Abstract
Purpose
Most common forms of human epilepsy result from a complex combination of polygenetic and environmental factors. Quantitative trait loci (QTLs) mapping is a first step toward the non-biased discovery of epilepsy related candidate genes. QTL studies of susceptibility to induced seizures in mouse strains have consistently converged on a distal region of chromosome 1 as a major phenotypic determinant; however, its influence on spontaneous epilepsy remains unclear. In the present studies we characterized the impact of allelic variations within this QTL, termed Szs1, on the occurrence of spontaneous Spike Wave Discharges (SWDs) characteristic of absence seizure in DBA/2 (D2) mice.
Methods
We analyzed SWDs occurrence and patterns in freely behaving D2, C57BL/6 (B6) and the congenic strains D2.B6-Szs1 and B6.D2-Szs1.
Key finding
We show that congenic manipulation of the Szs1 locus drastically reduced the number and the duration of SWDs in D2.B6-Szs1 mice, which are homozygous for Szs1 from B6 strain on a D2 strain background. However, it failed to induce the full expression of SWDs in the reverse congenic animals B6.D2-Szs1.
Significance
Our results demonstrate that the occurrence of SWDs in D2 animals is under a polygenic control and therefore, the couple D2 and B6 strains might be a useful model to dissect the genetic determinants of polygenic SWDs characteristic of typical absence seizures. Furthermore, we point to the existence of epistatic interactions between at least one modifier gene within Szs1 and genes within unlinked QTLs in regulating the occurrence of spontaneous non-convulsive forms of epilepsies.
Keywords: Epilepsy, Spike Wave Discharges, EEG, Quantitative trait loci, Congenic mice
INTRODUCTION
The etiology of the epilepsies is hypothesized to be influenced strongly by genetic factors. Mutations have been discovered for several rare Mendelian forms of epilepsy; however, because more common forms of epilepsy such as idiopathic generalized epilepsy (IGE) rely on polygenic and environmental interactions, advances are needed for understanding their biological basis and the underlying mechanisms (Steinlein, 2004).
One approach that allows the non-biased discovery of epilepsy related candidate genes is the use of quantitative trait loci (QTL) mapping in common inbred strains of laboratory mice that model complex heritable seizure traits (Frankel 2009).
Previous studies documented that the distal region of chromosome 1 contains a quantitative trait locus (QTL) termed Szs1 that is in large part responsible for the known difference in seizure susceptibility between C57BL/6 (B6) and DBA/2 (D2) mice (Ferraro, et al. 2001, Ferraro, et al. 1997, Ferraro, et al. 1999). However, the QTL studies that isolated Szs1 used induced convulsive seizures as phenotypic marker and, therefore, its influence on spontaneous epilepsy remains to be clarified.
A striking feature of the D2 strain, not present in B6, is the frequent occurrence of high-amplitude, 6-8 hertz spike and waves discharges (SWDs), which represents an endophenotype of of thalamo-cortically generated SWDs typical of absence seizures, one of the most common form of IGE (Crunelli & Leresche 2002, Ryan & Sharpless 1979). Indeed as in all models of absence seizure, SWDs in D2 mice are associated with behavioral arrest (Ryan 1984). They are strongly reduced by anti-absence medication (Marrosu, et al. 2007) and their occurrence is increased and suppressed by the administration of GABA-B agonists and antagonists, respectively (Marrosu, et al. 2006).
In the present study, using reciprocal congenic strains B6.D2-Szs1 and D2.B6-Szs1 that were generated previously (Ferraro et al. 2004), we have carried on a comprehensive evaluation of the impact of allelic variation(s) within the QTL Szs1 on the inherited occurrence of the generalized non-convulsive spontaneous SWDs of D2.
METHODS
Animals
Inbred mouse strains C57BL/6 (B6) and DBA/2 (D2) were bred in-house at the Research Service of the Veterans Affairs Medical Center in Coatesville, PA, using mating pairs purchased from The Jackson Laboratory. Congenic strains were obtained as described by Ferraro et al, 2004. D2.B6-Szs1 mice have a genetic background that is derived essentially from the D2 strain but they are homozygous for a fragment of distal chromosome 1 (about 40 cM between markers D1Mit390 and D1Mit 17) from the B6 strain. On the other hand, B6.D2-Szs1 mice are homozygous between markers D1Mit30 and D1Mit17 from the D2 strain on a B6 strain background.
Surgical procedures
Adult male mice, 10-14 weeks of age (26-30 g), were anesthetized with ketamine (80mg/kg) and xylazine (16 mg/kg) as one intraperitoneal injection. Two burr holes were drilled to house EEG electrodes over the frontal areas of both hemispheres (-2.0 mm bregma, 1.0 mm lateral). Teflon coated silver wire was used to make ball electrodes (1mm in diameter) for EEG recording. A stainless steel screw was used as the ground electrode. The EMG electrodes were sutured to dorsal nuchal muscle and the EMG was recorded in a bipolar configuration.
Recording procedures
Animals were allowed 10 days to recover from surgery. Experiments were carried out in a sound-attenuated sleep-recording chamber with a 12 hour lights-on and 12 hour lights-off. EEG signals were calibrated, amplified and filtered between 0.1 and 500 Hz. They were digitized at a sample rate of 1 kHz. EMG signals were filtered between 1 and 100 Hz. The signals were recorded for each mouse for > 4h per day over multiple days.
Data analysis
EEG traces were analyzed by performing power spectrum with a fast Fourier algorithm using custom software written in Igor (Wavemetrics, Oswego, OR). Quantification of spike wave discharges (SWDs) was done with a custom algorithm that detects the presence of at least two high amplitude spikes at 1 to 15 Hz, one of which is 2.5 the amplitude of the root-mean-square of the background activity. Characteristics of SWD (duration, frequency and occurrence) were compared between strains using Mann-Whitney U test.
RESULTS
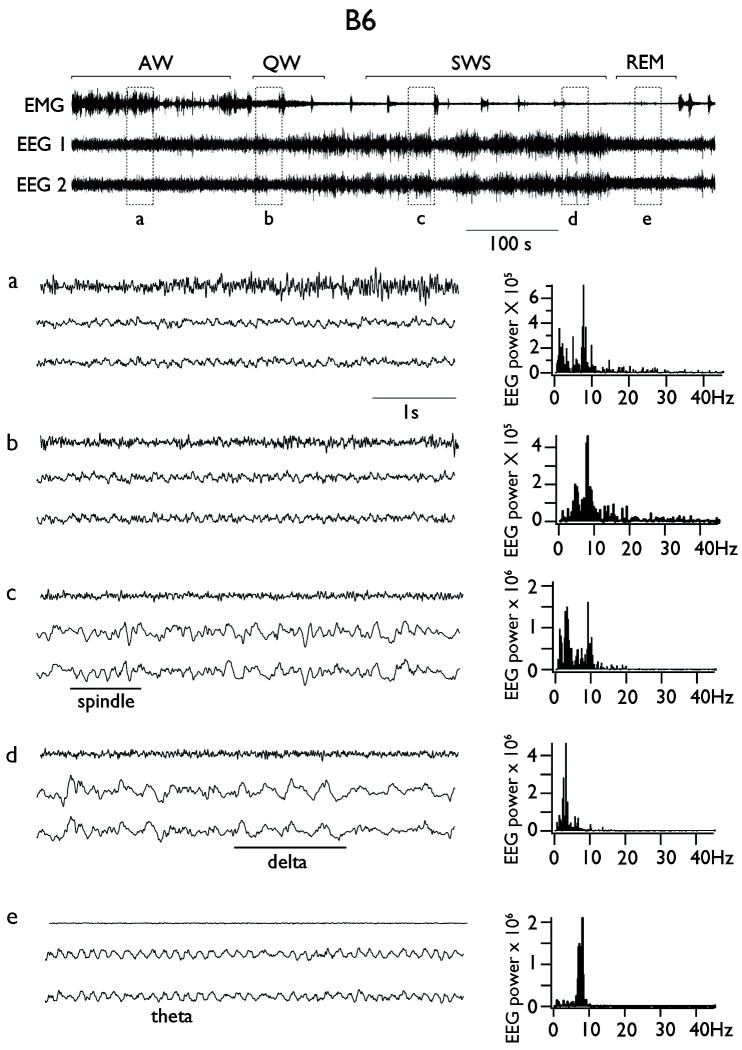
In order to characterize the influence of the locus Szs1 on the non-convulsive SWD of D2 mice, we recorded the baseline EEG from non-anesthetized, chronically implanted B6, D2 and their reciprocal congenic strains. In all four groups, the EEG showed spontaneous transitions from waking to sleep. We classified states of vigilance according to standard criteria (Erwin, et al. 1984) as illustrated in the example B6 mouse in Figure 1. During alert waking (AW) animals were behaviorally active and showed a high amplitude EMG (Fig. 1, upper panel and detail a). The EEG was rich in low-amplitude theta, with a spectral peak centered at 8 Hz (range: 6-9 Hz), as well as in fast frequencies above 15 Hz which included the beta and gamma bands (Fig. 1 detail a, right column, power spectrum). During quiet waking (QW) the EMG did not show overt signs of movement and the EEG remained of low amplitude and rich in theta and fast frequencies (Fig. 1, detail b). The transition to slow wave sleep (SWS) was characterized by an increase in the amplitude of the EEG, an increase in power in the delta (0.5-4 Hz) frequency band, a clear peak at the frequency of sleep spindles (~10 Hz) and the diminution of fast frequencies above 15 Hz and theta band activity (Fig. 1, detail c). Deepening of sleep was characterized by a further increase in EEG amplitude dominated by activity in the delta frequency range (Fig. 1, detail d). Finally, REM sleep was characterized by a dominant theta (6-8 Hz) frequency activity, visible by simple inspection of the EEG and by a large spectral peak centered at 8 Hz (Fig. 1, detail e).
Figure 1. Spontaneous transition from wake to sleep in freely behaving B6 mice.
Upper panel shows a representative continuous bilateral EEG recording from a B6 mouse alongside with EMG recording. Detail a: alert waking (AW). Detail b: quiet waking (QW). Detail c: epoch of slow wave sleep (SWS) with a boot of spindles oscillations. Detail d: epoch of slow wave sleep (SWS) with a boot of activity in the delta frequency range. Detail e: Rapid eye movements (REM) sleep. On the right are displayed the power spectra derived from the EEG traces situated on the left.
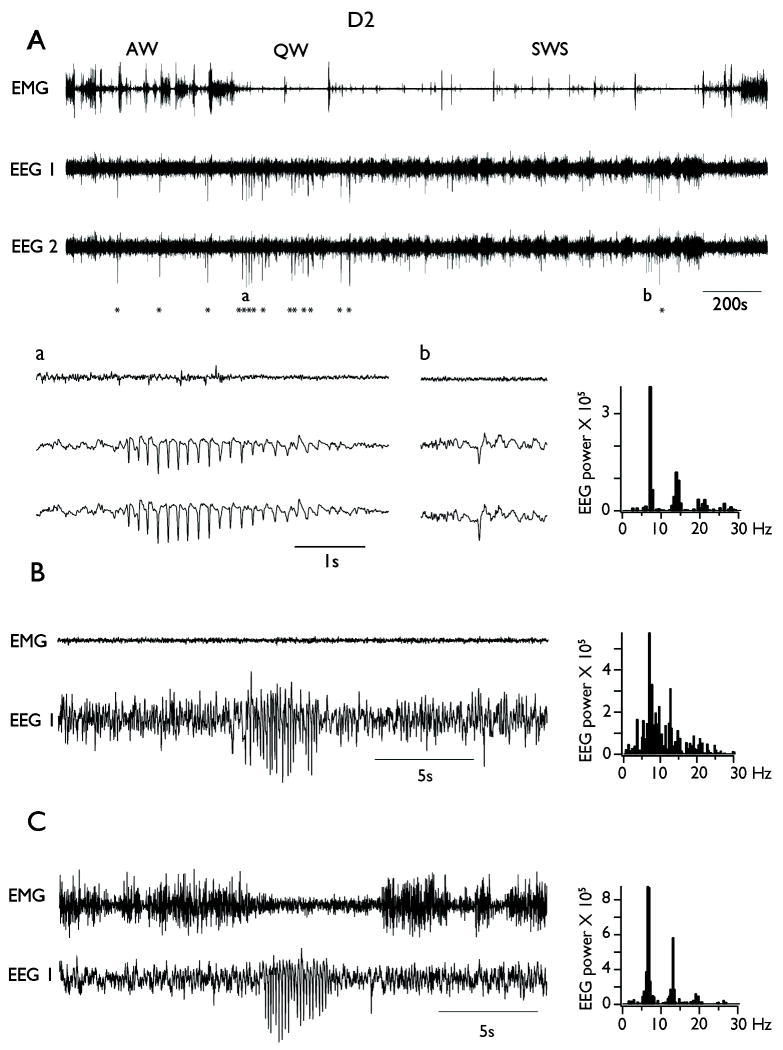
The EEG of D2 mice also showed the electrographic signs that characterize wake-sleep cycles (Fig. 2). However, the spontaneous EEG of D2 mice was distinguished by the presence of bilaterally expressed SWDs (Fig. 2A, detail a) and large amplitude single spikes (Fig. 2A, detail b; n = 11 out of 11 mice). SWDs consisted of large amplitude rhythmic waves at 6-9 Hz (7.4 ± 0.5 Hz, mean ± SD) that can last several seconds (mean=1.48 ± 0.31 s, range 1 to 5 s). Single spikes were large-amplitude isolated events lasting 50-100 ms and recurring irregularly. SWDs were easily distinguishable from sleep spindles by (i) their abrupt onset (in contrast with the waxing nature of spindles), (ii) their higher amplitude (spindles are 300-400 μV), and (iii) their prevalence during QW (spindles are the hallmark of slow wave sleep). Neither SWDs nor single spikes were ever observed in B6 mice (n=0 out of 9 mice).
Figure 2. Spike Wave Discharges (SWDs) occurrence in freely behaving D2 mice.
A. Upper panel shows a representative continuous bilateral EEG recording from a D2 mouse alongside with EMG recording. The stars indicate the occurrence of SWDs. Note the increase in the occurrence of SWDs during quiet wakefulness (QW) compared to active waking (AW) and slow wave sleep (SWS). Detail a: typical example of SWDs during QW. Detail b: typical example of isolated large amplitude single spike during SWS. B. Example of SWDs occurring during SWS. C. Example of SWDs occurring during AW. Note the decrease in EMG amplitude during SWDs. On the right are displayed the power spectra computed during the seizure episodes on the EEG traces situated on the left.
The overall rate of occurrence of SWDs in D2 mice was (7.7 ± 2.6 per hour). They appeared mostly during QW (46.1 ± 10.5 per hour; indicated by asterisks in Fig. 2A), and were less frequent during slow wave sleep (SWS; 4.3 ± 1.7; p<10-6), in which they seemed to develop during sleep spindles as an abnormal increase in amplitude and decrease in frequency of the ongoing spindling oscillation (Fig. 2B). When SWDs appeared during AW, they were associated with an arrest of movement (Fig. 2C), similar to SWDs characteristic of absence seizures in most species including humans (Blumenfeld 2005). The representative examples of SWDs depicted in Figure 3 show a clear peak in the frequency spectrum at 7 Hz.
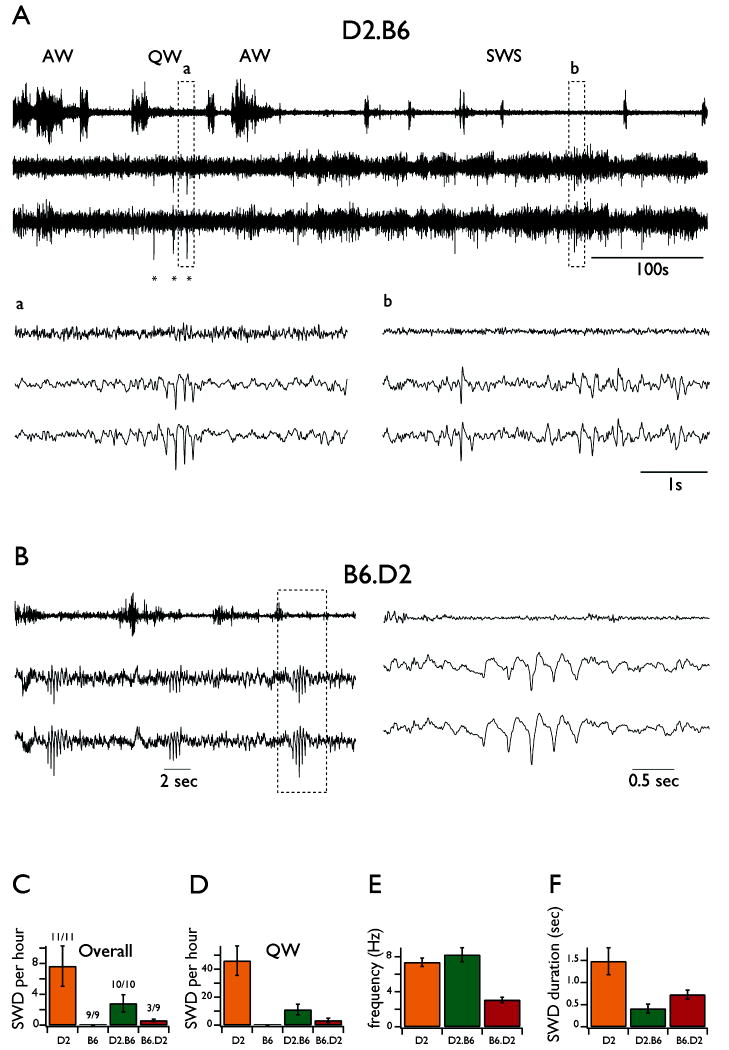
Figure 3. Spike Wave Discharges (SWDs) occurrence in freely behaving reciprocal congenic mice.
A. Upper panel shows a representative continuous bilateral EEG recording from a D2.B6-Szs1 mouse alongside with EMG recording. The stars indicate the occurrence of SWDs. Detail a: typical example of SWDs during QW. Detail b: typical example of isolated large amplitude single spike during SWS. B. The left panel shows a continuous EEG recording from a B6.D2-Szs1 mouse alongside with EMG recording during quiet wakefulness. The right panel corresponds to an enlargement of the traces on the left. C. Overall SWDs occurrence for all strains. D. SWDs occurrence during quiet wakefulness for all strains. E. Intra-SWDs frequency for all strains. F. SWDs duration for all strains. All error bars are SD.
In the congenic D2.B6-Szs1 mice (Fig. 3A), in which most of the genome is from the D2 strain except for the small portion of chromosome 1 containing Szs1 from B6, spontaneous SWDs (Fig. 3A asterisks, detail a) and single spikes (Fig. 3A, detail b) occurred in all mice (n=10 out of 10). However, compared to SWDs in the D2 strain, these occurred at much lower overall rates (2.8 ± 1.1 per hour; p<10-5; Fig. 3C). When analyzing only the periods of QW, their rate of occurrence was significantly lower as well (11.1 ± 3.8 per hour; p<10-5; Fig. 3D) and their duration was dramatically reduced (411 ± 101 msec; p<10-5; Fig. 3F). In addition, the intra SWD frequency was slightly higher (8.2 Hz ± 0.8; p<0.01; Fig. 3E). These differences were not due to variations of time spent in different behavioral states. Indeed, for comparisons, the EEG from each animal was analyzed for 2 hours of QW, 6 hours of AW, 13 hours of SWS and 1 hour of REM. Furthermore, all the data were extracted from recordings made at similar daily hours (between 2 and 6 pm).
In the congenic B6.D2-Szs1 mice, in which the same small portion of chromosome 1 is now from D2, spontaneous single spikes and SWDs (Fig. 3B) were present in 3 out of 9 animals. In the animals that presented SWDs, the events were seen only occasionally (0.6 ± 0.2 per hour) and thus were significantly less frequent than in D2 and D2.B6-Szs1 mice (p<0.003 and p<0.003, respectively; Fig 3C). When they occurred, SWD in B6.D2-Szs1 mice were of shorter duration (0.72 ± 0.10 sec; Fig. 3F) than in D2 but lasted longer than in D2.B6-Szs1 mice (p<0.003 and p<0.003, respectively; Fig 3F). Interestingly, the frequency of the SWDs in B6.D2-Szs1 mice was between 2 and 4 Hz (3.1 ± 0.3 Hz; Fig 4B and Fig. 3E), which is similar to human SWDs (Crunelli & Leresche 2002).
DISCUSSION
We show that congenic manipulation of the Szs1 locus drastically reduced the number and the duration of SWDs in D2.B6-Szs1 mice, however, it failed to induce the full expression of SWDs in B6.D2-Szs1. Our results demonstrate that the occurrence of SWDs in D2 animals is under a polygenic control and therefore, the couple D2 and B6 strains might be a useful model to dissect the genetic determinants of polygenic SWDs characteristic of typical absence seizures (Crunelli & Leresche 2002).
Indeed, while single gene manipulation can lead to SWDs in mice (reviewed in(Noebels 2003), several recent studies demonstrated the importance of the genetic background (Beyer, et al. 2008, Papale, et al. 2009, Strohl, et al. 2007). The present results point to the existence of epistatic interactions between at least one modifier gene within Szs1 and other genes within unlinked QTLs in regulating the occurrence this spontaneous non-convulsive epileptic trait. Considering the systematic involvement of the QTL-rich region on mouse distal chromosome 1 (Chr 1), that corresponds to human Chr1q21–23, in diverse epileptic traits; our results suggest that it might contains candidate target genes of particular importance for treating diverse types of epileptic disorders as well as understanding the epistatic interactions that determine phenotypic characteristics across syndromes.
High resolution mapping of Szs1 showed that out of 120 genes in the introgressed congenic interval, 12 genes satisfy both criteria of being expressed in the brain and having a missense single nucleotide polymorphism (SNP) (Ferraro, et al. 2004, Mozhui, et al. 2008). Among this latter group, one encodes a protein involved directly in the transport of ions across cell membranes: Kcnj10. This gene encodes an inward-rectifier potassium ion channel Kir4.1 predominantly expressed by glial cells (Takumi, et al. 1995). Homozygous Kcnj10 knockout mice show a severe demyelinating syndrome associated with multiple neurological defects including seizures (Djukic, et al. 2007) and short life span (Kofuji, et al. 2000, Marcus, et al. 2002, Neusch, et al. 2001).
Interestingly, activity of Kir4.1 channels in astrocytes of D2 mice is reduced compared to B6 mice and this deficit is associated with an impairment in potassium and glutamate buffering(Inyushin, et al. 2010). Furthermore, the Thr/Ser polymorphism in coding sequence of the (Kir4.1) at the position 262 correlates with electroshock induced seizure susceptibility among a variety of inbred strains (Ferraro et al., 2004) and translation of these results to human epilepsy led to the discovery of an SNP at the position 271 in the Kcnj10 protein predicting an Arg/Cys variation at amino acid position 271 in epilepsy patients with refractory mesial temporal lobe epilepsy, childhood absence and juvenile myoclonic epilepsy (Buono, et al. 2004, Heuser, et al. 2010, Lenzen, et al. 2005). Most recently, deleterious mutations of Kcnj10 have been shown to be causative in the SESAME and EAST syndrome which involves multiple organ systems as well as severe epilepsy as the prominent phenotype (Bockenhauer, et al. 2009, Scholl, et al. 2009). Taken together these results make Kcnj10 a strong candidate as a general seizure susceptibility gene and its mutation in D2 mice might explain the results observed in the congenic animals. Definitive confirmation would arise from the analysis of SWD in a D2 mice line with a knock-in of the allelic variant of the B6 Kcnj10.
Despite the compelling evidences with respect to the direct involvement of Kcnj10 gene product, roles for SNPs in the other genes harbored by the Szs1 locus cannot yet be ruled out. To our knowledge, except for Pxf which encodes for a peroxysomal receptor and has recently been shown to be mutated in a case study of a patient with a complex clinical syndrome that include abnormal EEG and epilepsy (Mohamed, et al. 2010), none of the others is documented to have a direct effect on cell excitability and/or seizures. However, other mechanisms might also account for their involvement. One particularly interesting hypothesis is that the phenotype of D2 mice results from interactions between SNPs of genes located within Szs1 and other genes situated within and/or outside this locus. In line with this hypothesis, analysis of 18 diverse array datasets from different mouse crosses revealed that Szs1 modulates the expression of at least 10 genes that have been implicated in seizure phenotypes including Cacna1g, Scn1b and Pnpo (Mozhui et al., 2008). In every case the D2 allelic variant has a positive additive effect, increasing the expression of the transcripts by 5 to 20 %. With respect to the control of SWD generation in D2 mice, an increase in Cacna1g transcripts, which underlies the expression of T-current in thalamocortical cells is of particular significance (Kim, et al. 2001). Indeed, T-current is either mutated or increased in animal models of absence seizures (Powell, et al. 2009, Tsakiridou, et al. 1995, Zhang, et al. 2002, Zhang, et al. 2004); knock-out of Cacna1g prevents the occurrence of SWDs induced by GBL administration (Kim et al., 2001) and spontaneous SWDs in some mutants mice (Song, et al. 2004). Alternatively, the over-expression of this gene (37 % increase in mRNA levels) is sufficient for the appearance of spontaneous SWDs (Ernst, et al. 2009). Even though the increase in the expression of this gene in D2 mice compared to B6 seems small (< 20 %), biophysical experiments revealed that slight variations in T-type current might have major impact on the excitability of thalamo-cortical cells depending on the weight of their synaptic inputs (Bessaïh, et al. 2008). Interestingly, D2 mice have been reported to present alterations of intrathalamic inhibition (Tan, et al. 2008), similar to what has been observed in other genetic rodent models of SWDs (Bessaïh, et al. 2006, Brockhaus & Pape 2011, Cope, et al. 2009). No evidence for the control of genes that regulate synaptic inhibition by allelic variations within Szs1 locus has been reported.
We propose that changes in genes within or regulated by Szs1 such as Kcnj10 or Cacna1g, might account for the drastic decrease on SWDs occurrence in D2.B6-Szs1 mice when compared to D2 mice. However, appearance of SWDs in B6 would necessitate concomitant abnormalities under the regulation of other loci, such as modifications of thalamo-cortical inhibition.
Acknowledgments
This work was supported by NIH grants NS041811 (DC) and NS40554 (TNF) and by a postdoctoral fellowship of the Foundation pour la Recherche Medicale (T.B.). This work does not represent the official views of the US government.
Footnotes
DISCLOSURE:
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bessaïh T, Bourgeais L, Badiu CI, Carter DA, Toth TI, Ruano D, Lambolez B, Crunelli V, Leresche N. Nucleus-specific abnormalities of GABAergic synaptic transmission in a genetic model of absence seizures. J Neurophysiol. 2006;96:3074–3081. doi: 10.1152/jn.00682.2006. [DOI] [PubMed] [Google Scholar]
- Bessaïh T, Leresche N, Lambert RC. T current potentiation increases the occurrence and temporal fidelity of synaptically evoked burst firing in sensory thalamic neurons. Proc Natl Acad Sci USA. 2008;105:11376–11381. doi: 10.1073/pnas.0801484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer B, Deleuze C, Letts VA, Mahaffey CL, Boumil RM, Lew TA, Huguenard JR, Frankel WN. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Human Molecular Genetics. 2008;17:1738–1749. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Pape H-C. Abnormalities in GABAergic synaptic transmission of intralaminar thalamic neurons in a genetic rat model of absence epilepsy. Mol Cell Neurosci. 2011;46:444–451. doi: 10.1016/j.mcn.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Buono RJ, Lohoff FW, Sander T, Sperling MR, O’Connor MJ, Dlugos DJ, Ryan SG, Golden GT, Zhao H, Scattergood TM, Berrettini WH, Ferraro TN. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res. 2004;58:175–183. doi: 10.1016/j.eplepsyres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Nneurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29:1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin CW, Somerville ER, Radtke RA. A review of electroencephalographic features of normal sleep. J Clin Neurophysiol. 1984;1:253–274. doi: 10.1097/00004691-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Longman RL, Snyder RL, DeMuth D, Szpilzak I, Mulholland N, Eng E, Lohoff FW, Buono RJ, Berrettini WH. Quantitative genetic study of maximal electroshock seizure threshold in mice: evidence for a major seizure susceptibility locus on distal chromosome 1. Genomics. 2001;75:35–42. doi: 10.1006/geno.2001.6577. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Martin JF, Lohoff FW, Gieringer TA, Zamboni D, Schwebel CL, Press DM, Kratzer SO, Zhao H, Berrettini WH, Buono RJ. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome. 2004;15:239–251. doi: 10.1007/s00335-003-2270-3. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Schork NJ, St Jean P, Ballas C, Choi H, Berrettini WH. Mapping murine loci for seizure response to kainic acid. Mamm Genome. 1997;8:200–208. doi: 10.1007/s003359900389. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, Ballas C, Schill J, Buono RJ, Berrettini WH. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci. 1999;19:6733–6739. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel WN. Genetics of complex neurological disease: challenges and opportunities for modeling epilepsy in mice and rats. Trends Genet. 2009;25:361–367. doi: 10.1016/j.tig.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser K, Nagelhus EA, Taubøll E, Indahl U, Berg PR, Lien S, Nakken S, Gjerstad L, Ottersen OP. Variants of the genes encoding AQP4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res. 2010;88:55–64. doi: 10.1016/j.eplepsyres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Inyushin M, Kucheryavykh LY, Kucheryavykh YV, Nichols CG, Buono RJ, Ferraro TN, Skatchkov SN, Eaton MJ. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia. 2010;51:1707–1713. doi: 10.1111/j.1528-1167.2010.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen KP, Heils A, Lorenz S, Hempelmann A, Höfels S, Lohoff FW, Schmitz B, Sander T. Supportive evidence for an allelic association of the human KCNJ10 potassium channel gene with idiopathic generalized epilepsy. Epilepsy Res. 2005;63:113–118. doi: 10.1016/j.eplepsyres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol, Cell Physiol. 2002;282:C403–407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Bortolato M, Frau R, Orrù M, Puligheddu M, Fà M, Muroni A, Tuveri A, Mereu G. Levetiracetam attenuates spontaneous spike-and-wave discharges in DBA/2J mice. Epilepsy Res. 2007;75:224–227. doi: 10.1016/j.eplepsyres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Santoni F, Fà M, Puligheddu M, Barberini L, Genugu F, Frau R, Manunta M, Mereu G. Beta and gamma range EEG power-spectrum correlation with spiking discharges in DBA/2J mice absence model: role of GABA receptors. Epilepsia. 2006;47:489–494. doi: 10.1111/j.1528-1167.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- Mohamed S, El-Meleagy E, Nasr A, Ebberink MS, Wanders RJA, Waterham HR. A mutation in PEX19 causes a severe clinical phenotype in a patient with peroxisomal biogenesis disorder. Am J Med Genet A. 2010;152A:2318–2321. doi: 10.1002/ajmg.a.33560. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Ciobanu DC, Schikorski T, Wang X, Lu L, Williams RW. Dissection of a QTL hotspot on mouse distal chromosome 1 that modulates neurobehavioral phenotypes and gene expression. Plos Genetics. 2008;4:e1000260. doi: 10.1371/journal.pgen.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels JL. Exploring new gene discoveries in idiopathic generalized epilepsy. Epilepsia. 2003;44(Suppl 2):16–21. doi: 10.1046/j.1528-1157.44.s.2.4.x. [DOI] [PubMed] [Google Scholar]
- Papale LA, Beyer B, Jones JM, Sharkey LM, Tufik S, Epstein M, Letts VA, Meisler MH, Frankel WN, Escayg A. Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Hum Mol Genet. 2009;18:1633–1641. doi: 10.1093/hmg/ddp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, Foote SJ, Snutch TP, O’Brien TJ. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LJ. Characterization of cortical spindles in DBA/2 and C57BL/6 inbred mice. Brain Res Bull. 1984;13:549–558. doi: 10.1016/0361-9230(84)90037-6. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sharpless SK. Genetically determined spontaneous and pentylenetetrazol-induced brief spindle episodes in mice. Exp Neurol. 1979;66:493–508. doi: 10.1016/0014-4886(79)90197-3. [DOI] [PubMed] [Google Scholar]
- Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAE syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Kim D, Choi S, Sun M, Kim Y, Shin H-S. Role of the alpha1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004;24:5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Gallaugher L, Lynn A, Friedman L, Hill A, Singer JB, Lander ES, Nadeau J. Sleep-related epilepsy in the A/J mouse. Sleep. 2007;30:169–176. doi: 10.1093/sleep/30.2.169. [DOI] [PubMed] [Google Scholar]
- Takumi T, Ishii T, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Tan HO, Reid CA, Chiu C, Jones MV, Petrou S. Increased thalamic inhibition in the absence seizure prone DBA/2J mouse. Epilepsia. 2008;49:921–925. doi: 10.1111/j.1528-1167.2008.01536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mori M, Burgess DL, Noebels JL. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci. 2002;22:6362–6371. doi: 10.1523/JNEUROSCI.22-15-06362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vilaythong AP, Yoshor D, Noebels JL. Elevated thalamic low-voltage-activated currents precede the onset of absence epilepsy in the SNAP25-deficient mouse mutant coloboma. J Neurosci. 2004;24:5239–5248. doi: 10.1523/JNEUROSCI.0992-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]