Abstract
Oxidative stress remains one of the most well studied, albeit somewhat contentious, causes of aging-related changes in humans. Consequently, a large number of putative antioxidant compounds are freely available in myriad formulations that are often not tested for their efficacy or regulated for quality control. Following the development of a Drosophila model of oxidative-stress dependent aging (p38 MAP Kinase (p38K) mutants) in our laboratory, we attempted to test the protective effect of some of these commonly available formulations against oxidative stress, stress induced motor defects and reduced life span in the p38K model. As environmental exposure to oxidizing toxins has been linked to a variety of human diseases, we also tested the efficacy of these supplements on chemically-induced models of oxidative stress (Paraquat and Hydrogen Peroxide exposure). Our results suggest that when added as a dietary supplement, some of these over-the-counter compounds, notably containing Açai extracts, confer significant protection for both the p38K-dependent genetic model as well as the toxin-induced model. These products were also remarkably effective at dampening stress-induced expression of the detoxifying enzyme GSTD1 and eliminating Paraquat induced circadian rhythm deficits. Overall, our results suggest potential benefits of dietary supplementation with some of these compounds, especially under conditions of elevated oxidative stress. These findings should be assessed in the context of other studies that seek to identify active principles in these extracts, determine their effective dosage for human consumption and evaluate the safety of long-term prophylactic applications.
Keywords: Antioxidants, p38MAPK, Açai, oxidative stress, Paraquat
1. Introduction
Oxidative stress has been implicated in a number of diseases including Alzheimer’s disease, cancer and Retinitis Pigmentosa (Yan et al., 1996, Benz and Yau, 2008, Tuson et al., 2009, Clark et al., 2010). Though how oxidative stress relates to these disorders is not always fully understood, it is thought that oxidative stress results in increasing cellular damage and eventual cell death if not properly managed. Furthermore, animals either exposed to oxidizing agents, such as the Paraquat and Rotenone models of Parkinson’s disease, or harboring mutations in oxidative stress response genes generally have a shortened life span and progressive locomotor behavior defects (Betarbet et al., 2000, Melov et al., 2001, Blander et al., 2003, Holzenberger et al., 2003, Kieran et al., 2004, Brys et al., 2007, Chaudhuri et al., 2007, Paul et al., 2007, Bernard et al., 2011, Vrailas-Mortimer et al., 2011). Therefore, a great deal of interest has been generated in the effectiveness of antioxidants to treat oxidative stress related diseases. For instance, melatonin (Cardinali et al., 2010), diets rich in polyphenols (Morillas-Ruiz et al., 2010), Vitamin E (Sano et al., 1997) and Vitamin C (Engelhart et al., 2002, Morris et al., 2002) have all been shown to have ameliorating effects for Alzheimer’s Disease patients, whereas, the antioxidants derived from Gingko biloba (DeKosky et al., 2008) and Curcumin (Baum et al., 2008) were not found to be as effective. Similarly, the relationship between antioxidant supplements and cancer has also been largely inconclusive with a few trials indicating minor protective effects (Goodman et al., 2011). In order to utilize the full potential of the multitude of antioxidant supplements available, a greater understanding of the efficacy, safety and mode of action of these compounds is necessary. Given the large number of compounds with potential antioxidant properties (as measured by chemical assays), rapid testing in animal models has emerged as a viable screening platform
The fruit fly Drosophila melanogaster has proven to be a rapid and powerful genetic platform for assessing the properties of various antioxidant supplements. For example, dietary supplementation of vitamin C (ascorbic acid, Bahadorani et al., 2008), and apple phenols (Peng et al., 2011) have been shown to extend adult life span in wild type flies. Interestingly, other dietary constituents may also play an important role in life span extension as Resveratrol is able to extend life span in wild type animals on a rich diet (Bauer et al., 2004, Wood et al., 2004), but not when the amount of sugar and yeast is reduced (Wood et al., 2004). Similarly, dietary supplementation of Resveratrol during larval developmental rescues short-lived Drosophila lines (Chandrashekara and Shakarad, 2011), suggesting that Resveratrol may only extend life span for specific genetic backgrounds. Further evidence for the role of base diet is that Açai pulp extends life span in wild type as well as oxidatively stressed SOD1 mutant flies only on a high fat diet (Sun et al., 2010).
Studies using Drosophila may further reveal how different antioxidants have protective effects against specific sources of oxidative stress. For example, Vitamin E is protective against chemically induced lipid peroxidation (Miquel et al., 1982) but is ineffective against exposure to the oxidizing agent Paraquat (Driver and Georgeou, 2003), whereas a variety of other antioxidant compounds including vitamin C (ascorbic acid) and melatonin are effective treatments against Paraquat toxicity (Bonilla et al., 2006). Genetic models of oxidative stress have also demonstrated that particular antioxidants can extend life span and reduce reactive oxygen species (ROS) in specific genetic backgrounds. As an example, loss of the detoxifying enzyme SOD1 (Cu/Zn SOD) results in a shortened life span, which is ameliorated by dietary supplementation with either Vitamin E (α-tocopherol) or Vitamin A (retinol) dietary supplementation (Bahadorani et al., 2008). Additionally, supplementation with a Resveratrol complex was able to rescue the locomotor behavior defects and reduced life span of the alpha-synuclein induced Drosophila Parkinson’s disease model (Long et al., 2009), but not Paraquat induced models of PD (Bagatini et al., 2011).
Although antioxidants have become increasingly popular in commercial health and beauty products, the effectiveness of these compounds is often not assessed in vivo. In an effort to test the efficacy of some of these commercially available antioxidant compounds in an intact animal, we have utilized Drosophila models of both genetic and chemically-induced oxidative stress in the current study. We recently reported a new Drosophila model of oxidative stress and aging, in which reduced levels of p38 MAP Kinase results in a reduced lifespan, age-dependent locomotor behavior defects, and increases sensitivity to the oxidizing agents Hydrogen Peroxide and Paraquat. Furthermore, p38K mutants also have elevated levels of endogenous oxidative stress and a reduction in the expression of the antioxidant enzyme SOD2 (MnSOD). In addition, over-expression of p38K specifically in the muscle extends lifespan and increases resistance to oxidizing agents. We have found that p38K functions in the muscle to regulate the expression of SOD2 through the transcription factor Mef2. Overexpressing SOD2 in the p38K mutant background rescues the mutant lifespan defect, suggesting that oxidative stress contributes to p38K aging phenotypes (Vrailas-Mortimer et al., 2011). Thus the p38K model is an ideal system in which to test the efficacy of antioxidant supplements against genetically induced oxidative stress. In addition to the p38K model, we also tested two chemical exposure models of oxidative stress, exposure to Hydrogen Peroxide and Paraquat to determine the protective effect of commercially available antioxidants supplements containing Açai and/or Resveratrol against oxidative stress.
2. Methods
2.1 Drosophila strains, husbandry and genetics
Oregon R was used as the wild type control strain. p38bΔ45 (Vrailas-Mortimer et al., 2011), GST-GFP (Gift of D. Bohmann) and GST-GFP, p38bΔ45 (Vrailas-Mortimer et al., 2011) have been generated previously. Flies were raised at 25°C on standard media until eclosion.
2.2 Media preparation
Oregon R or p38bΔ45 virgin females were raised on standard media (yeast (3.2%, Lynside Lesaffre), cornmeal (8%, LabScientific), agar (2.6%, Genesee Scientific), molasses (8%, Golden Barrel), propionic acid (0.6%, Acros Organics) and Tegosept (1.6%, 10% in ethanol, Apex Bioresearch Products), aged one day and then starved for 6 ½ hours before being transferred to test food sources to remove standard media from the digestive tract. Sucrose food consisted of 1% sucrose (Fisher Scientific), 1.3% agarose alone or supplemented with one of the following supplements: Super Fruits (Açai, Goji, Pomegranate, Mangosteen, Noni, vegetable cellulose, microcrystalline cellulose, natural colors, stearic acid, magnesium stearate, slica, and purified water, Nature’s Plus), Açai (Açai, microcrystalline cellulose, stearic acid, magnesium stearate, silica, Activessence (cellulose, pectinase, hemicellulose and xylanase), natural color, vegetable cellulose, and purified water, Nature’s Plus), Resveratrol (Resveratrol, cellulose, vegetable cellulose capsule, silica, maltodextrin, and magnesium steaeate, Solray), Ginger (Ginger, silica, di-calcium phosphate, vegetable cellulose, and purified water, Nature’s Plus) and Açai and Resveratrol (Açai, Resveratrol, modified cellulose gum, silica, vegetable stearate, and hydroxypropyl methylcellulose, Michael’s Naturopathic Programs). Complex food consisted of Dextrose (5.2%, Fisher Scientific), Sucrose (2.6%, Sigma), Yeast (1.5%, Lynside Lesaffre), cornmeal (8.58%, LabScientific), agar (0.465%, Genesee Scientific) and Tegosept (1.6%, 10% in ethanol, Apex Bioresearch Products). Concentrations for all supplements were equalized to the amount of Açai (50mg/ml, 25mg/ml, or 12.5mg/ml) or Resveratrol (14.3mg/ml, 7.18mg/ml or 3.59mg/ml). Ginger was used at the concentrations of 50mg/ml, 25mg/ml, or 12.5mg/ml to control for nutritional content. As far as possible, we tried to account for variation by using the formulations that belonged to the same lot number from the same company. Survival was assessed daily. Flies were transferred to fresh food every 2–3 days.
2.3 Hydrogen Peroxide Treatment
Oregon R flies were raised on standard media. Virgin females were aged one day and then starved for 6 ½ hrs before being transferred to sucrose food and 1% H2O2 (mixed in the food, Fisher Scientific) alone or with one of the following supplements: Super Fruits (Nature’s Plus), Açai (Nature’s Plus), Resveratrol (Solray), Ginger (Nature’s Plus) and Açai and Resveratrol (Nature’s Plus). Concentrations for all supplements were equalized to the amount of Açai (50mg/ml, 25mg/ml, or 12.5mg/ml) or Resveratrol (14.3mg/ml, 7.18mg/ml or 3.59mg/ml). Ginger was used at the concentrations of 50mg/ml, 25mg/ml, or 12.5mg/ml to control for nutritional content. Survival was assessed daily. Flies were transferred to fresh food every 2–3 days.
For pre-treatment experiments virgin Oregon R flies were aged one day and starved for 6 ½ hrs before being transferred to sucrose food that contained 1% H2O2 mixed within the food for 24hrs. Flies were then transferred to sucrose food alone or supplemented with either Super Fruits (50mg/ml Açai) or Açai (50mg/ml). Flies were transferred to fresh food every 2–3 days.
2.4 Paraquat Treatment
Oregon R flies were raised on standard media. Virgin females were aged one day and starved for 6 ½ hrs before being transferred to sucrose food mixed with 3mM, 5mM or 10mM Paraquat (Sigma) with either Super Fruits (50mg/ml) or Açai (50mg/ml). Alternatively, virgin females or males were aged one day and starved for 6 ½ hours before being transferred to complex food mixed with 5mM Paraquat with either Super Fruits (50mg/ml) or Açai (50mg/ml). Flies were transferred to fresh food every 2–3 days.
2.5 Western Blot Analysis
GST-GFP, p38bΔ45 virgin females were exposed to Sucrose, Super Fruits (50mg/ml) or Açai (50mg/ml) for 3 days. Three heads and thoraces were homogenized in 1X laemmli buffer. 5μl of each sample were loaded on an SDS-PAGE gel. For Paraquat exposure, GST-GFP flies were exposed to Sucrose, Super Fruits (50mg/ml) or Açai (50mg/ml) with and without 5mM Paraquat. Flies were exposed for 2 days. Three heads and thoraces were homogenized in 1X laemmli buffer. 5μl of each sample were loaded on an SDS-PAGE gel. Antibodies: rabbit anti-GFP 1:1000 (Molecular Probes), mouse anti-actin 1:50,000 (Millipore), goat-anti-rabbit-HRP (Jackson Labs), and goat-anti-mouse-HRP (Jackson Labs).
2.6 Drosophila activity measurements
Oregon R virgin females were aged one day, starved 6 ½ hours and then exposed to Sucrose, Super Fruits (50mg/ml) or Açai (50mg/ml) with and without 5mM Paraquat in the Drosophila Activity Monitor (TriKinetics, Inc) for a 12:12hr light:dark cycle for 6 days. Food was changed after 6 days. To determine if longer exposure may affect circadian rhythm further, Oregon R virgin females were aged one day, starved 6 ½ hours and then exposed to Sucrose, Super Fruits (50mg/ml) or Açai (50mg/ml) with and without 5mM Paraquat in standard vials for 6 days. The animals were then placed in the Drosophila Activity Monitor for 6 days on the same Paraquat containing food. The number of animals tested are as follows young sucrose, 30; young sucrose plus Paraquat, 24; young Super Fruits, 32; young Super Fruits plus Paraquat, 32; young Açai, 29; young Açai plus Paraquat, 26; aged Super Fruits, 40; aged Super Fruits plus Paraquat, 36; aged Açai, 31; aged Açai plus Paraquat, 36. Locomotor behavior was analyzed using DAMFileScan102X in 30minute bins. Actograms were generated using the ImageJ (rsbweb.nih.gov/ij/) plugin ActogramJ (actogramj.neurofly.de).
2.7 Statistical analysis of Drosophila Activity Monitor data
Data acquired from the Drosophila Activity Monitor were analyzed for the length of the period and strength of rhythmicity. Maximum Entropy Spectral Analysis (Levine et al., 2002, Dowse, 2009) was employed to determine the length of the period (Tau). The presence of significant rhythmicity was determined by autocorrelation analysis (Levine et al., 2002, Dowse, 2009) taking multiple peaks that exceed the 95% confidence interval that match the spectral peak as evidence of a robust rhythm. The strength of the rhythm is assessed by further interpretation of the autocorrelogram. The height of the third peak, counting the one at lag zero as #1 is the Rhythmicity Index. Since many of the fly rhythms were bimodal, we looked only at the peaks occurring at approximately 24-h intervals to establish RI. This was not possible in the case of the flies consuming sucrose with 5mM paraquat. By these criteria, only 2 animals would have had significant rhythmicity. To make comparisons possible, we relaxed the criterion demanding a peak in the 48-h range and took RIs from the third peak wherever it fell for this group. This is justified, as it allows flies with clear rhythmicity to be scored even if they do not live long enough to generate RIs by the more stringent criterion. Tau and RI were compared among groups using nonparametric analysis (Kruskal Wallis χ2, R statistics package).
3. Results
3.1 Açai containing formulations extend life span in p38 MAP Kinase mutants on minimal food
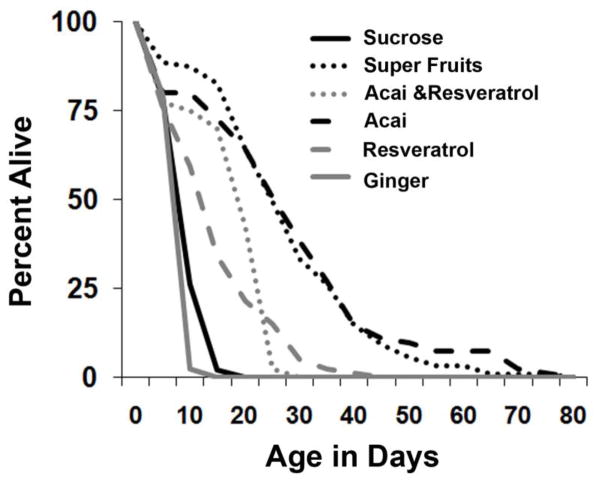
Adult p38Kb null animals were grown on controlled Sucrose minimal food alone or supplemented with either Açai, Resveratrol, Açai+Resveratrol or Super Fruits (a combination advertised to contain Açai, Goji, Noni, Pomegranate, and Mangosteen, without Resveratrol; SF). Ginger was used as a reasonable control for “inert” compounds that might otherwise impact the life span of p38Kb mutant flies. Compounds that contained Açai were administered such that the concentration of Açai was maintained uniformly across vials (see section 2.2). Our results clearly indicated a beneficial effect of adding Açai to the food (Figure 1) as either SF or Acai alone increased life span (average 26.5 and 26.8 days respectively, as compared to 8 days for sucrose). Resveratrol alone resulted in a mild extension of life span (average life span 13 days), whereas a combination of Açai and Resveratrol conferred significant life span extension (average life span of 16 days). As predicted, Ginger did not prove beneficial for p38Kb mutant animals (average life span of 6 days). Overall, in spite of the potential for non-standardized formulations in these commercial products, our results seemed to suggest a clear beneficial effect of dietary Açai supplementation. In fact, it is possible that Açai, in combination with other fruit extracts might provide added benefits as seen from the effect of SF on life span extension (Figure 1). We further tested different doses of these compounds and found that all concentrations of Açai containing compounds were protective (Table 1).
Figure 1. Açai containing compounds extend life span of p38Kb mutants.
p38Kb mutant animals were continuously reared on a variety of antioxidant formulations. All compounds are normalized for either Açai (50mg/ml of food) or Resveratrol (14.3mg/ml of food) concentration. The non-antioxidant compound Ginger was used as control and had no effect on life span.
Table 1.
Life span parameters when p38Kb mutant flies are reared on different concentrations of putative antioxidant supplements and control compounds.
| Compound | Average lifespan in days | Median lifespan in days | Oldest living fly | Logrank test | n |
|---|---|---|---|---|---|
| Sucrose | 8.21 | 8 | 19 | N/A | 145 |
| Super Fruits higha | 26.51 | 25 | 76 | 146.5453* | 87 |
| Super Fruits mediumb | 23.41 | 25.5 | 62 | 43.2002* | 34 |
| Super Fruits lowc | 26.40 | 27 | 53 | 83.4848* | 29 |
| Acai & Resveratrol higha,d | 16.30 | 20 | 28 | 63.42* | 40 |
| Acai & Resveratrol mediumb,e | 20.24 | 23 | 51 | 69.101* | 39 |
| Acai & Resveratrol lowc,f | 20.17 | 20 | 61 | 66.6204* | 38 |
| Acai higha | 26.83 | 26 | 78 | 111.1801* | 47 |
| Acai mediumc | 20.90 | 26 | 40 | 54.2644* | 40 |
| Acai lowb | 17.22 | 18 | 41 | 49.234* | 41 |
| Resveratrol highd | 14.32 | 14.5 | 44 | 54.2897* | 77 |
| Resveratrol mediume | 13.35 | 13 | 43 | 39.254* | 79 |
| Resveratrol lowf | 9.00 | 9.5 | 17 | 3.0658 | 30 |
| Ginger high | 6.03 | 7 | 11 | 27.5854* | 39 |
| Ginger medium | 8.66 | 9 | 19 | 0.0964 | 38 |
| Ginger low | 7.47 | 7 | 15 | 2.9005 | 36 |
is 50mg/ml of Açai,
is 25mg/ml of Açai,
is 12.5mg/ml,
is 14.3mg/ml Resveratrol,
is 7.18mg/ml Resveratrol, and
is 3.59mg/ml Resveratrol.
denotes p value <0.001
3.2 “Super Fruits” and Açai can protect against Hydrogen Peroxide induced oxidative stress
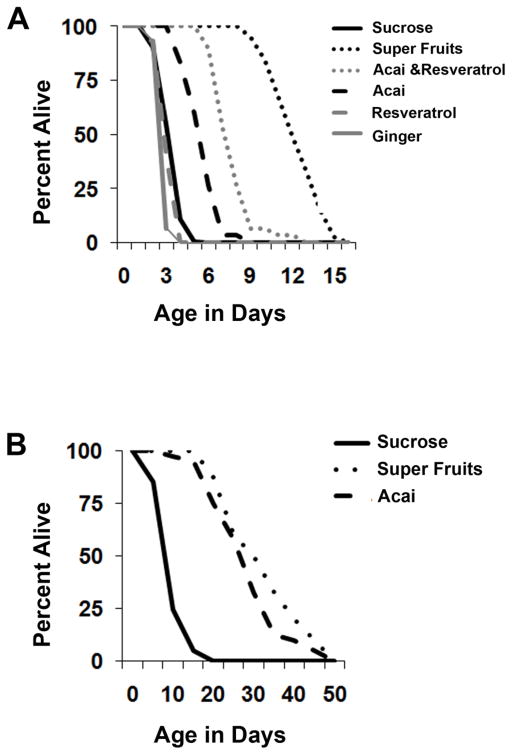
Açai and SF can alleviate reduced life span phenotypes due to a genetic source of oxidative insult (p38K mutants). To further confirm that this protection is due to the ability of these compounds to antagonize oxidative stress, we directly tested Açai and SF for their protective potential against Hydrogen Peroxide insult. These experiments would more directly document an oxidative stress-related role for Açai and SF since it is formally possible that life span rescue observed in p38Kb mutants could be due to factors other than oxidative stress. When adult wild type flies are treated with 1% Hydrogen Peroxide concurrently with or without antioxidants in Sucrose food they display a mean life span of 4 days. As shown in Figure 2A, both Açai and SF significantly extend the life span of animals that are challenged with Peroxide (6 days and 12 days respectively, Table 2). This result is consistent with the idea that Açai and SF can protect against oxidative stress. To test whether Açai and SF can confer protection to animals that have already undergone insult from oxidative stress, we pretreated wild type animals with Hydrogen Peroxide in the absence of supplements. Flies were exposed to 1% Hydrogen Peroxide for 24 hours and were then transferred to food containing Sucrose alone, Açai or SF. As compared to control Sucrose-only food, both Açai and SF were capable of ameliorating the negative impact of Hydrogen Peroxide on life span (Figure 2B, Table 3). Next, we tested whether pre-feeding with Açai and SF could preemptively protect against oxidative stress. We reared wild type flies on food containing Açai or SF for 24 hours, followed by transfer to food containing 1% Hydrogen Peroxide. In this case, we observed no significant rescue of life span suggesting that the beneficial effects of these supplements do not last much beyond the feeding period (data not shown). Taken together, results described in this section support a positive role for Açai and SF in combating the effects of oxidative stress and also suggest a temporal contingency between the intake of these supplements and stress.
Figure 2. Açai containing antioxidant compounds are protective against Hydrogen Peroxide.
Wild type flies were exposed to Hydrogen Peroxide (A) concurrently with antioxidant supplements or (B) pre-exposed to Hydrogen Peroxide followed by antioxidant feeding. Life spans were measured and plotted to evaluate the effect of Hydrogen Peroxide and antioxidant compounds.
Table 2.
Life span measurements for wild type flies in response to Hydrogen Peroxide exposure concurrent with different concentrations of antioxidant supplements.
| Compound | Average lifespan in days | Median lifespan in days | Oldest living fly | Logrank test | n |
|---|---|---|---|---|---|
| Sucrose + Hydrogen Peroxide | 3.56 | 4 | 6 | N/A | 167 |
| Super Fruits higha + Hydrogen Peroxide | 12.46 | 12 | 16 | 134.8432* | 40 |
| Super Fruits mediumb + Hydrogen Peroxide | 10.95 | 11 | 15 | 130.2839* | 38 |
| Super Fruits lowc + Hydrogen Peroxide | 9.08 | 9 | 15 | 136.5059* | 41 |
| Acai & Resveratrol higha,d + Hydrogen Peroxide | 3.61 | 4 | 5 | 0.2039 | 31 |
| Acai & Resveratrol mediumb,e + Hydrogen Peroxide | 7.90 | 8 | 13 | 112.4486* | 31 |
| Acai & Resveratrol lowc,f + Hydrogen Peroxide | 5.73 | 6 | 8 | 70.3814* | 31 |
| Acai higha + Hydrogen Peroxide | 5.77 | 6 | 9 | 74.6008* | 31 |
| Acai lowb + Hydrogen Peroxide | 3.87 | 4 | 5 | 2.5537 | 31 |
| Acai mediumc + Hydrogen Peroxide | 3.57 | 3.5 | 5 | 0.0836 | 29 |
| Resveratrol highd + Hydrogen Peroxide | 3.33 | 3 | 4 | 3.8174 | 40 |
| Resveratrol mediume + Hydrogen Peroxide | 3.26 | 3 | 4 | 6.0690* | 39 |
| Resveratrol lowf + Hydrogen Peroxide | 3.29 | 3 | 4 | 4.7020* | 39 |
| Ginger high + Hydrogen Peroxide | 3.00 | 3 | 4 | 17.7847* | 30 |
| Ginger medium + Hydrogen Peroxide | 3.53 | 4 | 4 | 0.3995 | 31 |
| Ginger low + Hydrogen Peroxide | 2.87 | 3 | 3 | 25.9502* | 31 |
is 50mg/ml of Açai,
is 25mg/ml of Açai,
is 12.5mg/ml,
is 14.3mg/ml Resveratrol,
is 7.18mg/ml Resveratrol, and
is 3.59mg/ml Resveratrol.
denotes p value <0.05.
Table 3.
Life span measurements for wild type flies in response to Hydrogen Peroxide pretreatment followed by exposure to 50mg/ml of Açai per antioxidant supplements.
| Compound | Average lifespan in days | Median lifespan in days | Oldest living fly | Logrank test | n |
|---|---|---|---|---|---|
| Sucrose | 8.81 | 9 | 18 | N/A | 81 |
| Super Fruits | 30.88 | 30.5 | 48 | 108.7226* | 40 |
| Acai | 27.40 | 27 | 50 | 99.3480* | 40 |
denotes p value <0.0001.
3.3 “Super Fruits” and Açai in minimal media protect against Paraquat induced stress in wild type Drosophila
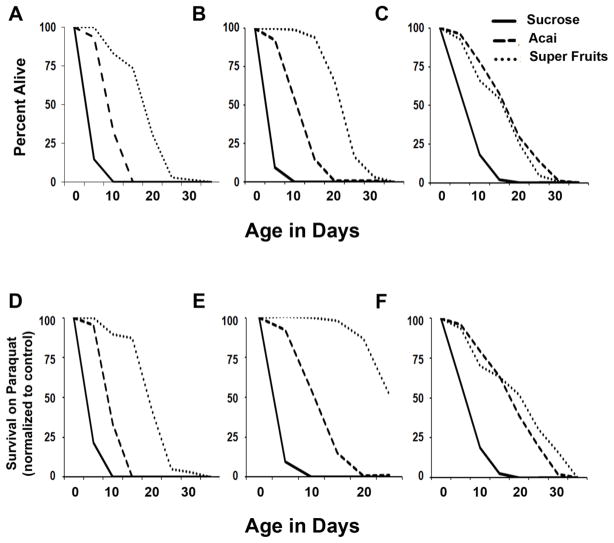
Once we had confirmed that Açai and SF confer life span extension to Hydrogen Peroxide treatment, we were curious to see if these compounds could protect against Paraquat induced stress, which results in an excess of Superoxide radicals. Paraquat is a widely used herbicide and the toxicity of this environmental xenobiotic has been demonstrated in many studies (Bismuth et al., 1990, Suntres, 2002) including several in which Paraquat has been linked to the loss of nigral dopaminergic neurons in the mammalian brain and therefore, to the incidence of sporadic Parkinson’s disease (Thiruchelvam et al., 2000, Dinis-Oliveira et al., 2006, Berry et al., 2010, Cannon and Greenamyre, 2010, Nistico et al., 2011, Jackson-Lewis et al., 2012). Wild type flies were treated with 3mM, 5mM or 10mM Paraquat together with Sucrose, Açai, or SF (Table 4). Açai and SF were both protective against Paraquat induced oxidative stress at all concentrations. Feeding 5mM Paraquat reduced the mean life span to 4 days as compared to control animals that have a mean life span of 8 days. If Açai and SF protect against Paraquat, we should observe significant rescue of this life span. As shown in Figure 3A and D, both Açai and SF showed dramatic protection against Paraquat. When 50% animals were dead following Paraquat treatment, 94% animals were alive with Açai and 100% with SF. These results, together with life span extension seen in p38Kb mutants, strongly suggest that Açai and SF can neutralize oxidative stress in Drosophila resulting in a significant improvement in life span.
Table 4.
Life span measurements for wild type flies in response to Paraquat treatment in sucrose food with simultaneous exposure to different concentrations of antioxidant supplements.
| Compound | Average lifespan in days | Median lifespan in days | Oldest living fly | Logrank test | n |
|---|---|---|---|---|---|
| Sucrose 0mM Paraquat | 7.80 | 7 | 18 | N/A | 172 |
| Sucrose 3mM Paraquat | 2.73 | 2 | 7 | 203.1465* | 102 |
| Sucrose 5mM Paraquat | 3.55 | 3 | 7 | 162.8590* | 107 |
| Sucrose 10mM Paraquat | 2.73 | 2 | 7 | 203.1465* | 102 |
| Super Fruits 0mM Paraquat | 32.08 | 31 | 98 | N/A | 182 |
| Super Fruits 3mM Paraquat | 24.98 | 23 | 35 | 20.7336* | 57 |
| Super Fruits 5mM Paraquat | 17.88 | 18 | 33 | 116.1874* | 136 |
| Super Fruits 10mM Paraquat | 19.08 | 22 | 30 | 53.9028* | 59 |
| Acai 0mM Paraquat | 39.61 | 35 | 115 | N/A | 166 |
| Acai 3mM Paraquat | 11.20 | 12 | 16 | 244.1654* | 40 |
| Acai 5mM Paraquat | 9.13 | 9 | 15 | 326.5166* | 107 |
| Acai 10mM Paraquat | 4.23 | 5 | 9 | 274.1095* | 39 |
denotes p value <0.0001.
Figure 3. Paraquat Toxicity is ameliorated by Açai containing antioxidant formulas.
Wild type animals were exposed to 5mM Paraquat concurrently with antioxidant supplements in minimal media (A, D) or in complex media with females (C, F) or males (B and E). Super Fruits has a greater protective effect than Açai alone when comparing animals treated with 5mM Paraquat (A–C) or when treated animals were normalized to untreated controls (D–F).
3.4 Açai containing formulations in a complete media protect against Paraquat induced oxidative stress in wild type Drosophila
As Açai and SF increased survival in response to Paraquat in the simple sucrose media, we next tested if these supplements are also effective in a nutritionally complete media (complex food). Animals were reared on a standard cornmeal-molasses media and then transferred to the better defined and less variable complex food described in the methods section alone or supplemented with either Açai or SF. Concurrent with antioxidant treatment, animals were exposed to 5mM Paraquat. As observed with the simple sucrose media, both Açai and SF supplementation in complex food was protective against Paraquat induced oxidative stress (Figure 3B, C and E, F). We and others have previously described that males and females respond to Paraquat toxicity differently, with males having increased sensitivity (Chaudhuri et al., 2007, Vrailas-Mortimer et al., 2011). Therefore, we tested if treatment with antioxidants differentially affects males and females in regards to Paraquat sensitivity. Açai and SF were equally protective in females with an average lifespan of 17 and 15 days, respectively, compared to 7 days for complex food alone (Figure 3C and F, Table 5). Strikingly in males, SF is significantly more protective than Açai against Paraquat with SF treated animals surviving for 22 days as compared to 11 days for Açai and 3 days for complex food alone (Figure 3B and E, Table 5).
Table 5.
Life span measurements for wild type flies in response to Paraquat treatment in complex food with simultaneous exposure to antioxidant supplements.
| Compound | Average lifespan in days | Median lifespan in days | Oldest living fly | Logrank test exposed | Logrank test unexposed | n |
|---|---|---|---|---|---|---|
| Males | ||||||
| Complex | 20.59 | 17 | 65 | N/A | N/A | 122 |
| Complex 5mM Paraquat | 3.49 | 3 | 9 | 284.9600** | N/A | 126 |
| Super Fruits | 23.03 | 24 | 30 | N/A | 8.2224* | 123 |
| Super Fruits 5mM Paraquat | 21.93 | 22 | 33 | 3.6444 | N/A | 122 |
| Acai | 39.87 | 35 | 77 | N/A | 103.9616** | 126 |
| Acai 5mM Paraquat | 10.93 | 11 | 35 | 275.2173** | N/A | 129 |
| Females | ||||||
| Complex | 30.05 | 23 | 99 | N/A | N/A | 321 |
| Complex 5mM Paraquat | 6.81 | 6 | 19 | 490.7614** | N/A | 160 |
| Super Fruits | 20.48 | 20 | 36 | N/A | 64.6175** | 250 |
| Super Fruits 5mM Paraquat | 15.30 | 17 | 32 | 40.4173** | N/A | 156 |
| Acai | 33.62 | 34 | 66 | N/A | 0.5414 | 140 |
| Acai 5mM Paraquat | 16.65 | 16 | 31 | 127.6946** | N/A | 78 |
| Resveratrol | 37.43 | 40 | 75 | N/A | 6.3692* | 136 |
denotes p value <0.01,
denotes p value <.0001.
Furthermore, sex differences were also observed when animals were fed a complex diet supplemented with antioxidants in the absence of any oxidizing agents. In wild type males, Açai extends lifespan from 21 days for complex food alone to 40 days (Table 5), whereas, SF has a negligible effect on lifespan. Though Açai also extends lifespan in wild type females, this effect is not as pronounced as that seen in males (30 days for complex food versus 34 days for Açai, Table 5). Interestingly, Resveratrol also extends the lifespan of wild type females to 37 days even though this compound had little protective effect against oxidative stress.
3.5 “Super Fruits” and Açai lower oxidative stress induced expression of GST
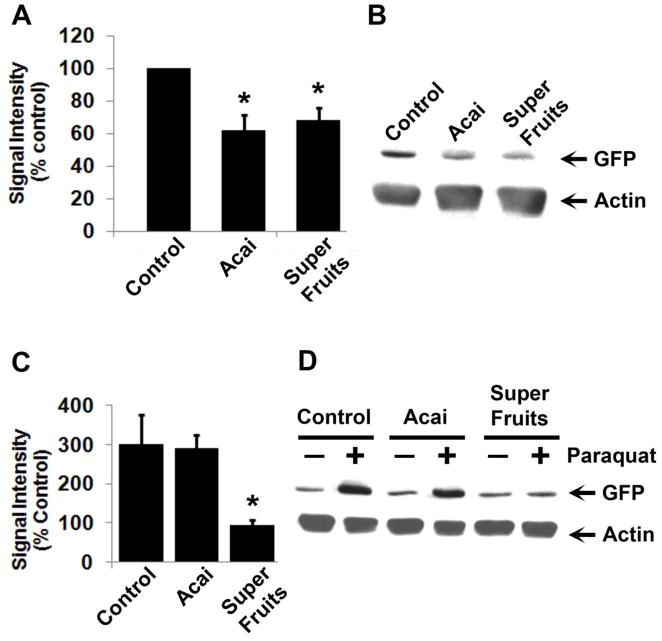
Another useful measure of oxidative stress relies on quantifying the native antioxidant response in vivo. To this end transgenic flies have been generated in the Bohmann laboratory that report transcription of the GSTD1 enzyme using a promoter fusion technique (Sykiotis and Bohmann, 2008, Vrailas-Mortimer et al., 2011). The GST-GFP transgenic carries antioxidant response elements (AREs) from the GSTD1 promoter placed upstream of the coding region for GFP. Following oxidative stress, transcription is activated at the AREs leading to increased GFP expression. Our previous results have shown increased GFP expression in p38Kb mutants as well as in Paraquat treated wild type animals (Vrailas-Mortimer et al., 2011). Therefore, we used this system to evaluate the effect of Açai and SF on oxidative stress. Both Açai and SF effectively reduce basal GFP expression from the GSTD1 based reporter in a p38Kb mutant background (Figure 4A and B). However, only SF and not Açai, significantly reduce GFP expression in response to 5mM Paraquat (Figure 4C and D). While these results molecularly confirm the antioxidant potential of Açai and SF, they also reveal the limitations of Açai in these paradigms. It is interesting to note that consistent with this result, SF performed better than Açai alone in all assays tested in this study. Given that we have calibrated dosage keeping Açai concentrations constant, these results further suggest that other extracts present in SF might potentiate the beneficial effects of Açai.
Figure 4. Antioxidant supplementation reduces expression of oxidative stress response reporters.
GFP is a readout of GST-D1 reporter expression in p38Kb mutant animals (A, B) and in wild type animals exposed to 5mM Paraquat (C, D). Antioxidant exposure with either Açai or Super Fruits reduces reporter expression in p38Kb mutants whereas only Super Fruits was effective at reducing elevated reporter expression in response to Paraquat exposure. Representative western blots from adult head and thorax protein lysates are shown in B and D. Quantitative measurements and statistical significance are depicted in A and C. Asterisks denote p < 0.05. Actin is used as a loading control.
3.6 Paraquat induced destabilization of circadian rhythm is rescued by “Super Fruits” and Açai
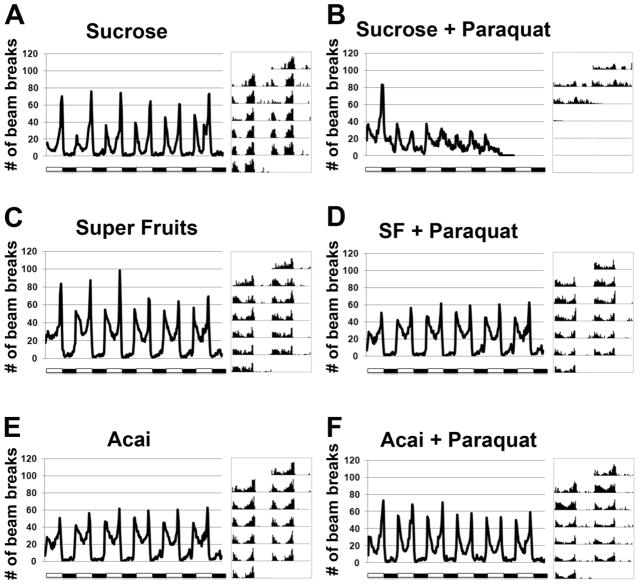
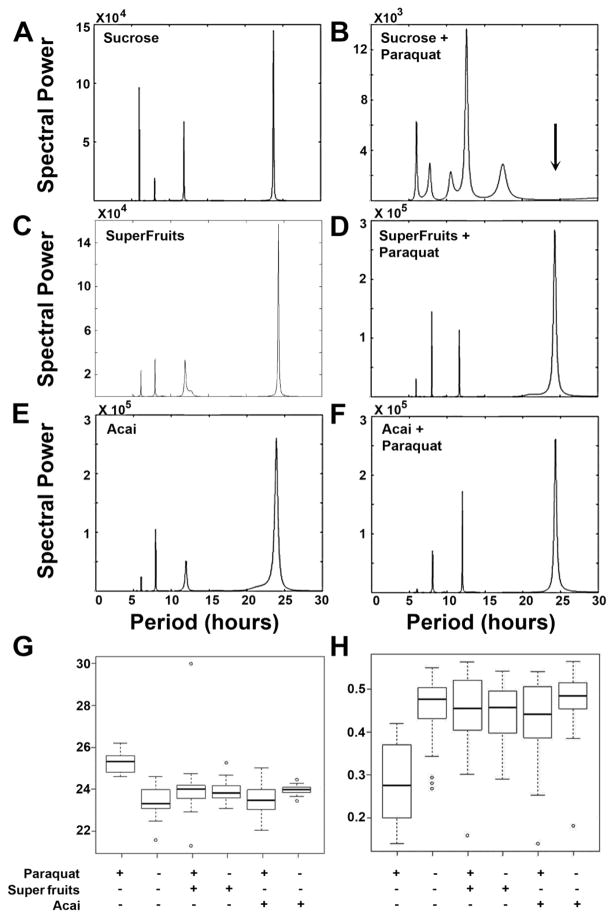
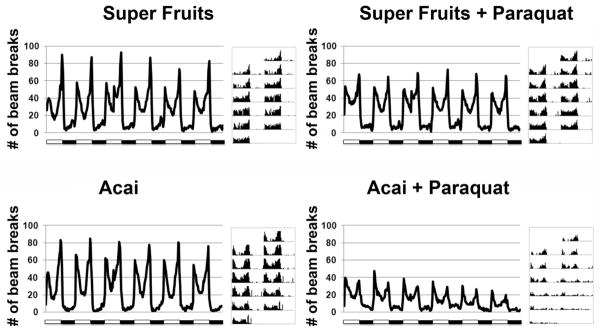
The beneficial effects of Açai and SF on life span and molecular markers of oxidative stress are encouraging. However, a simple extension of life span does not reveal any useful effect on normal physiological functioning or “health span.” To directly test if antioxidant supplementation improves health span, we turned to a previously reported phenotype in flies that is the outcome of Paraquat exposure. Flies, like most organisms, possess a robust light-entrained circadian rhythm that is best seen by following their locomotor activity over 24 hour light:dark cycles (Rosbash and Hall, 1989). Upon chronic treatment with Paraquat, this locomotor rhythm degenerates rapidly (Koh et al., 2006) with a reduction in both the amplitude and rhythmicity of movement until animals eventually die (Figure 5A and B). When flies are treated with Paraquat in the presence of Açai or SF, a remarkable improvement in their circadian rhythm is noticed in addition to the previously documented effect on life span (Figure 5C–F). Autocorrelation analysis, that can be used to measure rhythmicity indices, confirms that the rescue of circadian rhythm is dramatic. While the normal ~24 hour rhythm is completely abolished by Paraquat, Açai and SF restore this 24 hour rhythm (Figure 6 show the Maximum Entropy Spectra for the various experimental and control groups). Even the amplitude of locomotion during a circadian day is near normal. To further test the efficacy of Açai and SF, we asked if these extracts are protective against prolonged exposure to Paraquat. Indeed, animals treated with SF did not develop any defects in rhythm or amplitude over a longer period of exposure (Figure 7). However, Açai treatment was not as effective over the longer term as rhythm amplitude decreased with exposure time (Figure 7). These results provide additional evidence in support of the notion that Açai and SF not only reduce the deleterious effects of oxidative stress on life span, but they are also quite effective at restoring normal physiological function in the presence of toxic levels of Paraquat. Thus Açai, particularly in combination with other fruit extracts in SF, is capable of normalizing both the life span and health span of Drosophila in the face of increased oxidative stress.
Figure 5. Antioxidant supplements protect against deterioration of circadian rhythms in response to Paraquat.
(A, B) Cumulative activity of flies over a 6 day light:dark cycle when flies are reared on Sucrose medium or exposed to 5mM Paraquat. Representative actograms next to each graph show the circadian periodicity in activity patterns. (C, D) Activity of flies reared on Sucrose medium with “Super Fruits” supplement or exposed to both “Super Fruits” and 5mM Paraquat. (E, F) Activity of flies reared on Sucrose medium with Açai supplements or exposed to both Açai and 5mM Paraquat.
Figure 6. Super Fruits and Açai restore normal circadian rhythm periodicity and rhythmicity in Paraquat treated animals.
(A–F) MESA spectra from typical records of fly activity in each test group. A: sucrose alone; B sucrose with 5mM Paraquat. The spectrum from the control animal is simple with a major peak at about 24 h (Tau) with a rhythmicity index (RI) of 0.54. In B, the fly given 5mM Paraquat in sucrose has a spectrum with multiple peaks. Using the convention making comparisons of these very ill flies (assuming bimodality) the dominant peak at 12.6 h was doubled to yield 25.2. RI was only 0.32. C: Flies fed Super Fruits had a clean spectrum with Tau = 24.3 h, RI = 0.52. D: Flies given Super Fruits and 5 mM Paraquat has a good clean spectrum with Tau = 24.3 h and RI = 0.42. E: Flies given Açai, Tau = 23.9 h and RI = 0.52. F: Flies given Açai and 5 mM Paraquat has a clean spectrum with Tau = 24.3 h and RI = 0.50. (G) Boxplot of Tau for all results, dark lines are the medians, the boxes are ± the first quartiles and the whiskers are ± the largest and smallest within the normal range. Small circles are outliers. Treatments are shown below. (H) Boxplot, as in G, for RI.
Figure 7. Super Fruits, but not Açai, protects against prolonged Paraquat exposure.
Activity rhythms are shown from extended (2 week) recordings made from animals that are treated with 5mM Paraquat and are fed either Açai or Super Fruits. The last 6 days of recording are shown. SF supplementation maintains a robust circadian rhythm while Açai is incapable of alleviating the effects of Paraquat treatment over this time period. Representative actograms are shown next to the cumulative activity profiles.
4. Discussion
Since the harmful consequences of high oxidative stress have deeply permeated the public psyche, a large number of antioxidant formulations have been presented by numerous “health food” companies as panacea. It is generally true that most of these compounds have antioxidant potential at least as measured by chemical assays that directly assess the redox properties of a test substance (Juranic and Zizak, 2005, Kaur and Geetha, 2006). However, their efficacy in vivo remains a matter of debate. Animal models have been widely used to test a number of antioxidant compounds, and though these studies have been relatively promising, clinical trials with antioxidant compounds have not always lived up to expectations (Schwedhelm et al., 2003, Kamat et al., 2008, Klein, 2009, Wojcik et al., 2010, Floyd et al., 2011). One reason why antioxidants have performed poorly in clinical trials might have to do with the fact that these compounds may only be effective in a narrow temporal window in relation to oxidative damage. Once serious oxidative damage has occurred, further treatment with antioxidants may not be able to reverse that damage. Similarly, antioxidants delivered as prophylactic may be insufficient to combat subsequent oxidative stress. Indeed, our experiments suggest that adult fruit flies that are fed antioxidants prior to oxidative damage do not perform better than control animals. To investigate these possibilities systematically and continue to screen for effective antioxidant compounds, animal models still offer the most rapid and useful platform. Drosophila with its powerful genetic tools, short generation times and ease of experimental manipulation lends itself admirably to these demands.
The most widely available antioxidant formulations available to the general public are numerous non-FDA tested over-the-counter products sold in health food stores. We asked whether some of these products are actually effective at combating oxidative stress in a Drosophila model. To narrow down the myriad compounds available, we focused on Açai, either by itself or in combination with other potential antioxidant extracts. As experimental models we chose either a p38 MAP Kinase mutant (shown previously by us to have a reduced lifespan and increased oxidative stress) or a more conventional paradigm in which wild type flies are challenged with either Hydrogen Peroxide or the herbicide Paraquat, which results in a shortened lifespan (Reveillaud et al., 1992, Chaudhuri et al., 2007, Vrailas-Mortimer et al., 2011) and circadian rhythm defects (Koh et al., 2006). Overall, our results suggest that Açai, in particular, is quite effective at prolonging life span in the face of oxidative challenge. Açai is similarly effective at normalizing GSTD1 expression and is protective against the deleterious effect of oxidative stress on circadian rhythm in wild type animals. We further noted that while Resveratrol did not potentiate these beneficial effects of Açai, one or more compounds present in a mixture (Super Fruits) containing Açai, did augment Açai-dependent benefits significantly in both simple and complex media. Furthermore, we observed that Açai has differentially protective effects against oxidative stress in males and females. This sex difference may be due to the increased sensitivity of males to oxidative stress or possible differences in metabolic rates between males and females. Intriguingly, Açai also had beneficial effects for wild type animals, particularly males, even in the absence of Paraquat, suggesting that Açai may either provide a protective effect against the endogenous mitochondrially produced oxidative stress in the body or is involved in some as yet unidentified process that promotes the health of the animal. Interestingly, Super Fruits, which often performs better than Açai alone in promoting resistance to oxidative stress does not provide any beneficial effect to unexposed wild type animals, and is even detrimental for females. It may be that Super Fruits reduces endogenous levels of oxidative stress in untreated animals to a degree in which oxygen radical mediated cellular signaling is impaired. Another key observation is that both Açai and SF are more effective against Paraquat than Hydrogen Peroxide induced oxidative stress (compare 25 days for 3mM Paraquat and 12 days for 1% Hydrogen Peroxide in response to SF treatment). As these oxidizing agents produce different forms of oxidative stress (O2− and H2O2 respectively), it is possible that the antioxidant properties of Açai are more efficient at detoxifying superoxide radicals and hence the animals survive longer than when exposed to Hydrogen Peroxide. Thus, our data suggest that in multiple tests, these readily available compounds did in fact protect against the adverse effects of oxidative stress and improved both the life span and health span of these animals. This study also highlights the need to understand the mode of action of commercially available antioxidant supplements and how they interact in the body in stressful and non-stressful conditions.
In general, it seems that at least in the formulations tested, there are active principles present. Both Ginger and Resveratrol were ineffective. Thus, it is unlikely that inert compounds present in the mixture were responsible for the phenotypes observed. Given that one of the main goals of this study was to assess widely available supplements, we note that these relatively crude extracts might retain some of the advertised beneficial effects of Açai (and potentially some other compounds). Additionally, dietary supplementation is sufficient for the observed benefits. It may be argued that antioxidants present in these mixtures neutralize oxidants such as Hydrogen Peroxide in the media, thus reducing the load outside the animal. However, we find that these antioxidants protect p38K mutants and also that they are able to protect animals when delivered immediately after Hydrogen Peroxide exposure. These results would suggest that their antioxidant activity occurs in vivo. Both basal and stress induced GSTD1 transcription, as measured by an anti-oxidant response element (ARE) based reporter transgene, are reduced by these antioxidants. Interestingly, this contrasts with earlier findings where Açai feeding increased GSTD1 transcription (Sun et al., 2010). While we do not know the precise reason for this apparent discrepancy, we speculate that since animals in our experiments are in a state of increased oxidative stress, GSTD1 transcription is already augmented. Under these conditions, Açai probably leads to a reduction in oxidative stress followed by a lowering of GSTD1 transcription in response. Whether Açai directly promotes GSTD1 transcription remains unclear.
Antioxidants used in this study not only rescue life span deficits seen in mutants and oxidant treated animals, but they are also able to promote the maintenance of normal circadian locomotor patterns in the continued presence of Paraquat. This observation is promising since it is often unclear (especially in invertebrate experiments) whether life span extension occurs together with an improvement in health span. Finally, it is important to reiterate that our antioxidant treatments have proved effective in a sensitized background. The effect of Açai on normal lifespan has already been tested previously and these experiments suggest that Açai is incapable of extending the life span of wild type flies that are reared under normal laboratory conditions on classical media. However, when flies are reared on a high fat diet, Açai is able to rescue the negative effect of high fat on overall lifespan (Sun et al., 2010). Consistent with these results, our data indicate that Açai is protective under certain conditions of increased oxidative stress and might not offer any benefits under naïve conditions. The extent to which these findings translate to mammalian models and to humans, will likely form a topic for future studies. At the very least our study highlights the utility of multiple assays that include lifespan measurement, biochemical readouts of oxidative stress and age-dependent motor performance that can be easily combined when using Drosophila as a screening platform.
5. Conclusions
We have found that commercially available antioxidant supplements containing Açai are protective against both genetically and chemically induced models of oxidative stress. Dietary supplementation with Super Fruits or Açai was sufficient to rescue the lifespan defect observed in p38K mutants as well as in wild type animals treated with the oxidizing agents Hydrogen Peroxide or Paraquat. Furthermore, both Super Fruits and Açai also resulted in a reduction of GSTD1 reporter expression, suggesting that these supplements are able to reduce the oxidative stress generated in both p38K mutants and Paraquat exposed animals. Finally, Paraquat-induced circadian rhythm destabilization is also rescued by dietary supplementation of either Super Fruits or Açai. Though the results reported here are not exhaustive analyses of all commercial products or meant to substitute for future analyses of purified principles from these plant sources, this study suggests that Açai containing antioxidant supplements may be effective against multiple sources of oxidative stress.
Highlights.
p38K mutants are rescued by dietary supplementation with Açai containing compounds
Açai containing compounds are protective against oxidative stress induced death
Paraquat induced GST expression is reduced by Açai containing compounds
Circadian defects induced by Paraquat are rescued by Açai containing compounds
Acknowledgments
We would like to thank Ali M. Ahmed for technical assistance in initial life span experiments. We also thank members of the Center for Neurodegenerative Disease (CND) at Emory University and members of the Sanyal laboratory for discussions and useful criticisms.
Abbreviations
- p38K
p38 MAP Kinase
- SF
Super Fruits
- PD
Parkinson’s disease
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper. AVM was supported by NIH T32 training grants administered by the PD-CERC, Emory University. SS thanks funding from the Cell Biology Department and the University Research Council at Emory University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alysia Vrailas-Mortimer, Email: avraila@emory.edu.
Rosy Gomez, Email: rmgomez@emory.edu.
Harold Dowse, Email: dowse@maine.edu.
Subhabrata Sanyal, Email: ssanya2@emory.edu.
References
- Bagatini PB, Saur L, Rodrigues MF, Bernardino GC, Paim MF, Coelho GP, Silva DV, de Oliveira RM, Schirmer H, Souto AA, Vianna MR, Xavier LL. The role of calcium channel blockers and resveratrol in the prevention of paraquat-induced parkinsonism in Drosophila melanogaster: a locomotor analysis. Invert Neurosci. 2011;11:43–51. doi: 10.1007/s10158-011-0116-3. [DOI] [PubMed] [Google Scholar]
- Bahadorani S, Bahadorani P, Phillips JP, Hilliker AJ. The effects of vitamin supplementation on Drosophila life span under normoxia and under oxidative stress. J Gerontol A Biol Sci Med Sci. 2008;63:35–42. doi: 10.1093/gerona/63.1.35. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard KE, Parkes TL, Merritt TJ. A model of oxidative stress management: moderation of carbohydrate metabolizing enzymes in SOD1-null Drosophila melanogaster. PLoS One. 2011;6:e24518. doi: 10.1371/journal.pone.0024518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson’s disease. Cell Death Differ. 2010;17:1115–1125. doi: 10.1038/cdd.2009.217. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning. An overview of the current status. Drug Saf. 1990;5:243–251. doi: 10.2165/00002018-199005040-00002. [DOI] [PubMed] [Google Scholar]
- Blander G, de Oliveira RM, Conboy CM, Haigis M, Guarente L. Superoxide dismutase 1 knockdown induces senescence in human fibroblasts. J Biol Chem. 2003;278:38966–38969. doi: 10.1074/jbc.M307146200. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, Bohorquez A. Paraquat-induced oxidative stress in drosophila melanogaster: effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem Res. 2006;31:1425–1432. doi: 10.1007/s11064-006-9194-8. [DOI] [PubMed] [Google Scholar]
- Brys K, Vanfleteren JR, Braeckman BP. Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol. 2007;42:845–851. doi: 10.1016/j.exger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol. 2010;8:218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66:965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TA, Lee HP, Rolston RK, Zhu X, Marlatt MW, Castellani RJ, Nunomura A, Casadesus G, Smith MA, Lee HG, Perry G. Oxidative Stress and its Implications for Future Treatments and Management of Alzheimer Disease. Int J Biomed Sci. 2010;6:225–227. [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, Carvalho F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006;27:1110–1122. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Dowse HB. Analyses for physiological and behavioral rhythmicity. Methods Enzymol. 2009;454:141–174. doi: 10.1016/S0076-6879(08)03806-8. [DOI] [PubMed] [Google Scholar]
- Driver C, Georgeou A. Variable effects of vitamin E on Drosophila longevity. Biogerontology. 2003;4:91–95. doi: 10.1023/a:1023347803932. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med. 2011;51:931–941. doi: 10.1016/j.freeradbiomed.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Bostick RM, Kucuk O, Jones DP. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic Biol Med. 2011;51:1068–1084. doi: 10.1016/j.freeradbiomed.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S183–185. doi: 10.1016/S1353-8020(11)70057-8. [DOI] [PubMed] [Google Scholar]
- Juranic Z, Zizak Z. Biological activities of berries: from antioxidant capacity to anti-cancer effects. Biofactors. 2005;23:207–211. doi: 10.1002/biof.5520230405. [DOI] [PubMed] [Google Scholar]
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur IP, Geetha T. Screening methods for antioxidants-a review. Mini Rev Med Chem. 2006;6:305–312. doi: 10.2174/138955706776073448. [DOI] [PubMed] [Google Scholar]
- Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- Klein EA. Selenium and vitamin E: interesting biology and dashed hope. J Natl Cancer Inst. 2009;101:283–285. doi: 10.1093/jnci/djp009. [DOI] [PubMed] [Google Scholar]
- Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- Long J, Gao H, Sun L, Liu J, Zhao-Wilson X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson’s disease model. Rejuvenation Res. 2009;12:321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel J, Fleming J, Economos AC. Antioxidants, metabolic rate and aging in Drosophila. Arch Gerontol Geriatr. 1982;1:159–165. doi: 10.1016/0167-4943(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Morillas-Ruiz JM, Rubio-Perez JM, Albaladejo MD, Zafrilla P, Parra S, Vidal-Guevara ML. Effect of an antioxidant drink on homocysteine levels in Alzheimer’s patients. J Neurol Sci. 2010;299:175–178. doi: 10.1016/j.jns.2010.08.050. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- Nistico R, Mehdawy B, Piccirilli S, Mercuri N. Paraquat- and rotenone-induced models of Parkinson’s disease. Int J Immunopathol Pharmacol. 2011;24:313–322. doi: 10.1177/039463201102400205. [DOI] [PubMed] [Google Scholar]
- Paul A, Belton A, Nag S, Martin I, Grotewiel MS, Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128:706–716. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Chan HY, Huang Y, Yu H, Chen ZY. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J Agric Food Chem. 2011;59:2097–2106. doi: 10.1021/jf1046267. [DOI] [PubMed] [Google Scholar]
- Reveillaud I, Kongpachith A, Park R, Fleming JE. Stress resistance of Drosophila transgenic for bovine CuZn superoxide dismutase. Free Radic Res Commun. 1992;17:73–85. doi: 10.3109/10715769209061090. [DOI] [PubMed] [Google Scholar]
- Rosbash M, Hall JC. The molecular biology of circadian rhythms. Neuron. 1989;3:387–398. doi: 10.1016/0896-6273(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Maas R, Troost R, Boger RH. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin Pharmacokinet. 2003;42:437–459. doi: 10.2165/00003088-200342050-00003. [DOI] [PubMed] [Google Scholar]
- Sun X, Seeberger J, Alberico T, Wang C, Wheeler CT, Schauss AG, Zou S. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp Gerontol. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuson M, Garanto A, Gonzalez-Duarte R, Marfany G. Overexpression of CERKL, a gene responsible for retinitis pigmentosa in humans, protects cells from apoptosis induced by oxidative stress. Mol Vis. 2009;15:168–180. [PMC free article] [PubMed] [Google Scholar]
- Vrailas-Mortimer A, Del Rivero T, Mukherjee S, Nag S, Gaitanidis A, Kadas D, Consoulas C, Duttaroy A, Sanyal S. A Muscle-Specific p38 MAPK/Mef2/MnSOD Pathway Regulates Stress, Motor Function, and Life Span in Drosophila. Dev Cell. 2011;21:783–795. doi: 10.1016/j.devcel.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik M, Burzynska-Pedziwiatr I, Wozniak LA. A review of natural and synthetic antioxidants important for health and longevity. Curr Med Chem. 2010;17:3262–3288. doi: 10.2174/092986710792231950. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]