Abstract
Purpose
This contemporary tutorial will introduce general principles of molecular biology, common DNA, RNA and protein assays and their relevance in the field of communication sciences and disorders (CSD).
Methods
Over the past two decades, knowledge of the molecular pathophysiology of human disease has increased at a remarkable pace. Most of this progress can be attributed to concomitant advances in basic molecular biology and, specifically, the development of an ever-expanding armamentarium of technologies for analysis of DNA, RNA and protein structure and function. Details of these methodologies, their limitations and examples from the CSD literature are presented.
Results/Conclusions
The use of molecular biology techniques in the fields of speech, language and hearing sciences is increasing, facilitating the need for an understanding of molecular biology fundamentals and common experimental assays.
Keywords: molecular biology, DNA, RNA, transcription, translation, genomics, proteomics
Introduction
Recent advances in basic research, genomics and proteomics have improved our understanding of the molecular processes governing normal and disordered voice and swallowing. With an approximate 80% growth in basic science publications related to voice alone over the past 7 years (Benninger, in press), it is now more than ever essential to have an understanding of basic biological processes to be able to interpret the increasingly specialized literature. In particular, it is necessary to have knowledge of cellular processes at the molecular level, as well as the knowledge of the principles behind commonly used analytic techniques. This contemporary tutorial is intended for consumers of research in the field of communication sciences and disorders (CSD), whom have not worked in the molecular biology field. We will explain basic concepts related to DNA, RNA and protein, followed by descriptions of common molecular assays, discussing their limitations, and highlighting utilization of these techniques from the voice and swallowing literature. This tutorial is not meant to serve as an exhaustive review of all molecular methodologies; we have chosen those techniques that are the most common.
Central dogma of molecular biology
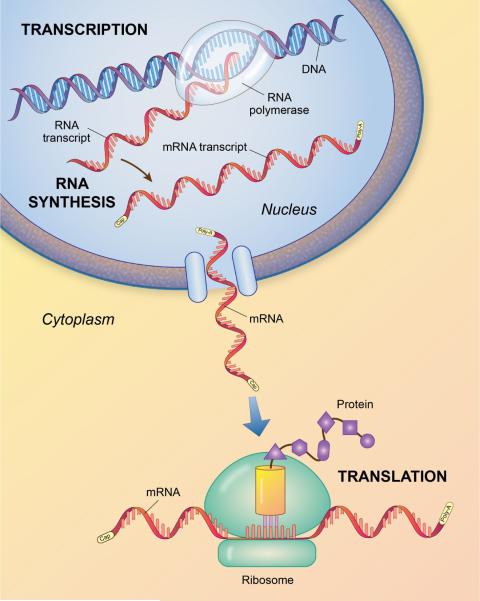
The central dogma of molecular biology is the transcription of deoxyribonucleic acid (DNA) to ribonucleic acid (RNA) which then through translation synthesizes proteins (Crick, 1970) (Figure 1). Most organisms use DNA to encode their genetic information within the nucleus of the cell. Viruses (e.g. retroviruses) utilize RNA as their genetic material. DNA is a double stranded, helical structure formed by two complementary chains of nucleotides. Each nucleotide is made up of one of four heterocyclic nitrogenous bases – adenine (A), guanine (G), cytosine (C) and thymine (T). DNA is present in every cell of the body, with the exception of red blood cells and its sequences are organized into genes, which can be transcribed to RNA in the nucleus. RNA has three of the same nucleotides as DNA (i.e., adenine, guanine, cytosine), and replaces thymine with uracil (U). Of the three types of RNA, only one type, messenger RNA (mRNA) encodes proteins in the cytoplasm of the cell. At any given time, only a fraction of the genes found within the DNA are transcribed to mRNA. Specific mRNA that is expressed or transcribed and subsequently translated into proteins determines the cell's chemical and physical properties. For example, fibroblasts in the lamina propria of the vocal fold express mRNA for fibrous proteins such as collagen and elastin. Even though fibroblast cells in the lamina propria have the genes encoded in DNA for rhodopsin (color pigment in the retina of the eye), they are more than likely not expressed or transcribed (Thibeault, Hirschi & Gray, 2003, p. 492). Translation of mRNA generates polypeptides, which are amino acid sequences of protein. It was once thought that a single gene made a single protein. We now know that one gene can make more than one protein via post translational modifications. Proteins fold into specific structures depending on the makeup of their amino acid sequence (Alberts, Bray, Lewis, Raff, Roberts & Watson, 1989).
Figure 1.
The central dogma of molecular biology. RNA is transcribed by RNA polymerase II from DNA in the nucleus of the cell. Messenger RNA leaves the cell nucleus and in the cytoplasm is translated to protein through the actions of a ribosome. Adapted from Alberts, et al., (1989).
This central dogma of molecular biology is the basis of two commonly used methodologies -- gel electrophoresis and hybridization. The large majority of molecular biology assays utilize one or both of these techniques with gel electrophoresis being the most widely used (Parra, Osgood, & Pappas, 2010). This methodology exploits the concept that positive charges attract negative charges and vice versa. DNA and RNA are negatively charged, proteins can be coated with negative charges and all three can migrate toward a positive electrode when dissolved in solution, placed in a gel and an electric current is applied. A gel is a semisolid polymer meshwork, typically made of agarose, through which these molecules migrate based on size. Larger molecules will move slowly through the gel, whereas smaller molecules will move quickly, resulting in variable sized bands formed on the gel surface (Figure 2). A UV spectrophotometer is used to detect ethidium bromide staining which is added to the gel to visualize DNA, RNA, or protein bands. Ethidum bromide intercalates itself into the nucleic acids of the molecules and fluoresces under UV light. In addition to ethidium bromide, radioactive labeling is another method used to visualize nucleic acids. Markers with designed molecular weights are used as a reference to determine the sizes of the molecules of interest. Hybridization, also known as annealing, is the second most common molecular biology technique and involves the binding of a radioactive or fluorescent probe to a corresponding DNA or RNA strand. The probe has a genetically similar sequence of amino acids that are complementary to the DNA or RNA. Complementary nucleotides are A to T for DNA, A to U for RNA and C to G for both RNA and DNA. The radioactive or fluorescent nucleotide probes once hybridized to its match can be visualized and measured. Numerous molecular techniques use hybridization, including Southern and northern blot analysis and polymerase chain reaction (PCR).
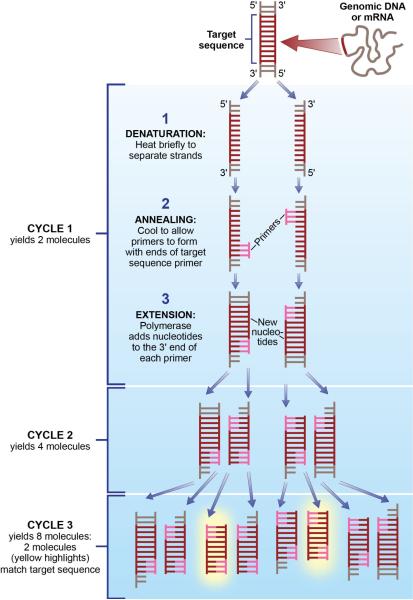
Figure 2.
Schematic representation of polymerase chain reaction (PCR). There are three major steps in a PCR, which can be repeated for 30 or 40 cycles – denaturation, annealing and extension. Because both strands are copied during PCR, there is an exponential increase of the number of copies of the gene. For example, if there is only one copy of the wanted gene before the cycling starts, after one cycle, there will be 2 copies, after two cycles, there will be 4 copies, three cycles will result in 8 copies and so on. Adapted from Lawley, R. A revolution in the microbiology laboratory. Retrived July 2011 from http://www.foodsafetywatch.com/public/1050
DNA
DNA extraction, PCR, and in situ hybridization are three examples of molecular techniques commonly used to assist investigators in better understanding of different disease processes at the DNA level.
DNA Extraction
The gold standard technique for extracting high-purity DNA from cells or tissue is a phenol-chloroform method (Sun, 2010). This procedure can be adapted to process a wide variety of tissue specimens including paraffin-embedded tissue and fluids. DNA extracted using phenol-chloroform has high purity, concentration, and yield; however, the process is labor intensive and requires user expertise. Prior to the extraction process, cells are lysed or broken open. DNA is separated and purified from proteins and other organic matter with the application of various buffers and centrifugation. Other methods of DNA extraction include salting-out (Howe, Klimstra, & Cordon-Cardo, 1997), solid-phase adsorption-based extraction (Wolfe et al, 2002), and magnetic DNA extraction (Hawkins, O'Connor-Morin, Roy, & Santillan, 1994), all of which can be completed with the use of commercially available kits. The salting-out method has an advantage in that it does not use toxic chemicals (i.e. phenol, chloroform) that are potentially harmful to the technician; however, it is mostly used to extract DNA from whole blood specimens. Solid-phase adsorption extraction can be used for tests that require a lower concentration of DNA, as yields are much lower than other extraction assays. While easily automated, magnetic DNA extraction is similar to solid-phase adsorption extraction in that it results in a lower concentration of DNA. The purity of DNA extracted with this method is typically less abundant than the other methods.
DNA extraction in CSD
DNA extraction is a required first step when performing many molecular assays. Poeta et al. (2007) studied the role of the gene TP53, one of the most common molecular alterations in squamous cell carcinoma of the head and neck, as a prognostic marker in 560 patient samples. DNA was extracted from primary tumor specimens using phenol-chloroform extraction and ethanol precipitation. A DNA microarray, called the GeneChip p53 assay was used to screen TP53 mutations, and these mutations were found to be significantly associated with decreased overall survival in the patients from whom the specimens were collected. It was suggest that based on these results, routine screening for TP53 gene mutations in head and neck cancer patients might be beneficial for determining long-term prognosis and subsequently developing an appropriate treatment plan.
Just as the interactions of bacteria within the gut can trigger gastric disease, it has been hypothesized that there are similar mechanisms within the larynx that lead to laryngeal pathology. To investigate this notion, Gillett, Rees, Coogan, Birchall, and Bailey (2006) extracted bacterial DNA from laryngeal biopsies obtained from pigs. They used polymerase chain reaction (PCR) to amplify the extracted DNA, which they then sequenced in order to identify a variety of different bacterial species resident in the laryngeal tissue. In the future, identifying potentially harmful pathogens within human laryngeal tissue may help guide medical treatment, such as the use of probiotics or antibiotics.
PCR
PCR is a technique used for amplifying specific DNA sequences - even when they are present at extremely low levels in a complex mixture - generating millions of copies of a DNA segment of interest (Figure 3). PCR is based upon hybridization and allows for a measurement of the amount of specific DNA of interest. PCR or endpoint PCR utilizes thermal cycling, a temperature modulation process that consists of cycles of heating and cooling. The heating process is used to denature or separate the double-stranded DNA complex, whereas the cooling process allows two DNA fragments known as primers to bind to their complementary sequences through hybridization on the separated strands of DNA. Polymerase allows the primers to extend into new complementary strands and as PCR progresses; the strands of DNA that are generated are used as a template for replication, resulting in a chain reaction and exponential amplification of the DNA template. In order to analyze the DNA amplification generated by PCR, DNA gel electrophoresis is typically performed. This type of PCR is considered semi-quantitative as it does not give an exact measure of DNA but rather a relative measure compared to control or other experimental conditions.
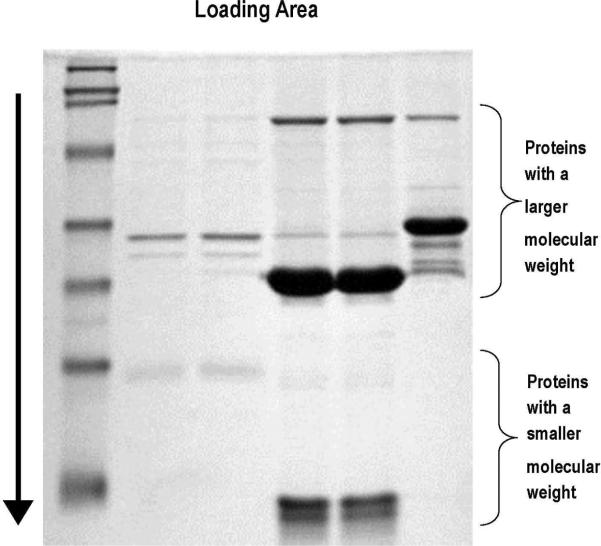
Figure 3.
An SDS-PAGE gel electrophoresis. Proteins have been labeled and loaded at the top of the gel. The first column at the left represents a ladder, which aids in identifying and quantifying the proteins in the other columns by a comparison of the protein size. Proteins run through the gel in the direction of the arrow. Proteins with a smaller molecular weight run faster and further down the gel and proteins with a larger molecular weight run slower and less far down the gel. Each lane represents a separate sample of interest.
PCR in CSD
PCR has been used in research investigating recurrent respiratory papillomatosis (RRP), a variant of human papillomavirus (HPV) that is the most common benign laryngeal neoplasm in children. While the disease is not typically fatal, affected children often require prolonged, extensive medical and surgical treatment that has severe emotional and physical consequences. HPV is an encapsulated, non-enveloped double-stranded DNA virus of the family Papillomaviridae. This family contains well over 100 subtypes, based on differences in ability to infect mucosal surfaces and DNA sequence. Some HPV types have been reported to cause more aggressive disease than others; infection with HPV-11, for example, predicts a more aggressive and severe clinical course for children with RRP (Wiatrak et al., 2004). In order to determine HPV type, PCR is used to amplify a single sequence of HPV DNA to several orders of magnitude. It can also be used to detect as little as one copy of HPV DNA sequence per cell making it extremely sensitive. Specificity for a particular HPV DNA sequence comes by the use of DNA primers complimentary to the region of interest, and primers can be designed to amplify any region of DNA for which the sequence is known. Ultimately, information about HPV type can help clinicians plan treatment and counseling for pediatric patients and their families.
In Situ Hybridization
Hybridization techniques (Southern blot, dot blot, and in situ hybridization) have been used to identify specific viral DNA sequences in cells and tissues. In situ and filter in situ hybridization do not require DNA isolation and extraction from tissue, but rather probe directly for the presence of viral sequences in tissue and smears. The use of in situ hybridization (ISH) allows determination of the presence as well as localization of DNA in a specimen. To perform ISH, complimentary nucleic acid probes with either radioactive, dye-labeled, or fluorescent-labeled bases localize DNA via direct microscopic visualization. DNA is quantified colorimetrically. Probes can be designed to detect sequences of DNA common to many disease subtypes or to sequences of DNA unique to individual subtypes. New innovation in ISH technology has made it more practical, as visualization can be achieved with nonfluorescent chromagens using a conventional light microscope and adding signal amplification steps allows viral detection down to one viral copy per cell. One disadvantage of ISH in comparison to PCR may be decreased sensitivity, resulting in false negatives (Allen, Lewis, El Mofty, Haughey, & Nussenbaum, 2010).
In situ hybridization in CSD
As with PCR, ISH is commonly used to perform HPV typing from tissue samples. Singhi & Westra (2010) used ISH to evaluate head and neck cancer specimens for the presence of 13 different HPV types, and found that 82% of their specimens contained HPV. These results support the notion of routine testing for HPV in patients with head and neck cancer in order to guide treatment strategies, predict treatment outcomes, and educate patients and their partners.
RNA
Messenger RNA (mRNA) is typically measured in molecular biology. As mRNA is single stranded and prone to denature, it is often converted into complementary DNA (cDNA) prior to completing reverse transcription (RT). The original “message” of the RNA is preserved in the cDNA code. Downstream analysis can be performed with either mRNA or cDNA using techniques such as northern blot, in situ hybridization, PCR or gene microarray.
RNA Extraction
In order to obtain valid and reliable results in downstream assays, RNA of high purity and integrity must first be obtained from experimental samples. Common techniques used for RNA isolation in the voice and swallowing literature include both the phenol-chloroform method (described in the DNA section) and affinity purification with chaotrophic agents. In the affinity purification technique, tissue is first mechanically homogenized and cell membranes are lysed open to release the nucleic acids. The lysate is applied to a silica membrane which is enclosed within a small vial. Following a series of centrifugation and washing steps, the nucleic acids will bind to a silica membrane in the presence of high concentrations of chaotrophic salts.
There are several considerations when selecting an RNA extraction method. A critical concern for all RNA isolation methods for maintaining RNA purity is the inactivation of ribonucleases (RNases). RNases are naturally occurring enzymes present within samples and the laboratory environment which degrade RNA. As such, special care (i.e., sterile bench, RNase-free tubes and pipette tips, etc) must be taken to prevent contamination during RNA extraction. Advantages of affinity purification are that it eliminates the need for organic solvents, is compatible with a variety of tissue sample types, typically yields excellent RNA purity and integrity and can be performed by relatively novice users with commercially available kits. Advantages of the phenol-chloroform method are that it is compatible with a variety of sample types, is inexpensive and can be used with small or large samples.
Semi-quantitative and Quantitative PCR
PCR was originally developed as a technology for DNA analysis (explained above), and was later used to detect mRNA levels by assessing the cDNA generated during Reverse Transcription PCR (RT-PCR). Following transcription of the mRNA sequence into cDNA using a reverse transcriptase enzyme, RT-PCR employs the same denaturing, annealing and extending phases as found in traditional endpoint PCR. The product created after 30–40 cycles of cDNA synthesis can be analyzed with agarose gel electrophoresis. RT-PCR is known as a semi-quantitative tool and has the same limitations as those described for semiquantitative PCR of DNA.
The advent of real-time or quantitative PCR (qPCR) (Higuchi, Fockler, Dollinger & Watson, 1993) in the early 1990s has greatly enhanced the analysis of mRNA. qPCR improved upon traditional endpoint PCR technology by not only allowing an investigator to determine if a cDNA sequence was expressed in a sample, but also to determine the number of copies present with a high sensitivity and specificity (Wang & Brown, 1999). qPCR utilizes the same process to amplify cDNA as semi-quantitative RT-PCR, yet it is also able to measure the exact amount of product generated after each amplification cycle. This requires a specialized thermal cycler working in concert with a product detection method, such as a fluorescent tag. In this method, the tag is attached to each new cDNA sequence replication and the fluorescence intensity can be detected.
qPCR has several distinct advantages over other methods for assessing mRNA levels. First, this technology is sensitive enough to detect even a single copy of a gene sequence found within a sample (Palmer et al., 2003). Second, qPCR is high throughput, allowing for the detection of a greater number of gene targets in less time as compared with traditional methods. Finally, the generation of quantitative data allows for more rigorous results and conclusions than was possible with the traditional, semi-quantitative methods. A limitation of qPCR is the variation in results that can occur with pipette error, however, the use of reference dyes can reduce or eliminate the pipette error. Additionally, a limitation of all assays which measure mRNA rather than protein levels is that they are an indirect indication of protein regulation and function. There is not a one to one correspondence between the amount of mRNA expression and subsequent production of protein.
qPCR in CSD
Voice scientists have employed qPCR for a variety of investigative pursuits which has contributed to a better understanding of the underlying pathophysiology of vocal fold disorders as well as to the evaluation of potential treatments. For example, vocal fold scarring is the primary cause of chronically poor voice following phonosurgery (Woo, Casper, Colton, Brewer, 1994), yet until the last decade little has been known about the native wound healing response post injury in vocal folds. Using qPCR, investigators have quantified the expression of inflammatory cytokines at 0, 4, 8, 16, 24, and 72 hours following a vocal fold scarring procedure (Lim, Tateya, Tateya, Munoz-Del-Rio, & Bless, 2006). These data mark the molecular underpinnings of the acute phase of wound healing which precedes extracellular matrix (ECM) remodeling and mature vocal fold scar formation. An understanding of these elegant processes is essential for developing and evaluating treatments to attenuate scar. Researchers have also used qPCR to evaluate outcomes of bioengineered strategies for treatment of vocal fold scarring. Variations in hyaluronic acid hydrogel compositions were prophylactically injected into injured rabbit vocal folds in order to evaluate their potential for preventing scar (Duflo, Thibeault, Li, Shu & Prestwich, 2006). Using qPCR, the investigators quantified the expression of ECM proteins in the tissues post-injury and used this information to compare the therapeutic potential of the various biomaterials for encouraging favorable ECM remodeling.
Genome Arrays
Gene or DNA microarray, first introduced in the early 1990s (Fodor, Read, Pirrung, Stryer, Lu & Solas, 1991; Fodor, Rava, Huang, Pease, Holmes & Adams, 1993), is a powerful, microscale tool that has enabled investigators to assess the expression of thousands of genes simultaneously a single sample. Using this method, one cDNA sample is fluorescently labeled green (e.g., sample A), and another is fluorescently labeled red (e.g., sample B). Samples are mixed and washed over a slide or chip that is dotted with probes which will detect the expression of thousands of genes. Each spot corresponds to a single gene. Labeled samples will then hybridize to the genes for which they have corresponding sequences, and the spot on the chip will glow the color of the fluorescent label. Eventually chips will have green, red, yellow and black dots. The green and red dots indicate gene expression of sample A and B, respectively. The yellow dots indicate genes for which both sample A and sample B express at similar levels, and the black dots indicate genes which neither sample express. A plate reader will detect the color and intensity of each dot, yielding gene expression measurements for all genes in both cDNA samples. As a single microarray chip can simultaneously assess the expression of over 30,000 genes, new challenges of data storage and analysis are presented to the scientific community. Powerful statistical software and expertise are being developed to manage these data repositories.
An advantage of microarray is that it is extremely high throughput, and thus useful as a screening tool for applications such as disease detection and drug development. Limitations include that they have decreased sensitivity to low abundance genes and require costly equipment.
Microarray in CSD
Microarray has been used in voice literature in the clinical differential diagnosis of two visually ambiguous laryngeal lesions, Reinke's edema and vocal fold polyps (Duflo et al., 2006). Arrays of nearly 9,000 genes comparing lesions of both types identified 65 genes which statistically distinguished them. As treatments for Reinke's edema and vocal fold polyp are distinct, accurate diagnosis is important. Microarray testing may assist in this effort through the identification of genes which most robustly mark specific pathologies.
Protein
Every tissue within the body contains distinct proteins that help them to grow, maintain structure, repair damage, and aid in movement. As a result of their unique structure, proteins can be extracted from tissue throughout the body and analyzed to determine the health, viability, and function of that specific tissue (Alberts, Bray, Lewis, Raff, Roberts & Watson, 1989). A variety of techniques are used to analyze the protein make-up of tissue within a sample; however, this tutorial will focus on six techniques that have been used throughout the CSD literature. These assays include two-dimensional gel electrophoresis using sodium dodecyl sulfate-polyacrylamide gel electrophoretic separation (SDS-PAGE), immunohistochemistry (IHC), western blot, flow cytometry, enzyme linked immunoabsorbant assay (ELISA) and full proteome assays.
The majority of the assays discussed in this section rely on the specificity of an antigen binding to an antibody. Antibodies are proteins in the body that are able to identify and neutralize foreign “non-self” substances/molecules (antigens) in the body. Antigens provoke the production of antibodies. Researchers can take advantage of the specificity of an antibody binding to an antigen to measure a target protein within a tissue of interest. The binding of an antigen and antibody can be thought of as the simple analogy of a key fitting into a lock. Just as certain keys match specific locks, only certain antibodies will bind to certain antigens. However, it is important to remember that the specificity of each antibody needs to be adequately tested with each experiment to make sure that the antibody binds selectively to the protein of interest. In addition, positive and negative controls should be included in every assay to prevent false positives due to non-specific binding, which may occur from additional proteins or artifacts within a sample (Renshaw, 2007).
Protein Extraction
There are several methods available for isolating proteins which can be differentially selected based on the starting material and the protein's location and function. As with RNA extraction, mechanical homogenization is the most common disruption technique for cells and tissue. Another method used to disrupt cells involves repeated freezing and thawing of cell suspensions, which causes cells to swell and eventually break open due to formation of ice crystals. Buffers and reagents can also be used alone or in conjunction with physical cell or tissue lysis methods. Such buffers and reagents account for the molecular mass, net charge, hydrophobicity, location and function of a particular protein, improving the protein purification process. Once the cell membranes are broken and the sample is homogenized, the samples are centrifuged. Centrifugation will separate the membrane associated proteins into the supernatant and the insoluble proteins into the solid residue at the bottom of the vial. For in vitro cell cultures, intracellular proteins can be extracted from cells and extracellular proteins expressed by cells can be directly obtained from their culture medium. In addition, protein can be measured leaving the morphology of the sample intact by using frozen or paraffin tissue sections.
ELISA
This technique is commonly used for both research and clinical diagnosis in numerous medical specialties due to ease of use and cost effectiveness. Importantly, it is associated with greater safety for the user as compared with radioimmunoassay. ELISA detect and measure the concentration of specific antibodies, soluble antigens or cell-surface molecules in a sample of interest (i.e. cell supernatant, mucous, serum, homogenized tissue). This method requires samples to be in suspension and capture antibodies or antigens to be immobilized on a polystyrene plate. To detect proteins bound to antibodies, a secondary antibody is incubated in each well. Attached to this antibody is an enzyme that can metabolize colorless labels (i.e. peroxidase). A colorimetric substrate is then added to elicit a color change that is measured using a spectrophotometer. An ELISA can take 5 to 7 hours to complete for one antibody or antigen and because of the risk of pipetting erros should be run in duplicate. ELISAs require
ELISA in CSD
Laryngopharyngeal reflux (LPR) is a common, yet unclearly understood diagnosis in the otolaryngology clinic. Pepsin is thought to be a major contributor to LPR symptoms in the absence of detectable acidic events. Knight et al (2005) designed and used an ELISA to detect pepsin concentrations in sputum samples of patients with LPR. Researchers purified different forms of pepsin (isoforms) from gastic juice and synthesized antibodies to multiple antigens to develop a pepsin “sandwich” ELISA. This type of ELISA requires the use of two primary antibodies to improve the specificity to the target, which is a commonly used technique in pregnancy tests. Sputum samples were collected from patients when they were experiencing symptoms of LPR and compared to information obtained from a pH probe. ELISA results showed that pepsin detection in the sputum was correlated with significant decreases in esophageal and pharyngeal pH levels. These results suggest the capability of the pepsin ELISA as a screening tool for LPR.
Flow Cytometry
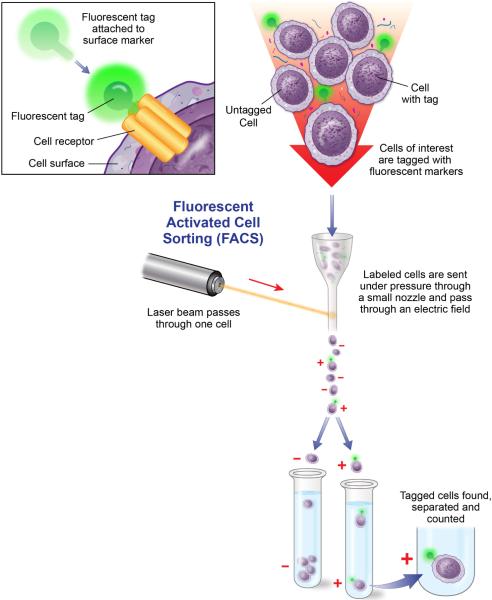
Flow cytometry is an innovative, high throughput technique allowing researchers to separate cells and assess multiple cell characteristics (i.e. surface receptors, cytokines, chemokines, growth factors) within a heterogeneous population of cells or particles. In this technology, fluidics, optics and electronics systems are combined. Procedurally, samples of cells or isolated proteins are suspended in fluid and incubated with specific target antibodies linked with a fluorescent label (Figure 4). If the cells are expressing the target protein, its antibody pair will bind to it. Analysis of isolated proteins requires the use of antibodies attached to synthetic polymer microbeads to bind the target protein and a secondary antibody linked with a fluorescent label. The cells and microbeads in suspension are streamed individually through fluidic chambers in the flow cytometer, passing multiple laser beams that excite the fluorescently-labeled antibodies and emit wavelengths that can be measured. Unlike many of the other assays described in this tutorial, flow cytometry analysis can be highly subjective. Therefore, researchers need to be adequately trained in study design and instrumentation. In addition, positive and negative controls should be included in every experiment. Another limitation is that the instrumentation can be costly and not readily available in all institutions.
Figure 4.
Flow cytometry. Cells are tagged with a fluorescent tag that is attached to the surface marker/receptor of interest (inset). Cells are then sorted and counted based as they are sent under pressure through a small nozzle and pass through an electric field. Adapted from Winslow, T. Kibiuk, L., and Duckwall, C. Looking for a Needle in a Haystack: How Researchers Find Stem Cells. Retrieved July 2011 from http://stemcells.nih.gov/info/2001report/appendixE.asp.
Flow Cytometry in CSD
Currently, only a handful of publications in the field of voice have reported using flow cytometry and many of these studies used the assay to isolate cell populations through multiple phenotypic markers (Hanson et al, 2010; 2011; Ling et al, 2010). Stem cell therapies are emerging in the literature which have demonstrated promise for the treatment of a variety of chronic disorders. Successful vocal fold tissue engineering constructs will likely require a cell source which mimics vocal fold fibroblasts. Research by Hanson et al (2010) used flow cytometry to compare specific cell markers between human vocal fold fibroblasts, adipose derived mesenchymal stem cells (MSC), and bone marrow derived MSC. Results showed that vocal fold fibroblasts positively expressed characteristic MSC markers, including CD29, CD44, CD90, and CD105. These results show that vocal fold fibroblasts have phenotypic characteristics of MSC, thereby identifying both adipose and bone marrow derived MSC as potential cell sources for vocal fold tissue engineering.
SDS-PAGE
SDS-PAGE is a type of electrophoretic analysis that separates proteins according to size (molecular weight) allowing researchers to measure the relative abundance of proteins in a sample. This method is based upon the gel electrophoresis method described at the beginning of this tutorial. An advantage of SDS-PAGE compared to other protein assays is that it does not require the use of costly antibodies to determine specificity. The limitation of this technique is that it operates on the assumption that the molecular weight of the protein of interest is well known and can be easily distinguished from other proteins within the sample. However, this assumption is not always true and additional testing with antibodies is often required to confirm the results.
SDS-PAGE in CSD
SDS-PAGE with densitometric analysis has been used in a number of different studies that provide useful information for professionals working in CSD. This assay has allowed researchers to study the make-up of many of the cranial muscles involved in voice and swallowing, particularly in disease, aging, and exercise models. For example, studies have analyzed the amount and type of different muscle fibers within the cranial muscles using a protein called myosin heavy chain (MHC) (Shiotani & Flint, 1998; Suzuki et al., 2002; Volz et al., 2007). MHC protein constitutes the majority of a key component of muscles, called myosin. In addition, MHC protein has been shown to be a major player in muscle contraction (Liu, Eriksson, Thornell, & Pedrosa-Domellöf, 2005). By analyzing the change in MHC proteins within the voice and swallowing musculature in different age, disease, and exercise models investigators are better able to understand the underlying muscular changes that occur within different populations and with different types of exercise therapies. Clinicians can then use this information to design treatment to combat the underlying changes associated with their different patient populations.
Immunohistochemistry (IHC)
IHC is a technique used to study protein location and distribution, in addition to tissue morphology. IHC uses antibodies that have been engineered to bind specifically to proteins of interest in tissue (Figure 5). To begin, a primary antibody that binds only to the protein of interest is applied to the tissue. Then, a secondary antibody (that contains a dye or fluorescent molecule label) is applied. The secondary antibody binds specifically to the primary antibody and the label on the secondary antibody allows scientists to see the exact site of the protein in the tissue. Thus, IHC takes advantage of this two-step process to allow scientists to visualize proteins of interest in a tissue sample (Renshaw, 2007). A limitation of this technique is that it can often be time consuming and costly to establish the correct antibody protocol for a protein of interest. As stated previously, each antibody must be tested and proper controls must be used to rule out non-specific binding within the sample.
Figure 5.
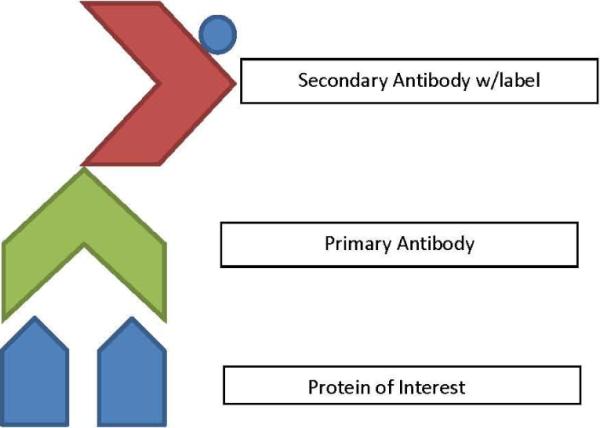
Antibody/antigen bases for identification of a number of protein assays. The protein of interest is identified by the presence of its antigens by a primary antibody. Primary antibodies are then tagged with a secondary antibody that has a label (either color of fluorescent) that will allow for identification of the protein.
IHC in CSD
Researchers within the CSD field have used the IHC assay to identify specific MHC proteins within laryngeal muscles. Rhee and colleagues (2004) used IHC to determine the type of MHC proteins located in the thyroarytenoid (TA) and cricothyroid (CT) muscles of the rat larynx. They also studied changes that occurred within these proteins after cutting and re-uniting the recurrent laryngeal nerve. Based on their analysis they found that the TA and CT muscles contain their own individual set of MHC proteins, corresponding to different fiber type distributions that help to carry out their unique motor demands. Additionally they found there were changes in the types of MHC proteins present in the muscles following their denervation/rennervation experiment. It was hypothesized that this indicated that nerves from adjacent muscle fibers were crossing to innervate the dennervated muscle fibers, thereby changing the MHC proteins present through changes in neural input.
Western Blot
Western blot is a technique that is a combination of both SDS-PAGE and IHC approaches allowing scientists to analyze proteins within a cell, tissue or muscle with greater specificity. During a western blot target experiment proteins are loaded into a gel and individual proteins separate by moving to different locations in the gel based on their molecular weight. However, instead of using a dye or stain to see all of the proteins present at a particular molecular weight within the gel, specific proteins can be detected using antibodies, as in IHC (Towbin, Staehelin, & Gordon, 1979). This method improves specificity of protein detection and can serve as a method for validating the results of an SDS-PAGE experiment. In fact, many scientists will use both SDS-PAGE and western blotting in a single experiment to leave no doubt that the proteins identified by molecular weight are in fact the proteins of interest. By using both molecular weight and antibody specificity, scientists can be certain that they are accurately quantifying their proteins. However, as with IHC alone, it is important to thoroughly test each antibody for specificity to rule out non-specific binding. Limitations include the high cost of primary antibodies, low flexibility in choice of primary antibody label and little signal amplification.
Western Blot in CSD
This combination of techniques has been used in a number of studies quantifying MHC proteins within the cranial muscles involved in voice and swallowing (Kingham, Birchall, Burt, Jones, & Terenghi, 2005; Schaser, Wang, Volz, & Connor, 2009; Shiotani, Westra, & Flint, 2010; Wu, Baker, Crumley, Blanks, & Caiozzo, 1998). Based on this information, researchers can be confident that the results of previous studies using only SDS-PAGE accurately identified MHC proteins within the cranial muscles, both in normal and disordered populations. Because MHC proteins are linked to changes in muscle contractile properties, changes in MHC proteins with age may be responsible for the age related changes reported in cranial musculature and could underlie the functional changes measured in the tongue musculature and the aging swallow.
Proteome Assays
Proteomics is the large scale study of the complete set of proteins expressed within a cell, tissue, organ or organism at a given time and condition. Proteome assays provide large-scale representation of the functional output of a biological system. While the number of assays available for full proteome assessment is growing everyday, several key technologies underlie the ability to capture and characterize the proteome. Two dimensional (2D) SDS-PAGE (O'Farrell, Goodman, & O'Farrell, 1977) is a powerful technology allowing the resolution and characterization of thousands of proteins from a complex sample. Using this technique, proteomes of interest can be visualized, studied and compared. 2D SDS-PAGE involves the separation of proteins according to their independent properties of isoelectric point (pI) and molecular weight (MW). Once separated in two-dimensions, proteins are visualized using protein-specific staining (e.g., Coomassie blue, silver, various fluorescent stains) or autoradiography and may be subjected to quantization using image analysis software (Voordijk, S., Walther, D., Bouchet, G., Appel, R.D., 2003). Careful analysis of high quality gel images allows the identification of both individual protein and proteome-wide variations across experimental conditions. Protein identification can be performed by excision, enzyme digestion, and mass spectrometry (MS) combined with sequence database analysis. Proper sample preparation is critical for valid results. Proteome analysis are time consuming, requires specialized equipment and expertise making the technology not readily available to all.
Liquid Chromatography-Mass Spectrometry (LC-MS/MS) in CSD
The rat is a well accepted and commonly used model for vocal fold mucosa research. Welham, Yamashita, Choi & Ling (2011) used a proteomic assay to characterize a wide range of structural and functional proteins in the rat vocal fold mucosa. Results identified proteins involved in several functions, including the circulation, DNA binding, immune defense, membrane structure, metabolism, motility, fate, signaling, translation and synthesis. Due to the wealth of data generated in this high throughput proteomic characterization, this paper will be a reference tool for voice scientists with a variety of research questions about the vocal fold mucosa.
Conclusions
Discoveries in the fields of genomics and proteomics related to the mechanisms of voice and swallowing have ushered in a new generation of scientists and clinicians committed to translational research. The use of molecular biology analyzation techniques in CSD is facilitating a more comprehensive understanding of a number of diseases and normal function. As noted in the introduction, this tutorial is not an exhaustive list of assays available but rather focused on those that have been principal in the field. It should be noted that the burgeoning fields of genomics and proteomics are poised to take a leap in the generation of clinical applications for molecular-based research. The identification of new molecular markers and the development of high throughput assays are ongoing processes to provide the tools necessary for revolutionizing clinical practice in CSD.
Table 1.
Commons Acronyms
| Acronym | Definition |
|---|---|
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
| mRNA | Messenger RNA |
| PCR | polymerase chain reaction |
| HPV | human papillomavirus |
| ISH | situ hybridization |
| cDNA | complementary DNA |
| RT-PCR | reverse transcription PCR |
| qPCR | quantitative PCR |
| LPR | laryngopharyngeal reflux |
| MSC | mesenchymal stem cell |
| hVFF | human vocal fold fibroblasts |
| CD | cluster differentiation |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoretic separation |
| IHC | immunohistochemistry |
| MHC | myosin heavy chain |
| TA | thyroarytenoid |
| CT | cricothyroid |
| MS | mass spectrometry |
Acknowledgements
The authors would like to acknowledge Gary Weismer, PhD for his encouragement and the original tutorial conception. This work was sponsored by NIH NIDCD R01 DC4336, R01 DC9600, R01 DC008149, T32 DC009401.
Footnotes
Publisher's Disclaimer: This is an author-produced manuscript that has been peer reviewed and accepted for publication in the Journal of Speech, Language, and Hearing Research (JSLHR). As the “Papers in Press” version of the manuscript, it has not yet undergone copyediting, proofreading, or other quality controls associated with final published articles. As the publisher and copyright holder, the American Speech-Language-Hearing Association (ASHA) disclaims any liability resulting from use of inaccurate or misleading data or information contained herein. Further, the authors have disclosed that permission has been obtained for use of any copyrighted material and that, if applicable, conflicts of interest have been noted in the manuscript.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular biology of the cell. 2nd ed. Garland Publishing, Inc; New York, NY: 1989. [Google Scholar]
- Allen CT, Lewis JS, Jr, El Mofty SK, Haughey BH, Nussenbaum B. Human papillomavirus and oropharynx cancer: Biology, detection and clinical implications. The Laryngoscope. 2010 doi: 10.1002/lary.20936. [DOI] [PubMed] [Google Scholar]
- Benninger MS. Quality of the voice literature: What is there and what is missing. Journal of Voice. doi: 10.1016/j.jvoice.2010.09.005. in press. [DOI] [PubMed] [Google Scholar]
- Crick F. Central dogma of molecular biology. Nature. 1970;227(5258):561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Engineering. 2006;12(8):2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- Duflo SM, Thibeault SL, Wenhua L, Smith ME, Schade G, Hess MM. Differential gene expression profiling of vocal fold polyps and reinke's edema by complementary DNA microarray. The Annals of Otology, Rhinology & Laryngology. 2006;115(9):703–714. doi: 10.1177/000348940611500910. [DOI] [PubMed] [Google Scholar]
- Fodor SP, Read JL, Pirrung MC, Stryer L, Lu AT, Solas D. Light-directed, spatially addressable parallel chemical synthesis. Science. 1991;251(4995):767. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- Fodor S, Rava RP, Huang XC, Pease AC, Holmes CP, Adams CL. Multiplexed biochemical assays with biological chips. Nature. 1993;364:555–556. doi: 10.1038/364555a0. [DOI] [PubMed] [Google Scholar]
- Gillett S, Rees L, Coogan T, Birchall M, Bailey M. Characterisation of the bacterial flora of the larynx. Clinical Otolaryngology. 2006;31(6):583–583. [Google Scholar]
- Hanson SE, Kim J, Johnson BHQ, Bradley B, Breunig MJ, Hematti P, Thibeault SL. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. The Laryngoscope. 2010;120(3):546–551. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SE, King SN, Kim J, Chen X, Thibeault S, Hematti P. The effect of mesenchymal stromal cell - hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue engineering. Part A. 2011;17(19):2463–71. doi: 10.1089/ten.tea.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins TL, O'Connor-Morin T, Roy A, Santillan C. DNA purification and isolation using a solid-phase. Nucleic Acids Research. 1994;22(21):4543. doi: 10.1093/nar/22.21.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: Real-time monitoring of DNA amplification reactions. Nature Biotechnology. 1993;11(9):1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- Howe J, Klimstra D, Cordon-Cardo C. DNA extraction from paraffin-embedded tissues using a salting-out procedure: A reliable method for PCR amplification of archival material. Histology and Histopathology. 1997;12(3):595. [PubMed] [Google Scholar]
- Kingham PJ, Birchall MA, Burt R, Jones A, Terenghi G. Reinnervation of laryngeal muscles: A study of changes in myosin heavy chain expression. Muscle & Nerve. 2005;32(6):761–766. doi: 10.1002/mus.20409. [DOI] [PubMed] [Google Scholar]
- Knight J, Lively MO, Johnston N, Dettmar PW, Koufman JA. Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope. 2005;115(8):1473–1478. doi: 10.1097/01.mlg.0000172043.51871.d9. [DOI] [PubMed] [Google Scholar]
- Levi JE, Delcelo R, Alberti VN, Torloni H, Villa LL. Human papillomavirus DNA in respiratory papillomatosis detected by in situ hybridization and the polymerase chain reaction. The American Journal of Pathology. 1989;135(6):1179. [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tateya I, Tateya T, MUNOZ-DEL-RIO A, BLESS DM. Immediate inflammatory response and scar formation in wounded vocal folds. The Annals of Otology, Rhinology & Laryngology. 2006;115(12):921–929. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- Ling C, Yamashita M, Zhang J, Bless DM, Welham NV. Reactive response of fibrocytes to vocal fold mucosal injury in rat. Wound Repair Regeneration. 2010;18(5):514–23. doi: 10.1111/j.1524-475X.2010.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellöf F. Fiber content and myosin heavy chain composition of muscle spindles in aged human biceps brachii. Journal of Histochemistry & Cytochemistry. 2005;53(4):445. doi: 10.1369/jhc.4A6257.2005. [DOI] [PubMed] [Google Scholar]
- O'Farrell PZ, Goodman HM, O'Farrell PH. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12(4):1133–1142. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JAM, Polis M, Metcalf JA. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. Journal of Clinical Microbiology. 2003;41(10):4531. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra KJ, Osgood MP, Pappas DL., Jr A research based laboratory course designed to strengthen the research teaching nexus. Biochemistry and Molecular Biology Education. 2010;38(3):172–179. doi: 10.1002/bmb.20358. [DOI] [PubMed] [Google Scholar]
- Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Saunders J. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. The New England Journal of Medicine. 2007;357(25):2552. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Lucas CA, Hoh JFY. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. Journal of Histochemistry & Cytochemistry. 2004;52(5):581. doi: 10.1177/002215540405200503. [DOI] [PubMed] [Google Scholar]
- Renshaw S, editor. Immunohistochemistry. Scion Publishing Ltd; Oxfordshire, UK: 2007. [Google Scholar]
- Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2010:1–8. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani A, Flint PW. Myosin heavy chain composition in rat laryngeal muscles after denervation. The Laryngoscope. 1998;108(8):1225–1229. doi: 10.1097/00005537-199808000-00023. [DOI] [PubMed] [Google Scholar]
- Shiotani A, Westra WH, Flint PW. Myosin heavy chain composition in human laryngeal muscles. The Laryngoscope. 1999;109(9):1521–1524. doi: 10.1097/00005537-199909000-00030. [DOI] [PubMed] [Google Scholar]
- Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- Sun W. Nucleic extraction and amplification. In: Grody WW, Nakamura RM, Kiechle FL, Strom C, editors. Molecular Diagnostics. Academic Press; London, UK: 2010. pp. 35–49. [Google Scholar]
- Suzuki T, Connor NP, Kyungah L, Bless DM, Ford CN, Inagi Age-related alterations in myosin heavy chain isoforms in rat intrinsic laryngeal muscles. The Annals of Otology, Rhinology & Laryngology. 2002;111(11):962–967. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- Thibeault SL, Hirschi SD, Gray SD. DNA microarray gene expression analysis of a vocal fold polyp and granuloma. Journal of Speech, Language, and Hearing Research. 2003;46(2):491. doi: 10.1044/1092-4388(2003/040). [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Sciences. 1979;76(9):4350. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz LM, Mann LB, Russell JA, Jackson MA, Leverson GE, Connor NP. Biochemistry of anterior, medial, and posterior genioglossus muscle in the rat. Dysphagia. 2007;22(3):210–214. doi: 10.1007/s00455-006-9075-y. [DOI] [PubMed] [Google Scholar]
- Voordijk S, Walther D, Bouchet G, Appel RD. Encyclopedia of the human genome. Macmillan Publishers Ltd., Nature Publishing Group; London: 2003. Image analysis tools in proteomics; pp. 404–410. [Google Scholar]
- Wang T, Brown MJ. mRNA quantification by real time TaqMan polymerase chain reaction: Validation and comparison with RNase protection. Analytical Biochemistry. 1999;269:198–200. doi: 10.1006/abio.1999.4022. [DOI] [PubMed] [Google Scholar]
- Welham NV, Yamashita M, Choi SH, Ling C. Cross-sample validation provides enhanced proteome coverage in rat vocal fold mucosa. PLoS One. 15. 2011;6(3) doi: 10.1371/journal.pone.0017754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiatrak BJ, Wiatrak DW, Broker TR, Lewis L. Recurrent respiratory paillomatosis: A longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114(S104):1–23. doi: 10.1097/01.mlg.000148224.83491.0f. [DOI] [PubMed] [Google Scholar]
- Wolfe KA, Breadmore MC, Ferrance JP, Power ME, Conroy JF, Norris PM, Landers JP. Toward a microchip based solid phase extraction method for isolation of nucleic acids. Electrophoresis. 2002;23(5):727–733. doi: 10.1002/1522-2683(200203)23:5<727::AID-ELPS727>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wu YZ, Baker MJ, Crumley RL, Blanks RHI, Caiozzo VJ. A new concept in laryngeal muscle: Multiple myosin isoform types in single muscle fibers of the lateral cricoarytenoid. Otolaryngology--Head and Neck Surgery. 1998;118(1):86. doi: 10.1016/S0194-5998(98)70380-8. [DOI] [PubMed] [Google Scholar]