expression of Equarin is coupled to down- and up-regulation of p27, respectively. These results indicate that Equarin is important for cell cycle exit and early differentiation of primary lens fiber cells. Consistently, during adipocyte differentiation in preadipocyte cell lines, the Ccdc80 mRNA expression was increased 10-fold before the initiation of differentiation, followed by subsequent reduction. Moreover, inhibition of Ccdc80 by RNA interference in this cell assay suppresses adipocyte differentiation (Tremblay et al., 2009). It was also suggested that Ccdc80 is down-regulated during osteoblast differentiation of bone marrow stromal cells (Liu et al., 2004). Taken together, these findings suggest that Equarin might act as a general modulator of cell differentiation in various cell types.
Our results indicate that the onset of Equarin requires both FGF and BMP activity. Moreover, BMP activity is sufficient to induce Equarin expression even in the absence of FGF signals, whereas FGF activity is not sufficient to induce ectopic Equarin expression. Thus, our results suggest that BMP activity induces Equarin expression, whereas FGF signals play a role in restricting the expression of Equarin to the equatorial region of the lens. In correlation with this, the expression of gap junction–mediated intracellular coupling (GJIC), located at the equatorial region of the lens, is also regulated by FGF and BMP activity in a lens cell culture assay (Boswell et al., 2008a). In support of our results, GJIC expression can be induced by BMP activity in the absence of FGF signals (Boswell et al., 2008a). These results point to a common regulatory mechanism in which BMP activity is critical for the onset of markers in the equatorial region of the lens.
Crystallin expression is associated with lens fiber differentiation (Wistow and Piatigorsky, 1988), in which δ-crystallin is the first crystallin protein up-regulated in chicks (Shinohara and Piatigorsky, 1976; Bower et al., 1983). We previously showed that after L-Maf up-regulation at HH13, the onset of δ-crystallin expression is BMP independent (Pandit et al., 2011), which also is confirmed in this study. Our results now extend this knowledge and show that inhibition of BMP activity at this stage disturbs cell cycle exit and blocks Equarin expression. In addition, after inhibition of FGF signals in lens explants, cell cycle exit and the generation of Equarin-positive cells are suppressed, whereas δ-crystallin expression can still be detected. Consistently, δ-crystallin expression can be detected in late HH13/early HH14 lens placodes, when most lens cells are in a proliferative state (Reza and Yasuda, 2004). These results indicate that after L-Maf up-regulation, δ-crystallin can be induced in lens cells regardless of cell cycle exit, whereas Equarin expression is restricted to cells that are committed to leave the cell cycle. In contrast, in a secondary lens fiber cell differentiation assay using lens epithelial cell cultures, inhibition of BMP activity resulted in reduced δ-crystallin synthesis (Boswell et al., 2008b). Thus, these results suggest that the mechanism of δ-crystallin up-regulation during primary and secondary fiber differentiation might be slightly different or that in our assay the presence of retinal cells promotes δ-crystallin expression in lens cells even in the absence of BMP or FGF signals.
In chicks and mice, BMP and FGF signals have been shown to play important roles during lens induction (Faber et al., 2001; Sjodal et al., 2007). A recent study focused on the generation of lens progenitor cells from human embryonic stem (ES) cells presented data that after neural differentiation of human ES cells, coactivation of BMP and FGF signals stimulated formation of lens progenitor cells (Yang et al., 2010). In this study we provide evidence that FGF activity is the key pathway to regulate proliferation of cells in the early-developing lens, whereas interaction between FGF and BMP signals promotes cell cycle exit and early differentiation of primary lens fiber cells. This is in agreement with many studies focusing on either BMP or FGF signals, which provided evidence that both BMP and FGF signals are required for proper development of the lens (Furuta and Hogan, 1998; Wawersik et al., 1999; Robinson, 2006; Sjodal et al., 2007; Zhao et al., 2008; French et al., 2009; Loren et al., 2009; Rajagopal et al., 2009; Pandit et al., 2011). Now our results suggest that an interaction between FGF and BMP signals is critical for cell cycle regulation during early lens development. Our results show that inhibition of either FGF or BMP activity suppresses cell cycle exit and blocks Equarin expression in lens cells, indicating that a balance of FGF and BMP signals is required for proper cell cycle exit and early differentiation of primary lens fiber cells.
Of interest, by use of a lens epithelial cell assay in chicks that mimics secondary fiber cell differentiation, it has been shown that BMP signals induce markers of lens fiber cells in the same manner as FGF activity or vitreous conditioned medium (Boswell et al., 2008b). Furthermore, in this lens epithelial cell assay, inhibition of BMP activity suppresses the FGF-induced secondary fiber differentiation (Boswell et al., 2008b). Taken together, these findings suggest that the same or similar mechanisms involving cross-talk between FGF and BMP signals is regulating primary and secondary fiber cell differentiation. Moreover, our finding that FGF signals are required for both proliferation and differentiation of lens cells during early stages of development had previously been shown in chicks and rats during lens development at stages for secondary fiber cell differentiation (Chamberlain and McAvoy, 1987; McAvoy and Chamberlain, 1989; Le and Musil, 2001; Iyengar et al., 2009). One difference, however, is that during secondary lens fiber cell differentiation, different levels of FGF activity have been suggested to control cell proliferation versus differentiation (McAvoy and Chamberlain, 1989), which is not observed in our primary fiber cell differentiation assays. Thus, how similar the regulatory mechanisms are between primary and secondary fiber cell differentiation remains to be studied in more detail.
Our gain- and loss-of-function experiments provide evidence that BMP activity promotes cell cycle exit and the generation of Equarin-positive lens cells. Furthermore, our results show that BMP signals play a role in proliferation in the lens. In the absence of BMP activity, BrdU incorporation is increased, whereas pHistone H3+ mitotic cells are decreased in cultured lens cells. Because BrdU is incorporated in proliferating cells in the S phase of the cell cycle and pHistone H3 labels cells in late G2 and M phases, these results indicate that in the absence of BMP activity, lens cells have an increased S phase length or are arrested in the early G2 phase, thereby explaining the decreased level of mitosis and cell cycle exit during these conditions. Conversely, elevated BMP exposure causes decreased BrdU incorporation, without, however, affecting mitosis in lens cells. The latter is explained by our finding that BMP inhibition in lens/retina cultures leads to a suppression of Fgf8 expression in retinal cells, indicating that the reduction of mitotic lens cells observed after BMP inhibition is caused by decreased FGF activity. Accordingly, our data show that in the absence of BMP activity, FGF signals restore the levels of mitosis in lens cells. Our finding that Fgf8 expression in chick retinal cells is dependent on BMP activity is supported by results in mice. In Bmp4 mouse mutants and mice that lack Bmpr1a/Bmpr1b in the retina, Fgf15 expression is drastically reduced or absent in the optic vesicle (Murali et al., 2005). Furthermore, in Bmp4−/− optic vesicle explant cultures, Fgf15 expression is restored by exogenous Bmp4 protein (Murali et al., 2005).
The results taken together indicate that BMP activity plays an important role in cell cycle exit in the lens but in the presence of FGF signals appears to be dispensable for mitosis. Consistently, pSmad1 expression, indicative of BMP activity, is localized to cells at the equator of the lens, the region where epithelial cells differentiate into secondary lens fiber cells (Belecky-Adams et al., 2002). In addition, in whole chick embryo assays, BMP signals are crucial for the differentiation process of lens cells, detected by delayed lens fiber cell elongation after BMP inhibition (Belecky-Adams et al., 2002). In agreement, a previous study showed that BMP4 induces p27, indicative of cell cycle exit and osteoblast differentiation in osteoblast-like cell lines (Chang et al., 2009), further supporting our finding that BMP activity plays an important role in cell cycle exit. In summary, we determined the requirement of BMP and FGF activity during proliferation, cell cycle exit, and early differentiation of primary lens fiber cells and provided evidence that FGF activity is the key pathway to regulation of proliferation, whereas interaction between FGF and BMP signals promotes cell cycle exit and early differentiation of primary lens fiber cells.
MATERIALS AND METHODS
Embryos
Fertilized white Leghorn chicken eggs were obtained from Agrisera AB (Umeå, Sweden). Chick embryos were staged according to Hamburger and Hamilton stages (Hamburger and Hamilton, 1951). The use of chick (Gallus gallus) embryos in this study was approved by the Ethical Committee on Animal Experiments for Northern Sweden.
Lens and retina tissue explants
Prospective LR explants were isolated from stage 13 chick embryos and cultured for 12 or 26 h in collagen (Advanced BioMatrix, San Diego, CA). Serum-free OPTI-MEM medium (Life Technologies, Carlsbad, CA) containing N2 supplement (Invitrogen, Carlsbad, CA) and fibronectin (Sigma-Aldrich, St. Louis, MO) was used. Human BMP4 (R&D Systems, Minneapolis, MN) was used at 3.5–35 ng/ml, and recombinant soluble human bFGF2 (Invitrogen) and FGF8b (R&D Systems) was used at 50–250 ng/ml with 0.5 μg/ml heparin (Sigma-Aldrich). SU5402 (Calbiochem, La Jolla, CA) was used at 5 μM. Soluble noggin and control-conditioned medium (CM) were obtained from stably transfected or untransfected Chinese hamster ovary cells (Lamb et al., 1993) and cultured in CHO-S-SFM II media (Life Technologies). The concentration of the Noggin-CM was estimated on SDS gel with different concentrations of bovine serum albumin as a control. Noggin-CM was used at an estimated concentration of 50–100 ng/ml. Explants cultured in the presence of control-CM generated the same combination of cells as explants cultured alone (unpublished data).
In situ hybridization and immunohistochemistry
Explants and embryos were fixed at 4°C for 30 min (explants) or 2 h (embryos) in 4% paraformaldehyde in phosphate-buffered saline, and embryos were transferred to 30% sucrose at 4°C overnight. Thereafter, explants and embryos were frozen and serially sectioned at 10 μm. The consecutive sections from the same explants and embryos were analyzed with in situ hybridization and immunohistochemistry. In situ RNA hybridization using chick digoxigenin-labeled Equarin–long probe (Mu et al., 2003) and p57 probe (B. Novitch, University of California, Los Angeles, Los Angeles, CA) was performed essentially as described (Wilkinson and Nieto, 1993). For the immunochemistry study, antibodies used were as follows: anti-p27 (1:750; BD Biosciences, San Diego, CA) and anti-BrdU (1:50; BD Biosciences) mouse antibodies, sheep anti–δ-crystallin (1:1000; Beebe and Piatigorsky, 1981), and rabbit anti–phosphorylated-histone H3 (1:500; Millipore, Billerica, MA). As a secondary antibodies, fluorescent dye–coupled anti-mouse Alexa Fluor 594 (Invitrogen) and anti-sheep or anti-rabbit mouse Alexa Fluor 488 (Invitrogen) was used. 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) was used to mark nuclei of the cells.
BrdU analysis
Explants were incubated for the last 20 min of the 26-h culture period with 10 μM BrdU (Sigma-Aldrich), fixed, frozen, and sectioned as described. Sections were blocked for 1 h in 10% fetal calf serum and then treated for 20 min with 2 M HCl to denature the nucleic acids to facilitate epitope recognition. Sections were rinsed for 5 min in 1 M sodium borate buffer, pH 8.5, followed by overnight incubation with a monoclonal anti-BrdU antibody (BD Biosciences) and standard immunohistochemistry protocol as described.
Statistical analyses
To determine the percentage of pHistone H3–, BrdU-, and p27-expressing cells in explant, the number of antigen-expressing cells was quantified and compared with the total number of cells, determined by DAPI-stained nuclei. The graphs represent the mean number of cells positively stained for pHistone H3, BrdU, and p27, respectively. Error bars represent ± SEM. Statistical differences between groups were analyzed using analysis of variance and Student's t test. **p < 0.02.
Supplementary Material
Acknowledgments
We thank J. Piatigorsky K. Ohta, and B. Novitch for kindly providing the δ-crystallin antibody and Equarin and p57 plasmids, respectively. We are grateful to Michael Wride for comments on the manuscript and members of the Gunhaga laboratory for helpful discussions. L.G. is supported by Umeå University, Sweden, the Swedish Research Council, and Kronprinsessan Margaretas Foundation.
Abbreviations used:
- BMP
bone morphogenetic protein
- FGF
fibroblast growth factor
- LR
lens/retina
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-01-0075) on June 20, 2012.
REFERENCES
- Beebe DC, Piatigorsky J. Translational regulation of delta-crystallin synthesis during lens development in the chicken embryo. Dev Biol. 1981;84:96–101. doi: 10.1016/0012-1606(81)90374-2. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Boswell BA, Lein PJ, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell. 2008a;19:2631–2641. doi: 10.1091/mbc.E08-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008b;324:202–212. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower DJ, Errington LH, Pollock BJ, Morris S, Clayton RM. The pattern of expression of chick delta-crystallin genes in lens differentiation and in trans-differentiating cultured tissues. EMBO J. 1983;2:333–338. doi: 10.1002/j.1460-2075.1983.tb01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruelle D, Groux-Muscatelli B, Gaudric A, Sestier C, Coscas G, Caruelle JP, Barritault D. Immunological study of acidic fibroblast growth factor (aFGF) distribution in the eye. J Cell Biochem. 1989;39:117–128. doi: 10.1002/jcb.240390204. [DOI] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res. 1987;6:1165–1169. doi: 10.3109/02713688709034890. [DOI] [PubMed] [Google Scholar]
- Chang SF, Chang TK, Peng HH, Yeh YT, Lee DY, Yeh CR, Zhou J, Cheng CK, Chang CA, Chiu JJ. BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21 CIP1 and p27 KIP1 regulation. Mol Endocrinol. 2009;23:1827–1838. doi: 10.1210/me.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development. 2000;127:3593–3605. doi: 10.1242/dev.127.16.3593. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- French CR, Erickson T, French DV, Pilgrim DB, Waskiewicz AJ. Gdf6a is required for the initiation of dorsal-ventral retinal patterning and lens development. Dev Biol. 2009;333:37–47. doi: 10.1016/j.ydbio.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc Lond B Biol Sci. 2011;366:1193–1203. doi: 10.1098/rstb.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. Series of embryonic chicken growth. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, McAvoy JW, Lovicu FJ. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors. 2009;27:50–62. doi: 10.1080/08977190802610916. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- Liu Y, Monticone M, Tonachini L, Mastrogiacomo M, Marigo V, Cancedda R, Castagnola P. URB expression in human bone marrow stromal cells and during mouse development. Biochem Biophys Res Commun. 2004;322:497–507. doi: 10.1016/j.bbrc.2004.07.161. [DOI] [PubMed] [Google Scholar]
- Loren CE, Schrader JW, Ahlgren U, Gunhaga L. FGF signals induce Caprin2 expression in the vertebrate lens. Differentiation. 2009;77:386–394. doi: 10.1016/j.diff.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW, de Iongh RU. Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci. 2011;366:1204–1218. doi: 10.1098/rstb.2010.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- Modak SP, Morris G, Yamada T. DNA synthesis and mitotic activity during early development of chick lens. Dev Biol. 1968;17:544–561. doi: 10.1016/0012-1606(68)90004-3. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Mu H, Ohta K, Kuriyama S, Shimada N, Tanihara H, Yasuda K, Tanaka H. Equarin, a novel soluble molecule expressed with polarity at chick embryonic lens equator, is involved in eye formation. Mech Dev. 2003;120:143–155. doi: 10.1016/s0925-4773(02)00423-9. [DOI] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Ogino H, Yasuda K. Induction of lens differentiation by activation of a bZIP transcription factor, L-Maf. Science. 1998;280:115–118. doi: 10.1126/science.280.5360.115. [DOI] [PubMed] [Google Scholar]
- Pandit T, Jidigam VK, Gunhaga L. BMP-induced L-Maf regulates subsequent BMP-independent differentiation of primary lens fibre cells. Dev Dyn. 2011;240:1917–1928. doi: 10.1002/dvdy.22692. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza HM, Yasuda K. Lens differentiation and crystallin regulation: a chick model. Int J Dev Biol. 2004;48:805–817. doi: 10.1387/ijdb.041863hr. [DOI] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Piatigorsky J. Quantitation of delta-crystallin messenger RNA during lens induction in chick embryos. Proc Natl Acad Sci USA. 1976;73:2808–2812. doi: 10.1073/pnas.73.8.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl-Franco A, Fragel-Madeira L, Macama Ada C, Linden R, Ventura AL. ATP controls cell cycle and induces proliferation in the mouse developing retina. Int J Dev Neurosci. 2010;28:63–73. doi: 10.1016/j.ijdevneu.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Sjodal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell. 2007;13:141–149. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Sullivan CH, Marker PC, Thorn JM, Brown JD. Reliability of delta-crystallin as a marker for studies of chick lens induction. Differentiation. 1998;64:1–9. doi: 10.1046/j.1432-0436.1998.6410001.x. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Revett T, Huard C, Zhang Y, Tobin JF, Martinez RV, Gimeno RE. Bidirectional modulation of adipogenesis by the secreted protein Ccdc80/DRO1/URB. J Biol Chem. 2009;284:8136–8147. doi: 10.1074/jbc.M809535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Pino G, Lovicu FJ. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Prog Retin Eye Res. 2010;29:135–143. doi: 10.1016/j.preteyeres.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- Yang C, Yang Y, Brennan L, Bouhassira EE, Kantorow M, Cvekl A. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB J. 2010;24:3274–3283. doi: 10.1096/fj.10-157255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.