Abstract
Age-related changes in cancer mortality risk are important for understanding the processes of disease and aging interaction. The extent to which these age changes differ by sex further contributes to this understanding but has not been well studied to date. We conducted a systematic examination of dynamics and heterogeneity of age changes in cancer mortality rates for the top 14 cancer sites using vital statistics from the NCHS and SEER between 1969 and 2007. We assessed patterns of age changes in site-specific mortality rates in terms of both increase (age slope) and acceleration (change in age slope) as measured by the log-log acceleration rate (LLA). We assessed sex differences in mortality rates through sex mortality rate ratios and sex differences in age changes through comparisons of the LLA by sex. The logged male-to-female mortality ratios are positive but vary substantially with age in magnitude. And the age patterns of sex ratios also vary across sites. The LLA values show similar declines and hence slowdowns of mortality increment into or during old age for both sexes for most sites and periods. Post-reproductive changes in sex differences in cancer mortality are not entirely consistent with the estrogenic hypothesis about the anticarcinogenic effects of sex hormones and suggest the utility of the multistage model of disease progression for some tumor sites. Analysis of age dynamics and sex differences in cancer mortality may modify extant aging-related theories of carcinogenesis and frame future searches for specific explanatory factors.
Keywords: aging, sex differences, cancer mortality, multistage progression, estrogen
1. Introduction
As the second leading cause of death in the United States and the disease most prevalent in older people, cancer is killing an ever increasing number of Americans as the population ages. The relationship between cancer and aging, in this context, becomes increasingly important for the understanding of not only cancer etiology but also the prospect of human longevity. The fact that cancer incidence rates increase dramatically in late life in mammalian species suggests potential common causative mechanisms shared by cancer and aging such as genetic instability leading to improper gene regulation (Cutler and Semsei 1989). There is also considerable evidence that increases in cancer incidences slow at advanced age and further suggest that the processes of cancer and senescence might be closely related (Frank 2007; Manton and others 2009). To more fully understand this relationship in humans, however, we need more direct assessment of the age dynamics of cancer mortality rates at the population level. In addition, population heterogeneity and cancer site provide clues to physiological and behavioral processes leading to mortality but have not been systematically examined in relation to aging. This study focuses on sex differences and mortality from leading cancer sites that can further inform the specific mechanisms underlying the process of disease and aging interaction.
Sex differentials in mortality, namely female advantages in survival, have been widely observed across time, space, cause of death, and even species (Austad 2006; Carey and Liedo 1995). This pattern applies to cancer related mortality. It has been shown that males are at much higher risks of cancer incidence and mortality for a vast majority of sites (Cook and others 2009; Cook and others 2011; Manton and others 2009) that largely result from cancer etiology instead of prognosis (Cook and others 2011). The higher male incidence rates, from the point of view of evolutionary biology of aging, may reflect sex differences in strategies of “fighting external stress” in males and “fighting physiological aging” in females (Arbeev and others 2005). In addition, the prominent male excess in cancer mortality risk has been expected to result from sex differences in physiological risk factors such as gonadal hormones. Specifically, estrogen may protect women at the cellular level in terms of immune competence, fat and glucose metabolism, and carcinogenic susceptibility (Cook and others 2011; Yang and Kozloski 2011). While a number of physiological and behavioral factors have been proposed as potential explanations for this pattern, a full understanding of the origins and mechanisms in sex differences in cancer as well as all-cause mortality has remained elusive (IOM 2001). We argue that studying how sex differences in cancer mortality risk unfold with aging provides one important avenue toward an improved account of why women live longer than men.
Whereas prior research on this topic focused on the differences in mortality risk within age groups instead of across age groups, there appear to be age-associated changes in sex mortality differentials that remain poorly documented and understood. For instance, studies of both historical data from vital statistics and recent nationally representative survey data show that the positive male-to-female mortality rate ratios in young adulthood decreased in older age for all causes of death except cancer, in which case the ratios increased indicating greater male excesses in cancer-related deaths in late life (Austad 2006; Yang and Kozloski 2011). Given the physiological pathways to intrinsic mortality shared by degenerative diseases, this difference seems an anomaly that requires further investigations. Taking age changes into account may modify extant understanding of carcinogenesis and cancer progression in different sites of origin. For instance, the same “estrogenic hypothesis” (EH thereafter) that is used to explain the female advantage in cancer survival also implies female increases in cancer mortality risk after middle ages due to the loss of those physiological advantages after the reduction of estrogen. That is, sex differences in age pattern of fecundity decline should lead to reductions of sex cancer mortality gaps. Persistent or increasing sex gaps in cancer mortality in post-reproductive life span would be inconsistent with the hypothesis on the anticarcinogenic effects of estrogen.
While the sex disparities mentioned above are based on the metrics of mortality rate at a given age and increase in the rate with age (age slope), a closely related but distinct question that has been less examined is whether sex differentials also reside in cancer mortality acceleration with age (change in age slope). Data on cancer incidence and mortality, for example, show patterns of age acceleration that are different from age increase. While incidence and mortality risks increase with age, the increases occur more slowly or decelerate after the age of 60 (Frank 2007; Horiuchi 1997). This raises the possibility that the potential effect of menopause on cancer mortality risk is not simply reflected by age increases, which are continuous and independent of fecundity declines, but reflected by age acceleration of the mortality increase (Horiuchi 1997). The absence of sex difference in mortality acceleration would then suggest alternative mechanisms that apply to both sexes such as the multistage cancer progression and aging model. Therefore, age variations contribute to the complexity of explanations of sex differences in cancer mortality and need to be systematically examined in population-based studies. Such examinations are particularly useful for testing extant hypotheses or generating new ones on the causes of sex disparities in mortality in relation to cancer etiology and exposures and vulnerabilities to carcinogens related to constitutional endowment, reproductive biology, and social behaviors.
In sum, there is a lack of studies that compare sex differences in both mortality increase and acceleration. And empirical findings from a few prior studies are suggestive of old age changes in the sex gap in cancer mortality but are limited in the coverage of historical period, cancer sites, or number of cancer related deaths (Cook and others 2011; Horiuchi 1997; Yang and Kozloski 2012). This study fills these gaps through systematic examinations of dynamics and heterogeneity of age changes in cancer mortality using vital statistics that span four decades. We assess both age increase and age acceleration in cancer mortality rates and sex differences in these age dynamics for the top cancer sites with diverse etiologies and age trajectories of progression to mortality.
2. Material and Methods
2.1 Data
We obtained the U.S. cancer mortality data collected by the National Center for Health Statistics (NCHS) using the NCI's Surveillance, Epidemiology, and End Results (SEER)*Stat software for the period 1969 to 2007 (Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD). We examined all cancer sites combined, all cancer sites excluding sex-specific and breast, and 14 leading causes of cancer deaths (Jemal and others 2008), including cancers of the lung and bronchus, colon and rectum, pancreas, esophagus, urinary bladder, liver and intrahepatic bile duct, kidney and renal pelvis, stomach, brain and other nervous system, oral cavity and pharynx and melanoma of the skin, leukemia, non-Hodgkin lymphoma, and myeloma. The International Classification of Disease (ICD) versions changed over this time period (ICD-8 to ICD-10). The underlying causes of death are based on the death certificate information reported to the CDC National Vital Statistics and categorized according to SEER site groups to ensure comparability among ICD versions (Jemal and others 2008). The SEER cause of death recode is listed at http://seer.cancer.gov/codrecode/1969+_d09172004/. Denominators in the death rate computation are from county-level population estimates that were summed to the state and national level. More detailed description of the methodologies used for the population estimates by the Census Bureau and the NCI is available elsewhere (Jemal and others 2008).
2.2 Death Rates and Sex Mortality Ratio
For each period spanning a decade apart (1969-1979, 1980-1989, 1990-1999, 2000-2007), we constructed age-specific death rates by site for each sex as the number of deaths per 100,000 person years at exposure between the ages of x and x + 5, which we denote as 5Mx. We present results for ages 30 to 85+ due to much smaller numbers of deaths in younger ages and correspondingly low and unstable death rates. To compare sex differences in mortality rates, we calculated the sex mortality ratio (SMR) as logged male to female ratios of death rates at each age. Use of logarithms provides a scale-free measure of difference. Because the size of the sex ratio will depend on the choice of numerator, taking the log of the ratio makes it symmetrical. Positive values of the SMR indicate higher male cancer mortality rates relative to female mortality rates; negative values indicate lower male relative to female rates. Increases in the SMR with age suggest widening of the sex gap in cancer mortality rates.
2.3 Measure of Aging Rate: Log-log Acceleration Rate
We further compare sex differences in mortality acceleration as measured by the log-log acceleration rate (LLA). The LLA is defined as the percentage change in mortality rate relative to percentage change in age (Frank 2004a, 2004b):
| (1) |
where μ(x) is the force of mortality at exact age x. The LLA relates to the life table aging rate (LAR) commonly adopted in studies of overall mortality (Carey and Liedo 1995) as a measure of relative mortality increase. While the LAR is a scale-free rate relative to scale-specific age, the LLA is scale-free in both rate and age. Additional analyses show highly consistent results using the LAR and LLA. We only present those based on the LLA in the interest of space. The value of the LLA at a given age indicates the rate of mortality increase with respect to age. A higher LLA for the male population than for the female population thus indicates faster male than female relative mortality increase. The slope of the LLA indicates rate of change in mortality increment. That is, an increase in the LLA indicates acceleration in relative mortality increase, whereas a decline in the LLA indicates deceleration in the increase. Similar to the LAR, the LLA can be estimated with data on age-specific death rate by (Horiuchi 1997; Horiuchi and Coale 1990):
| (2) |
The LLA can also be related to the multistage model of carcinogenesis and lends itself to the depiction of cancer progression and the related aging and mortality processes. Genetic and morphological studies of cancer suggest that a tumor progresses through a sequence of stages. The multistage theory of carcinogenesis (MTC thereafter), initially developed in the 1950s (Fisher and Hollomon 1951; Nordling 1953), has been used to mathematically describe the progression of cancer (Frank 2007; Manton and others 2009). Assuming that the development of cancer requires n independent stages at the probability p of experiencing each, it has been shown that the age-specific cancer incidence or mortality rates can be fit to a Weibull model: . Taking log transformation to both sides of the Weibull function, one can derive that the LLA is equal to the slope n-1. Therefore, the deceleration in cancer incidence or mortality rate with age indicated by the decline in the LLA has been proposed to result from surviving population concentrating in the fewer and fewer remaining stages (Frank 2004a).
3. Results
In Figures 1-3, we present the numerical results from the two analyses on mortality increase and mortality acceleration for each cancer site. Table 1 provides a reader's reference of major empirical findings from these analyses.
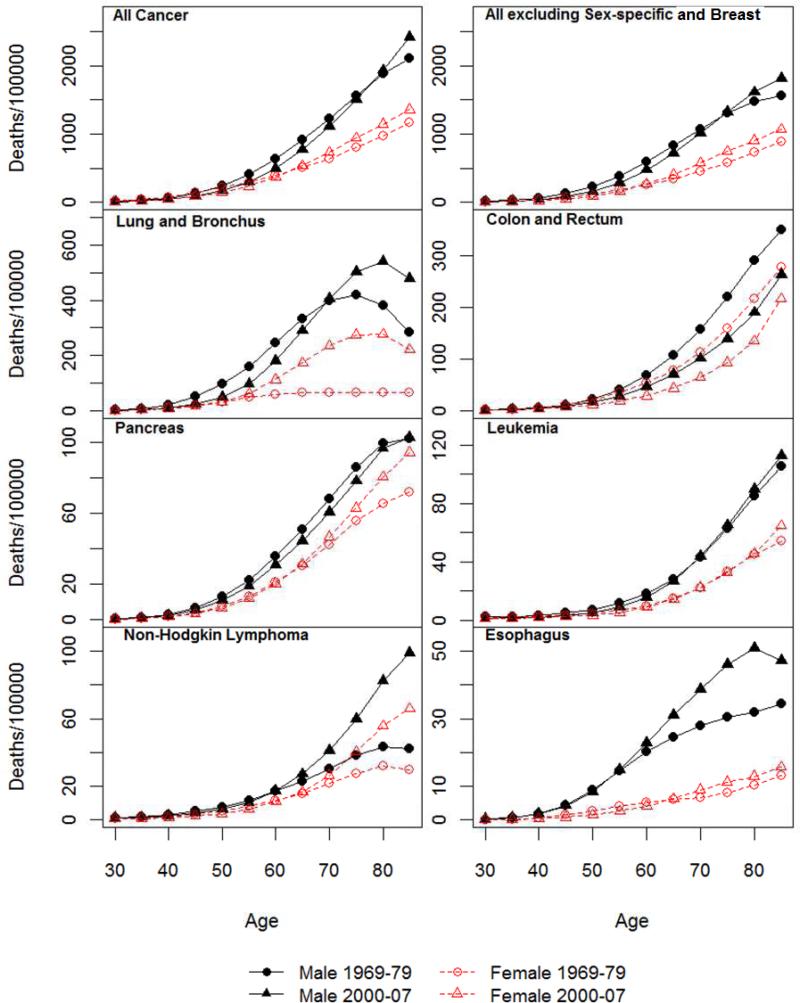
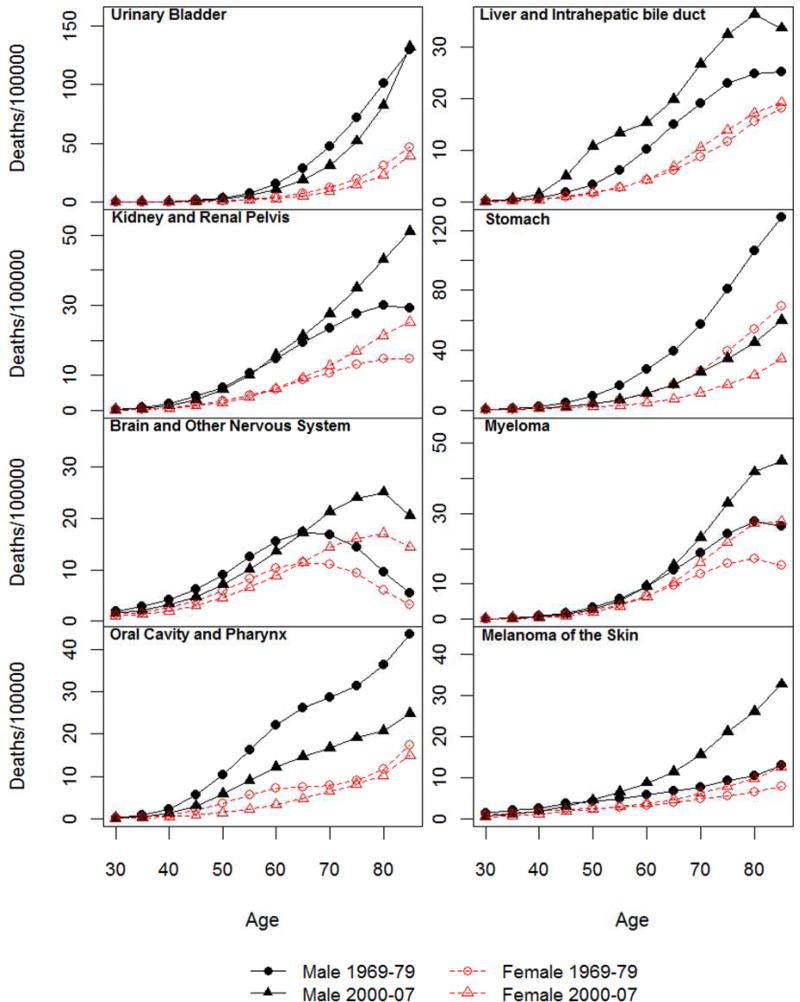
Figure 1.
Age-specific mortality rate for all cancer and 14 non sex-specific leading cancer sites: 1969-1979 and 2000-2007.
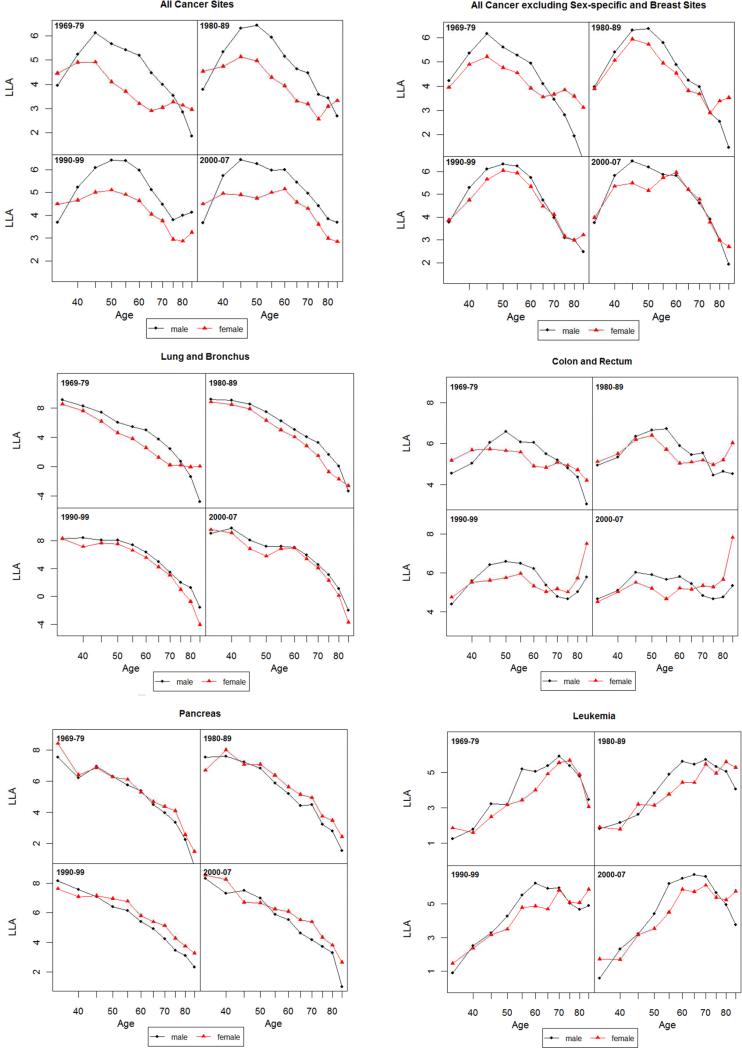
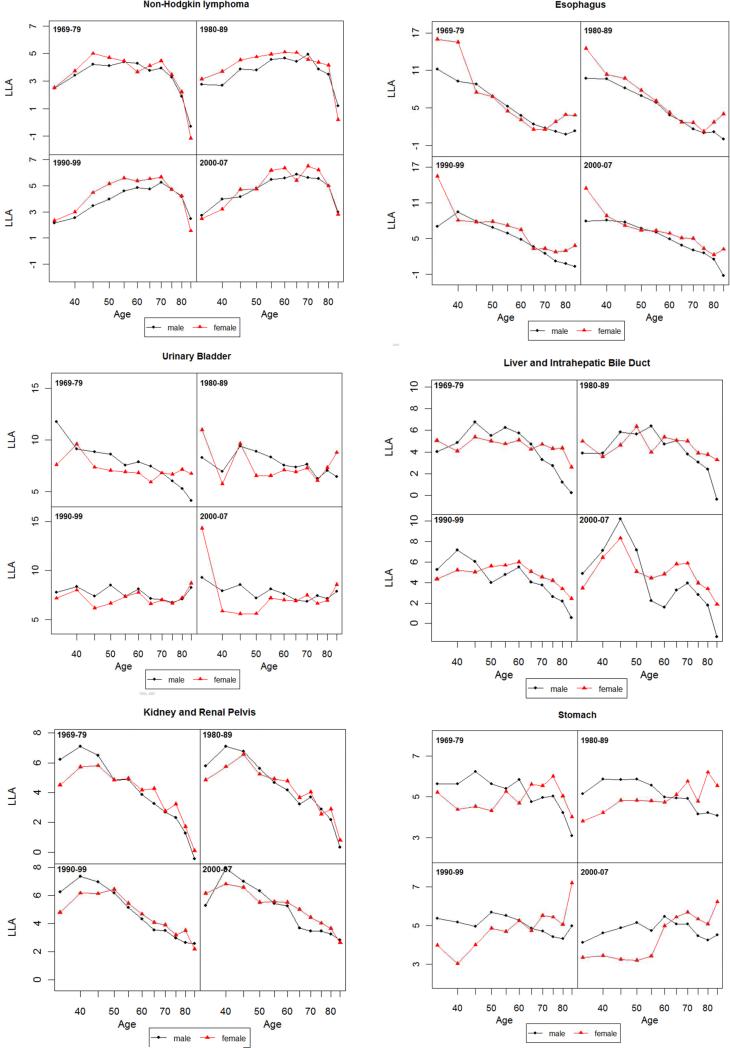
Figure 3.
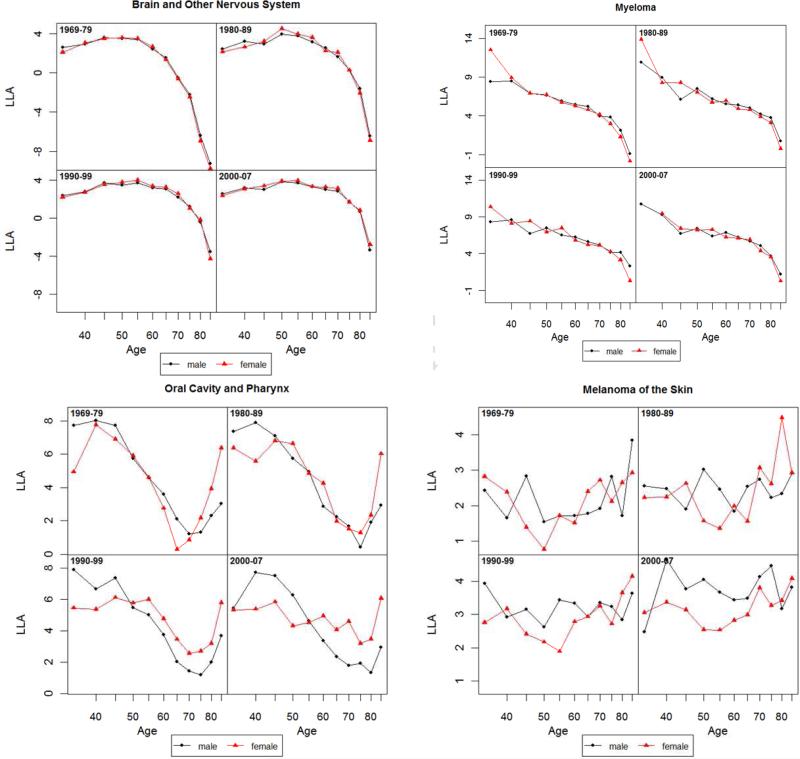
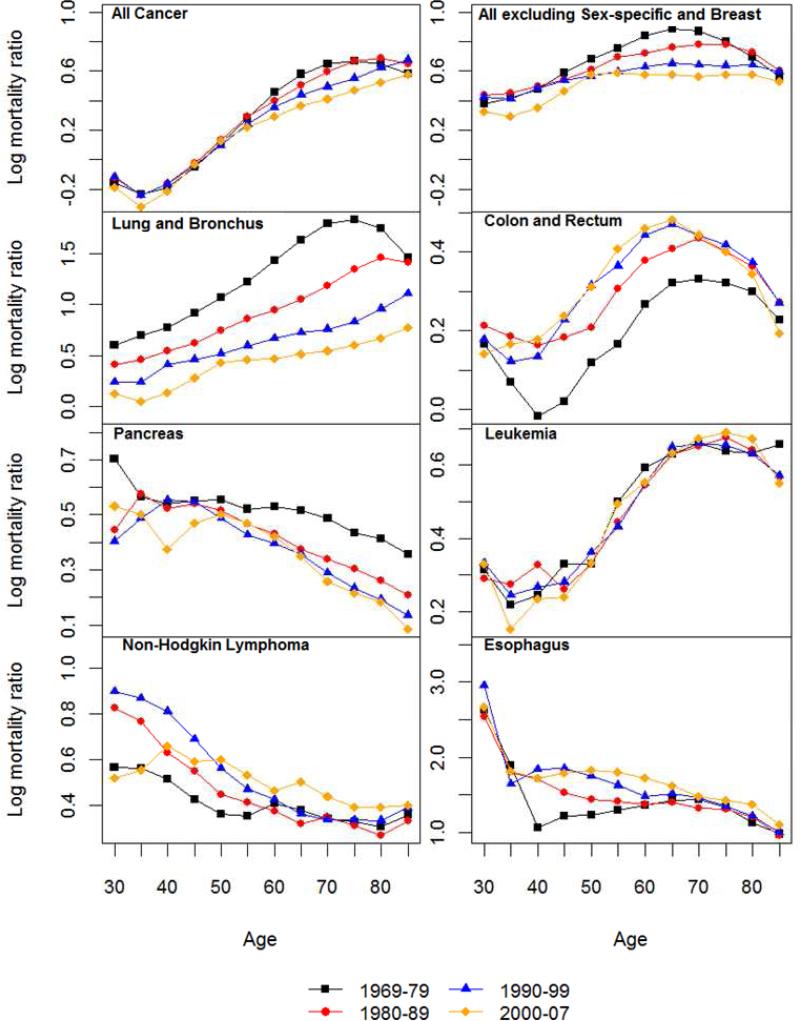
Log-log acceleration rate (LLA) for all cancer and 14 non sex-specific leading cancer sites: 1969-1979, 1980-1989, 1990-1999 and 2000-2007.
Table 1.
Summary of Findings on Age Dynamics and Sex Differences in Cancer Mortality.
| Patterns | Cancer Sites | Implications |
|---|---|---|
| Mortality increase | ||
| 5Mx continuous increase until age 70 | All examined | Higher risk of cancer mortality at older age |
| Sex Differences | ||
| SMR > 0 for most ages | All examined | Male excess at most ages |
| SMR changes with age | ||
| Continuous increase | All combined, lung, skin | Widening sex gap with age |
| Increase followed by decrease | ||
| Decrease in old age | All excluding sex-specific and breast, colorectal, stomach, leukemia | Widening and then narrowing sex gap with age |
| Decrease prior to old age | Urinary bladder, liver, kidney, oral cavity | |
| Decrease followed by increase | Brain and nervous system, myeloma | Narrowing and then widening sex gap with age |
| Continuous decrease | Esophagus, pancreas, non-Hodgkin lymphoma | Narrowing sex gap with age |
| Mortality Acceleration | ||
| LLA decrease with age | All examined | Deceleration of relative cancer mortality increase |
| Sex Differences | ||
| Male LLA > Female LLA | All combined | Relative cancer mortality increase greater for males |
| Lung, colorectal, leukemia, urinary bladder, stomach | ||
| Male LLA = Female LLA | All excluding sex-specific | Greater relative mortality increase for males due to male-only cancers |
| Brain and nervous system, myeloma | ||
| Male LLA < Female LLA | Pancreatic, non-Hodgkin lymphoma, kidney | Relative cancer mortality increase greater for females |
| No difference in LLA decrease | Most sites | Deceleration of relative cancer mortality increase similar to both sexes |
| Less female decrease in old age | Lung and urinary bladder (1970s), liver (1970-80s), colorectal and stomach (after 1970s), esophagus | Female mortality acceleration after middle age |
3.1 Age-specific Cancer Mortality Rates
Age-specific mortality rates for all cancer sites combined and by site for periods of 1969-79 and 2000-07 are shown in Figure 1. Those for the middle two periods are largely similar in shape and omitted in the interest of space. For both sexes, the age-specific mortality rates generally increase steadily from the age of 30 to the 70s. Old-age decreases in mortality rates across both periods are evident for some sites such as lung cancer, brain cancer, and myeloma. Patterns of age acceleration or deceleration can be discerned but will be better described by the analysis of the LLA. Figure 1 indicates prominent male excesses in cancer mortality rates. Correspondingly, the SMRs shown in Figure 2 are positive for all ages and sites.
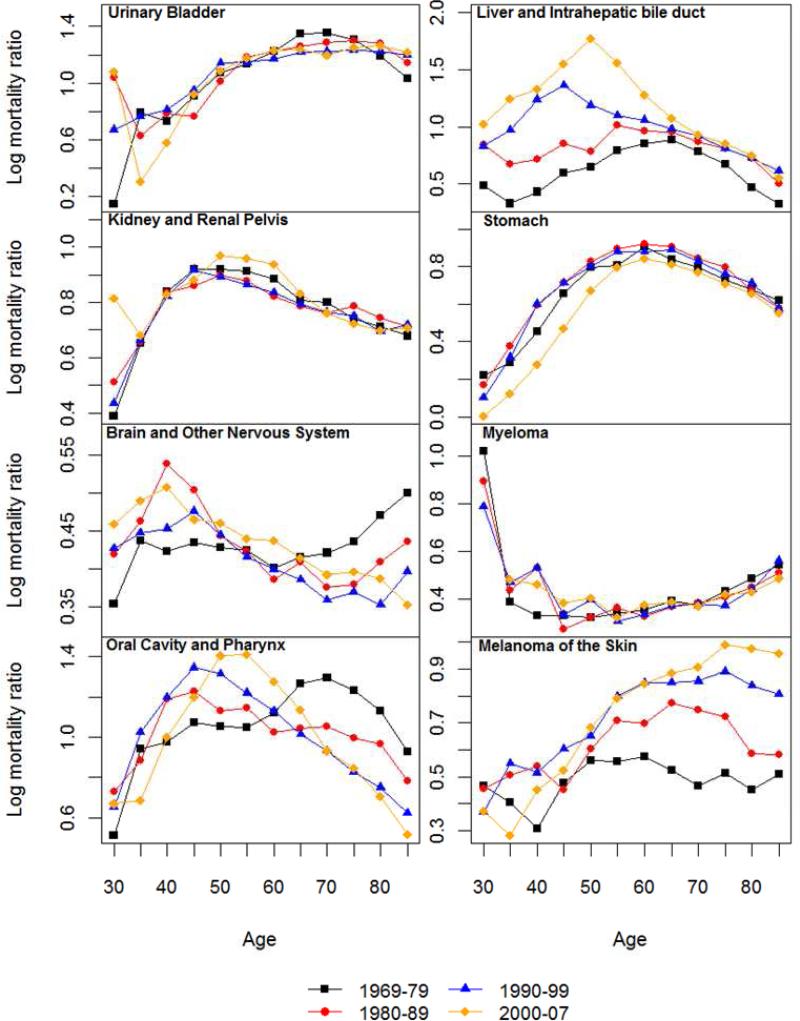
Figure 2.
Logged sex (male-to-female) mortality ratios (SMR) for all cancer and 14 non sex-specific leading cancer sites: 1969-1979, 1980-1989, 1990-1999 and 2000-2007.
3.2 Sex Differences in Cancer Mortality Rates
Figure 2 also suggests that there are substantial age variations in the sex differences in mortality rates for all sites and historical periods. The age patterns of the SMRs follow four types of trajectories by cancer site that are summarized in Table 1. Consistent with previous research of total cancer mortality (Cook and others 2011), the male excess increased continuously from younger adulthood into old ages. For all cancer sites excluding sex-specific and breast, an old age decrease or leveling of the sex gap becomes more apparent. This suggests that the old age increases in male excesses for all cancer sites combined are partly due to differences in cancers of reproductive tissues, especially prostate cancer mortality that increased markedly in old age for men and female breast cancer mortality that increased more before old ages than the other cancers. Site specific results show nearly linear increases in the male excess for lung cancer mortality that apparently contribute to the pattern of total cancer mortality as noted above. Similar continuous increases in SMRs with age are also observed in the two most recent periods for skin cancer mortality. The old age decreases in SMRs occurred for cancers of colon and rectum, stomach, and leukemia. A pattern of increases followed by decreases prior to old age is evident for a number of cancer sites, including urinary bladder, liver, kidney, oral cavity, with varying peak ages. The opposite pattern of change marked by decreases followed by increases in old age is found for brain cancer and myeloma. Continuous decreases are found for cancers of esophagus, pancreas, and non-Hodgkin lymphoma.
The considerable regularities in the shapes of age patterns of the SMRs across periods suggest both a developmental and a biological basis for the sex differences in cancer mortality. Relatively large changes in the magnitude of sex gaps over time have occurred for a few cases that suggest additional period-specific influences on exposures to carcinogens and detrimental lifestyle factors. The SMRs for all cancer deaths combined decreased across time, largely because of the period pattern in mortality from lung, pancreatic and stomach cancers due to female increases in cigarette smoking in more recent decades (Yang 2008). Period increases in male excesses are evident for colorectal, esophageal, liver, and skin cancer mortality, which suggest disproportionate male increases in relevant risk factors such as high fat diet, alcohol consumption, Hepatitis B virus and/or Herpes simplex virus infections, and UV exposures (Manton and others 2009). For the remaining sites, the SMRs remained relatively constant or fluctuated slightly with time.
Evidence so far is not entirely consistent with the EH in that only 3 out of the 14 cancer sites (colorectal, stomach cancers, and leukemia) show post-reproductive decreases in male excesses in mortality across all periods, whereas age changes in the SMRs for the other sites are either in the opposite direction or show initiations of declines before middle ages that cannot be attributed to the hypothesized effects of estrogen.
3.3 Mortality Acceleration: Age Patterns of LLA and Sex Differences
The SMRs based on mortality rates concern mortality levels and increase in absolute terms. We next examine male and female dynamics of mortality change in relative terms. Figure 3 presents the age patterns of mortality acceleration as measured by the LLA.
3.3.1 All Cancer Sites Combined
As shown in Figure 3 for all cancer sites, an LLA for males at the age of 40 in the 1970s is estimated to be 5, meaning that the total cancer death rate is increasing at the rate of 5% per percentage change of age. The LLA for both sexes decreased after the age of 45, indicating that the relative cancer mortality increase decelerated. According to the MTC hypothesis of cancer progression, the rise in acceleration in early to middle life is due to increasing rates of transition between stages of the disease, whereas the late-life decline in acceleration of cancer mortality rates may result from the concentration of older individuals with few stages remaining (Frank 2004b; Frank 2007). The LLA for the 1970s show a more pronounced female slowdown in mortality deceleration after the age of 60 that was also observed in other national populations such as England, Wales and Italy during the same historical period (Horiuchi 1997), but the female slowdown largely disappeared in subsequent periods. The lower LLA for females compared to males at most ages indicate that the percentage mortality increase of all cancer sites was slower for females relative to percentage change in age. But the similar slope changes in the LLA indicate that the extent of cancer mortality acceleration does not differ much between the two sexes. The lack of a consistent postmenopausal mortality acceleration for females across time cautions against a straightforward explanation of the correlation between estrogen and malignancies.
3.3.2 All Cancer Sites Excluding Sex-specific and Breast
The next set of results on all cancer sites, excluding sex-specific and breast, helps to further isolate the carcinogenic or anticarcinogenic effects of sex hormone profiles. The two sexes show closer values of LLA and hence mortality increase in two recent decades, suggesting the much greater cancer mortality increase for males (as mentioned before for all sites combined) is due to prostate and possibly other male-only cancers. However, the slope changes in LLA estimates for cancer deaths common to both sexes suggest a remarkable resemblance with those for all cancer sites combined. The sex differences, or the lack thereof, in the age patterns of mortality acceleration in post-reproductive life span are therefore not likely due to the age patterns of cancers in reproductive or breast tissues.
3.3.3 Cancer by Site
Results from site-specific analysis show greater male mortality increases with age (higher male LLA) for lung, colorectal, leukemia, urinary bladder, and stomach cancers for most ages but greater female mortality increases with age (higher female LLA) for pancreatic, non-Hodgkin lymphoma, and kidney cancers. The sex differences in mortality increase are ambiguous for other sites. Most individual sites show age patterns of mortality acceleration that indicate continuous declines (deceleration of mortality increase) into or during old age for both sexes in the LLA. A greater degree of female mortality acceleration after middle age, as predicted by the EH, is found in a few cases, including lung and urinary bladder cancers in the 1970s, liver cancer in the 1970-1980s, colorectal and stomach cancers after 1970s, and esophageal cancer. It is also possible that the gradual disappearance of female disadvantage in more recent periods is related to the widespread use of hormone replacement therapy in postmenopausal women in the U.S. (Hemminki and others 1988). However, that same therapy has been found to increase the risks of female cancers (Manton and others 2009). Period differences across cancer sites suggest the need to further examine the role of hormone replacement therapy as one potential factor responsible for the post-reproductive convergence in male and female patterns of mortality acceleration.
4. Discussion
The processes underlying cancer and aging are undoubtedly more complex than what this single study is able to capture. Variation across cancer sites further contributes to this complexity as each site involves specific risk factors. Our aim, however, is to identify some regularities among all cancer sites that may bear theoretical relevance to the study of aging and chronic disease. Through a systematic analysis of the age dynamics of cancer mortality rates and sex heterogeneity therein, this study has expanded previous studies on age changes in cancer incidence to shed new light on the relationship between aging and cancer mortality. Using measures of both mortality increase and mortality acceleration, we found complex age patterns of cancer mortality.
While the shapes of the age changes in mortality risks vary by tumor site, the mortality acceleration followed by the old-age deceleration is common to both sexes across all time periods for most sites, suggesting the similar aging related processes underlying cancer incidence (Manton and others 2009). The multistage theory of disease progression, derived from incidence data, can thus be applied to explain the subsequent mortality deceleration in older ages. If, as suggested by the theory, the LLA is closely related to the stage parameter n and thus has underlying biological meanings, the finding that the age patterns of cancer mortality acceleration vary much more across sites than within sites imply that the pathologic process leading to mortality is quite robust, yet unique, for each cancer site. It should be noted, however, that the age patterns of cancer mortality are more varied than those of incidence fit to the MTC model. Somewhat unexpected from the multistage point of view explaining the old age decline in incidence acceleration, mortality rates of colorectal (in 1990s and 2000s), urinary bladder (after 1970s), stomach (after 1970s for females) and oral cavity cancers show accelerations during old age, while skin cancer mortality does not suggest a clear age pattern of mortality acceleration or deceleration. It is thus possible that mortality risks associated with these three cancers are not entirely explained by their incidence patterns. Additional factors related to cancer prognosis and survival need to be considered in future research toward a more general theory of disease progression and mortality.
To the extent that the old age decline of cancer mortality acceleration resembles the age pattern of all-cause mortality (Horiuchi and Wilmoth 1998), the potential mechanisms that underlie these seemingly related phenomena merit considerations. Different causes for the deceleration in overall mortality in the oldest-old ages (85+) have been proposed. One major candidate explanation is the selective survival process that decreases heterogeneity in the surviving population later in life (Vaupel and others 1979). That is, selection of the elite eliminates the frail early on, so that the remaining subgroup consists of individuals who are more robust and longer-lived. Some recent study, however, suggests that the deceleration is an artifact due to poor data quality at old ages and the Gompertz function (exponential increase) of mortality can be reliably extended above age 100 after correcting for age misreporting (Gavrilov and Gavrilova 2011). While demographic selection can certainly contribute to the deceleration of cancer mortality at old age in addition to the MTC model, the sex differences in the age patterns call for more advanced explanations as we discuss below. The vital statistics data to which we had access are capped at age 85, therefore, it is difficult to assess the patterns beyond that age. But the old-age deceleration pattern seems to agree with a recent study of data on the 85+ population in the Utah Cancer Registry that shows declines in cancer incidence rates for ages 90-99 (Hanson and others 2012). The topic of old-age deceleration has been a subject of controversy in demographic research on overall mortality. It is not the intention of this study to engage in this controversy. Rather, we focus on cancer mortality and its age dynamics that may be related to the overall mortality but are not necessarily subject to the same explanations or problems. The new empirical results shown here highlight the relevance of these issues and invite future investigations to better address them using more detailed data.
Two major findings emerge regarding the sex differences in cancer mortality in relation to aging that are not completely understood and thus require further research. First, the male excess in level of cancer mortality rates, previously conceived as universal across the life course, actually varies greatly with age (as shown in the upper half of Table 1). The myriad of distinct age trajectories of sex gaps in cancer mortality rates by site and period discourage a simplified and static view of male disadvantages in cancer survival and frame future searches for explanatory factors that are more selective and specific. Second, although the age patterns of mortality acceleration vary considerably from one cancer site to another, they are relatively invariant to sex within each site. It follows that changes in sex differences of cancer mortality largely reside in changes in the absolute levels of male and female cancer mortality rates but not changes of their shapes for most sites (as shown in the lower half of Table 1). These results on sex differences and their age variations provide interesting puzzles for future investigations to solve.
First, an increasing male excess in level of mortality rates (measured by the SMR) in some sites and the lack of sex differences in old age cancer mortality acceleration (measured by the LLA) are not entirely consistent with the EH that predicts a postmenopausal acceleration of female mortality increase and reductions of sex differences. At the population level, the small numbers of cancer sites and time periods in which the post-reproductive reductions of sex mortality gaps occurred do not strongly support the anticarcinogenic role of estrogen as hypothesized in prior research (see, e.g., a review by Cook and others 2011). Instead, the findings here suggest that the influences of hormone profiles on non-reproductive cancer mortality are relatively weak and much less widely applicable across sites than conventionally assumed.
The post-reproductive reductions of sex differentials in mortality found in previous studies are, however, consistent for causes such as cardiovascular, infectious, and other chronic diseases which are more closely related to circulatory and immune functions regulated by sex hormone profiles (Horiuchi 1997). It thus indicates that cancer is a unique body of diseases which bear more complex relationships to reproductive apparatus. It also implies that cancer is related to the aging process in ways that are not necessarily sexually dimorphic as in the case of other diseases. Reviews of explanations of aging mechanisms across levels of organization and organisms suggest that aging is a process of passive damage accumulation and dysregulation (Yin and Chen 2005). The mortality acceleration pattern revealed in this, as well as other studies, (Horiuchi 2003) can thus be attributed to deterioration of the damage repair and prevention system according to this view. Furthermore, genetic theories of senescence expect age related increases in cancer mortality to be the result of dysregulation of cell proliferation and protooncogenes during the post-reproductive period consistent with an antagonist pleiotropy manner (Carnes 2011; Carnes and others 2008; Cutler and Semsei 1989).
We further recognize that because aging is characterized by a process of progressive dysregulation of the homeostatic network and accelerated decline in function across multiple physiological systems, the patterns of mortality from cancer in late life can be affected by competing risks such as comorbidity of cardiovascular disease (CVD). The aggregate data on death counts by the underlying cause of death based on the ICD codes from the vital statistics do not record comorbid conditions and hence do not permit a more in-depth investigation of cancer trends with, versus without, CVD. A comparison of our results with recently published findings from individual-level clinical examination data (Yang and Kozloski 2012: Figure 1), however, suggests highly consistent age patterns of sex differences in both CVD and cancer mortality as well as other major causes of deaths. So it is reasonable to expect that the findings shown here are not artifacts of competing risks but reflect actual population processes. Nonetheless, additional research utilizing comorbidity data in older adults is needed to formally test this possibility and examine its potential impact on age variations in sex ratios of cancer mortality. In addition, future research along this line should also benefit from a stronger focus in social behavioral factors such as smoking, substance use, and neighborhood conditions that may alter exposures to environmental carcinogens as well as genetic factors, morphological indicators of tissue growth, damage, and repair that determine cancer progression.
Additionally, the results on sex differences in cancer mortality change in late life suggest alternative or more complicated explanations than selective survival that has been mostly applied to explain overall mortality deceleration. That is, the sex gaps generally increased in level of cancer mortality rates and persisted in rate of acceleration/deceleration. Exclusion of sex-specific sites partially explained the increases in sex gaps in level of mortality rates with age but not persistent sex differences in acceleration/deceleration. The absence of convergences in sex gaps in cancer mortality, therefore, is not expected from the point of view of the selection process that should reduce population heterogeneity. The differential selection processes by sex remain a possibility for future studies.
The existence of cohort effects on cancer mortality may confound period patterns, particularly for sites influenced by behavioral risk factors such as smoking which show pronounced sex and cohort differences (Yang 2008). We compared the SMR and LLA patterns between male and female cohorts born between 1916 and 1939 for whom the age-specific data are the most complete. The cohort SMRs are very similar to period SMRs. And similar to the finding from a previous analysis of overall mortality (Horiuchi 1997), we also found that the LLA estimates for male and female cohorts are lower than the period estimates due to the declining mortality levels in more recent cohorts and that cohort patterns of sex differences in cancer mortality are consistent with the period patterns, thereby not distorting the results presented. Additional data on complete age trajectories of cancer mortality rates across all relevant birth cohorts are desirable in future investigations for a better test of the potential cohort confounding effect.
Highlights.
Systematic examinations of both age increase and age acceleration in cancer mortality
Analyses based on the best population level data on cancer mortality available
Document sex disparities in cancer mortality in relation to aging
Shed new light on the processes of aging and disease interaction
Have implications for origins and mechanisms of sex differences in life expectancy
Acknowledgements
We thank Igor Akushevich for assistance with data preparation.
Funding
This work was supported by the National Institute of Aging grant number 1K01AG036745-01 to Y.Y. and University Cancer Research Funds (UCRF) at Lineberger Comprehensive Cancer Center (LCCC) to Y.Y., T.L., and M.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbeev K, Ukraintseva S, Arbeeva L, Yashin A. Mathematical models for human cancer incidence rates. Demographic Research. 2005;12:237–260. [Google Scholar]
- Austad SN. Why women live longer than men: Sex differences in longevity. Gender medicine. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- Carey J, Liedo P. Sex-specific life table aging rates in large medfly cohorts. Experimental Gerontology. 1995;30:315–325. doi: 10.1016/0531-5565(94)00041-z. [DOI] [PubMed] [Google Scholar]
- Carnes BA. What is lifespan regulation and why does it exist? Biogerontology. 2011:1–8. doi: 10.1007/s10522-011-9338-3. [DOI] [PubMed] [Google Scholar]
- Carnes BA, Staats DO, Sonntag WE. Does senescence give rise to disease? Mechanisms of ageing and development. 2008;129:693–699. doi: 10.1016/j.mad.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiology Biomarkers & Prevention. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiology Biomarkers & Prevention. 2011;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Semsei I. Development, cancer and aging: possible common mechanisms of action and regulation. Journal of gerontology. 1989;44:25–34. doi: 10.1093/geronj/44.6.25. [DOI] [PubMed] [Google Scholar]
- Fisher JC, Hollomon JH. A hypothesis for the origin of cancer foci. Cancer. 1951;4:916–918. doi: 10.1002/1097-0142(195109)4:5<916::aid-cncr2820040504>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Frank S. A multistage theory of age-specific acceleration in human mortality. BMC biology. 2004a;2:16–24. doi: 10.1186/1741-7007-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA. Age-specific acceleration of cancer. Current biology. 2004b;14:242–246. doi: 10.1016/j.cub.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Frank SA. Dynamics of cancer. Princeton University Press; 2007. [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. Mortality measurement at advanced ages: a study of the Social Security Administration Death Master File. North American actuarial journal: NAAJ. 2011;15:432. doi: 10.1080/10920277.2011.10597629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HA, Smith KR, Stroup AM, Harrell J, Rull RP. Cancer Incidence in the Oldest Old: Disentangling Age, Period, and Cohort Effects.. Population Association of America Annual Meeting.; San Francisco, CA. 2012. [Google Scholar]
- Hemminki E, Kennedy DL, Baum C, McKinlay SM. Prescribing of noncontraceptive estrogens and progestins in the United States, 1974-86. American journal of public health. 1988;78:1479–1481. doi: 10.2105/ajph.78.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S. Postmenopausal acceleration of age-related mortality increase. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1997;52:B78–B92. doi: 10.1093/gerona/52a.1.b78. [DOI] [PubMed] [Google Scholar]
- Horiuchi S. Interspecies differences in the life span distribution: humans versus invertebrates. Population and Development Review. 2003;29:127–151. [Google Scholar]
- Horiuchi S, Coale AJ. Age patterns of mortality for older women: an analysis using the age-specific rate of mortality change with age. Mathematical Population Studies. 1990;2:245–267. doi: 10.1080/08898489009525312. [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Wilmoth JR. Deceleration in the age pattern of mortality at older ages. Demography. 1998;35:391–412. [PubMed] [Google Scholar]
- IOM . Exploring the biological contributions to human health: does sex matter? National Academies Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- Jemal A, Thun MJ, Ries LAG, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. Journal of the National Cancer Institute. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG, Akushevich I, Kravchenko J. Cancer mortality and morbidity patterns in the US population: an interdisciplinary approach. Springer Verlag; 2009. [Google Scholar]
- Nordling CO. A new theory on the cancer-inducing mechanism. British journal of cancer. 1953;7:68. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, A.W.S., Total U.S. (1969-2007) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released June 2010. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- Vaupel J, Manton K, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979:439–454. [PubMed] [Google Scholar]
- Yang Y. Trends in US adult chronic disease mortality, 1960–1999: age, period, and cohort variations. Demography. 2008;45:387–416. doi: 10.1353/dem.0.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:493–500. doi: 10.1093/gerona/glr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, Chen K. The essential mechanisms of aging: Irreparable damage accumulation of biochemical side-reactions. Experimental Gerontology. 2005;40:455–465. doi: 10.1016/j.exger.2005.03.012. [DOI] [PubMed] [Google Scholar]