Abstract
The number of prescriptions for hydrocodone-containing opioid analgesics has greatly increased over the past decade. This increase has led to an associated enhancement in the non-medical use of hydrocodone products. There is a lack of evidence to determine the extent of the rewarding effects and signal transduction properties of hydrocodone. Therefore, the present study aimed to examine the rewarding properties of hydrocodone (1 and 5 mg/kg) and morphine (1 and 5 mg/kg) using the conditioned place preference paradigm (CPP) in rats. Both hydrocodone and morphine produced a CPP at the 5mg/kg dose, but not the lower 1mg/kg dose, suggesting that both drugs possess similar rewarding properties in the CPP paradigm. Moreover, hydrocodone and morphine equally reduced phosphorylation levels of ERK and CREB proteins in the nucleus accumbens, suggesting that both drugs exert their effects through signal transduction pathways known to be involved in drug reward and reinforcement. These findings suggest that hydrocodone should be viewed as similarly capable of producing rewarding and euphoric properties as morphine.
Keywords: Conditioned place preference, addiction, opioid analgesics, drug abuse
Introduction
The prevalence of prescription opioid analgesic abuse continues to grow at an astonishing rate in the United State (SAMHSA, 2008a). Opioid analgesics are the most commonly prescribed drugs, with hydrocodone-containing formulations (e.g. hydrocodone with acetaminophen) topping the list of most prescribed generic drug in the U.S. in 2008 with over 121 million prescriptions filled (SDI/Verispan, 2008). This translates to Americans consuming approximately 99% of the global hydrocodone supply (International Narcotics Control Board, 2005). The high use is in part due to hydrocodone-containing products being classified as Schedule III controlled substances by the Drug Enforcement Administration, allowing health care professionals to prescribe up to five refills in a six month time frame, as opposed to Schedule II drugs (e.g. morphine) where refills are not permitted. This has lead to overprescription of opioid analgesics (Bates et al., 2011), which has increased the availability of the drug for use in non-medical contexts.
Hydrocodone is a semi-synthetic codeine analog with binding affinity for mu, delta, and kappa opioid receptors, similar to morphine (Peckham and Traynor, 2006). Hydrocodone and morphine also have similar analgesic ED50 values, 2.23mg versus 2.17mg, respectively. Both drugs are prescribed in similar analgesic dose ranges orally (hydrocodone 5–10mg, morphine 5–30mg) (Baumann et al., 2011). Hydrocodone and morphine also possess similar drug discriminatory properties (Meert and Vermeirsch, 2005). Morphine administration modulates the phosphorylation of the extracellular signal-regulated kinases (ERK) and its downstream transcription factor the cyclic-AMP response element binding protein (CREB), which are involved in the neuroadaptations that contribute to the development of drug dependence and addiction (for a review see Robison and Nestler, 2011). For instance, acute morphine reduces phosphorylation of ERK and CREB in the nucleus accumbens (Eitan et al., 2003; Morón et al., 2010; Muller and Unterwald, 2004). We are not aware of any studies where the effects of hydrocodone on signaling molecules related to drug abuse have been measured. Moreover, there has not been a direct comparison between the rewarding and signal transduction properties of hydrocodone and morphine. Considering the high number of hydrocodone prescriptions and the non-medical abuse in the U.S., it is important to determine whether hydrocodone produces similar rewarding and intracellular signaling effects to morphine, the gold standard in opioid therapy with well characterized rewarding and abuse potential.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–400 grams were pair-housed in standard Plexiglas® cages and placed on a 12-hour light/dark cycle (lights on at 6 a.m.) with unrestricted access to food and water. All rats were handled for 5 days prior to the start of the experiments. Experiments took place between 9 a.m. and 2 p.m. Animal care and use was in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication 85–23, Bethesda, MD, USA) and approved by the Institutional Animal Care and Use Committee of Western University of Health Sciences.
Apparatus
The conditioned place preference (CPP) apparatus was a truncated T-maze, consisting of two adjacent conditioning chambers (L: 35 x W: 30 x H: 55 cm, each chamber) and a small start box (L: 19 x W: 17 x H: 55cm). The conditioning chambers possessed distinct visual and tactile cues. One chamber had alternating black and white horizontally striped walls and a metal rod floor, whereas its adjacent conditioning chamber had alternating black and white vertically striped walls and a metal grid (mesh) floor. The start box had gray walls, a smooth floor texture, and was located on the side of the apparatus, towards the center point where the two adjacent conditioning chambers share a wall. Thus on preconditioning and postconditioning test days, when the start box door was opened, rats had the option to enter either one of the two conditioning chambers. Once the rat exited the start box, the door would close and prevent reentry into the start box. Rats would then be able to travel from one conditioning chamber to the other without traveling through the start box. Rats were considered to have entered a compartment when their head, shoulders and forearms had entered a given compartment. These body parts combined occupy a space of approximately 225 pixels when quantifying the behavior by our video tracking software. The tactile and visual cues were chosen to ensure the apparatus to be balanced, where rats would not exhibit a preference to a given conditioning chamber. Indeed, we have previously shown that rats do not show an initial preference to either one of the conditioning compartments, indicating a balanced apparatus (Nazarian et al., 2011). Place preference was digitally recorded and then quantified using Ethovision XT 5.0 software (Noldus Information Technology, Leesburg, VA).
Procedures
A 6-day CPP paradigm was used, consisting of a preconditioning day, 4 conditioning days with one pairing session per day counterbalanced and alternating between saline and drug (i.e. 2 drug pairing and 2 saline pairings), and a postconditioning test day. On preconditioning day, rats were placed in the start box for 3 min and then permitted to enter the conditioning chambers once the start box door was opened. Upon exiting the start box, the door was closed at which time rats were given 15 min to travel and explore the two conditioning chambers. On conditioning days, rats were injected with saline, hydrocodone (1.0 or 5.0 mg/kg) or morphine (1.0 or 5.0 mg/kg) and placed in one of two conditioning chambers for 30 min. Given our balanced apparatus, drug pairing to a particular compartment was assigned in an unbiased arrangement. On the postconditioning test day, rats were placed in the start box for 3 min and then permitted to enter the conditioning chambers. The amount of time spent in the drug-paired or saline-paired chamber was measured for 15 min. Conditioned place preference was defined as greater time spent in the drug-paired chambers on the postconditioning test day between rats conditioned with drug versus rats conditioned with saline.
To assess changes in phosphorylated ERK (pERK) and phosphorylated CREB (pCREB) proteins, a separate group of rats were administered hydrocodone (5mg/kg) or morphine (5mg/kg). Twenty minutes after administration, rats were lightly anesthetized with isoflurane, decapitated, brains removed, flash frozen in liquid nitrogen and maintained at −80°C.
Drugs, Reagents and antibodies
Hydrocodone bitartrate and morphine sulfate pentahydrate were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). Drugs were dissolved in saline and injected through the intraperitoneal route (i.p.) at a volume of 1ml/kg. Primary antibodies for phosphorylated ERK (Cat#4370), total ERK (Cat#4696), phosphorylated CREB (Cat#4276) and total CREB (Cat#9104) were purchased from Cell Signaling Technology (Danvers, MA, USA). The primary antibody for alpha-tubulin (Cat#8035), secondary antibodies and radioimmunoprecipitation assay (RIPA) lysis buffer kit (Cat#24948) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phosphatase inhibitor cocktail 1 (Cat#P2850) and cocktail 2 (Cat#P5726) were purchased from Sigma-Aldrich (St. Louis, MO). Bicinchoninic acid (BCA) protein assay kit and Supersignal west pico chemiluminescent substrate were produced by Thermo Scientific (Rockford, IL). Laemmli buffer, tris-glycine TGX gels and nitrocellulose membranes were purchased from Bio-Rad Laboratories (Hercules, CA).
Tissue Preparation
Brains were dissected while frozen and the nucleus accumbens (NAc) was removed. Tissues were homogenized using a handheld homogenizer in RIPA lysis buffer containing PMSF (1%), sodium orthovanadate (1%), protease inhibitor cocktail (2%) and phosphatase inhibitor cocktails 1 and 2 (1%). Homogenized samples were incubated in the buffer for 30 min on ice and then centrifuges at 14,000rpm for 10 min. The lysates were then used to quantify protein concentration using a BCA protein assay kit.
Western Blotting
Western blots were performed using SDS-PAGE. Specifically, 10 or 25μg samples were combined with Laemmli buffer containing beta-mercaptoethanol (5%). Samples were then boiled for 3 min, centrifuged, loaded onto gels and electrophoresed at 100V constant. Proteins were then transferred to nitrocellulose membranes. Membranes were blocked for 1hr at room temperature in 5% non-fat dry milk in TBST. For pERK and pCREB detection, membranes were incubated in 5% BSA with the antibodies (1:500) overnight at 4°C. For total ERK, CREB and α-Tubulin detection, membranes were incubated in 5% non-fat dry milk in TBST with the antibodies (1:1000) overnight at 4°C. After several washes in TBST, membranes were then incubated in their corresponding secondary antibodies (1:1000) for 2hrs at room temperature. Membranes were then washed and visualized in Supersignal west pico chemiluminescent substrate using a Kodak digital imaging system. For quantifying band intensities, densitometry was conducted using the Carestream software (Woodbridge, CT).
Statistical Analysis
All data were analyzed using one-way analysis of variance (ANOVA), followed by Dunnett’s posthoc analysis for pairwise comparisons with the saline group. Statistical significance was set at the 5% probability level (p<0.05).
Results
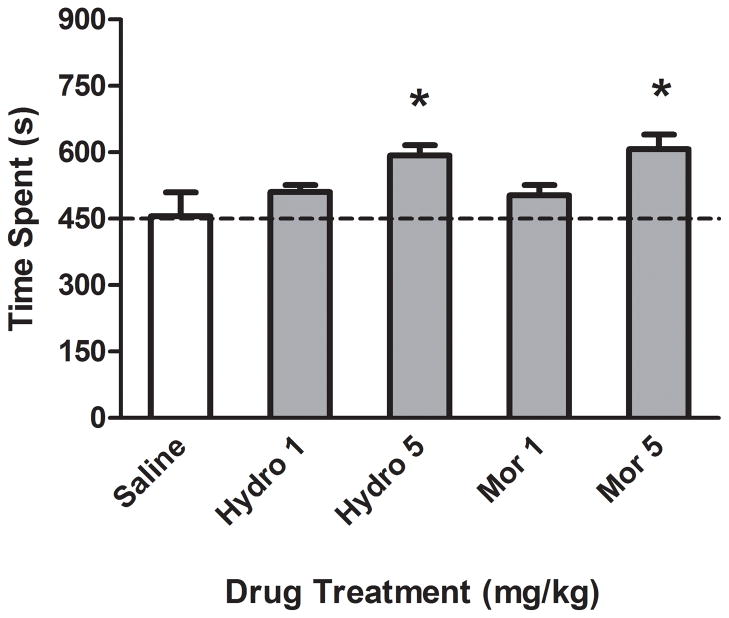
On the preconditioning day, rats used in this experiment exhibited 439±54 sec on the horizontally striped side and 461±54 sec on the vertically striped side, demonstrating no significant initial preference for either side of the conditioning compartments. The rewarding effects of hydrocodone and morphine are illustrated in Figure 1. Analysis of variance demonstrated a significant effect of Drug, F(4,30)=3.82, p< 0.05. Follow-up Dunnett’s posthoc analysis indicated that rats conditioned with 5 mg/kg of hydrocodone or morphine exhibited greater time spent on the drug-paired side as compared to rats conditioned with saline, indicating a CPP to hydrocodone and morphine. However, rats conditioned with the lower dose (1mg/kg) of hydrocodone or morphine did not exhibit a CPP.
Figure 1.
Time spent (s) in the drug-paired chamber on the postconditioning test day following hydrocodone or morphine pairing in the CPP paradigm. Rats conditioned with the highest dose of hydrocodone (5mg/kg) or morphine (5mg/kg) spent more time on the drug side as compared to rats conditioned with saline. *Denotes a significant difference in time spent on the drug-paired side between drug treated versus saline treated rats, n=7 rats per group.
The effects of hydrocodone and morphine on changes in phosphorylation ERK and CREB proteins are illustrated in Figure 2. Both hydrocodone and morphine (5mg/kg) reduced the levels pERK and pCREB in the NAc, F(2,12)=10.64, p<0.05 for pERK, and F(2, 15)=4.79, p<0.05 for pCREB. Lastly, Table 1 illustrates the levels of total ERK, CREB, α-Tubulin and their ratios. Saline, hydrocodone or morphine did not alter total protein levels in the NAc.
Figure 2.
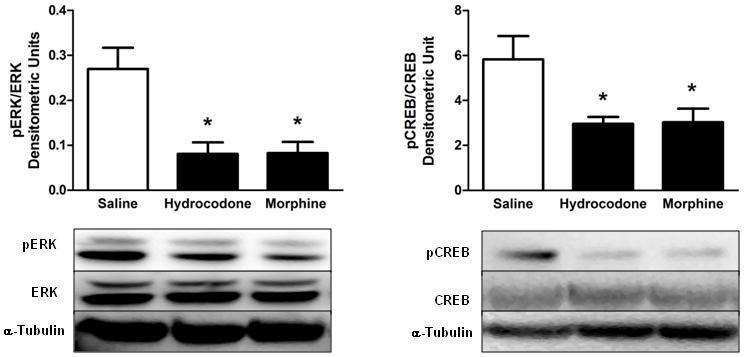
Effects of hydrocodone and morphine on pERK and pCREB levels. Twenty minutes after hydrocodone (5mg/kg) or morphine (5mg/kg) administration, pERK and pCREB levels were significantly reduced in the NAc. *Denotes a significant difference in densitometric units between saline and hydrocodone or morphine, n=4–6 rats per group for pERK, n=5–7 rats per group for pCREB data.
Table 1.
Levels of ERK, CREB and α-Tubulin proteins in the NAc after acute drug administration.
| Drug | ERK | Tubulin | ERK/Tubulin | CREB | Tubulin | CREB/Tubulin |
|---|---|---|---|---|---|---|
| Saline | 1035790±77130 | 73964±10512 | 15.71±2.26 | 3730±354.2 | 14102±2247 | 0.28±0.03 |
| Hydrocodone | 1100460±57549 | 77115±10947 | 16.58±2.88 | 4134±465.5 | 18336±2144 | 0.23±0.02 |
| Morphine | 1173252±37685 | 75710±11432 | 18.44±3.63 | 4844±680.2 | 14057±1347 | 0.37±0.07 |
Mean±S.E.M. of arbitrary densitometric units of total ERK, CREB, α-Tubulin and their ratios obtained from the NAc by western blotting procedures. Data represents the effects of saline, hydrocodone (5mg/kg) or morphine (5mg/kg) on protein levels 20min after injection. When comparing the data within a column, no significant differences were detected as a function of drug treatment.
Discussion
The use of hydrocodone-containing analgesics has significantly increased over the past decade. This increase has led to a concomitant increase in the non-medical abuse of hydrocodone-containing product. A lack of studies assessing the rewarding effects of hydrocodone has limited our understanding of the rewarding and abuse potential of this drug. Therefore, the present study sought to compare the rewarding properties of hydrocodone to morphine. Previous findings have shown that hydrocodone and morphine have similar analgesic and drug discriminatory potencies (Meert and Vermeirsch, 2005; Peckham and Traynor, 2006). In extension to these studies, our findings demonstrated for the first time that the rewarding effects of hydrocodone are also similar to that of morphine using the CPP paradigm. Hydrocodone was also compared with morphine on its ability to modulate signal transduction molecules known to be critical to drug reward and reinforcement. Our findings also showed that both hydrocodone and morphine equally reduced pERK, as well as pCREB levels in the NAc. These findings are consistent with previous studies demonstrating that acute morphine reduces pERK and pCREB levels in the NAc (Eitan et al., 2003; Morón et al., 2010; Muller and Unterwald, 2004), but see (Valjent et al., 2004). Altogether, the present study indicates that hydrocodone produces similar rewarding and signal transduction changes as morphine.
We have previously shown that acetaminophen is unable to enhance the rewarding effects of hydrocodone in physiologically relevant analgesic doses (Nazarian et al., 2011). Therefore, when considering our findings together, the rewarding effect of analgesic formulations containing hydrocodone is indeed due to hydrocodone, which appears to possess similar rewarding properties as compared to morphine. Future studies require comparison of other doses of hydrocodone and morphine in the CPP and self-administration paradigms to further characterize their potency and efficacy relative to reward and reinforcement. Such data is critical to consider for determining the rewarding and abuse potential of analgesic products containing hydrocodone. Given the high rate of prescriptions containing hydrocodone and its non-medical abuse, it is important for the medical community to consider hydrocodone to possess similar rewarding effects, and possibly similar abuse potential as morphine.
Acknowledgments
This work was supported by National Institute of Health research grant DA027943 (AN) and funds provided by the Western University of Health Sciences.
References
- Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. The Journal of urology. 2011;185(2):551–555. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- Baumann TJ, Strickland JM, Herndon CM. Pain Management. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A pathophysiological approach. 8. New York: McGraw-Hill; 2011. pp. 1045–1060. [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23(23):8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Narcotics Control Board. Annual Report from 2004 Data. 2005. [Google Scholar]
- Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav. 2005;80(2):309–326. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Morón JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA. Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(4):955–966. doi: 10.1038/npp.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther. 2004;310(2):774–782. doi: 10.1124/jpet.104.066548. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Are D, Tenayuca JM. Acetaminophen modulation of hydrocodone reward in rats. Pharmacol Biochem Behav. 2011;99(3):307–310. doi: 10.1016/j.pbb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther. 2006;316(3):1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature reviews Neuroscience. 2011;12(11):623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. (Substance Abuse and Mental Health Services Administration), office of applied studies, Results from the 2007 National Survey on Drug Use and Health: National Findings. 2008a. [Google Scholar]
- SDI/Verispan. [Last accessed, May 14, 2012. Drug Topics.];2008 Top 200 generic drugs by total prescriptions. 2008 http://drugtopics.modernmedicine.com/top200gen.
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19(7):1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]