Abstract
Background
Optimal surgical management of digital nerve lesions remains uncertain despite the publication of numerous studies. The purposes of this review were primarily to analyze whether there is a superior surgical technique for digital nerve repair and secondarily to statistically verify the variables to be predictors of sensory recovery.
Methods
A literature search was performed using PubMed including citation from MEDLINE. Studies were included if they involved patients with digital nerve lacerations in whom end-to-end neurorrhaphy, nerve grafts, conduits, or end-to-side neurorrhaphy were performed. Further, the sensory outcome had to be assessed according to the modified American Society for Surgery of the Hand guidelines to stratify for two-point discrimination in millimeters. The variables age, follow-up, delay in repair, type of trauma, and gap length were extracted. The association between each predictor and response was assessed using a linear mixed model and corrected for heterogeneity between studies. Significance was considered present at p ≤ 0.05.
Results
Of the 34 articles found, 14 articles were included giving appropriate individual data for 191 nerves. There was no statistically significant difference in outcome between operation techniques. Age and follow-up were verified predictors of sensory recovery.
Conclusion
In this review, the type of operation for digital nerve repair does not influence sensory outcome. However, we verified outcome to be influenced by the patient’s age and the follow-up period. To add more scientific evidence to our results, larger cohort prospective studies need to be done with better detailed description of data.
Keywords: Conduits, Digital nerve, Grafts, Neurorrhaphy, Sensory recovery
Introduction
Digital nerve lacerations are common in hand trauma surgery and are even the most frequently injured of the peripheral nerves. Digital nerve injury can result from simple cuts or from severe hand trauma. In case a digital nerve is left unrepaired, the axonal growth will disperse and could lead to neuroma formation which then would interfere with rehabilitation, functional recovery, and sensory deficit especially in the thumb and the second digit which are involved in pinch and gnostic function [43]. Sensibility is an essential factor, concerning a normal hand function. Since Moberg [36] studied and described the sensory function of the fingers, many studies reported the treatment and the evaluation of the sensibility after treatment of nerve injuries.
Primary repair by end-to-end neurorrhaphy can be performed in about 82 % of the cases [28]. Brown recommended primary repair when there is a clean, sharply incised wound and both nerve ends are easily seen and mobilized without extension of the wound and with availability of a well-trained team with adequate facilities [10]. In about 18 % of the cases, when gaps exist, nerve reconstruction is required by grafting or tubulization [28]. The gold standard for these nerve type injuries is the nerve autograft [9, 27, 32, 38, 46]. Usually, the sural nerve or the antebrachial cutaneous nerve serve as favorable donor nerves to bridge the nerve defect. However, the morbidity caused by sacrificing a functioning nerve resulted in searching for suitable non-autologous graft materials. Effective alternatives were found, like synthetic and autogenous non-nervous conduits which function as a guide for axonal sprouting, a barrier against scar tissue ingrowths and maintain an internal milieu for nerve regeneration [26]. Recently, the end-to-side neurorrhaphy has been described to solve avulsion injuries or when the proximal stump is not available for traditional end-to-end repair. In 1991, this type of neurorrhaphy was reintroduced by Viterbo et al. and reconnects a distal injured nerve stump laterally to a neighboring nerve [2, 7, 17, 28, 51, 52].
Predictors of sensory recovery have been evaluated in several studies. Weinzweig et al. [57] found mechanism of injury and age to be predictors of sensory recovery after digital nerve injury [1, 3, 16, 20, 31, 41, 47, 57]. They did not observe a significant correlation between gender, involved digit, level of injury, time from injury till repair, and gap length. Numerous other studies did state that there is a correlation between the latter factors and sensory recovery. However, none of these studies performed a statistical analysis to confirm an actual correlation between these factors.
In conclusion, we noticed a lack of consensus regarding the most optimal management of digital nerve repair. Also, it is remarkable that there is still no agreement on which variables are predictors of a successful sensory recovery. The aim of this review is to present an update and statistical evidence for digital nerve repair. We review the surgical techniques, the outcomes, and the variables to be predictors of sensory recovery.
Materials and Methods
A literature search was performed in March 2011 using the PubMed service that includes citations from MEDLINE and other life science journals. Initial search focused on the text words: digital nerve, trauma, injury, surgery, repair, and sensory. In a secondary search, the medical subject heading term “peripheral nerve” was also used. The limit was set on abstracts in English, Dutch, German, French, and Spanish dating back to 1980. If the title of a study was relevant, the abstract was reviewed by the first and second author. Full-text articles were obtained and reviewed if the abstracts suggested that the study met the inclusion criteria. In addition, footnote chasing of references cited in previous studies of sensibility outcome in patients with digital nerve repair was performed. The studies were classified according to their level of evidence [12].
Articles were eligible for inclusion if they met the inclusion criteria. Inclusion criteria included (1) patients with clinically identified peripheral digital nerve lesion, (2) the surgical intervention was either microsurgical end-to-end neurorrhaphy, end-to-side/terminolateral neurorrhaphy, non-nervous conduits repair, or grafts surgical repair, and (3) the outcome assessment was postoperative sensory recovery in static two-point discrimination (s2PD) expressed in millimeters and stratified into groups according to the modified American Society for Surgery of the Hand guidelines (modified ASSH) (Table 1) [4]. Studies were excluded when they (1) did not meet the eligibility criteria, (2) did not present the individual data per patient, or (3) were descriptive reviews.
Table 1.
Modified ASSH guidelines for stratification of s2PD results
| Rating | s2PD (mm) |
|---|---|
| Excellent | <6 |
| Good | 6–10 |
| Fair | 11–15 |
| Poor | >15 or protective sensation |
Following the literature analysis, it was possible to reanalyze the individual data per patient. We extracted the next variables from each study meeting the inclusion criteria: the type of repair, the age at the time of injury, the type of injury, the delay between injury and repair in weeks, the gap length in centimeters, the follow-up period in months, and the sensory outcome expressed in millimeters. None of these articles presented individual data for all mentioned variables. Therefore, we included articles with minimal six out of seven variables. We did not include the variables gender and site of injury because, for this, data was not specified per patient most of the time.
In addition, we performed a statistical analysis of individual data from the selected articles. First, the association between each predictor and response was assessed separately using a linear mixed model. The above-described risk factors were all analyzed on p value, 95 % confidence interval, estimate, and standard error of the mean. Further, the variables that were significantly associated with sensory recovery (p ≤ 0.05) were included in a linear mixed model to evaluate their independent contribution to the prediction of recovery. The linear mixed model was chosen because we needed to correct for correlation between subjects from the same study. The heterogeneity between different studies could affect the relation between risk factors and outcome.
Results
Trial Selection
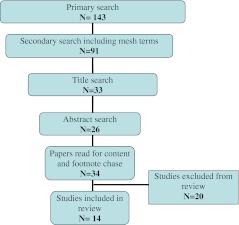
A total of 34 papers were recovered after the PubMed search. Among these, 20 did not meet the inclusion criteria because of different reasons (Table 2). Eventually, 14 studies were appropriate for inclusion. A total of 191 nerves were evaluated for sensory outcome. The numbers of nerve repair for each operation technique were 21 with grafts, 71 with end-to-end neurorrhaphy, 33 with biological conduits, 42 with synthetic conduits, and 24 with end-to-side/terminolateral neurorrhaphy. The selection process is described in Fig. 1. The 20 excluded studies are mentioned in Table 2 with the description of each article and the reason of exclusion in Tables 3, 4, 5, 6, and 7.
Table 2.
Excluded articles
| Study | Year |
|---|---|
| Altissimi et al. [3] | 1991 |
| Berger et al. [8] | 1991 |
| Efstathopoulos et al. [16] | 1995 |
| Wang et al. [55] | 1996 |
| Weinzweig et al. [57] | 2000 |
| Cheng et al. [13] | 2001 |
| Tadjalli et al. [48] | 1995 |
| Al-Ghazal et al. [1] | 1994 |
| Kallio et al. [24] | 1993 |
| Goldie et al. [19] | 1992 |
| Mackinnon et al. [30] | 1988 |
| Weber et al. [56] | 2000 |
| Battiston et al. [6] | 2006 |
| Rinker et al. [42] | 2011 |
| Walton et al. [54] | 1989 |
| Tang et al. [50] | 1993 |
| Risitano et al. [43] | 2002 |
| Meek et al. [33] | 2004 |
| Meek et al. [34] | 2004 |
| Mennen [35] | 2003 |
Fig. 1.
Flow diagram of the selection process of included articles
Table 3.
End-to-end primary repair group studies excluded
| Study | Year | Follow-up method | Predictors | Not predictors | Type of study |
|---|---|---|---|---|---|
| Altissimi et al. [3] | 1991 | No individual data | Age | Postoperative treatment | Prospective |
| Time to repair (primary versus delayed) | |||||
| Type of trauma (sharp versus crush) | |||||
| Berger et al. [8] | 1991 | No individual data | Age | Associated injuries | Prospective |
| Surgeon experience | |||||
| Efstathopoulos et al. [16] | 1995 | No individual data | Age | Presence of associated injuries | Retrospective |
| Type of injury (sharp versus crush) | |||||
| Wang et al. [55] | 1996 | No individual data | Primary versus secondary repair | Contralateral nerve block | Prospective |
| Age | |||||
| Injury mechanism | |||||
| Weinzweig et al. [57] | 2000 | No individual data | Age (child versus adult) | Gender | Retrospective |
| Type of injury (sharp versus avulsion | Involved digit | ||||
| Level of injury | |||||
| Radial or ulnar side | |||||
| Time injury til repair (acute versus delayed | |||||
| Cheng et al. [13] | 2001 | No individual data | Tactile stimulation after repair | Mechanism of injury | Prospective randomized |
| Age | Site of injury | ||||
| Tadjalli et al. [48] | 1995 | No individual data, British scale | Severity of injury | – | Retrospective |
| Al-Ghazal et al. [1] | 1994 | Highet method and no individual data | Smoking | Associated injuries | Retrospective |
| Type of injury (sharp versus crush) | |||||
| Kallio et al. [24] | 1993 | No individual data | Age | Level of injury | Retrospective |
| Time of repair (acute versus delayed) | Type of trauma | ||||
| Goldie et al. [19] | 1992 | No individual data | – | No correlation between the used assessments tests | Retrospective |
Table 4.
PGA conduit group studies excluded
| Study | Year | Follow-up methods | Predictors | Not predictors | Type of study |
|---|---|---|---|---|---|
| Mackinnon et al. [30] | 1988 | Lack of individual data | − | Type of tube | Prospective |
| Contralateral nerve block | |||||
| Weber et al. [56] | 2000 | No individual data | Type of repair (PGA versus nerve grafts and primary repair) | Age | RCT |
| Gap length | Location of injury | ||||
| Mechanism of injury | Worker’s compensation status | ||||
| Time to repair | |||||
| Battiston et al. [6] | 2006 | m2PD | Type of repair (autogenous versus synthetic conduits) | Type of tube | Prospective |
| Age | |||||
| Rinker et al. [42] | 2011 | No individual data | Type of repair (PGA versus vein conduits) | Type of tube | RCT |
| Smoking |
m2PD moving two point discrimination in millimeters
Table 5.
Autogenous conduit group studies excluded
| Study | Year | Follow-up methods | Predictors | Not predictors | Type of study |
|---|---|---|---|---|---|
| Walton et al. [54] | 1989 | m2PD | Time till repair (acute versus delayed) | Laceration versus avulsion | Retrospective |
| Tang et al. [50] | 1993 | m2PD | Secondary repair with vein conduits after primary repair | – | Prospective |
| Battiston et al. [6] | 2006 | m2PD | Type of repair (autogenous versus synthetic conduits) | Type of tube | Prospective |
| Age | |||||
| Risitano et al. [43] | 2002 | Mackinnon and Dellon no millimeters given | Vein grafts satisfactory for gap length <30 mm, poor for gaps >30 mm | – | Retrospective |
m2PD moving two point discrimination in millimeters
Table 6.
Nerve graft group studies excluded
| Study | Year | Follow-up methods | Predictors | Not predictors | Type of study |
|---|---|---|---|---|---|
| Meek et al. [33, 34] | 2004 | No individual data/review | PGA permits reconstruction of longer defects | – | Literature review of peripheral nerve repair |
| Incorporation of muscle in vein superior results than vein conduits alone in same defect | |||||
| Wang et al. [55] | 1996 | Not enough individual data | Primary versus secondary repair | Length of graft | Prospective |
| Age | Contralateral nerve block | ||||
| Injury mechanism | |||||
| Berger et al. [8] | 1991 | No individual data | Age | − | Prospective |
| Surgeon experience | |||||
| Tension on nerve graft | |||||
| Associated injuries | |||||
| Kallio et al. [24] | 1993 | No individual data | Age | Level of injury | Retrospective |
| Time of repair (acute versus delayed) | Type of trauma | ||||
| Length of nerve graft |
Table 7.
End-to-side group study excluded
| Study | Year | Follow-up methods | Predictors | Not predictors | Type of study |
|---|---|---|---|---|---|
| Mennen [35] | 2003 | Highet British Medical Research Council | Elimination of tension | – | Prospective |
| Postoperative immobilization |
Assessment of Level of Evidence
The 14 included studies had a design classification of II to IV. Five studies were level II, two studies were level III, and seven studies were level IV.
Description of Included Studies
Table 8 provides the clinical characteristics of the 14 included studies, which incorporates the amount of repaired nerves, the study design, the study year, and the type of repair. All studies were published between 1985 and 2010. The patient cohort age ranged from 8 to 72 years, with a mean of 39.52 years. The patient cohort follow-up time after surgery ranged between 3 and 144 months, with a mean of 28.31 months. The patient cohort time till repair ranged between 0 and 148 weeks (mean, 3.28 months). The nerve cohort gap length ranged between 0 and 4 cm, with a mean of 1.16 cm. An excellent sensory outcome was achieved in 25.13 % of the patients. Table 9 gives a crude association between the predictor age and an excellent sensory recovery.
Table 8.
Included studies
| Author | Year | No. of nerves repaired | Age (years) | Follow-up (months) | Delay (weeks) | Type of repair | Type of study | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |||||
| Hirasawa et al. [21] | 1985 | 12 | 29.4 | 8–47 | 33.1 | 19–70 | 1.1 | 0–8 | End-to-end/graft | Prospective |
| Segalman et al. [44] | 2001 | 19 | 65 | 60–72 | >12 | – | – | – | End-to-end | Retrospective |
| Sullivan et al. [47] | 1985 | 42 | – | 20–65 | 13 | 9–96 | 5.86 | 0–88 | End-to-end | Retrospective |
| Marcoccio et al. [32] | 2010 | 21 | 38 | 11–61 | 43 | 18–96 | Delayed (not further specified) | Autogenous conduit | Retrospective | |
| Lee et al. [26] | 2008 | 3 | 32.33 | 19–52 | 103 | 33–144 | 48.8 | 2.42–104 | Autogenous conduit | Case series |
| Norris et al. [37] | 1988 | 8 | 40.9 | 15–61 | 7.63 | 3–11 | 68 | 0–120 | Grafts | Prospective |
| Nunley et al. [39] | 1990 | 19 | 27.77 | 16–52 | 55.15 | 24–89 | 22.15 | 0–44 | Grafts | Prospective |
| Bushnell et al. [11] | 2008 | 11 | 33 | 18–55 | 11 | 12–22 | – | – | Synthetic conduit | Prospective |
| Lochmeyer et al. [28] | 2009 | 12 | 38 | 12–66 | 12 | – | 21.6 | 0–148 | Synthetic conduit | Prospective |
| Battiston et al. [7] | 2007 | 19 | 40.05 | 15–67 | 29.7 | 6–74 | 5.5 | 0–72 | Synthetic conduits | Prospective |
| Artiaco et al. [5] | 2010 | 7 | 45 | 20–62 | 35. 71 | 8–60 | 19.42 | 0–48 | End-to-side | Prospective |
| Pelissier et al. [40] | 2001 | 6 | 32.83 | 13–46 | 12.2 | 6–15 | – | – | End-to-side | Retrospective |
| Frey et al. [18] | 2003 | 2 | – | 12–42 | – | 37–48 | – | – | End-to-side | Case series |
| Voche et al. [53] | 2004 | 10 | 30 | 9–55 | 16.8 | 9–29 | 0 | 0–0 | End-to-side | Retrospective |
Table 9.
Crude association of predictors
| Predictors | Groups | Excellent sensory recovery, % (n)a |
|---|---|---|
| Age (years) | <16 | 36.4 (4/11) |
| 16–25 | 21.7 (5/23) | |
| 26–40 | 25.5 (12/47) | |
| >40 | 29.4 (20/68) | |
| Total no. | 149 | |
| Gap length (cm) | <1 | 32.2 (27/84) |
| >1 | 21.8 (19/87) | |
| Total no. | 26.9 (46/171) | |
| Follow-up (months) | <6 | 0 (0/7) |
| 6–12 | 26.9 (17/63) | |
| >12 | 25.6 (31/121) | |
| Total no. | 191 | |
| Trauma | Sharp | 27.8 (27/97) |
| Avulsion/circular saw | 28.3 (17/60) | |
| Iatrogenic | 0 (0/5) | |
| Secondary | 0 (0/2) | |
| Total no. | 164 | |
| Delay | No delay | 23.6 (13/55) |
| 1 day–1 month | 26.6 (8/30) | |
| 1–6 months | 12 (3/25) | |
| 6–12 months | 30 (4/12) | |
| >12 months | 27.3 (3/11) | |
| Total no. | 133 | |
| Type of surgery | End-to-end | 31 (22/71) |
| Biological conduits | 24.3 (8/33) | |
| Synthetic conduits | 31 (13/42) | |
| End-to-side | 8.3 (2/24) | |
| Grafts | 14.3 (3/21) | |
| Total no. | 191 |
aResults of this table are not adjusted for the type of studies included in this review
Risk Factors Associated with Sensory Recovery
For sensory recovery, the type of surgery did not significantly influence outcome (p = 0.707). A longer time of follow-up after trauma was predictive for better recovery. The estimated average monthly decrease in 2PD is 0.035 (slope = −0.035). This reduction in 2PD is statistically significant (p = 0.042) (Table 10).
Table 10.
Result adjusted for type of surgery
| Parameter | Estimated change in s2PD | p value | 95 % CI |
|---|---|---|---|
| Follow-up | −0.034961 | 0.042 | [−0.068565–(−0.001357)] |
| Age | 0.057806 | 0.040 | [0.002590–0.113022] |
| Gap length | 1.067788 | 0.120 | [−0.280003–2.415578] |
A younger age is significantly predictive for better sensory recovery. For every year, the estimated average increase of 2PD is 0.058 (slope = 0.058). This increase in 2PD is statistically significant (p = 0.040) (Table 10).
A broader gap length, although borderline significant (p = 0.120), showed a tendency for a worse sensory recovery. For every centimeter of gap length, the estimated average increase in 2PD is 1.07 (slope = 1.07) for all types of operations (Table 10).
In the second analysis, we tested age adjusted for follow-up and type of surgery. The 2PD increases over time (slope = 0.066). This increase in 2PD is highly statistically significant (p = 0.017) (Table 11). There was no significant difference in mean sensible outcome in regard to time to repair (p = 0.803) and type of trauma (p = 0.165).
Table 11.
Results age adjusted for follow‐up and type of surgery
| Parameter | Estimated change in s2PD | p value | 95 % CI |
|---|---|---|---|
| Age | 0.066475 | 0.017 | [0.012612–0.120337] |
Discussion
The aim of this review was to provide an update of the current surgical interventions for digital nerve repair. With this, we wanted to analyze whether there is evidence for the superiority of one technique above the other and statistically verify the prognostic factors for sensory recovery after reconstruction.
Our study showed no significant difference in sensory recovery between the types of operation for digital nerve repair. There are only two randomized controlled trials (RCTs) comparing surgical interventions for digital nerve repair. One of these, recently published by Rinker et al. studied 76 nerves randomized in two groups. One group underwent repair with a vein conduit and the other group underwent repair with polyglycolic acid conduits (PGA) [42]. The other RCT, published by Weber et al., studied 136 digital nerve transections. They randomized the patients into two groups, the first consisting of end-to-end neurorrhaphy or nerve grafts and the second consisting of PGA conduits repair [56]. Rinker et al. observed no difference in sensory recovery between both groups [42]. In contrast and not in line with our paper, Weber et al. did find a significant difference between groups. They found improved sensory outcome when using a conduit for nerve gaps of 4 mm or less, compared with end-to-end repair. They further showed a significantly better sensory recovery for synthetic conduits compared to nerve-grafted repairs with a gap length of 8 mm or more [56]. The reason Weber et al. [56] did observe a difference, unlike us, may be explained due to the type of studies included in this review, which were all level of evidence II to IV. Battiston et al. presented their personal clinical experience on tubulization repair of digital nerves, using biological and synthetic tubes. Good clinical results were seen in both groups [6].
In the past decade, numerous studies proposed several variables to predict sensory recovery [1, 16, 47, 57]. We observed a tendency of worse sensory recovery with broader gap length. This seems logical because a bigger distance for axonal growth has to be bridged, even though the type of surgery is the important predictive factor in case of gap length. Literature states that if a gap has to be bridged, tensionless repair with end-to-end neurorrhaphy can be difficult and, as a result, other types of surgical interventions have to be performed [11, 22, 28, 32]. The critical distance for nerve regeneration through a conduit was reported as 1.0 to 1.5 cm in a rat model [49]. In our review, 65 % of the nerve gaps were below 1.5 cm. The critical distance for nerve regeneration in humans has not yet been established. According to Walton et al. [54], Chiu et al. [14], and Mackinnon et al. [30], the results of digital nerve reconstruction with nerve grafts were comparable to those of nerve defects repaired by a vein conduit for defects <3.0 cm [14, 30, 54]. These results and the small sample size for nerve gaps above 3 cm in our data (4 %) could explain why we found no significant difference in sensible recovery between vein conduits and synthetic conduits in gap length above and under 1 cm.
A trend towards better outcome for sharp injuries was observed. The nonsignificance of this result could be explained according to the variable gap length. The type of trauma is related to the gap length that has to be bridged; as in big crush injuries, normally there is more tissue damage and, as a consequence, after trimming the dead nerve ends, a bigger gap length is left for repair. The reason why the variable gap length was not significant has been discussed above.
No association between delay in repair and outcome was seen. Weinzweig et al. [57] and Weber et al. [56] did neither find any correlation between time from injury to repair and outcome. On the other hand, they did find a significant correlation between type of trauma and outcome at a young age. In the older patient, this correlation disappeared [48, 56, 57].
In general, age was found to be a main factor for sensory recovery [1, 3, 8, 16, 25, 41, 47]. Berger et al. [8] and Steinberg et al. [45] emphasize that the better results in young patients are not only caused by a better axonal regeneration and a greater adaptability [8, 44, 45]. They suggest that older patients have fewer receptors because of age correlated centrally occurring changes, as uninjured digits in younger patients compared with older patients have superior sensibility [8, 31, 44, 56].
Finally, follow-up is verified to be positively correlated with sensory recovery. The recovery period of an injured nerve is based on functional reorganizational changes in the somatosensory cortex, mainly because of misdirection of regenerating axons. Regardless of how accurate the repair technique is, axonal misdirection is unavoidable [23, 29, 58]. According to the literature, a stable level of sensibility is reached by 6 months, which could be the time needed for functional reorganization. According to our crude analysis, excellent results are only seen after a period of 6 months [5, 13, 31].
Our review study differs from most others cited in the literature because we incorporated a statistical analysis from the included data. Most reviews in the literature are descriptive reviews and none of them covers all of the above-described available operation techniques for digital nerve injuries [5, 33, 57].
In addition, we want to mention that we included the end-to-side/terminolateral group because we wanted to give a comprehensive overview of all operation techniques used for digital nerve repair in the past 30 years. While documentation concerning the end-to-side/terminolateral group experience is limited, we still found 24 repairs performed according to this technique. Therefore, we think it is important to include these results in our analysis. We realize though that it is not a first-choice technique when doing a digital nerve repair. But Artiaco et al. [5] reported their clinical experience along with a literature review of digital nerve repair with the end-to-side technique, and the technique has encouraging results. It can be used in case of loss of substance and as an alternative to biological and synthetic conduits when digital nerve repair by means of nerve autograft is declined by the patient.
In conclusion, we state that the type of surgery, if selected accurately, does not influence the sensory outcome in our analysis but we verified that the time of follow-up and age of injury are predictive factors concerning the sensibility. However, this study still has a few limitations. Firstly, one major limitation is the reduced number of included nerves in this study. To perform a statistical analysis, and with this to obtain a good functional comparison of sensibility after digital nerve reconstruction, a uniform outcome measurement has to be used. Most authors survey the ASSH to stratify the results of s2PD but other surveys rely on the criteria set by the Nerve Injuries Committee of the British Medical Research Council, as modified by Mackinnon and Dellon to stratify the results for s2PD and moving two-point discrimination (m2PD) [28]. For this study, we intentionally choose the s2PD to be our uniform measurement outcome. The s2PD is a valid and reliable measurement of functional sensibility [15]. Despite the fact that most surveys used this measurement as an expression of their outcome, we had to exclude a significant amount of nerves from studies using Highet and Sander’s criteria as modified by Mackinnon and Dellon if they did not specify the exact value of s2PD and m2PD in millimeters.
Secondly, most studies performed concerning this subject were of small sample size, lacking statistical analysis, or with a low level of evidence (level III or IV). Therefore, it was not possible to only include studies of a higher sample size or higher level of evidence as this would reduce our sample size to a minimal level. Finally, we excluded the articles without presentation of individual data which could have led to selection bias if other predictors of recovery were present in the excluded patients.
Therefore, as a final and major remark, we want to point out one important recommendation for future research in this area. We think that a large cohort of patients should be followed up and randomized in the different types of operation techniques with a better detailed description of individual data in order to find answers to the lacunas in knowledge regarding digital nerve repair.
Acknowledgments
Conflict of Interest
The authors declare that they have no conflicts of interest, commercial associations, or intent of financial gain regarding this research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Al-Ghazal SK, McKiernan M, Khan K, et al. Results of clinical assessment after primary digital nerve repair. J hand Surg. 1994;19B:255–7. doi: 10.1016/0266-7681(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qattan MM. Terminolateral neurorrhaphy: review of experimental and clinical studies. J Reconstr Microsurg. 2001;17:99–108. doi: 10.1055/s-2001-12698. [DOI] [PubMed] [Google Scholar]
- 3.Altissimi M, Mancini GB, Azzarà A. Results of primary repair of digital nerves. J Hand Surg. 1991;16B:546–7. doi: 10.1016/0266-7681(91)90111-z. [DOI] [PubMed] [Google Scholar]
- 4.American Society for Surgery of the Hand . History and general examination. The hand examination and diagnosis. 2. New York: Churchill Livingstone; 1983. pp. 3–10. [Google Scholar]
- 5.Artiaco S, Tos P, Conforti LG, et al. Termino-lateral nerve suture in lesions of the digital nerves: clinical experience and literature review. J Hand Surg. 2010;35B:109–14. doi: 10.1177/1753193409337959. [DOI] [PubMed] [Google Scholar]
- 6.Battiston B, Geuna S, Ferrero M, et al. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258–67. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 7.Battiston B, Tos P, Conforti LG, et al. Alternative technique for peripheral nerve repair: conduits and end-to-side neurorraphy. Acta Neurochir Suppl. 2007;100:43–50. doi: 10.1007/978-3-211-72958-8_10. [DOI] [PubMed] [Google Scholar]
- 8.Berger A, Mailänder P. Advances in peripheral nerve repair in emergency surgery of the hand. World J Surg. 1991;15:493–500. doi: 10.1007/BF01675646. [DOI] [PubMed] [Google Scholar]
- 9.Berger A, Millesi H. Nerve grafting. Clin Orthop Relat Res. 1978;133:49–55. [PubMed] [Google Scholar]
- 10.Brown PW. Factors influencing the success of the surgical repair of peripheral nerves. Surg Clin North. 1972;52A:1137–55. doi: 10.1016/s0039-6109(16)39832-2. [DOI] [PubMed] [Google Scholar]
- 11.Bushnell BD, McWilliams AD, Whitener GB, et al. Early clinical experience with collagen nerve tubes in digital nerve repair. J Hand Surg. 2008;33A:1081–7. doi: 10.1016/j.jhsa.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Cook DJ, Guyatt GH, Laupacis A, et al. Clinical recommendations using levels of evidence for antithrombotic agents. Chest 1995; 108(4 Suppl):227S–230S. [DOI] [PubMed]
- 13.Cheng AS, Hung L, Wong JM, et al. A prospective study of early tactile stimulation after digital nerve repair. Clin Orthop Relat Res. 2001;384:169–75. doi: 10.1097/00003086-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg. 1990;86:928–34. doi: 10.1097/00006534-199011000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Dellon AL, Mackinnon SE, Crosby PM. Reliability of two point discrimination measurements. J Hand Surg. 1987;12:693–6. doi: 10.1016/s0363-5023(87)80049-7. [DOI] [PubMed] [Google Scholar]
- 16.Efstathopoulos D, Gerostathopoulos N, Misitzis D, et al. Clinical assessment of primary digital nerve repair. Acta Orthop Scand Suppl. 1995;264:45–7. doi: 10.3109/17453679509157166. [DOI] [PubMed] [Google Scholar]
- 17.Ferdindandez E, Lauretti L, Tufo T, et al. End-to-side nerve neurrorraphy: critical appraisal of experimental clinical data. Acta Neurochir Suppl. 2007;100:77–84. doi: 10.1007/978-3-211-72958-8_17. [DOI] [PubMed] [Google Scholar]
- 18.Frey M, Giovanoli P. End-to-side neurorraphy of sensory nerves. Eur J Plast Surg. 2003;26:85–8. doi: 10.1007/s00238-003-0502-0. [DOI] [Google Scholar]
- 19.Goldie BS, Coates CJ, Birch R. The long term results of digital nerve repair in no-man’s land. J Hand Surg. 1992;17B:75–7. doi: 10.1016/0266-7681(92)90016-u. [DOI] [PubMed] [Google Scholar]
- 20.Green DP, Hotchkiss RN, Pederson WC. Operative hand surgery, 4th ed., vols. 1 and 2. Philadelphia: Churchill Livingstone; 1998.
- 21.Hirasawa Y, Katsumi Y, Tokioka T. Evaluation of sensibility after sensory reconstruction of the thumb. J Bone Joint Surg. 1985;67B:814–9. doi: 10.1302/0301-620X.67B5.4055885. [DOI] [PubMed] [Google Scholar]
- 22.Johnson EO, Soucacos PN. Nerve repair: experimental and clinical evaluation of biodegradable artificial nerve guides. Injury. 2008;39(Suppl 3):S30–6. doi: 10.1016/j.injury.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–56. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- 24.Kallio PK. The results of secondary repair of 254 digital nerves. J Hand Surg. 1993;18B:327–30. doi: 10.1016/0266-7681(93)90054-j. [DOI] [PubMed] [Google Scholar]
- 25.Kallio PK, Vastamaki M. An analysis of the results of late reconstruction of 132 median nerves. J Hand Surg. 1993;18B:97–105. doi: 10.1016/0266-7681(93)90205-t. [DOI] [PubMed] [Google Scholar]
- 26.Lee YH, Shieh SJ. Secondary nerve reconstruction using vein conduit grafts for neglected digital nerve injuries. Microsurgery. 2008;28:436–40. doi: 10.1002/micr.20517. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Acad Orthop Surg. 2000;8A:243–52. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lohmeyer JA, Siemers F, Machens HG, et al. The clinical use of artificial nerve conduits for digital nerve repair: a prospective cohort study and literature review. J Reconstr Microsurg. 2009;25:55–61. doi: 10.1055/s-0028-1103505. [DOI] [PubMed] [Google Scholar]
- 29.Lundborg G, Rosén B. Hand function after nerve repair. Acta Physiol. 2007;189:207–17. doi: 10.1111/j.1748-1716.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon SE, Dellon AL. A study of nerve regeneration across synthetic (maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J Reconstr Microsurg. 1990;6:117–21. doi: 10.1055/s-2007-1006810. [DOI] [PubMed] [Google Scholar]
- 31.Mailänder P, Berger A, Schaller E, et al. Results of primary nerve repair in the upper extremity. Microsurgery. 1989;10:147–50. doi: 10.1002/micr.1920100218. [DOI] [PubMed] [Google Scholar]
- 32.Marcoccio I, Vigasio A. Muscle-in-vein nerve guide for secondary reconstruction in digital nerve lesions. J Hand Surg. 2010;35A:1418–1126. doi: 10.1016/j.jhsa.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Meek MF, Coert JH. Clinical use of nerve conduits in peripheral nerve repair: review of the literature. J Reconstr Microsurg. 2002;18:97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- 34.Meek MF, Coert JH, Robinson PH. Poor results after nerve grafting in the upper extremity: quo vadis? Microsurgery. 2005;25:396–402. doi: 10.1002/micr.20137. [DOI] [PubMed] [Google Scholar]
- 35.Mennen U. End-to-side suture in clinical practice. Hand Surg. 2003;8:33–42. doi: 10.1142/S0218810403001352. [DOI] [PubMed] [Google Scholar]
- 36.Moberg E. Criticism and study of methods for examining sensibility in the hand. Neurology. 1962;12:8–19. doi: 10.1212/WNL.12.1.8. [DOI] [PubMed] [Google Scholar]
- 37.Norris RW, Glasby MA, Gattuso JM, et al. Peripheral nerve repair in humans using muscle autografts. A new technique. J Bone Joint Surg. 1988;70B:530–3. doi: 10.1302/0301-620X.70B4.3403592. [DOI] [PubMed] [Google Scholar]
- 38.Nunley JA, Saies AD, Sandow MJ, et al. Results of interfascicular nerve grafting for radial nerve lesions. Microsurgery. 1996;17:431–7. doi: 10.1002/(SICI)1098-2752(1996)17:8<431::AID-MICR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Nunley JA, Ugino MR, Goldner RD, et al. Use of the anterior branch of the medial antebrachial cutaneous nerve as a graft for the repair of defects of the digital nerve. J Bone Joint Surg. 1989;71A:563–7. [PubMed] [Google Scholar]
- 40.Pélissier P, Riahi R, Casoli V, et al. Terminal–lateral nerve anastomoses. Preliminary clinical report of two cases. Ann Chir Plast Esthet. 2001;46:129–33. doi: 10.1016/S0294-1260(01)00009-7. [DOI] [PubMed] [Google Scholar]
- 41.Poppen NK, McCarroll HR, Doyle JR, et al. Recovery of sensibility after suture of digital nerves. H Hand Surg. 1979;4A:212–25. doi: 10.1016/s0363-5023(79)80156-2. [DOI] [PubMed] [Google Scholar]
- 42.Rinker B, Liau JY. A prospective study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. J Hand Surg. 2011;36A:775–81. doi: 10.1016/j.jhsa.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Risitano G, Cavallaro G, Cavallaro T, et al. Clinical results and thoughts on sensory nerve repair by autologous vein grafts in emergency hand reconstruction. Chir Main. 2002;21:194–7. doi: 10.1016/S1297-3203(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 44.Segalman KA, Cook PA, Wang BH, et al. Digital neurorrhaphy after the age of 60 years. J Reconstr Microsurg. 2001;17:85–8. doi: 10.1055/s-2001-12695. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg DR, Koman LA. Factors affecting the results of peripheral nerve repair. In: Gelberman RH, editor. Operative nerve repair and reconstruction. Philadelphia: JB Lippincott; 1991. pp. 349–64. [Google Scholar]
- 46.Strauch B. Use of nerve conduits in peripheral nerve repair. Hand Clin. 2000;16:123–30. [PubMed] [Google Scholar]
- 47.Sullivan DJ. Results of digital neurorraphy in adults. J Hand Surg. 1985;10B:41–4. doi: 10.1016/s0266-7681(85)80013-9. [DOI] [PubMed] [Google Scholar]
- 48.Tadjalli HE, McIntyre FH, Dolynchuk KN, et al. Digital nerve repair: relationship between severity of injury and sensibility recovery. Ann Plast Surg. 1995;35:36–40. doi: 10.1097/00000637-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Tang JB. Vein conduits with interposition of nerve tissue for peripheral nerve defects. J Reconstr Microsurg. 1995;11:21–6. doi: 10.1055/s-2007-1006506. [DOI] [PubMed] [Google Scholar]
- 50.Tang JB, Gu YQ, Song YS. Repair of digital nerve defect with autogenous vein graft during flexor tendon surgery in zone 2. J Hand Surg. 1993;18B:449–53. doi: 10.1016/0266-7681(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 51.Viterbo F, Trinidade JC, Hoshino K, et al. Latero-terminal neurorraphy without removal of epineural sheath. Experimental study in rats. Rev Paul Med. 1992;110:267–75. [PubMed] [Google Scholar]
- 52.Viterbo F, Trinidade JC, Hoshino K, et al. Two end-to-side neurorrhapies and nerve grafts with removal of epineural sheaths: experimental study in rats. J Plast Surg Br. 1994;47:75–80. doi: 10.1016/0007-1226(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 53.Voche P, Ouattara D. End-to-side neurorraphy for defects of palmar sensory digital nerves. Br J Plast Surg. 2005;58:239–44. doi: 10.1016/j.bjps.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Walton RL, Brown RE, Matory WE, Jr, et al. Autogenous vein graft repair of digital nerve defects in the finger: a retrospective clinical study. Plast Reconstr Surg. 1989;84:944–9. doi: 10.1097/00006534-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Wang WZ, Crain GM, Baylis W, et al. Outcome of digital nerve injuries in adults. J Hand Surg. 1996;21A:138–43. doi: 10.1016/s0363-5023(96)80167-5. [DOI] [PubMed] [Google Scholar]
- 56.Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–45. doi: 10.1097/00006534-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Weinzweig N, Chin G, Mead M, et al. Recovery of sensibility after digital neurorraphy: a clinical investigation of prognostic factors. Ann Plast Surg. 2000;44:610–7. doi: 10.1097/00000637-200044060-00006. [DOI] [PubMed] [Google Scholar]
- 58.Witzel C, Rohde C, Bushart TM. Pathways sampling by generating peripheral axons. J Comp Neurol. 2005;485:183–90. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]