Summary
Purpose
Pilocarpine induces prolonged status epilepticus (SE) in rodents that results in neurodegeneration and cognitive deficits, both commonly observed to be associated with human temporal lobe epilepsy. The multifunctional neuronal modulator, cyclooxygenase-2 (PTGS2 or COX-2), is rapidly induced after SE, mainly in principal neurons of the hippocampal formation and cortex. We used mice in which COX-2 is conditionally ablated in principal forebrain neurons to investigate the involvement of neuron-derived COX-2 in delayed mortality and performance in the Barnes maze.
Methods
Using the COX-2 conditional knockout mouse (nCOX-2 cKO) and their littermate wildtype controls, we compared motor behavior and performance in the Barnes maze before and three weeks after the induction of SE by pilocarpine. Mortality rate was also measured during SE and in the week following SE.
Key Findings
nCOX-2 cKO mice showed less delayed mortality than wildtype mice in the week after SE. Although motor behavior and most cognitive measures were not different in the nCOX-2 cKO, upon re-exposure to the maze three weeks after pilocarpine the latency to find the previously-learned target hole was significantly shorter in the nCOX-2 cKO than their wildtype littermate controls. By this measure pilocarpine-treated nCOX-2 cKO mice were identical to mice that had not experienced SE.
Significance
Results point to a role for neuronal COX-2 in delayed mortality in mice during the week following SE and suggest that neuronal COX-2 contributes to selected cognitive deficits observed after SE. Taking into consideration our previous findings that neurodegeneration and neuroinflammation after SE are reduced in the nCOX-2 cKO, and opening of the blood-brain barrier after pilocarpine is prevented, we conclude that neuronal COX-2 induction is an early step in many of the deleterious consequences of SE.
Keywords: Seizure-induced neuronal COX-2, pilocarpine, retrograde memory, anterograde memory, Barnes maze
Introduction
Cognitive deficits are common in epileptic patients, especially patients that suffer from temporal lobe epilepsy (TLE), in which seizure foci are frequently located within the critical memory structures of the medial temporal lobe (Bergin et al., 2000; Preston & Gabrieli, 2002; Leritz et al., 2006). TLE patients can present retrograde and anterograde amnesia, which are respectively defined as the loss of memories formed prior to brain insult and the inability to form new memories after the insult (Kapur et al., 1997; Milner et al., 1998; Bergin et al., 2000; Lah et al., 2006; Lah et al., 2008). Long-lasting yet subtle cognitive deficits are also produced by over-activation of the cholinergic system, as occurred in the Tokyo subway sarin attack (Miyaki et al., 2005). In rodents, status epilepticus triggered by the cholinergic agonist pilocarpine has been widely used to study TLE-related pathologies, including behavior modifications related to anxiety, depression, and learning and memory (Persinger et al., 1993; Hort et al., 1999; Mohajeri et al., 2003; Mohajeri et al., 2004; Muller et al., 2009).
It is well known that during seizures COX-2 is rapidly induced in principal forebrain neurons, including hippocampal pyramidal cells and dentate granule cells (Yamagata et al., 1993; Marcheselli & Bazan, 1996; Sandhya et al., 1998; Ciceri et al., 2002; Takemiya et al., 2006; Serrano et al., 2011). It is also well established that COX-2 plays an important role in synaptic plasticity (Murray & O'Connor, 2003; Chen & Bazan, 2005; Slanina & Schweitzer, 2005; Yang & Chen, 2008). Moreover, COX-2 inhibitors are reported to improve working memory after traumatic brain injury (Gopez et al., 2005) and in transgenic models of Alzheimer’s Disease (Melnikova et al., 2006; Echeverria et al., 2009), but in none of these studies was the relevant source of COX-2 (neurons, glia, etc) identified. In this study we used a mouse in which COX-2 had been conditionally ablated in a subset of forebrain neurons (Serrano et al., 2011) to investigate the role of neuronal COX-2 induction in spatial learning and memory deficits following SE. These mice were created by crossing a floxed COX-2 mouse line with a line expressing cre recombinase under the control of a synapsin I promoter to create the nCOX-2 cKO line. The nCOX-2 cKO mice lack immunoreactivity for COX-2 in hippocampal pyramidal cells, dentate granule cells, and some neocortical neurons. To test spatial learning and memory we used the well-established Barnes maze, which is similar to the Morris water maze but with less stress created by cold-water swimming (Barnes, 1979; Morris, 1984; Harrison et al., 2009). We observed retrograde memory impairment in mice treated with pilocarpine, consistent with previous reports (Persinger et al., 1993; Hort et al., 1999; Mohajeri et al., 2003; Mohajeri et al., 2004; Muller et al., 2009). However neuronal COX-2 deletion improved some aspects of performance in a retrograde amnesia task, and delayed mortality in mice during the week following SE was nearly abolished in the nCOX-2 cKO mice. Relief from SE-associated delayed mortality and memory deficits complements the neuroprotection observed in the nCOX-2 cKO months following SE, demonstrating a broad detrimental role of neuronal COX-2 induction after the intense seizures of SE.
Methods
Subjects
All animals were housed in a temperature-controlled environment with a 12 h light/dark schedule and had access to food and water ad libitum. Both nCOX-2 cKO mice and their WT littermates were generated in the C57Bl/6 (CR) strain and genotyped according to Serrano et al. (2011). Briefly, mice with a loxP element inserted into introns 5 and 8 of the COX-2 gene (Wang et al., 2009) were bred with females expressing cre recombinase under the control of a synapsin 1 promoter to generate females expressing cre and heterozygous for floxed COX-2. These females were further bred with heterozygous floxed COX-2 males to generate WT and nCOX-2 cKO offspring. Both transgenic mouse lines were bred for six to eight generations from the C57Bl/6 (Jackson) strain into the C57BL/6 (Charles River) strain. In nCOX-2 cKO mice the PTGS2 gene is ablated in hippocampal pyramidal cells, dentate granule cells, and some cortical neurons as judged by the lack of constitutive and seizure-induced COX-2 immunohistochemistry (Serrano et al., 2011). The postnatal conditional ablation of neuronal COX-2 avoids the known developmental defects associated with the global COX-2 knockout (see Serrano et al., 2011), but homeostatic adjustments of gene expression cannot be entirely ruled out. Testing began at 8 ± 2 weeks of age. On day 8 of testing mice were treated with either saline or pilocarpine resulting in four groups: WT Saline, nCOX-2 cKO Saline, WT Pilocarpine, nCOX-2 cKO Pilocarpine and these groups were then subjected to further testing 21 days post-treatment. All mice were on a C57BL/6CR background. All experiments were approved by the Institutional Animal Care and Use Committee of Emory University and conducted in accordance with its guidelines. Every effort was made to minimize animal suffering.
Pilocarpine treatment
Pilocarpine injections were performed in nCOX-2 cKO and WT littermates as previously described for C57Bl/6 mice (Borges et al., 2003; Borges et al., 2004; Serrano et al., 2011). Mice were injected with methylscopolamine and terbutaline (3 mg/kg each i.p. in 0.9% NaCl) 15–30 min prior to pilocarpine (275–310 mg/kg, i.p.) to minimize peripheral side effects. Every mouse injected with pilocarpine experienced behavioral SE as defined by continuous behavioral seizure activity consisting mainly of whole body clonic seizures (see Borges et al., 2003 for a more detailed description of seizure behavior including a modified Racine scale). Seizure intensity was evaluated behaviorally rather than by EEG. One hour after continuous SE, a mixture of pentobarbital (30 mg/kg, i.p.) and diazepam (10 mg/kg, i.p.) was administered to interrupt SE. Mice showing behavioral seizure activity for less than one hour were not included in our analysis. Two to four hours after terminating SE, mice were injected with 0.5 ml lactated dextrose Ringers to improve hydration. Control mice received terbutaline, methylscopolamine, pentobarbital, and lactated dextrose Ringers, but no pilocarpine.
Light Dark Exploration
Animals were habituated to the investigator and experimental room one week prior to behavioral testing. Light dark preference and locomotor ability were examined in the same cohorts of mice 7 d before SE and 21 d after SE using a 42 × 42 cm2 Plexiglas box divided evenly into a light side with clear walls and a dark side with black walls as represented schematically in Figure 1A. Each animal was placed in the light side of the box and allowed to explore the entire apparatus for 10 min. Movements of the mice were recorded by VersaMax Animal Activity Monitors (AccuScan Instruments, Inc., Columbus, OH). Data were collected in 1 min intervals using VersaMax version 1.3 software (AccuScan Instruments, Inc., Columbus, 7 OH). Percent time in dark was used as a measure of anxiety and preference for the dark. Number of transitions, total distance traveled, percent time spent in motion and speed were used to assess locomotor activity.
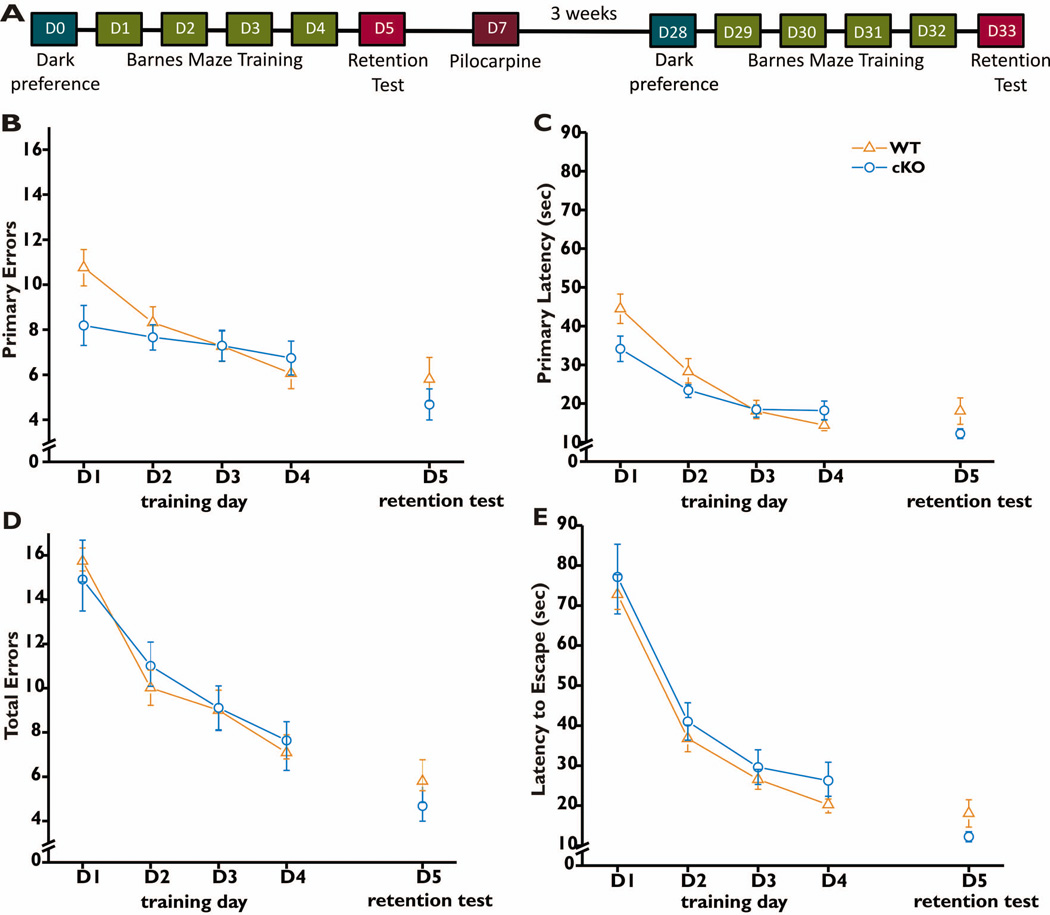
Figure 1. Barnes maze performance pre-treatment.
Behavioral analysis was conducted before and after the induction of status epilepticus according to the timeline shown (A). On Day 0 dark preference and motor behavior was measured. This was followed by Barnes maze training on Days 1–4 followed by a retention test on Day 5. The same timeline was repeated 3 weeks after induction of status epilepticus. For each of the first four days of training, mice (28 WT and 25 nCOX-2 cKO) were subjected to four 3-minute trials in the maze, and the data for all four trials were averaged for each mouse. On day 5 the target hole was covered and the mice were subjected to two 90-second trials to assess retention of spatial memory. The number of error holes explored before reaching the target hole (B), the latency to first reach the target hole (C), the total number of errors made before escaping into the target hole (D) and the time required to escape into the target hole (E) were measured. Data represent mean ±SEM. The two genotypes did not differ in any measure by two-way ANOVA with repeated measures (D1–D4): genotype × day interaction (B) F=2.101, p=0.102; (C) F=2.651, p=0.051; (D) F=0.323, p=0.089; (E) F=0.042, p=0.988. There was a substantial main effect of training for all measures: panel B, F=7.22, p=0.0001; panel C, F=30.27, p<0.0001; panel D, F=24.95, p<0.0001; panel E, F=62.2, p<0.0001. Comparison of the two genotypes on the test day (D5) was made by t-tests: (B,D) p=0.359; (C,E) p=0.132.
Barnes maze
Spatial memory was assessed with the Barnes maze 6 d before SE and 22 d after SE using the same cohorts of mice so both anterograde and retrograde memory could be assessed (Fig. 1A) (Barnes, 1979). The Barnes maze protocol and apparatus were modified from McLay et al. (1998) and Sunyer et al. (2007). Four days of training were followed by a retention test on Day 5. The maze was located in a bright room with visual cues and the apparatus was isolated from the experimenter during testing. Mice were individually trained in the apparatus, which consisted of a white, circular platform 92 cm in diameter with 20 equally spaced holes (5 cm diameter) located 2.5 cm from the edge. The platform was elevated 76 cm above the ground and was able to rotate 360°. During training, 19 holes were closed and one hole led to the dark escape box (20 cm × 9 cm × 7.5 cm) beneath the platform. From the surface of the maze, the open escape hole looked identical to the closed holes, therefore the mice could not visually identify the escape hole location until it was approached closely. The escape hole’s location was fixed in relation to the visual cues in the room but the table was rotated in between animals to eliminate possible odor cues. On each training day, mice were given four trials with an inter-trial interval of 15 min. Each trial began by placing the animal under a black start box (13 cm diameter, 7.5 cm height) in the middle of the platform for 10 s. After 10 s, the start box was lifted and mice were allowed to freely explore the apparatus. Mice that failed to find the escape hole within 3 min were gently guided there. All animals were allowed to remain in the escape box for 60 s after entering and were prevented from emerging prematurely. Primary latency, latency to escape, primary errors, and total errors were recorded. Primary latency was defined as the time it took each animal to make initial contact (nose or forepaws) with the escape hole regardless of whether they entered, and latency to escape was defined as the time it took for the animal to enter the escape hole (defined by all 4 paws leaving the surface of the table). Errors were defined by a nose poke into or forepaw contact with a false hole. Primary errors were the number of errors that occurred prior to the initial contact with the escape hole and total errors were the number of errors the mouse made before entering the escape hole. After four training days, on Day 5 two retention trials were performed with an inter-trial interval of 15 min i which all 20 holes were closed, including the escape hole. After 10 s in the start box mice were allowed 90 s to explore the maze. Latency to the target hole location (previously location of the escape box) and errors made before reaching the target hole location were recorded. All trials were recorded with a Sony Handycam and the videos were later analyzed by an observer unaware of treatment and genotype.
Statistical Outliers
On the retention test (Day 5) before or after pilocarpine treatment, mice that scored a number of errors or latency to reach the target hole location that were far from the mean values of their respective groups were identified as a statistical outlier (p<.05), as determined by the Grubb’s Test performed by GraphPad QuickCalcs with significance set to α = 0.05. By this means we identified one WT mouse that did not survive pilocarpine treatment, one WT Saline mouse, and one cKO Pilocarpine mouse as significant outliers. These three animals were excluded from all analyses.
Statistical analysis
For parametric values we calculated mean and standard error of the mean (SEM). Comparisons were made by Fisher’s exact test (Fig. 2), two-way ANOVA followed by post-hoc Bonferroni testing (Fig. 3,4 and to assess individual days for Fig. 1 and 5), or two-way ANOVA with repeated measures followed by post-hoc Bonferroni testing (Fig. 1 and 5). Graphpad Instat was employed for all statistical comparisons.
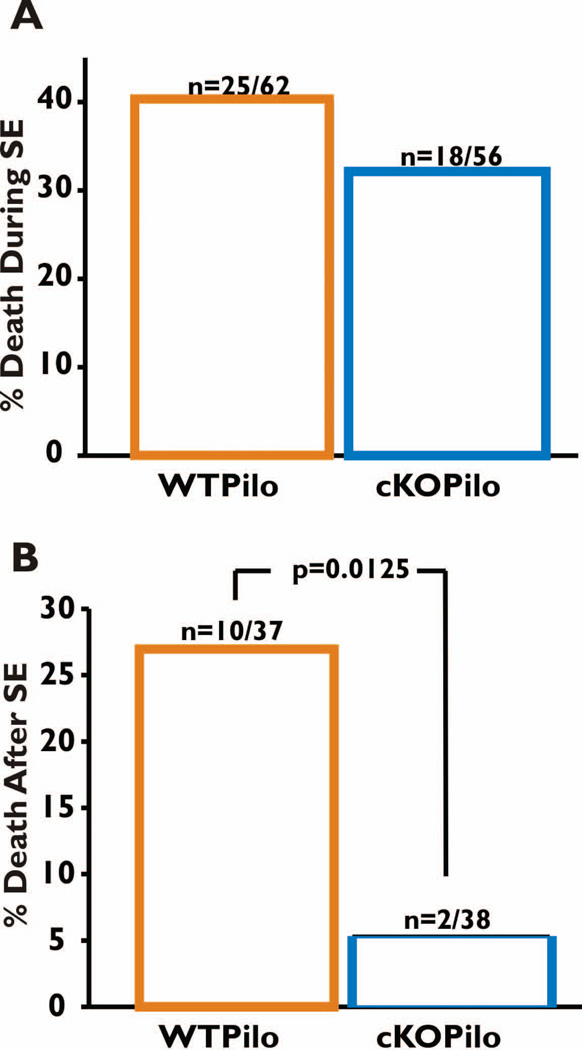
Figure 2. Survival rate of WT and nCOX-2 cKO mice during and after SE.
A. Mortality during SE was defined as deaths that occurred between the times of pilocarpine and pentobarbital/diazepam injections. Out of 62 WT mice, 25 died during SE, whereas a similar proportion (18 of 56) nCOX-2 cKO mice died during SE (Fischer’s exact test p = 0.45). B. Mortality after SE, defined as deaths that occurred during the seven days after termination of SE, was significantly different between the two genotypes (Fischer’s exact test p = 0.0125).
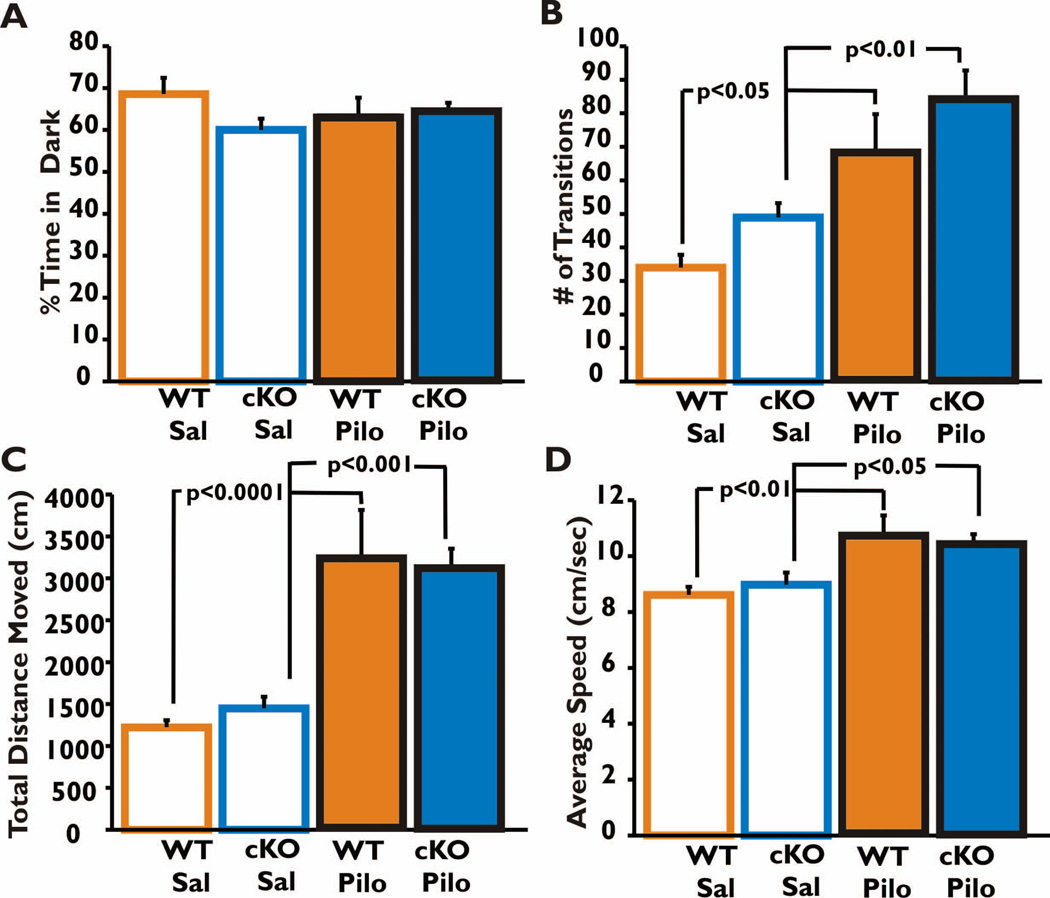
Figure 3. Anxiety and locomotor activity 21 days after SE.
Light-dark preference (A), the number of transitions between the light and dark sides of the box in the 10 min trial period (B), the total distance traveled (C) and average speed (D) were compared among the four groups of mice. All groups had similar preference for the dark (A). Both saline-treated control groups (WT and nCOX-2 cKO) were significantly less active than their respective pilocarpine-treated groups, but for both treatments the two genotypes behaved similarly (B, C). There was no interaction between genotype and treatment (in panel B, F=0.002, p=0.96; in panel C, F= 0.44, p=0.51; in panel D, F=0.72, p=.40), but there was a strong main effect of treatment (p<0.0001 for both panels B,C; p=.0004 in panel D). The data were analyzed by two-way ANOVA with Bonferroni post-test; the post-hoc p-values shown. WT Saline n=8, nCOX-2 cKO Saline n=9, WT Pilocarpine n=7, and nCOX-2 cKO Pilocarpine n=11.
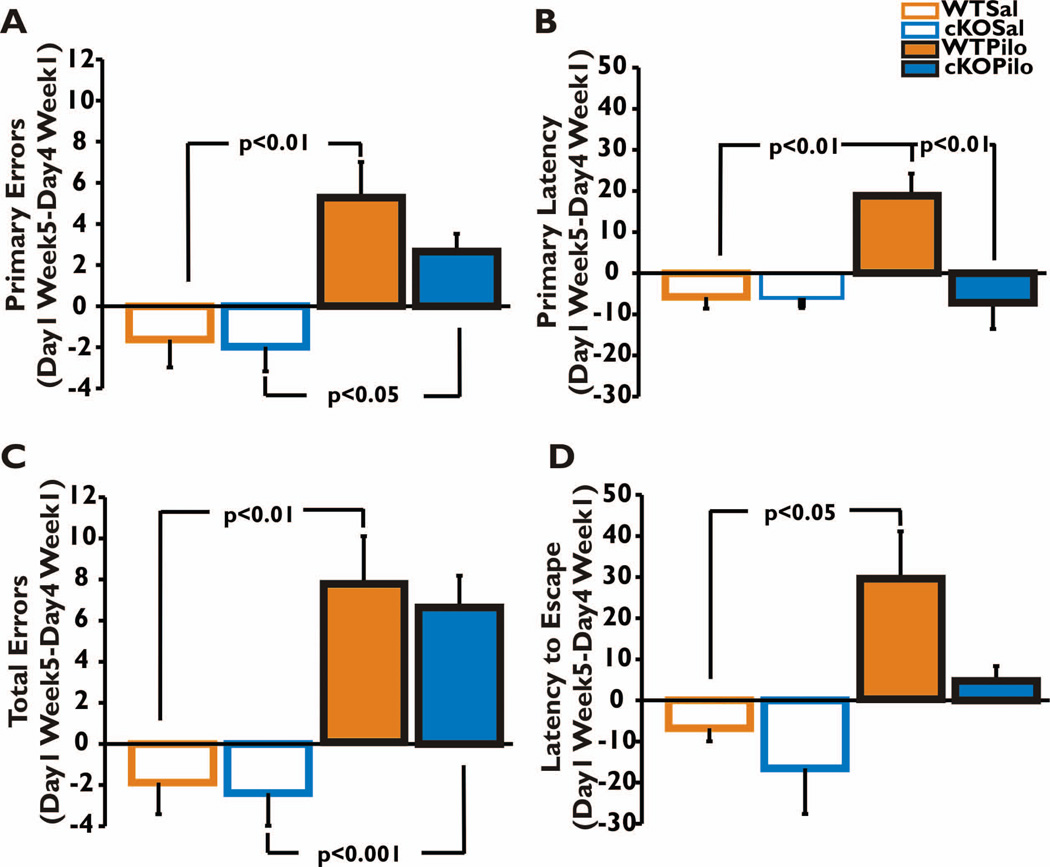
Figure 4. Retrograde amnesia: comparing Barnes maze performance before and after SE.
The difference in error and latency measures made between Day 1 of Week 5 and Day 4 of Week 1 were calculated and plotted for each of the four groups. The data were analyzed by two-way ANOVA with post-hoc Bonferroni tests. The difference in number of errors was significantly higher for both WT and nCOX-2 cKO mice that received pilocarpine compared to their respective saline groups (A,C). However, the difference in time it took to make initial contact with the hole (Primary Latency) was significantly higher only for the WT Pilocarpine group when compared to their respective saline group and to the nCOX-2 cKO Pilocarpine group (B). The WT Pilocarpine group also had a significantly higher difference in latency to enter the hole compared to the WT Saline but no other significant differences between groups were detected using this measure of memory (D). Main effect of treatment p<0.0001 in both panels A and C, p=0.022 in panel B, and p=0.001 in panel D; main effect of genotype p=0.244 in panel A, p=0.011 in panel B, p=0.620 in panel C and p=0.035 in panel D. The interaction between treatment and genotype was non-significant for every measure except primary latency (panel A, F=0.830, p=0.369; B, F=7.105, p=0.012; C, F=0.042, p=0.840; D, F=0.940, p=0.340). WT Saline n=8, nCOX-2 cKO Saline n=9, WT Pilocarpine n=7, and nCOX-2 cKO Pilocarpine n=11.
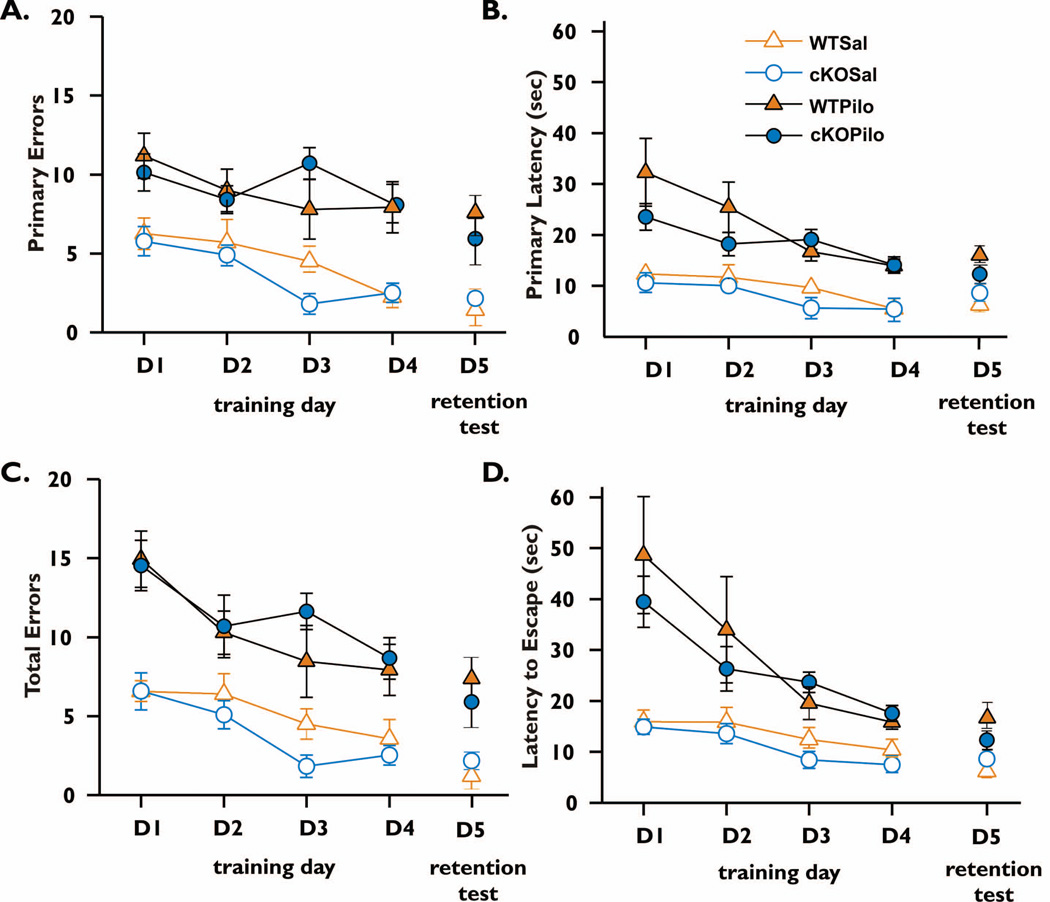
Figure 5. Barnes maze performance after SE.
For each of four days of training performed 21 days after pilocarpine, mice were subjected to four 3-minute trials in the maze as in Figure 1, and the data for all four trials were averaged for each mouse. After 4 days of training, on day 5 the target hole was covered to assess retention of spatial memory and mice were allowed to explore the maze during two 90 sec trials. The number of error holes explored before reaching the target hole (A), the latency to first reach the target hole (B), the total number of errors made before escaping into the target hole (C) and the time required to escape into the target hole (D) were measured. Data represent mean (±SEM) of four trials per day for D1–D4 and two trials on D5. Animals of both genotypes performed more poorly on all measures when treated with pilocarpine compared with saline (main effect of treatment in panel A WT, F=16.7, p=0.0013; panel A cKO F=65.6, p<0.0001; panel B WT F=16.7, p=0.0013; cKO F=45.9, p<0.0001; panel C WT, F=13.6, p=0.0027; cKO F=45.7, p<0.0001; panel D WT F=8.23, p=0.0132; cKO F=35.8, p<0.0001). Both genotypes performed similarly under both treatments (main effect of genotype Sal (A) F=1.592, p=0.2045; (B) F=0.7261, p=0.5417; (C) F=1.326, p=0.2777; (D) F=0.3587, p=0.7831; for pilocarpine (A) F=0.8554, p=0.4707; (B) F=2.064, p=0.1174; (C) F=0.4742, p=0.7017; (D) F=1.053, p=0.3777). A significant interaction between genotype and treatment was found on training day 3 for both primary errors (panel A, F=6.59, p=0.0153) and total errors made (panel C, F=5.36, p=0.0274). However, for other training days there was no interaction between treatment and genotype for any measure (panel A, F ranged from 0.00 to 0.02 and p ranged from 0.886 to 0.993; panel B, F=0.08 to 3.03 and p=0.092 to 0.777; panel C, F=0.02 to 0.53 and p= 0.474 to 0.887; panel D, F=0.26 to 3.69 and p=0.064 to 0.616). There was a strong main effect of training day under all conditions (in panels A-D, F ranged from 3.08 to 11.0 and p ranged from 0.0385 to <0.0001), except for panel A cKO mice treated with pilocarpine (F=2.63, p=0.0590), indicating that mice learned the maze at a similar rate. On D5, the retention trial, the WT Pilocarpine group made significantly more errors and took longer to locate the original escape hole location compared to their respective saline group (A/C,B/D). nCOX-2 cKO mice showed a trend to poorer performance after pilocarpine compared to control, but this did not reach significance (panels A/C,B/D). There was no interaction between treatment and genotype in the retention test: A, F=0.873, p=0.357; B, F=3.380, p=0.076. WT Saline n=8, nCOX-2 cKO Saline n=9, WT Pilocarpine n=7, and nCOX-2 cKO Pilocarpine n=11.
Results
Behavioral Baseline Before SE
Light Dark Exploration
Behavioral analysis was conducted before and after the induction of status epilepticus according to Fig. 1A. Baseline anxiety and locomotor behavior were examined prior to treatment with pilocarpine or saline. nCOX-2 cKO mice did not differ significantly from their WT littermates in their preference for the dark (data not shown). The two groups did not differ in the number of transitions made, total distance traveled, percent time spent in motion or average speed in the activity box. These results demonstrate that neuronal COX-2 deletion does not affect general locomotor ability or preference for the dark, suggesting similar baseline anxiety and activity levels in the two genotypes.
Memory testing
We used the Barnes maze paradigm to assess spatial memory (Barnes, 1979). Animals were allowed to explore the apparatus for a maximum of 3 minutes on days 1–4, and for 90 seconds when the target hole was covered on day 5. Prior to pilocarpine treatment, nCOX-2 cKO mice did not differ from WT mice in the number of primary or total errors made during training or test trials (Fig. 1B,D). There was also no difference between nCOX-2 cKO and WT in the time to make initial contact with the escape hole (primary latency) or in the latency to escape during training or retention trials (Fig. 1C,E). These results suggest that at baseline, constitutive neuronal COX-2 is not prominently involved in these measures of spatial learning and memory.
Pilocarpine Treatment
Mortality during and after SE
Once baseline behavior of both genotypes had been established we explored the effects of SE using the pilocarpine model in the same mice tested in Fig. 1. Four groups of mice were studied: WT and nCOX-2 cKO mice treated with either pilocarpine or saline. The mortality rate observed during SE did not differ significantly between nCOX-2 cKO animals and WT animals (Fig. 2A; Fisher’s exact test p=0.45). Moreover, in a previous study the latency to the first behavioral or electrographic seizure and the temporal evolution of behavioral seizures into status epilepticus following pilocarpine injection also were not different between genotypes. The intensity of electrographic seizures during SE (evaluated by the EEG coastline index, modified from Korn et al. 1987) was also not significantly different between the two genotypes (Serrano et al., 2011). From these observations we conclude that the postnatal ablation of COX-2 from a subset of forebrain neurons does not modify the threshold or intensity of seizures triggered acutely by pilocarpine injection. However, it is possible, perhaps likely (see Discussion), that the anti-inflammatory consequences of the nCOX-2 cKO exert an anticonvulsant effect in the days to weeks after pilocarpine administration.
In the week following SE a striking finding was that fewer nCOX-2 cKO mice died compared to WT (Fig. 2B; WT=27%, cKO=5.3%, Fisher’s exact test p=0.0125. Recurrent seizures were not observed in either group during the week after SE but could have occurred unnoticed at night. These results indicate that neuron-derived COX-2 induction increases the likelihood of delayed mortality in mice following SE. Mice surviving pilocarpine and saline treatment were subjected to further behavioral testing described below.
Behavioral Measurements After SE
Light Dark Exploration
Twenty-one days after SE all four groups (WT or nCOX-2 cKO treated with saline or pilocarpine) had a similar preference for the dark (Fig. 3A; two-way ANOVA F=2.103, p=0.157). This suggests that the four groups did not differ after SE in their anxiety levels based on treatment or genotype. Pilocarpine-treated animals of both genotypes made significantly more transitions between light and dark compartments (Fig. 3B) and traveled a significantly larger distance over the 10 min period allowed for exploration (Fig. 3C). Mice of both genotypes also moved around the enclosure faster after pilocarpine (Fig. 3D) spending 49% of their time in motion whereas saline-treated mice spent 24–26% time in motion. These data demonstrate increased locomotor activity three weeks after pilocarpine and show that neuronal COX-2 induction is not involved in this effect.
Improved performance in the Barnes maze in the nCOX-2 cKO following pilocarpine treatment
To investigate the animals’ ability to remember their initial training after treatment with pilocarpine or saline, we subtracted the average value of the four trials for each animal’s primary errors, primary latency, total errors, and latency to escape on Day 1 of training after SE (Week 5) from the average values during the last day of training before SE (Day 4 Week 1). Since no training was received in the intervening three weeks after pilocarpine or vehicle treatment, the difference between the two is an indicator of retention of an animal’s memory for events prior to SE, i.e., retrograde memory. Saline-treated nCOX-2 cKO and WT mice did not differ in any measure (Fig. 4A–D; p>0.05). Both genotypes treated with pilocarpine made more primary and total errors, in contrast to control saline-treated mice, which performed equally as they had three weeks earlier (Fig. 4A,C). WT pilocarpine-treated mice took longer to escape compared to saline-treated controls (Fig. 4B,D), reflecting the expected deficit in retrograde memory. However, a conspicuous observation was that the nCOX-2 cKO mice performed equally well as control mice after pilocarpine treatment, both in measures of primary latency and latency to escape (Fig. 4B,D).
Barnes maze acquisition after pilocarpine
We compared performance by genotype in the Barnes maze during the training period, which occurred 22–25 days after pilocarpine, in order to uncover potential differences in learning capabilities. Animals were again allowed to explore the apparatus for a maximum of 3 minutes. The two genotypes (nCOX-2 cKO and WT) treated with saline did not differ on any training day for any measure of learning in the Barnes maze but for each measure there was a significant effect of day (Fig. 5), indicating that both genotypes were able to learn the maze over the four days. Likewise, pilocarpine-treated mice of both genotypes did not significantly differ in any measure on any acquisition day but there was a significant effect of day for most measures again indicating that both genotypes were able to learn after pilocarpine treatment (Fig. 5A). In the retention test, which occurred the day after four training days described above, animals were allowed to explore the apparatus for a maximum of 90 seconds with all escape holes closed. WT pilocarpine-treated mice made more errors and took longer to identify the previous escape hole location than their WT saline-treated controls (Fig. 5 retention tests). Although the performance measures of the nCOX-2 cKO were similar for saline and pilocarpine treatments, nonetheless there was no genotype × treatment effect for any measure. Taken together with figure 4, these data suggest that memory acquisition three weeks after pilocarpine treatment is not reliably influenced by neuronal COX-2, but that one feature of retrograde memory – time to locate a previously learned target – is improved in nCOX-2 cKO mice that experienced SE.
Discussion
The very low delayed mortality in the week after SE observed in nCOX-2 cKO mice (Fig. 2) was an interesting and unexpected finding. Epileptic patients refractory to medication are at high risk for sudden unexplained death (Hirsch et al., 2011). The mechanisms underlying sudden unexplained death in epilepsy (SUDEP) are unknown and probably multifactorial, but are strongly correlated with the frequency of uncontrolled seizures (Shorvon and Tomson, 2011). To our knowledge neuronal COX-2 signaling is the first pathway to be implicated in the delayed mortality that follows status epilepticus that does not also noticeably attenuate stutus epilepticus itself. The cause of reduced delayed mortality after status epilepticus in mice has not been explored but could involve reduced inflammation and neurodegeneration experienced by the nCOX-2 cKO mice after SE (Serrano et al., 2011), perhaps resulting in fewer recurrent seizures in the days after SE. Whether the brain inflammation produced by neuronal COX-2 induction is important for seizure recurrence in the days after SE is worthy of study.
It is well established that rodents treated with pilocarpine experience difficulties in learning and memory and many studies correlate these cognitive impairments with the neurodegeneration and neuronal plasticity observed following SE (Persinger et al., 1993; Hort et al., 1999; Mohajeri et al., 2003; Mohajeri et al., 2004; Muller et al., 2009). Taking into consideration the finding that COX-2 plays an important role in synaptic plasticity (Murray & O'Connor, 2003; Chen & Bazan, 2005; Slanina & Schweitzer, 2005; Yang & Chen, 2008), we wanted to investigate the role of COX-2 induction in principal forebrain neurons in the motor and cognitive sequelae of SE.
Results from behavioral studies on untreated animals of either genotype demonstrated that spatial learning and memory (Fig. 1) as well as locomotion and anxiety are not noticeably modulated by neuronal COX-2. Three weeks following SE the survivors of both genotypes were also similar in most behavioral measures. However in their first training day nCOX-2 cKO mice were able to locate the target hole as quickly as control mice of either genotype treated with saline rather than pilocarpine, whereas WT mice treated with pilocarpine took much longer to locate the target hole (Fig 4B, p<.01). Although there was a visible trend towards genotype differences in primary errors (fig 4A) and latency to escape (Fig 4D), neither reached significance. The neuronal COX-2 dependent deficit in retrograde memory caused by status epilepticus thus does not appear robust. Whether the subtle improvement in performance of nCOX-2 cKO mice in the retrograde memory test (quicker to the target hole but no fewer errors) involves better executive function in picking a search strategy, less impulsivity, or simply improved retrograde memory after pilocarpine, cannot be determined from the data at hand. However, the results imply that some feature of seizure-induced memory deficit is dependent upon neuronal COX-2 pathways. It is well known that hippocampal damage, which is reduced in the nCOX-2 cKO (Serrano et al., 2011), produces retrograde memory deficits (Squire et al., 2001), but in some instances the measured deficits have been caused by performance rather than recall problems (Clark et al., 2005). A somewhat trivial explanation, namely that nCOX-2 cKO mice simply ran around the maze faster than WT mice, is contradicted by direct comparison (Fig 3C,D), and by the observation that pilocarpine-treated mice of both genotypes traveled faster than saline controls. It is also clear in our studies that the retrograde memory deficit after pilocarpine was not due to the inability to navigate, because after pilocarpine the mice could relearn the task at a similar rate as before pilocarpine (cf Fig. 5 and 1). Both saline-treated genotypes showed improved learning compared with pilocarpine-treated animals as expected from retained memory of training prior to treatment.
Although the effect of global inhibition of COX-2 on neurodegeneration observed after SE depends on the timing of drug administration (Baran et al., 1994; Baik et al., 1999; Kunz and Oliw, 2001; Jung et al., 2006; Takemiya et al., 2006; Kim et al., 2008; Toscano et al., 2008; Zhang et al., 2008; Polascheck et al., 2010), we have demonstrated that delayed apoptotic neuronal injury in CA1 pyramidal cells four days to four months after SE, as judged by FluoroJade, TUNEL and Nissl staining, was drastically reduced in the nCOX-2 cKO (Serrano et al., 2011) and similar results have been observed using COX-2 inhibitors (Jung et al., 2006; Polascheck et al., 2010). The nCOX-2 cKO mice also showed less gliosis, less proinflammatory cytokine formation, and an intact blood-brain barrier after SE. Taken together we can infer that numerous physiological responses are improved in the nCOX-2 cKO. By contrast, global pharmacologic inhibition of COX-2 before or after SE appears to worsen mortality (Polascheck et al., 2010; Holtman et al., 2010), suggesting that non-neuronal COX-2 may mediate protective effects that would argue against the use of COX-2 inhibitors after status epilepticus.
Evidence is mounting that brain inflammation is pro-epileptogenic and promotes spontaneous seizures (Vezzani et al., 2011, 2012). For example, the frequency of spontaneous seizures in the chronic phase of a kainate epilepsy model is reduced by inhibitors of caspase I (which synthesizes IL-1β) (Maroso et al., 2011) or inhibitors of toll-like receptor 4 (Maroso et al., 2010), both of which exert anti-inflammatory effects. It is possible that elements of the inflammatory cascade triggered by neuronal COX-2 induction (Serrano et al., 2011) promote the appearance of recurrent seizures, which are the key events responsible for delayed mortality and cognitive deficits. Alternatively the inflammatory pathways themselves downstream of COX-2 could be maladaptive and underlie the panoply of morbidity and delayed mortality after status epilepticus. Selection between these hypotheses would require quantitative EEG recordings in the days and weeks following status epilepticus.
The COX-2 enzyme sits atop an extensive signaling cascade that involves the cell-specific production of five different prostanoids and activation of at least nine G-protein coupled receptors (Smyth et al., 2009). Rather than blocking the whole cascade with a COX-2 inhibitor, it will be important to determine which prostaglandin receptors mediate retrograde memory loss, delayed neurodegeneration and delayed mortality after SE. The prostanoid receptors, or prostaglandin synthases, may offer more selective and effective therapeutic targets. To this end, we have recently shown that a novel selective antagonist of the EP2 receptor for PGE2 is neuroprotective in the mouse pilocarpine model (Jiang et al., 2012) and recapitulates most of the other effects of the nCOX-2 cKO (Jiang et al., submitted).
Acknowledgments
This work was supported by National Institutes of Health grants R21NS074169 (RD) and F32-NS064695 (GS), and the CounterACT Program, Office of the Director, National Institutes of Health (OD) and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number U01NS058158.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Baik EJ, Kim EJ, Lee SH, Moon C. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843:118–129. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- Baran H, Vass K, Lassmann H, Hornykiewicz O. The cyclooxygenase and lipoxygenase inhibitor BW755C protects rats against kainic acid-induced seizures and neurotoxicity. Brain Res. 1994;646:201–206. doi: 10.1016/0006-8993(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bergin PS, Thompson PJ, Baxendale SA, Fish DR, Shorvon SD. Remote memory in epilepsy. Epilepsia. 2000;41:231–239. doi: 10.1111/j.1528-1157.2000.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Borges K, McDermott DL, Dingledine R. Reciprocal changes of CD44 and GAP-43 expression in the dentate gyrus inner molecular layer after status epilepticus in mice. Exp Neurol. 2004;188:1–10. doi: 10.1016/j.expneurol.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Chen C, Bazan N. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;93:929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- Ciceri P, Zhang Y, Shaffer AF, Leahy KM, Woerner MB, Smith WG, Seibert K, Isakson PC. Pharmacology of celecoxib in rat brain after kainate administration. J Pharmacol Exp Ther. 2002;302:846–852. doi: 10.1124/jpet.302.3.846. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Burgess S, Gamble-George J, Zeitlin R, Lin X, Cao C, Arendash GW. Sorafenib inhibits nuclear factor kappa B, decreases inducible nitric oxide synthase and cyclooxygenase-2 expression, and restores working memory in APPswe mice. Neuroscience. 2009;162:1220–1231. doi: 10.1016/j.neuroscience.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch LJ, Donner EJ, So EL, Jacobs M, Nashef L, Noebels JL, Buchhalter JR. Abbreviated report of the NIH/NINDS workshop on sudden unexpected death in epilepsy. Neurology. 2011;76:1932–1938. doi: 10.1212/WNL.0b013e31821de7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res. 2010;91:49–56. doi: 10.1016/j.eplepsyres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Hort J, Brozek G, Mares P, Langmeier M, Komarek V. Cognitive functions after pilocarpine-induced status epilepticus: changes during silent period precede appearance of spontaneous recurrent seizures. Epilepsia. 1999;40:1177–1183. doi: 10.1111/j.1528-1157.1999.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, Qui M, Speigel I, Rojas A, Lelutiu N, Dingledine R. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A. 2012;109:3149–3154. doi: 10.1073/pnas.1120195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23:237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kapur N, Millar J, Colbourn C, Abbott P, Kennedy P, Docherty T. Very long-term amnesia in association with temporal lobe epilepsy: evidence for multiple-stage consolidation processes. Brain Cogn. 1997;35:58–70. doi: 10.1006/brcg.1997.0927. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chung JI, Lee SH, Jung YS, Moon CH, Baik EJ. Involvement of endogenous prostaglandin F2alpha on kainic acid-induced seizure activity through FP receptor: the mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res. 2008;1193:153–161. doi: 10.1016/j.brainres.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainite-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13:569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- Lah S, Lee T, Grayson S, Miller L. Effects of temporal lobe epilepsy on retrograde memory. Epilepsia. 2006;47:615–625. doi: 10.1111/j.1528-1167.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Lah S, Lee T, Grayson S, Miller L. Changes in retrograde memory following temporal lobectomy. Epilepsy Behav. 2008;13:391–396. doi: 10.1016/j.yebeh.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Grande LJ, Bauer RM. Temporal lobe epilepsy as a model to understand human memory: the distinction between explicit and implicit memory. Epilepsy Behav. 2006;9:1–13. doi: 10.1016/j.yebeh.2006.04.012. [DOI] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes Maze. Physiol Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Bazan NG. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem. 1996;271:24794–24799. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrande M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Iori V, Wright C, French J, Vezzani A. ICE/Caspase-1 inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, Kaufmann WE, Vehmas A, Andreasson KI. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer's disease in a sex-dimorphic pattern. Neuroscience. 2006;141:1149–1162. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, Omae K. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- Mohajeri MH, Madani R, Saini K, Lipp HP, Nitsch RM, Wolfer DP. The impact of genetic background on neurodegeneration and behavior in seizured mice. Genes Brain Behav. 2004;3:228–239. doi: 10.1111/j.1601-1848.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- Mohajeri MH, Saini K, Li H, Crameri A, Lipp HP, Wolfer DP, Nitsch RM. Intact spatial memory in mice with seizure-induced partial loss of hippocampal pyramidal neurons. Neurobiol Dis. 2003;12:174–181. doi: 10.1016/s0969-9961(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Muller CJ, Groticke I, Bankstahl M, Loscher W. Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice. Exp Neurol. 2009;219:284–297. doi: 10.1016/j.expneurol.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Murray HJ, O'Connor JJ. A role for COX-2 and p38 mitogen activated protein kinase in long-term depression in the rat dentate gyrus in vitro. Neuropharmacology. 2003;44:374–380. doi: 10.1016/s0028-3908(02)00375-1. [DOI] [PubMed] [Google Scholar]
- Persinger MA, Bureau YR, Kostakos M, Peredery O, Falter H. Behaviors of rats with insidious, multifocal brain damage induced by seizures following single peripheral injections of lithium and pilocarpine. Physiol Behav. 1993;53:849–866. doi: 10.1016/0031-9384(93)90261-d. [DOI] [PubMed] [Google Scholar]
- Polascheck N, Banksthal M, Löscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp. Neuro. 2010;224:219–233. doi: 10.1016/j.expneurol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Preston AR, Gabrieli JD. Different functions for different medial temporal lobe structures? Learn Mem. 2002;9:215–217. doi: 10.1101/lm.54702. [DOI] [PubMed] [Google Scholar]
- Sandhya TL, Ong WY, Horrocks LA, Farooqui AA. A light and electron microscopic study of cytoplasmic phospholipase A2 and cyclooxygenase-2 in the hippocampus after kainate lesions. Brain Res. 1998;788:223–231. doi: 10.1016/s0006-8993(97)01552-7. [DOI] [PubMed] [Google Scholar]
- Serrano G, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011;31:14850–14860. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina K, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50:S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378:2028–2038. doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sunyer B, Patil S, Höger H, Lubec G. Barnes Maze, a useful task to assess spatial reference memory in the mice. Nature Protocols. 2007;198:58–68. [Google Scholar]
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56:103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Kingsley PJ, Marnett LJ, Bosetti F. NMDA-induced seizure intensity is enhanced in COX-2 deficient mice. Neurotoxicology. 2008;29:1114–1120. doi: 10.1016/j.neuro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nature Rev. Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine R. The role of inflammation in epileptogenesis. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.04.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des. 2008;14:1443–1451. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Sun RP, Lei GF, Yang L, Liu CX. Cyclooxygenase-2 inhibitor inhibits hippocampal synaptic reorganization in pilocarpine-induced status epilepticus rats. J Zhejiang Univ Sci B. 2008;9:903–915. doi: 10.1631/jzus.B0820018. [DOI] [PMC free article] [PubMed] [Google Scholar]