Abstract
Airways diseases such as asthma, emphysema, and chronic bronchitis are, in part, characterized by reversible airflow obstruction and inflammation. In severe disease, marked decreases in lung function are associated with airway smooth muscle proliferation and airway neutrophilia. Inhaled glucocorticoids attenuate increased airflow obstruction and airway inflammation that occur, in part, due to increased smooth muscle migration and proliferation, as well as the airway neutrophilia. Glucocorticoids, however, have adverse side effects and, in some patients, are ineffective despite high doses. Recent research has explored the effects of non-traditional steroids on attenuation of inflammation associated with airways diseases. These non-traditional steroids have improved side effect profiles in comparison to glucocorticoid therapy. Our studies assessed effects of dehydroepiandrosterone-3-sulfate (DHEA-S) on migration of both human peripheral blood neutrophils (PMN) and human airway smooth muscle cells (HASM). DHEA-S dose-dependently inhibited chemotaxis of PMN and HASM while having no effect on the phosphorylation levels of Akt, ERK1/2, p38 MAPK or PKC, canonical positive regulators of cell migration. These studies demonstrate direct effects of DHEA-S on cell migration, thereby suggesting that DHEA-S may attenuate airway inflammation and cell migration.
Keywords: airway inflammation, DHEA-S, airway remodeling
1. Introduction
Asthma and other airways diseases are marked by inflammation of the airways, increased migration of airway smooth muscle cells, and inflammatory cell influx that contribute to airway remodeling. Current therapeutics that modulate these responses traditionally comprise glucocorticoids and β2 adrenergic receptor (β2AR) agonists. Unfortunately, some patients exhibit steroid insensitivity despite high doses of glucocorticoids.
Dehydroepiandrosterone-3-sulfate (DHEA-S) and its metabolite dehydroepiandrosterone (DHEA) are metabolites of cholesterol that can be further metabolized into testosterone and estradiol. Evidence suggests that levels of DHEA and DHEA-S in patients with asthma are decreased, both in the stable phase of the disease as well as during exacerbations [1]. Research in animal models and in clinical trials has demonstrated that DHEA-S and DHEA are effective in attenuating Th2, cytokines associated with allergic disease, cytokine elaboration and by inhibiting influx of inflammatory cells into the airways [2,3,4]. Therefore, supplementation of these naturally occurring steroids may be therapeutically beneficial and offer a steroid-based therapy without the adverse side effects observed with traditional glucocorticoid treatments. Although evidence supports the existence of both cell surface and nuclear receptors that bind DHEA [5,6,7], the signaling pathways by which DHEA or DHEA-S mediates its effects remain unclear.
We sought to determine the effects of DHEA-S on cells known to be involved in the pathogenesis of asthma and COPD, human airway smooth muscle cells (HASM) and primary human neutrophils (PMN). We found that treatment with DHEA-S inhibited chemokinesis and chemotaxis of PMN, and chemokinesis of HASM. To identify a mechanism by which DHEA-S inhibits cell migration, phosphorylation of Akt, ERK1/2, p38 MAPK and PKC was examined.
2. Methods
2.1. Neutrophil chemokinesis/chemotaxis
Peripheral blood (20–30 ml) was drawn from healthy donors, in accordance with the Declaration of Helsinki and approved by the Committee on Studies Involving Human Beings at the University of Pennsylvania, and layered over PMN isolation medium (Matrix) and spun at room temperature for 35 min at 1500 rpm. The plasma and mononuclear layers as well as half of the clear dextrose layer were aspirated off, and an equal volume of 0.45% NaCl was added, filling the remaining tube to the top with 0.9% NaCl. The resulting solution was centrifuged for 15 min at 1500 rpm at room temperature. Supernatant was removed, and the pellet was resusupended in 0.2% NaCl, and then an equal volume of 1.6% NaCl to ensure red blood cell lysis. The solution was centrifuged at 1500 rpm for 15 min, supernatant removed, and PMNs resuspended in media. Total number of cells was counted, and 100,000 cells were incubated with DHEA-S (Epi-12323, Epigenesis Pharmaceuticals, Cranbury, NJ) (0.01–10 µM) for 4 h prior to loading into 12-well transwell plates with or without 10 nM fMLP in the bottom chamber. Chemokinesis and chemotaxis were measured by counting the number of cells present in the bottom chamber 2 h after their addition onto the transwell insert. Cell viability was assessed by trypan blue exclusion and caspase activation. A single concentration of DHEA-S (10 µM) was chosen as the concentration to use for subsequent migration and signaling experiments, based on the dose responses performed in the neutrophils but also as a result of this concentration being used in clinical trials [3].
2.2. Human airway smooth muscle cell isolation and migration analysis
HASM cells were dissociated from human tracheas obtained from human lung transplant donors, in accordance with the Declaration of Helsinki and approved by the Committee on Studies Involving Human Beings at the University of Pennsylvania, as described previously [8]. Cells derived from a minimum of two healthy donors were cultured in Ham's F12 media (Life Technologies, Grand Island, NY), supplemented with 10% FBS (HyClone Laboratories, Logan, UT), 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Before the experiments, HASM cells were maintained for 48 h in serum-free Ham's F12 media, supplemented with 0.1% BSA. Cell migration was examined using a Boyden chamber apparatus as we described previously [9]. Briefly, HASM cells, pre-incubated with 10 µM DHEA-S or diluent for 18 h, were briefly trypsinized by 0.05% trypsin/0.53 mM EDTA, centrifuged at 900 rpm for 10 min and resuspended in serum-free media supplemented with BSA. Cells were then placed into the upper wells of the Boyden chamber (5 × 104 cells/well) fitted with an 8-µm pore membrane coated with Vitrogen (100 µg/ml). Vehicle or 10 ng/ml PDGF-BB in serum-free media supplemented with BSA was added to the lower chambers. Cells in the Boyden chamber were incubated for 4 h at 37°C in a 5% CO2 incubator. Non-migrated cells were scraped off, the membrane was fixed with methanol, stained with Hemacolor stain set (EM Industries, Inc., Gibbstown, NJ) and scanned. Cell migration was analyzed using the Gel-Pro analyzer program (Media Cybernetics, Silver Spring, MD). Cell viability was measured by trypan blue exclusion and caspase activation.
2.3. Cell lysate preparation and immunoblot analysis
HASM cells were cultured in Ham's F12 media, supplemented with 10% FBS, and primocin™ (Invivogen, San Diego, CA). Before the experiments, HASM cells were maintained for 48 h in serum-free Ham's F12 media, supplemented with 0.1% BSA. Cells were incubated for 18 h with 10 µM DHEA-S, 1 µM dexamethasone (Sigma Aldrich, St. Louis, MO), or for 40 min with 30 µM LY294002 (Cayman Chemicals, Ann Arbor, MI), treated with 10 ng/ml PDGF-BB or diluent for 10 min, and cell lysates were prepared as we described previously [10]. Cells were also stimulated with PDGF (1 ng/ml) for 2, 5 or 30 min in the presence and absence of 10 µM DHEA-S. Immunoblot analysis with anti-phospho-Akt, anti-phospho ERK1/2, anti-phospho p38 MAPK, anti-phospho PKC and anti-COX-4 (Cell Signaling Technology, Danvers, MA) was performed similarly as described [11].
2.4. Data analysis
Data points from individual assays represent mean values ± standard error. Statistically significant differences among groups were assessed with the analysis of variance (ANOVA) (with the Bonferroni-Dunn correction), with values of P < 0.05 sufficient to reject the null hypothesis for all analyses. All experiments were designed with matched control conditions within each experiment to enable statistical comparison as paired samples and to obtain statistically significant data.
3. Results
3.1. DHEA-S inhibits PDGF-induced migration of human airway smooth muscle cells (HASM)
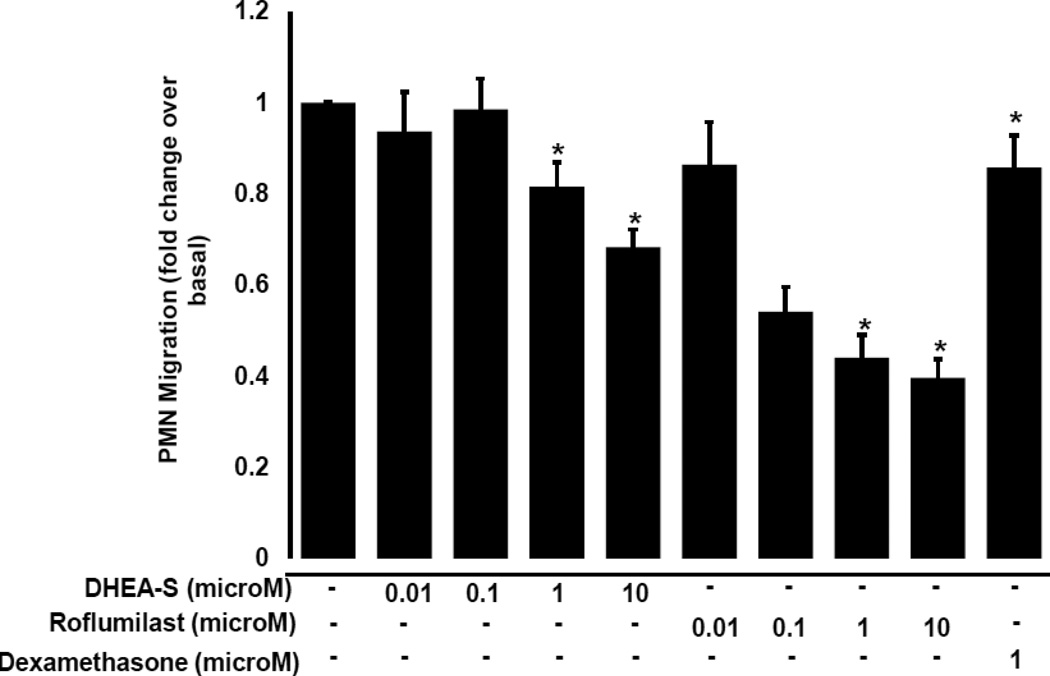
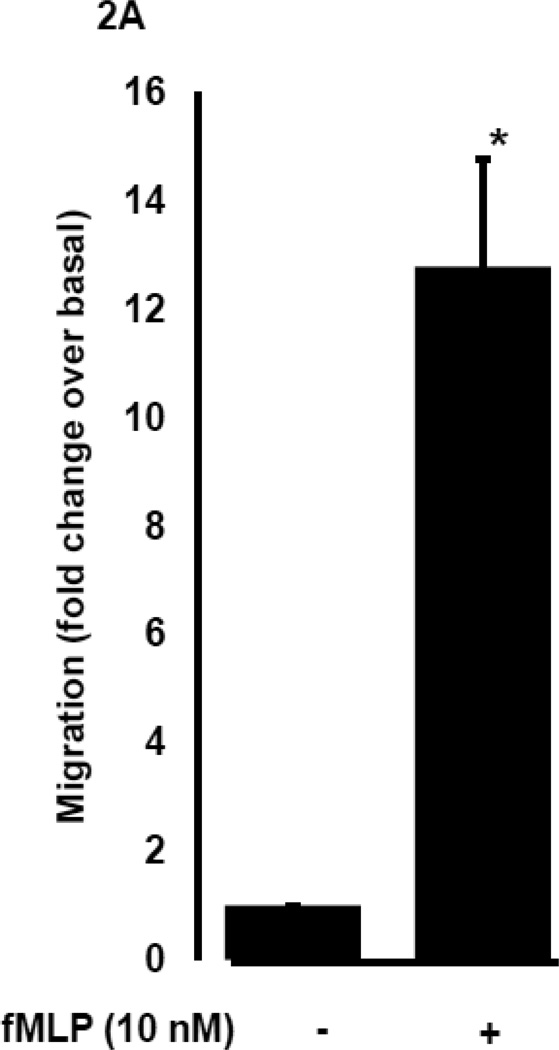
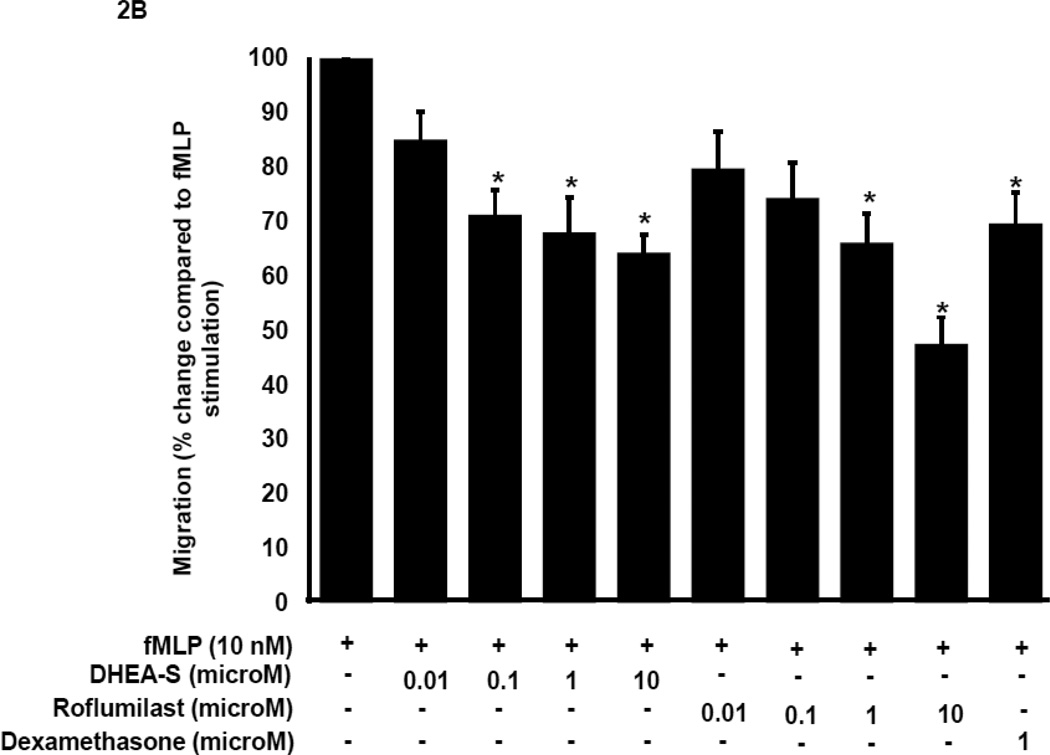
To determine whether DHEA-S modulates migration of peripheral blood neutrophils (PMN), we evaluated chemokinesis and fMLP-induced chemotaxis of human primary PMN. Cells were incubated for 4 h with 0.01–10 µM DHEA-S, 0.01–10 µM roflumilast (a selective phosphodiesterase 4 inhibitor), or 1 µM dexamethasone, and then PMN migration was assessed in the presence or absence of 10 nM fMLP. As seen in Fig. 1, DHEA-S, roflumilast and dexamethasone significantly inhibited PMN chemokinesis. Stimulation with fMLP induced a ~13-fold increase in PMN migration compared to diluent-treated cells (Fig. 2A). Chemotaxis to fMLP was significantly attenuated by preincubation with 0.1, 1, and 10 µM DHEA-S, 1 and 10 µM roflumilast, and 1 µM dexamethasone (Fig. 2B). Treatment with DHEA-S did not affect PMN viability (data not shown). Taken together, these data suggest that DHEA-S is effective at attenuating both chemokinesis and fMLP-induced chemotaxis.
Fig. 1. DHEA-S inhibits neutrophil chemokinesis.
Human peripheral blood neutrophils were incubated with 0.01–10 µM DHEA-S, 0.01 µM roflumilast or 1 µM dexamethasone for 4 h, transferred to transwell inserts and allowed to migrate in the absence of chemoattractant.
Fig. 2. DHEA-S inhibits fMLP-induced neutrophil migration.
Human peripheral blood neutrophils were incubated with 0.01–10 µM DHEA-S, 0.01 µM roflumilast or 1 µM dexamethasone for 4 h, transferred to transwell inserts and allowed to migrate in the presence of 10 nM fMLP for 2 h (A) and (B). Inhibition by treatment with compounds (B) was expressed as a percentage of fMLP stimulated alone (set at 100%). Data is representative of three to seven measurements per condition tested (* p<0.0001)
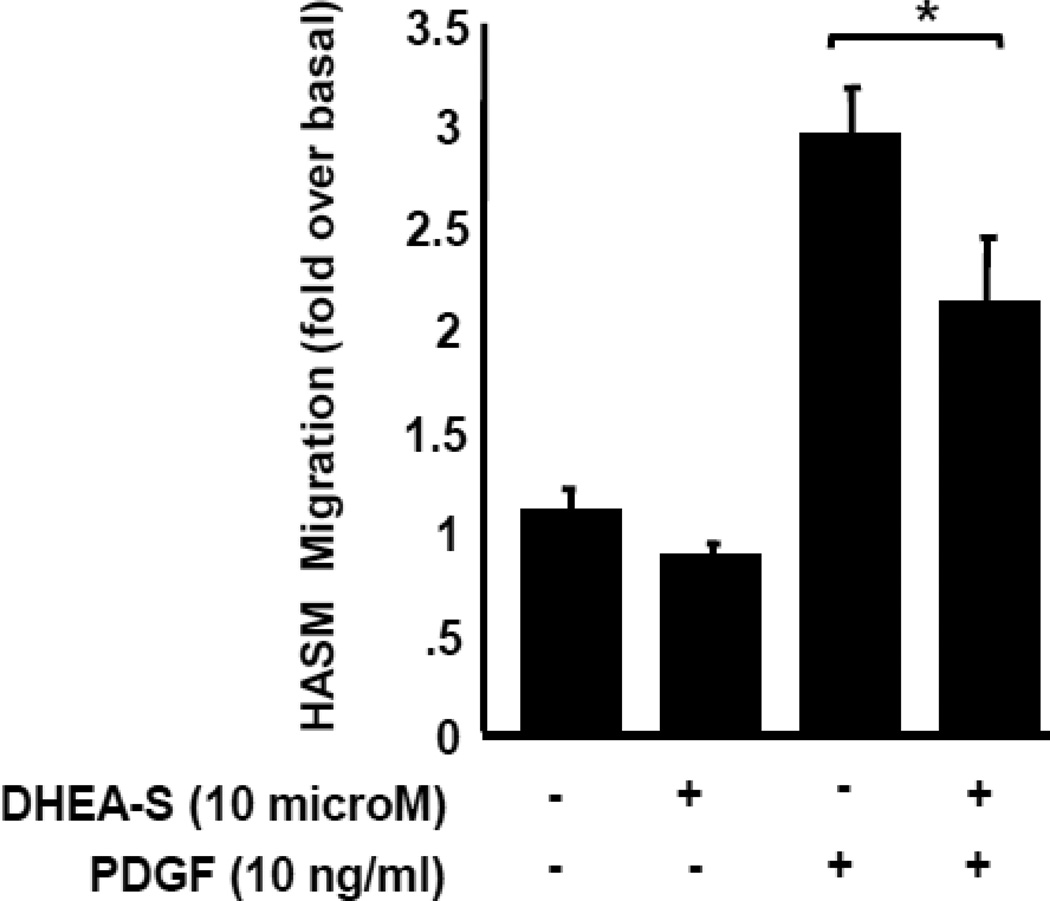
3.2. DHEA-S inhibits PDGF-induced migration of human airway smooth muscle cells (HASM)
To evaluate effects of DHEA-S on HASM cell migration, cells were pre-incubated for 18 h with DHEA-S, and then migration of HASM in the presence or absence of PDGF was examined. We found that DHEA-S had little effect on HASM cell chemokinesis (Fig. 3). PDGF increased migration of HASM three-old over basal, and pretreatment with DHEA-S significantly reduced PDGF-induced HASM migration (Fig. 3). These data show that DHEA-S inhibits PDGF-induced chemotaxis, but not chemokinesis of HASM cells.
Fig. 3. DHEA-S inhibits PDGF-mediated airway smooth muscle migration.
HASM cells were incubated with 10 µM DHEA-S overnight (18 h), loaded into a Boyden chamber and allowed to migrate to 10 ng/ml PDGF for 4 h. Data is representative of triplicate measurements from two different cell lines (* p<0.005)
3.3. DHEA-S has no effect on PDGF-induced Akt, ERK1/2, p38 MAPK and PKC phosphorylation
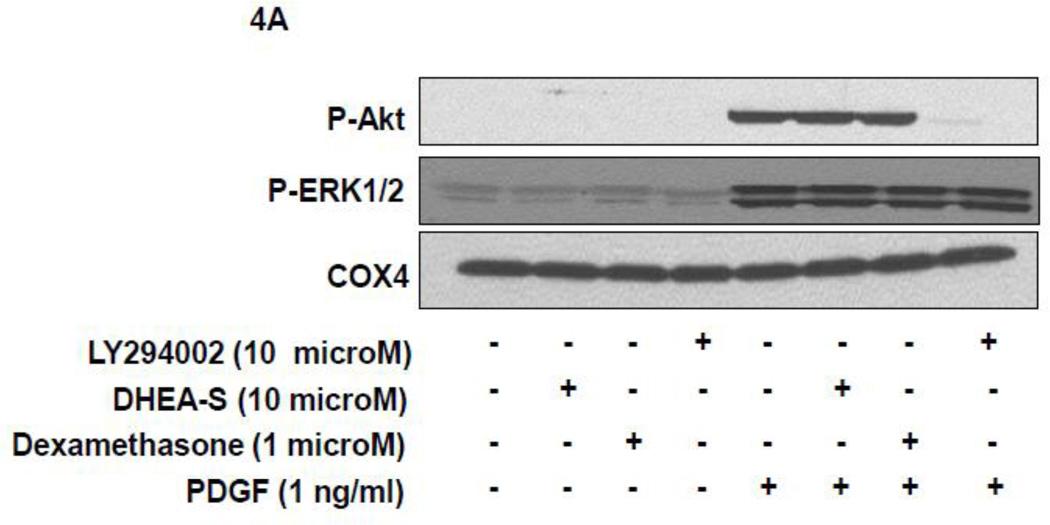
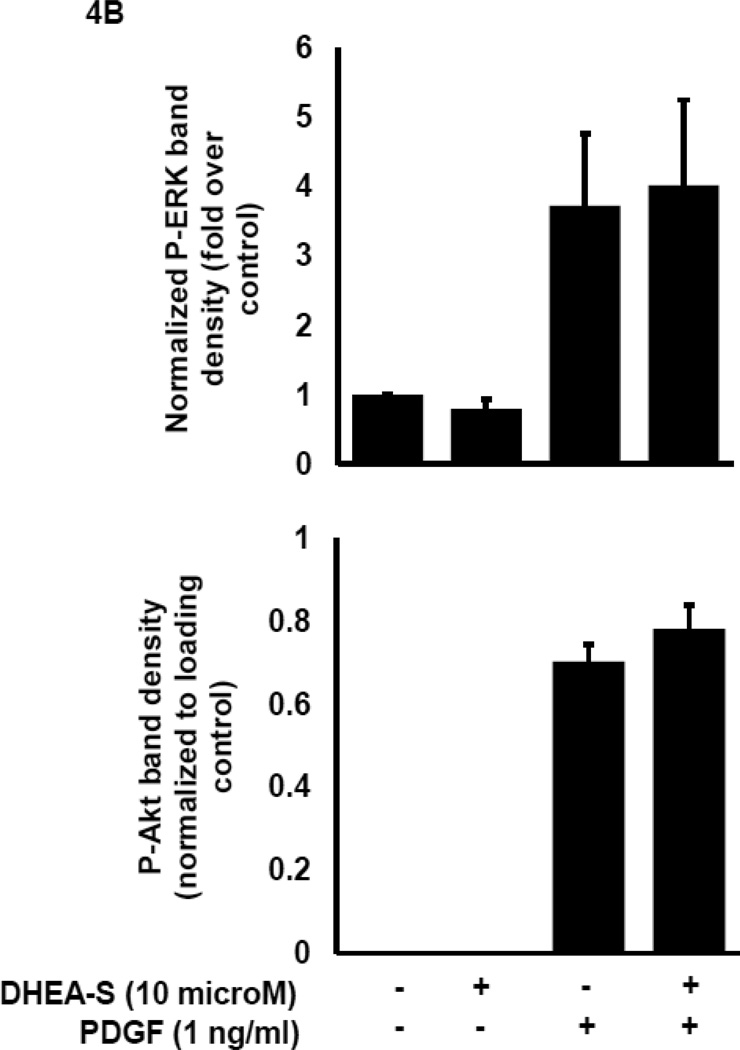
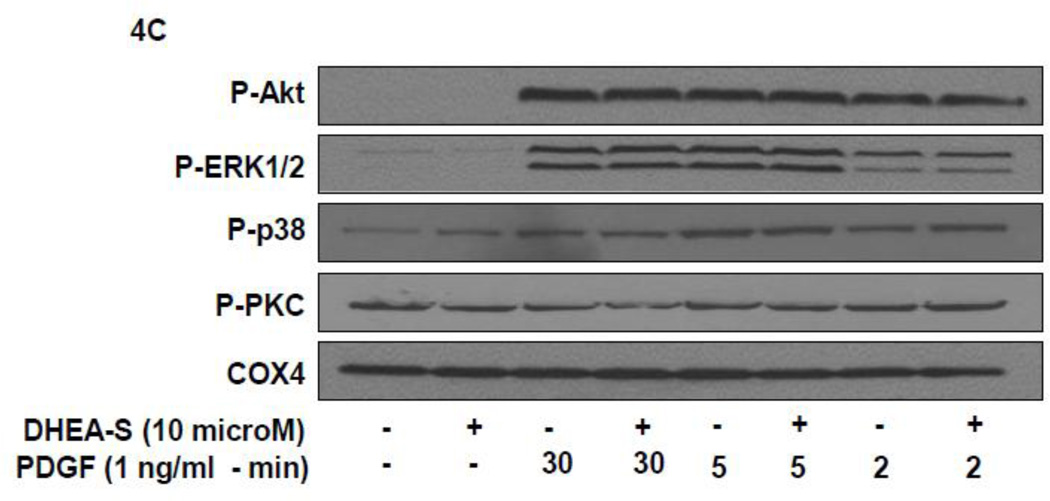
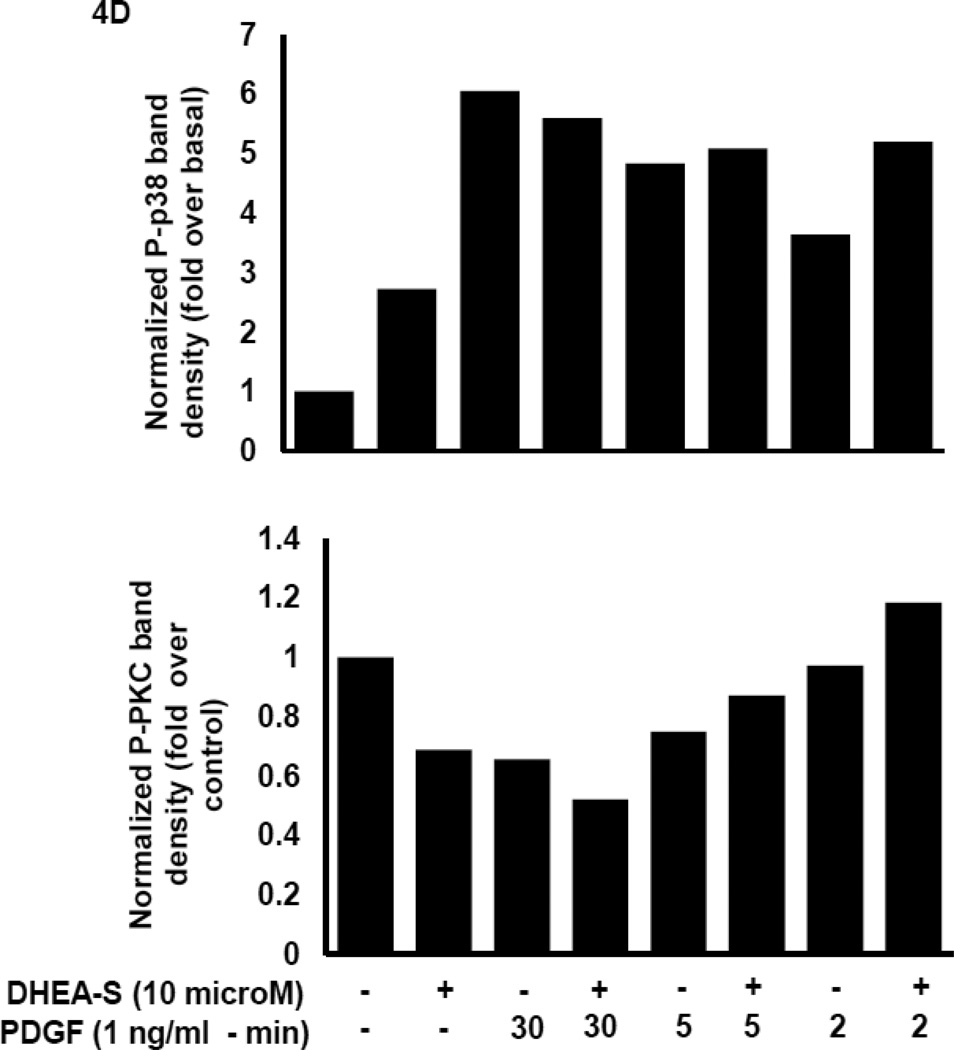
To delineate potential mechanisms whereby DHEA-S attenuates migration of HASM following PDGF stimulation, we first assessed phosphorylation of Akt as a downstream substrate for PI3 kinase activation. Previously, we reported that PI3 kinase (PI3K) activity is required for PDGF-induced HASM migration by utilizing the pharmacological inhibitor LY294002 to inhibit PI3K [11]. We reasoned that DHEA-S alters PI3K-Akt signaling pathways to decrease PDGF-induced migration of HASM. We found that pretreatment of HASM with 10 µM DHEA-S or 1 µM dexamethasone, which inhibited PDGF-induced HASM migration (Fig. 3 and [9]), has little effect on PDGF-induced phosphorylation of Akt. Pretreatment with PI3K inhibitor LY294002 under identical experimental conditions suppressed Akt phosphorylation (Fig. 4A and 4B). The phosphorylation state of other signaling molecules known to positively modulate cellular migration, including ERK1/2, p38 MAPK and PKC, were examined to assess a potential role for these in DHEA-S-mediated attenuation of PDGF-induced migration (Fig. 4A–D). The phosphorylation of all proteins tested was unaffected by pretreatment with DHEA-S and subsequent stimulation with PDGF. These data suggest that DHEA-S inhibits PDGF-mediated HASM migration independently of canonical pathways known to regulate migration.
Fig. 4. DHEA-S does not inhibit PDGF-dependent phosphorylation of Akt, ERK1/2, p38 MAPK or PKC.
(A–B) HASM cells were incubated with 10 µM DHEA-S (18 h), 1 µM dexamethasone (18 h) or 30 µM LY294002 (40 min) prior to stimulation with 1 ng/ml PDGF (10 min). Phosphorylation of Akt and ERK1/2 was assessed by immunoblotting. Total COX-4 was used as a loading control. (C-D) HASM cells were incubated with 10 µM DHEA-S (18 h) prior to stimulation with 1 ng/ml PDGF (2, 5 and 30 min). Phosphorylation of Akt, ERK1/2, p38 MAPK, and PKC was assessed, using total COX-4 as a loading control. Data is representative of three independent experiments in three different cell lines.
4. Discussion
In this study, we sought to examine the effects of DHEA-S on migration of human neutrophils (PMNs) and airway smooth muscle cells (HASM). We found that DHEA-S dose-dependently decreased chemokinesis and fMLP induced chemotaxis of PMNs, as well as PDGF-mediated chemotaxis of HASM. Two types of commonly used asthma and COPD therapeutics were used as comparison since dexamethasone, a glucocorticoid, and roflumilast, a phosphodiesterase 4 inhibitor, inhibit PDGF-induced airway smooth muscle migration [9]. We show that at 10 µM, but not at other concentrations, roflumilast is more effective than DHEA-S at inhibiting fMLP-induced chemotaxis. Given these dose responses, we show that roflumilast is more potent and effective at similar concentrations of DHEA-S. In an effort to elucidate potential mechanisms for DHEA-S-mediated attenuation of migration, we assessed Akt phosphorylation, a surrogate for PI3K activation, as well as ERK1/2, p38 MAPK and PKCα phosphorylation – signaling molecules known to modulate cell migration. We found that treatment with DHEA-S had little effect on PDGF-mediated Akt, ERK1/2, p38 MAPK and PKCα phosphorylation. Interestingly, a study of vascular smooth muscle demonstrated that DHEA inhibited phosphorylation of Akt at a concentration ten-fold higher than we utilized to inhibit cell migration [12], a finding that contrasts with effect of DHEA-S that we show. Our data suggests that other mechanisms governing cytoskeletal rearrangements necessary for coordinated cell movement are affected and attenuated by DHEA-S treatment, rather than canonical pathways governing cell migration.
Glucocorticoids, a mainstay in the treatment of lung disorders, decrease airway inflammation in asthma and COPD. Although effective at reducing inflammation, steroids have undesirable effects, and some patients are insensitive to glucocorticoids. Use of anti-inflammatory alternatives exhibiting fewer adverse effects may serve as alternative strategies. DHEA and DHEA-S offer potential options to traditional glucocorticoid therapy. In mice, OVA-induced allergic airway inflammation was markedly attenuated by DHEA-S, and this treatment decreased eosinophilic infiltration of the lungs as well as inhibited increases in eotaxin-1 and eotaxin-2, IL-5, IL-13, IL-6 and TNFα on. Interestingly, DHEA treatment also decreased levels of PGE2 [2]. Collectively, these data suggest that DHEA-S can attenuate cytokine-induced hyperresponsiveness of airway smooth muscle, but may increase the agonist-mediated bronchoconstriction by decreasing endogenous levels of PGE2. Others showed, using Der f, a house dust mite allergen, eosinophil influx, and IL-4 and IL-5 levels into the lungs were decreased following administration of DHEA [4]. Collectively, these data suggest that DHEA and DHEA-S may modulate inflammatory responses associated with allergen-induced airways diseases. In human studies, nebulized DHEA and DHEA-S attenuated allergen-induced late phase response in mild asthma subjects [3]. Using human PBMCs, DHEA also decreased IL-10, IL-5 and IFNγ production, and decreased cytokine production was more pronounced in individuals exhibiting airway hyperresponsiveness [13].
Radford et al. demonstrated that treatment of human neutrophils with DHEA-S increased superoxide formation, in both the presence and absence of priming with GM-CSF [14]. Others, utilizing neutrophils and endothelial cells, showed that DHEA treatment increased L-selectin expression, decreased LPS-induced CD11b expression, and had little effect on CD18 expression in neutrophils. On endothelial cells, there was also little effect on LPS-induced levels of VCAM or ICAM-1 expression. Levels of E-selectin, however, were decreased following DHEA alone or in combination with LPS stimulation [15]. In rat tracheal smooth muscle, DHEA treatment decreased proliferation of the smooth muscle following stimulation with FBS or PDGF [16]. Utilizing a rabbit vascular smooth muscle cell line, Furutama et al. demonstrated that DHEA-S administration inhibited PDGF-induced cell migration, proliferation and adhesion to fibronectin [5]. This same study noted by Scatchard analysis that a majority of DHEA-S was found to be bound in the nucleus, suggesting that it is indeed binding a nuclear receptor. Given current evidence, we hypothesized that DHEA-S may have direct effects on neutrophils and HASM that modulate inflammation observed in asthma. We found that treatment of human neutrophils with DHEA-S inhibited both chemotaxis and chemokinesis of the cells. In animal studies, DHEA-S inhibits immune cell recruitment to the lungs following allergen challenge. Our data also suggest that the effects of DHEA-S may not be exclusively a function of decreasing levels of inflammatory mediators necessary to recruit cells, but may also directly modulate cytoskeletal arrangements within cells. We demonstrated that migration of HASM to the growth factor PDGF was also inhibited by DHEA-S, suggesting a generalized effect of DHEA-S on cell migration. We also showed that a dose of DHEA-S that inhibited migration had little effect on PDGF-induced phosphorylation of Akt, which is a downstream substrate of PI3K that is required for PDGF-mediated HASM migration [11]. The effect of DHEA-S on phosphorylation of ERK1/2, p38 MAPK and PKCα was examined as other potential signaling molecules modulating cell migration. A study examining migration of neutrophils to fMLP noted that inhibition of myristoylated alanine-rich c-kinase substrate (MARCKS), a target of PKCα, abolished chemokine-dependent migration [17]. We examined phosphorylation of PKCα in our study and found that there was no modulation of phosphorylation of this molecule. Further studies are necessary to define the probable mechanism by which DHEA-S attenuates cell migration; we speculate that DHEA-S directly modulates cytoskeletal rearrangements. Collectively, DHEA-S may have benefit in the treatment of airways disorders such as asthma and COPD, not only by inhibiting inflammatory mediator secretion but also by inhibiting myocyte and immune cell migration.
Highlights.
DHEA-S attenuated migration of airway smooth muscle and neutrophils.
DHEA-S had little effect on PDGF-induced Akt phosphorylation.
Non-traditional steroids may offer novel therapy for airways disease.
Acknowledgements
We would like to thank Dr. Lisa R. Forbes for her assistance with blood draws. We would also like to thank Mary McNichol for assistance with preparation of the manuscript.
Role of funding source
This research was supported in part by funding from Epigenesis Pharmaceuticals, LLC, and the National Institutes of Health (ES013508 to RAP). Epigenesis Pharmaceuticals, LLC, contributed to the study design but had no other involvement. The National Institutes of Health had no involvement in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
Contributor Information
Cynthia J. Koziol-White, Email: kcynthia@mail.med.upenn.edu.
Elena A. Goncharova, Email: goncharo@mail.med.upenn.edu.
Gaoyuan Cao, Email: gaoyuan@mail.med.upenn.edu.
Vera P. Krymskaya, Email: krymskay@mail.med.upenn.edu.
Reynold A. Panettieri, Jr., Email: rap@mail.med.upenn.edu.
References
- 1.Kasperska-Zajac A. Asthma and dehydroepiandrosterone (DHEA): facts and hypotheses. Inflammation. 2010;33:320–324. doi: 10.1007/s10753-010-9188-1. [DOI] [PubMed] [Google Scholar]
- 2.Liou CJ, Huang WC. Dehydroepiandrosterone suppresses eosinophil infiltration and airway hyperresponsiveness via modulation of chemokines and Th2 cytokines in ovalbumin-sensitized mice. J. Clin. Immunol. 2011;31:656–665. doi: 10.1007/s10875-011-9529-3. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE, Robinson CB, Leonard JM, Panettieri RA., Jr Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010;31:461–471. doi: 10.2500/aap.2010.31.3384. [DOI] [PubMed] [Google Scholar]
- 4.Yu CK, Liu YH, Chen CL. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J. Microbiol. Immunol. Infect. 2002;35:199–202. [PubMed] [Google Scholar]
- 5.Furutama D, Fukui R, Amakawa M, Ohsawa N. Inhibition of migration and proliferation of vascular smooth muscle cells by dehydroepiandrosterone sulfate. Biochim. Biophys. Acta. 1998;1406:107–114. doi: 10.1016/s0925-4439(97)00085-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3) J. Biol. Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 7.Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab. Rev. 2006;38:89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am. J. Physiol. 1989;256:C329–C335. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 9.Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, Krymskaya VP, Panettieri RA., Jr Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am. J. Respir. Cell Mol. Biol. 2003;29:19–27. doi: 10.1165/rcmb.2002-0254OC. [DOI] [PubMed] [Google Scholar]
- 10.Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA, Jr, Amrani Y. Tumor necrosis factor alpha modulates airway smooth muscle function via the autocrine action of interferon beta. J. Biol. Chem. 2003;278:50615–50623. doi: 10.1074/jbc.M303680200. [DOI] [PubMed] [Google Scholar]
- 11.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L354–L363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-{beta}/NFAT axis. Circulation. 2009;120:1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 13.Choi IS, Cui Y, Koh YA, Lee HC, Cho YB, Won YH. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Korean J. Intern. Med. 2008;23:176–181. doi: 10.3904/kjim.2008.23.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radford DJ, Wang K, McNelis JC, Taylor AE, Hechenberger G, Hofmann J, Chahal H, Arlt W, Lord JM. Dehdyroepiandrosterone sulfate directly activates protein kinase C-beta to increase human neutrophil superoxide generation. Mol. Endocrinol. 2010;24:813–821. doi: 10.1210/me.2009-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkhausen T, Westphal BM, Putz C, Krettek C, van Griensven M. Dehydroepiandrosterone administration modulates endothelial and neutrophil adhesion molecule expression in vitro. Crit. Care. 2006;10:R109. doi: 10.1186/cc4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dashtaki R, Whorton AR, Murphy TM, Chitano P, Reed W, Kennedy TP. Dehydroepiandrosterone and analogs inhibit DNA binding of AP-1 and airway smooth muscle proliferation. J. Pharmacol. Exp. Ther. 1998;285:876–883. [PubMed] [Google Scholar]
- 17.Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am. J. Respir. Cell Mol. Biol. 2010;42:586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]