Abstract
Objective
To examine associations of central adiposity, serum adiponectin and clamp-derived insulin sensitivity in a single longitudinal cohort from early adolescence to young adulthood.
Methods
The cohort was examined three times at mean ages 15 years (n = 308), 19 years (n = 218) and 22 years (n = 163). Insulin sensitivity was measured with the euglycaemic hyperinsulinaemic clamp. Circulating adiponectin was measured by enzyme-linked immunosorbent assay. Computed tomography scans were used at mean age 22 years to compute subcutaneous and visceral abdominal fat volume. Partial Pearson correlations and linear regression were used to examine cross-sectional associations at each examination.
Results
The moderate negative correlation between waist circumference and adiponectin was significant and essentially unchanged from mean age 15 years (−0.32, P < 0.0001) to mean age 22 years (−0.29, P < 0.002), whereas the negative correlation between waist circumference and insulin sensitivity and the positive correlation between adiponectin and insulin sensitivity increased steadily in magnitude to mean age 22 years (−0.29, P = 0.0002; and 0.32, P < 0.0001, respectively). In regression models including both visceral and subcutaneous fat, only visceral fat was significantly associated with insulin sensitivity, while only subcutaneous fat was nearly significantly associated with adiponectin.
Conclusions
This study shows that the significant negative relationship between waist circumference and adiponectin predated the development of significant relationships between insulin sensitivity and both waist circumference and adiponectin. It also shows that adiponectin is more closely related to subcutaneous fat and insulin sensitivity is more closely related to visceral fat in young adults.
Keywords: adiponectin, adolescence, euglycaemic clamp, insulin sensitivity, visceral fat
Introduction
It is hypothesized that obesity-associated insulin resistance is mediated, in part, through altered production of adiponectin in the central fat mass [1-3]. However, it is unknown exactly how and when during maturation associations among central adiposity, adiponectin and insulin resistance develop. It also is not established whether specific components of the central fat mass (visceral or subcutaneous) drive these associations in young adults.
Previously, we reported on the changing relationships between waist circumference, adiponectin and insulin sensitivity during adolescence, showing an early significant negative relationship between waist circumference and adiponectin, without a significant adiponectin/insulin sensitivity or waist circumference/insulin sensitivity relationship [4]. In this report, we extend these findings to examine how these associations continue to change during the important developmental transition from adolescence (mean age 15 years) into young adulthood (mean age 22 years). Understanding how these relationships change during development may provide some of the information needed to address the obesity/metabolism interaction that begins in childhood and leads to adult cardiovascular disease.
Patients and methods
Subjects were drawn from a longitudinal study of obesity, insulin resistance and cardiovascular risk factors beginning in adolescence. The Human Subjects Committee of the University of Minnesota approved this study and details of the recruitment have been published previously [4,5]. Briefly, in 1995, white and African American 5th–8th grade Minneapolis school children were randomly recruited with stratification for age, race and systolic blood pressure. Four hundred and one children enrolled at baseline and were asked to attend three follow-up visits over the subsequent 9 years. Informed consent was obtained from the children’s parents and assent from the children prior to age 18 years and informed consent from the children 18 years and older.
The analyses for the present study involved the three follow-up visits where adiponectin was measured at mean ages 15, 19 and 22 years. Participants with waist circumference, adiponectin and insulin sensitivity all measured at mean age 15 years were included in age 15 years’ cross-sectional analyses (n = 308), those with all three measures at mean age 19 years were included in age 19 years’ cross-sectional analyses (n = 218) and those with all three measures as well as computed tomography (CT) measurements of adiposity at mean age 22 years were included in age 22 years cross-sectional analyses (n = 163).
Abdominal adiposity was estimated by measuring waist circumference to the closest 0.5 cm. For subjects without any indentation of the waist, measurements were made 1 cm above the umbilicus. Tanner stage was assessed in children during a physical examination by a paediatrician according to pubic hair in boys and pubic hair and breast development in girls. Blood for adiponectin analysis was drawn in the morning, after an overnight fast and before beginning the insulin infusion for the euglycaemic clamp. All serum samples were stored frozen at −70 °C until analysis at the cytokine reference laboratory at the University of Minnesota. Adiponectin at ages 19 and 22 years was measured with the commercial Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). At age 15 years, adiponectin was measured with a non-commercial ELISA developed by the cytokine laboratory prior to the availability of the kit. The intra-assay and interassay coefficients of variation for the age 15 years’ adiponectin assay were 2.8–7.4% and 14.4%, respectively. The intra-assay and interassay coefficients of variation for the ages 19 and 22 years’ adiponectin assays were 2.5-4.6% and 5.8–6.9%, respectively. As reported previously [4], two studies (involving a total of 33 stored samples from the age 15 years visit) showed high test–retest correlations between adiponectin measured with the commercial Quantikine kit and the non-commercial ELISA (r = 0.83 and 0.89), indicating adiponectin concentrations were correctly ranked using the non-commercial ELISA at the age 15 years visit.
Euglycaemic clamp studies were conducted in the University of Minnesota Clinical Research Center after a 12-h fast, as previously described [5]. Plasma glucose was measured at baseline and every 5 min during the clamp. The insulin infusion was started at time 0 and continued at 1 mU kg−1 min−1 for 3 h. An infusion of 20% glucose was started at time 0 and adjusted, based on plasma glucose levels, to maintain plasma glucose at 5.6 mmol/l (100 mg/dl). Insulin sensitivity, M, was determined from the amount of glucose administered over the final 40 min of the euglycaemic clamp and was expressed as MLBM mg/kg/min (glucose utilization per kg lean body mass per min). Percentage of body fat and lean body mass, or fat-free mass, were calculated by the skinfold formula method of Slaughter [6] at age 15 years, and by dual-energy X-ray absorptiometry (DEXA) at ages 19 and 22 years in children and in parents. The lean body mass values from age 15 years were adjusted to the DEXA values according to equations derived from studies in siblings of the present cohort, and within the same age range, as previously published [7].
Computed tomography was performed using a Siemens Sensation 16, 120-kv, 170-mA, 1-s exposure and two 10-mm slice thickness at L4-L5 interspace. The two images were subdivided into 5-mm slices and the first and third 5-mm slices were combined and analysed to determine adipose tissue volume. The upper limit of adipose tissue density was −30 Hounsfield units (HU) and the lower limit was −190 HU. A trained reader highlighted areas of visceral (within the abdominal cavity) and subcutaneous (outside the abdominal cavity) adipose tissue on each computerized CT image and a computer program (Fat Scan version 3.0; N2 System, Osaka, Japan) was used to sum these areas to obtain estimates of visceral, subcutaneous and total abdominal fat volume. Measurements were obtained in ml. Estimates of abdominal fat volume obtained using CT slices have been shown to correlate well with total imaging volumes on middle-aged Caucasian men and women [8].
All statistical analyses were performed on a PC in SAS v 9.1 (SAS Institute, Cary, NC, USA). Adiponectin was ln-transformed for all analyses. The Meng test was used to test statistical significance of the difference between Pearson correlations [9]; P < 0.05 was the set level for significance. Linear regression was used to assess the association of both visceral and subcutaneous fat with either adiponectin or insulin sensitivity. In these analyses, adiponectin or insulin sensitivity was the dependent variable, modelled continuously, and subcutaneous fat volume and visceral fat volume were included simultaneously in the model as predictor variables. These regression models were controlled for age, sex, race and height. To compare the strength of association for subcutaneous vs. visceral fat, predicted regression coefficients (betas) for one 1-sd deviation change in either visceral or subcutaneous fat volume are presented.
Results
Table 1 lists the mean characteristics of study participants included in the analyses, by visit. Because the adiponectin values were calculated at the times of each visit (ages 15, 19 and 22 years) and a different method was used at age 15 years than at ages 19 and 22 years, median levels across visits should not be compared, although, as noted in the Methods section [4], our analyses indicate the relative rankings across visits are valid, making comparisons of correlations with adiponectin across visits valid. Insulin sensitivity measured by the clamp was normally distributed while adiponectin was highly right-skewed. Mean BMI increased slightly from age 15 years to age 22 years, while per cent of body fat decreased, as described in greater detail in a prior publication from this cohort [10].
Table 1.
Characteristics of individuals, by visit
| Participants Mean age 15 years (n = 308) |
Participants Mean age 19 years (n = 218) |
Participants Mean age 22 years (n = 163) |
|
|---|---|---|---|
| Age (years) | 15.0 (1.2) | 18.6 (0.9) | 22.2 (0.5) |
| Waist circumference (cm) | 79.7 (12.3) | 82.4 (13.6) | 87.2 (14.7) |
| Per cent body fat (%) | 33.5 (14.4) | 27.2 (12.8) | 28.0 (11.9) |
| BMI (kg/m2) | 23.3 (4.9) | 25.1 (5.5) | 25.8 (5.5) |
| Insulin sensitivity | 12.7 (4.3) | 10.9 (3.7) | 11.6 (3.9) |
| (MLBM) (mg kg−1 min−1) | |||
| Adiponectin (μg/ml)* | 13.3 (9.6–17.0) | 6.1 (4.1–8.3) | 8.3 (6.0–10.8) |
| Systolic blood pressure (mmHg) | 108.9 (8.7) | 110.9 (9.6) | 110.6 (10.3) |
| Race (%) | |||
| White | 80.2 | 82.1 | 82.1 |
| African American | 19.8 | 17.9 | 17.9 |
| Male (%) | 56.8 | 59.5 | 56.0 |
| Tanner stage (%) | |||
| < 5 | 32.1 | 0.9 | Not assessed |
| 5 | 67.9 | 99.1 | Not assessed |
| Abdominal fat (ml)† | Not measured | Not measured | 306.4 (187.2) |
| Visceral fat (ml)† | Not measured | Not measured | 68.3 (38.7) |
| Subcutaneous fat (ml)† | Not measured | Not measured | 238.0 (159.4) |
Median (interquartile range)
Measured from two 5-mm CT slices as described in the Patients and methods section. Abdominal fat = visceral fat + subcutaneous fat.
LBM, lean body mass.
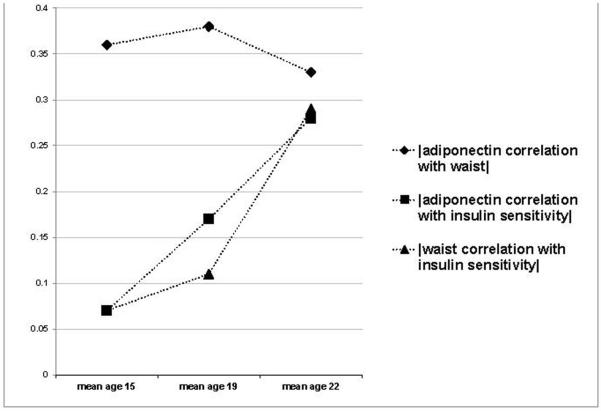
Table 2 shows cross-sectional adjusted Pearson correlations between waist circumference, adiponectin and insulin sensitivity in the cohort at mean ages 15, 19 and 22 years. The negative moderate correlation between waist circumference and adiponectin at mean age 22 years (r = −0.29) was significant and relatively unchanged from the correlation observed between waist circumference and adiponectin at mean ages 15 and 19 years. In contrast, the positive correlation between insulin sensitivity and adiponectin and the negative correlation between insulin sensitivity and waist circumference were not significant at mean age 15 years but increased in magnitude with ageing, becoming significant at age 19 years, and approaching the magnitude of the moderate correlation between waist circumference and adiponectin by age 22 years. As a result, the magnitude of correlations between waist circumference and adiponectin and between waist circumference and insulin sensitivity were not significantly different at age 22 years, unlike ages 15 and 19 years when the waist circumference/adiponectin correlation was significantly greater. Figure 1 graphically illustrates how the correlations changed as the cohort aged, with the data in the figure presenting correlations calculated in the subsample of 137 participants attending all three study visits at mean ages 15, 19 and 22 years. [Table S1 in the Supporting Information lists select mean age 15 years visit characteristics of the 137 individuals who were able to attend and successfully complete study visits at mean ages 15, 19 and 22 years (other participants may have missed one or more visits, or not successfully completed the clamp procedure at all visits). The subsample of participants that attended all visits had a slightly larger percentage of male participants, but was otherwise was very similar to the entire mean age 15 years’ sample (n = 308)]. The correlations calculated in this subsample were very similar to the correlations reported in Table 2, which were calculated using all available members of the cohort at each visit.
Table 2.
Adjusted* cross-sectional correlations of central adiposity, adiponectin and insulin sensitivity
| Age 15 years adiponectin | Age 15 years insulin sensitivity |
Meng test P-value† |
|
|---|---|---|---|
| Age 15 years adiponectin | 1 | 0.05 (0.40) | Not applicable |
| Age 15 years waist | −0.32 (< 0.0001)a | −0.09 (0.14) | 0.01 |
|
| |||
|
| |||
| Age 19 years adiponectin | Age 19 years insulin sensitivity |
Meng test P-value† |
|
|
| |||
| Age 19 years adiponectin | 1 | 0.22 (0.0012) | Not applicable |
| Age 19 years waist | −0.36 (< 0.0001) | −0.16 (0.02) | 0.002 |
|
| |||
|
| |||
| Age 22 years adiponectin | Age 22 years insulin sensitivity |
Meng test P-value† |
|
|
| |||
| Age 22 years adiponectin | 1 | 0.32 (< 0.0001) | Not applicable |
| Age 22 years waist | −0.29 (0.002) | −0.29 (0.0002) | 0.93 |
| Age 22 years abdominal fat | −0.25 (0.0013) | −0.29 (0.0002) | 0.65 |
| Age 22 years visceral fat | −0.21 (0.0077) | −0.37 (< 0.0001) | 0.03 |
| Age 22 years subcutaneous fat |
−0.25 (0.0013) | −0.23 (0.008) | 0.45 |
Values are Pearson correlation coefficients (P-value).
Adjusted for age, race, height, sex and Tanner stage (age 15 years only).
A test comparing the magnitude of the correlation with adiponectin and the magnitude of the correlation with insulin sensitivity. A P-value less than 0.05 indicates the correlations are significantly different.
Figure 1.
Magnitude of correlations between waist, adiponectin and insulin sensitivity from adolescence to young adulthood in the subset of participants participating in all three visits (n = 137). Points represent correlations (r) between two indicated variables at three different examinations in a single cohort. The absolute values of all correlations are presented so as to best compare magnitudes.
At mean age 22 years, visceral fat and subcutaneous fat were positively correlated (r = 0.78, P < 0.0001). Subcutaneous fat was positively correlated with BMI (r = 0.93) and waist circumference (r = 0.94). Visceral fat was also positively correlated with BMI (r = 0.76) and waist circumference (r = 0.80). The negative correlations of visceral fat with adiponectin (r = −0.211) and insulin sensitivity (r = −0.373) were both statistically significant, but the Meng test showed that the moderate negative correlation with insulin sensitivity was significantly (P = 0.03) larger. The negative modest correlations of subcutaneous fat with adiponectin (r = −0.253) and insulin sensitivity (r = −0.232) also were both statistically significant, and not statistically different from one another.
To better understand the negative relationships of subcutaneous and visceral fat with insulin sensitivity and adiponectin at age 22 years, regression models were run including both fat depots as independent variables in a single regression model. When ln(adiponectin) was regressed on visceral and subcutaneous fat simultaneously (adjusted for age, race, sex and height), only subcutaneous fat was (nearly) significant in the model [for subcutaneous fat, P = 0.07, β = −0.086 ln(adiponectin)units per 1-sc increase; for visceral fat, P = 0.76, β = −0.015 ln(adiponectin) units per 1-sc increase). In contrast, when insulin sensitivity was regressed on visceral and subcutaneous fat simultaneously (adjusted for age, race, sex and height), only visceral fat was a significant term in the model (for visceral fat P = 0.0004, β = −1.73 mg kg−1 m−1 per 1-sc increase; for subcutaneous fat, P = 0.58, β = 0.26 mg/kg−1 m−1 per 1-sc increase). Repeating all analyses excluding non-white individuals from the study sample yielded similar results.
Discussion
The present study shows that the negative relationship between waist circumference and adiponectin was significant during early adolescence and remained stable during the transition from adolescence to young adulthood (i.e. ages 15 to 22 years). In contrast, the negative relationship of insulin sensitivity and waist circumference and the positive relationship of insulin sensitivity and adiponectin were both not significant in early adolescence, but increased in magnitude during adolescence and reached a similar magnitude to the negative relationship between waist circumference and adiponectin by early young adulthood. We hypothesize that, as these young adults continue to age, the negative association between waist circumference and insulin sensitivity will continue to strengthen and eventually reach the reported moderate magnitude seen in their parents (r = −0.50) [4].
Abdominal CT studies at age 22 years confirmed the significant negative association of central fat with both adiponectin and insulin sensitivity and further suggested that the subcutaneous fat area was more strongly associated with adiponectin while the visceral fat area was more strongly associated with insulin sensitivity. Most prior studies in adolescents have not formally compared the magnitude of correlations to determine the relative importance of visceral and subcutaneous fat with clamp-derived insulin sensitivity or adiponectin. However, it has been previously reported in adolescents that the magnitude of the negative association between subcutaneous fat and adiponectin appeared larger than that of visceral fat and adiponectin [11,12], and that the magnitude of negative association in obese adolescents between visceral fat and insulin sensitivity appeared larger than that of subcutaneous fat and insulin sensitivity [13]. Reported negative correlations between central fat and insulin sensitivity vary considerably, based on age, obesity and race. Visceral adiposity was significantly negatively correlated with insulin sensitivity (derived from a frequently sampled intravenous glucose tolerance test) in white subjects but not in black African pre-menopausal women [14]. A small (n = 30) study in obese Italian (prepubertal) children found a significant negative correlation between subcutaneous fat and insulin sensitivity (derived from a frequently sampled intravenous glucose tolerance test), but not between visceral fat and insulin sensitivity [15]. The discrepant results from this study may be related to age or method of insulin sensitivity measurement (frequently sampled intravenous glucose tolerance test vs. clamp). However, the significant negative association between subcutaneous adiposity and adiponectin in the present study is supported by recent transcriptional work demonstrating greater expression of adiponectin in subcutaneous adipose tissue than in visceral adipose tissue (excised from humans during elective surgery) [16]. Recently published results from the Framingham study also suggest that visceral adipose tissue is a stronger correlate of insulin resistance than subcutaneous adipose tissue [17].
The differing associations of adiponectin and insulin sensitivity with visceral and subcutaneous fat in this cohort raise questions about the role adiponectin may play in the association of abdominal obesity and insulin resistance. The evidence that adiponectin and insulin sensitivity may be primarily associated with different central fat depots, combined with the fact that associations between waist circumference and adiponectin appear earlier in development than associations between waist circumference or adiponectin and insulin sensitivity may indicate that associations of central fat with adiponectin and insulin sensitivity are largely mediated through distinct biologic pathways. Understanding how metabolic associations develop from childhood into adulthood will result in a better understanding of these pathways and, thus, may prove a useful tool to explore hypothesized pathophysiologic pathways for disease. Although it is not clear why the significant relation between waist circumference and adiponectin predates their associations with insulin sensitivity, it seems reasonable to speculate that it may be related to the different associations of adiponectin and insulin sensitivity with visceral and subcutaneous fat and potential differences in development of the fat depots during adolescence. It has been suggested that an increase in the ratio of visceral to subcutaneous fat results in a decrease in insulin sensitivity [18-20], and this is supported by the finding that a higher ratio is found in obese adolescents with impaired glucose tolerance, consistent with reduced insulin sensitivity [20,21]. Prior studies from the present cohort have shown significant changes in insulin sensitivity during the second decade of life [10]. While the developmental pattern during adolescence for central body fat has not been reported, it is possible that increases in visceral fat, perhaps related to an overflow effect from limited subcutaneous fat storage [22], results in an increasing visceral fat/subcutaneous fat ratio, decreasing insulin sensitivity.
Strengths of this study include the longitudinal design permitting serial assessment of correlations from early adolescence into young adulthood; novel information about the changing associations between central adiposity, adiponectin and insulin sensitivity change in a single cohort over time; the use of CT to measure abdominal fat masses; the use of the gold-standard hyperinsulinaemic euglycaemic clamp to measure insulin sensitivity; and the use of the Meng test to statistically compare correlated correlations. Limitations include low sample diversity and lack of CT measurements at earlier study visits, which would have permitted evaluating the changes relative to changing patterns of visceral and subcutaneous fat. Also, recent studies suggest levels of adiponectin sub-fractions as well as the biological activity and function of adiponectin are likely more relevant than level of total circulating adiponectin in biological associations.
In summary, this study utilized repeated measures over time in an adolescent cohort to show that the significant negative relationship between waist circumference and adiponectin was present early in life and predated the development of the significant negative relationship between insulin sensitivity and waist circumference and the significant positive relationship between adiponectin and insulin sensitivity. In addition, the study showed that, in young adulthood, adiponectin was more closely related to subcutaneous fat and insulin sensitivity was more closely related to visceral fat. These results provide new information on the early development of the relation between adiponectin and insulin sensitivity, supporting an early onset of associations among factors associated with cardiovascular disease.
Supplementary Material
Acknowledgements
This study was supported by grants HL52851 and M01RR00400 from the National Institutes of Health.
Footnotes
Competing interests
Nothing to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- 1.Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 2.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 3.Krakoff J, Funahashi T, Stehouwer CD, Schalkwijk CG, Tanaka S, Matsuzawa Y, et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745–1751. doi: 10.2337/diacare.26.6.1745. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, Jr, Steinberger J, Moran AM, Sinaiko AR. Influence of waist on adiponectin and insulin sensitivity in adolescence. Obesity (Silver Spring) 2009;17:156–161. doi: 10.1038/oby.2008.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinaiko AR, Jacobs DR, Jr, Steinberger J, Moran A, Luepker R, Rocchini AP, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–707. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Human Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 7.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 2005;29:1346–1352. doi: 10.1038/sj.ijo.0803026. [DOI] [PubMed] [Google Scholar]
- 8.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34:781–787. doi: 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng X, Rosenthal R, Rubin D. Comparing correlated correlation coefficients. Psychol Bull. 1992;111:172–175. [Google Scholar]
- 10.Moran A, Jacobs DR, Jr, Steinberger J, Steffen LM, Pankow JS, Hong CP, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117:2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 13.Caprio S, Hyman LD, Limb C, McCarthy S, Lange R, Sherwin RS, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269:E118–126. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 14.Goedecke JH, Levitt NS, Lambert EV, Utzschneider KM, Faulenbach MV, Dave JA, et al. Differential effects of abdominal adipose tissue distribution on insulin sensitivity in black and white South African women. Obesity (Silver Spring) 2009;17:1506–1512. doi: 10.1038/oby.2009.73. [DOI] [PubMed] [Google Scholar]
- 15.Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–2128. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- 16.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 17.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danforth E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 19.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 22.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.