Abstract
Chronic HIV infection, which is primarily characterized by the progressive depletion of total CD4+ T cells, also causes persistent inflammation and immune activation. This is followed by profound changes in cellular and tissue microenvironments that often lead to prolonged immune dysfunction. The global nature of this immune dysfunction suggests that factors that are involved in immune cell survival, proliferation, differentiation and maturation are all affected. Of particular interest is the transcriptional factor Foxo3a that regulates a number of genes that are critical in the development and the maintenance of T and B cells, dendritic cells (DCs) and macrophages. Alterations in the microenvironment mediated by HIV infection cause significant increase in the transcriptional activity of Foxo3a; this has major impact on T cell and B cell immunity. In fact, recent findings from HIV infected individuals highlight three important points: 1) The alteration of Foxo3a signaling during HIV infection deregulates innate and adaptive immune responses; 2) Foxo3a-mediated effects are reversible and could be restored by interfering with the Foxo3a pathway; and 3) down-regulation of Foxo3a transcriptional activity in elite controllers (ECs) represents a molecular signature, or a correlate of immunity, associated with natural protection and lack of disease progression. In this review, we will discuss how HIV-infection altered microenvironments could result in impaired immune responses via the Foxo3a signaling pathway. Defining precisely the molecular mechanisms of how persistent inflammation and immune activation are able to influence the Foxo3a pathway could ultimately help in the development of novel approaches to improve immune responses in HIV infected subjects.
Keywords: Foxo3a, HIV, microenvironment, EC, apoptosis, memory
1. Introduction
During chronic HIV infection, lymphoid and mucosal tissues, and peripheral blood undergo substantial anatomical and microenvironmental alterations. These changes are highlighted by the aberrant production of cytokines, chemokines and interferons, all leading to the establishment of polyclonal immune activation (1–4). HIV infection is also characterized by the disruption of gut integrity and subsequent release of bacterial products within the bloodstream and lymphoid tissues leading to immune activation (5). Notably, persistent inflammation in infected lymph nodes (LNs) leads to the deposition of collagen (6), a phenomenon that has been attributed in part to increased levels of TGF-β1 (7). These changes result in profound reorganization of tissue architecture thereby leading to severe immune dysfunction that is illustrated by the inability to generate and maintain T cell and B cell immunity. These defects persist even in infected subjects undergoing highly active anti-retroviral treatment (HAART) suggesting that factors besides virus replication contribute to immune dysfunction during HIV infection (8–12). The immune defects observed in HIV infected subjects are the result of deregulation of key signaling pathways that are involved in normal physiological processes. In this context, CTLA-4 and PD-1, known to be implicated in T cell tolerance (13), have been associated with exhausted HIV-specific T cell responses in infected subjects (14–17). Furthermore, signaling through IL-7 and IL-2, two common γ-chain receptor cytokines that are important for survival and homeostatic proliferation of memory lymphocytes via the STAT5 pathway (18–20), is impaired in HIV infected subjects (11, 12, 21). While all of these pathways contribute to the immune dysfunction that is associated with HIV-1 infection, other key regulatory signaling pathways are also impacted including the Foxo3a pathway that will be the subject of this review.
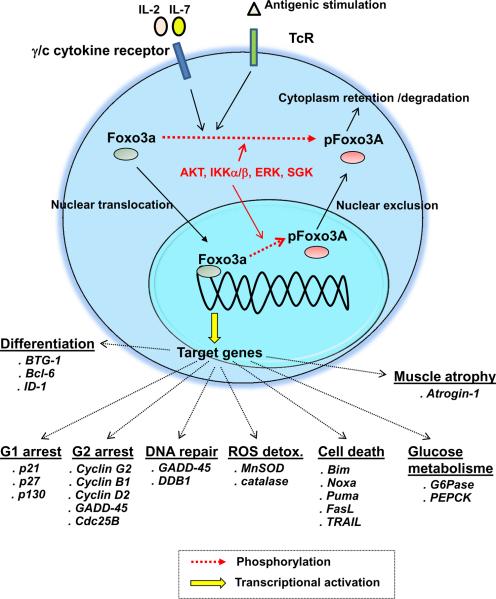
Foxo3a is a transcription factor that is constitutively expressed in hematopoietic cells. Foxo3a induces the transcription of pro-apoptotic genes (such as Bim, Noxa, Puma, FasL and TRAIL (22–26)), anti-proliferative genes (including p21, p27, p130, cyclin G2 and GADD45 (26–28)), genes implicated in the reactive oxygen species (ROS) detoxification (such as superoxide dismutase and catalase (29–31)), genes regulating glucose metabolism (such as glucose-6-phosphatase (32, 33)), and genes involved in differentiation processes (including Bcl-6 and BTG-1 (26, 27, 31, 34)) (Fig. 1). Foxo3a transcriptional activity is regulated by multiple mechanisms including acetylation, ubiquitination and phosphorylation (26). Phosphorylation of Foxo3a by several kinases including AKT, IKKα/β, ERK and serum glucose kinase (SGK) results in its exclusion from the nucleus and inhibition of its transcriptional activity (23, 26, 35, 36). We have previously shown that Foxo3a signaling is critical for central memory CD4+ T cell (TCM) survival and that Foxo3a is highly phosphorylated in this subset by AKT and IKK at multiple residues upon TCR and cytokine receptor engagement (19).
Figure 1. Regulation of Foxo3a transcriptional activity.
Triggering T cell receptor (TcR), B cell receptor (BCR) or common gamma chain cytokine receptors activates of several kinases such as AKT, IKK and ERK11,12,19. These kinases induce the phosphorylation of Foxo3a at multiple residues, leading to its sequestration into the cytoplasm and ultimately to its degradation23,24. On the other hand, when Foxo3a is unphosphorylated, it translocates to the nucleus, binds to the promotors of a number of target genes and induces their transcriptional activation23,24. Foxo3a drives the expression of proapoptotic and anti-proliferative molecules as well as proteins implicated in the ROS detoxification and glucose metabolism22–25.
Since CD4+ T cells constitute the primary target for HIV (37, 38), we will discuss experimental evidence linking the deregulation of Foxo3a activity with alterations in CD4 subsets. In addition, we will detail how aberrant Foxo3a activation affects other lymphocytes including memory B cells in HIV infected hosts.
2. Foxo3a is a critical hematopoietic modulator
Foxo3a regulates many normal physiological functions, including cell-cycle arrest (26–28), stress resistance during oxidative damage (29–31), aging (39), apoptosis (22–26) and glucose metabolism (32, 33). It has been shown in Foxo3a deficient mice and in vitro assays using human lymphocytes that the maintenance of CD34+ stem cells (40–42), T cell progenitors (43) and mature central memory CD4+ T cells (TCM) (19) are mediated by increased AKT, ERK and IKK kinase activities, whose functions are to induce the phosphorylation of Foxo3a (26, 27, 35). Control of Foxo3a transcriptional activity by phosphorylation is also required for differentiation of the erythroid lineage via expression of BTG-1 (44–46) and of B-cell progenitors via Bcl-6 and Bim (47–49). Several studies using Foxo3a deficient mice have revealed that Foxo3a promotes transcription of the FoxP3 gene that is essential for optimal regulatory T cell development and function (50, 51). Interestingly, the Foxo3a pathway has also been shown to regulate the function of dendritic cells by inducing the production of key pro-inflammatory molecules such as IL-6 and TNF-α (52) and to increase the survival of natural killer cells upon IL-15 stimulation (53). Overall, these observations demonstrate the involvement of Foxo3a signaling pathway at multiple levels of hematopoiesis.
3. Deregulation of Foxo3a is associated with disease pathogenesis
Foxo3a transcriptional activity is regulated at multiple levels and by multiple mechanisms. This is important to avoid aberrant expression of genes that are involved in cell survival, differentiation and maturation. In fact, deregulation of the Foxo3a pathway has been associated with several pathologies. For example, persistent phosphorylation of Foxo3a has been associated with in vitro and in vivo induction of tumors including breast, colorectal, gastric and pancreatic cancers as well as leukemia, lymphoma and myeloma (26, 54–56). Treatment of many tumors by chemical drugs such as microRNA-155, imatinib, tamoxifen, and all-trans retinoic acids, has been shown to be mediated by the induction of pro-apoptotic Foxo3a activity through increased expression of Bim, Puma and TRAIL(56–61). Essafi et al. have recently demonstrated that the delivery of exogenous Foxo3a proteins into cancer cells might constitute a novel potential therapeutic strategy in the treatment of several cancers (62). Down-regulation of Foxo3a transcriptional activity in mice and humans has furthermore been associated with the establishment of atherosclerotic plaques and autoimmune and inflammatory diseases such as arthritis (22, 63–65). Conversely, conditions that lead to increased Foxo3 activity cause neurodegenerative diseases such as Parkinson's and Alzheimer's, brain damage after hypoxia/ischemia or stroke, and cardiomyocyte dysfunctions (66–70).
In the next sections, we will focus on the role of Foxo3a during HIV infection, as alterations of its transcriptional activity could be associated with disease progression and natural protection against HIV infection. We will also present evidence showing that the deregulation of Foxo3a signaling during HIV infection occurs through two major mechanisms: one mediated by the viral proteins, and the second by the deregulated microenvironment within infected blood and tissues.
4. HIV viral proteins alter Foxo3a signaling
HIV expresses a number of viral proteins such as Env, Vpr and Tat that can have significant effects on host cellular function (38, 71–73). Apoptosis of HIV infected CD4+ T cells has been shown to be triggered by Foxo3a whose pro-apoptotic transcriptional function appears to be significantly up-regulated by HIV-1 Tat through the interference with AKT kinase activity (74, 75). Accordingly, silencing Foxo3a signaling within infected CD4+ T cells using small interfering RNAs (siRNAs) significantly improve their survival (74, 75). Kino et al. have reported that the HIV protein Vpr inhibits the ability of insulin to induce the phosphorylation of Foxo3a via AKT, thus interfering with its exclusion from the nucleus (76). Because viremia and long-term use of HAART have been linked to the establishment of severe metabolic abnormalities including hyperglycemia and insulin resistance (77), this may indicate that Vpr is involved in these processes by up-regulating Foxo3a in HIV infected subjects. Interestingly, decreasing Foxo3a activity and subsequent Bim expression by Insulin-like Growth Factor counteracts the high levels of neuronal apoptosis occurring in patients suffering from HIV encephalitis (78). Alterations of Foxo3a transcriptional activities have also been reported in macrophages and dendritic cells; this could have major implications on innate defenses during HIV infection. For instance, HIV infection of monocyte-derived macrophages by different HIV strains induces apoptosis through a Foxo3a dependent process (80, 81). Indeed, HIV infected macrophages display reduced levels of AKT and Foxo3a phosphorylated forms. This coincides with elevated expression levels of the Foxo3a pro-apoptotic target genes TRAIL and the subsequent TRAIL-mediated apoptosis (80, 81). It is worthy noting that the decrease of Foxo3a activity by siRNAs or by the over-expression of its dominant negative form leads to significant improvement of macrophage survival (80).
5. Foxo3a signaling contributes to global cell death during HIV infection
The direct cytopathic effects mediated by HIV cannot explain the massive CD4+ T cell depletion observed in infected patients. Therefore, extrinsic factors that are generated as a result of HIV infection, such as disruption of cellular anatomical niches, alterations of tissue and cellular microenvironments, affect bystander populations in infected hosts and contribute to global cell death. In this context, pro-inflammatory cytokines, interferons, and bacterial products, known to be increased during HIV infection (1, 4, 5), induce Foxo3a dephosphorylation and enhance its pro-apoptotic activity (11, 82), leading to global cell death. On the other hand, HIV infection is also characterized by the decrease of cytokines such as IL-2 (83) and IL-21 (9). Both of these cytokines have the ability to induce Foxo3a phosphorylation and block its pro-apoptotic function (11, 19). Accordingly, Foxo3a transcriptional targets TRAIL and FasL (26, 27), whose expression levels have been shown to be up-regulated by interferons in infected blood and tissues, are responsible for T cell and B cell death in HIV+ subjects (4, 84–88). Thus, the Foxo3a pathway integrates multiple signals that can influence lymphoid proliferation and survival. Understanding the role of Foxo3a in memory lymphocyte loss and disease progression during chronic HIV infection could shed some light on the underlying mechanisms of global T cell and B cell death during HIV infection (11, 12, 82).
It is worth mentioning that Foxo3a-mediated lymphocyte dysfunction is also apparent even in chronically HIV-infected subjects that are under HAART. This suggests that HIV-induced destruction and changes of tissue and cellular microenvironments occur early during HIV infection and persist in the chronic phase of the disease even in the absence of HIV viral replication. In this context, decreased persistence of peripheral TCM and TEM subsets has been reported in HIV infected subjects under HAART and this was directly linked to enhanced Foxo3a transcriptional activity and FasL expression (12, 19). Similarly, memory B cell survival has also been shown to be impaired during chronic HIV infection and that Foxo3a and its target gene TRAIL are strongly involved in this process (11). Bacterial products such as lipopolysaccharide (LPS), and soluble factors such as TGF-β1 and IFN-α are increased during HIV and SIV infection (4, 5, 7). All of these induce Foxo3a transcriptional activity and the expression of pro-apoptotic targets including TRAIL and Bim (11).
Interestingly, silencing Foxo3a by siRNA or by using a dominant negative mutant of Foxo3a, restores the survival of memory T and B cells to levels similar to those observed in uninfected subjects (11, 12), indicating that these defects are reversible even after many years of HIV infection.
6. Implication of Foxo3a in natural immune protection against HIV infection
Perhaps validation of the role of Foxo3a pathway in the immune response is best illustrated in a rare subset (< 0.3%) of chronic HIV-infected subjects, the elite controllers (ECs), that are able to fully control viral replication (plasma loads < 50 copies/ml) and maintain high CD4 cell counts (> 500 cells/μl) for many years without HAART (89). ECs do not exhibit immune activation and do not display any increase in inflammatory cytokines or products of bacterial translocation. More importantly, these subjects express increased levels of T cell and B cell survival cytokines including IL-2 and IL-21; these are normally reduced in chronic HIV infected subjects (5, 9, 90). Accordingly, we found that ECs display lower levels of Foxo3a-mediated pro-apoptotic transcriptional activity in both memory T cells and B cells when compared to HIV non-controller and uninfected subjects (11, 12). Thus, identifying selective pathways in ECs, that are known to be involved in the maintenance of memory T and B cells, provides critical information to better understand the underlying mechanisms of HIV control in these subjects (91).
7. Foxo3a in HIV infection: a friend or a foe
The Foxo3a transcription factor represents a key-signaling hub at the intersection of numerous pathways that are associated with apoptosis, cell cycle, quiescence, stress response, and cell metabolism. Thus, the Foxo3a pathway needs to be tightly regulated otherwise enhancement of disease pathogenesis could result.
During HIV infection, chronic immune activation has been shown to be associated with the release of bacterial products from the gut (5), coincided with elevated levels of interferon and pro-inflammatory cytokine expression (1, 4) as well as with viral replication in untreated HIV infected subjects. These induce alterations in the microenvironment and lead to persistent expression of several negative regulators of T cell activation such as PD-1, CD160 and CTLA-4 (92–94) (Fig. 2). This results in T-cell exhaustion characterized by the inability of CD4+ T helper cells to proliferate and to secrete pro-survival cytokines such as IL-2 and IL-21 (9, 83). Of note, IL-2 has been shown to drive the survival of TCM cells by a Foxo3a-dependent manner (19). We recently found that the loss of memory CD4+ T cell subsets in HIV chronically infected subjects involves pro-apoptotic Foxo3a activity (12). Accordingly, it remains to be seen if PD-1 triggering influences Foxo3a signaling pathway. Moreover, HIV proteins including Tat interfere with the Foxo3a pathway in infected CD4+ T cells and this results in their progressive depletion (74, 75, 92, 93) (Fig. 2). The lack of pro-survival cytokines, increased chronic immune activation, and the augmented PD-1 ligation also lead to memory B cell dysfunction. Indeed, we recently showed that the loss of memory B cells in chronically infected individuals occurs through the increase of Foxo3a transcriptional activity and likely involves the contribution of multiple extrinsic signals including LPS, IL-2, IL-21 and interferons (11). Of note, preliminary reports have suggested that CD4 helper cells from HIV infected subjects are not capable of providing B cell help as these cells have lost their capacity to secrete IL-4 and IL-21. This might impede the survival of memory B cells and antibody production in HIV non-controller subjects. In this context, it would be interesting to determine whether the impaired HIV microenvironment contributes to the dysfunction of CD4 helper T cells.
Figure 2.
Integrative and multi-lineage immune dysfunction during HIV infection.
Conversely, EC subjects, that fully control HIV replication without the intervention of anti-retroviral therapy, exhibit a pro-survival molecular advantage illustrated by lower Foxo3a transcriptional activity, when compared to HIV non-controllers and healthy subjects. Thus, it would be interesting to examine whether polymorphisms within the genes that are part of the Foxo3a pathway exist in ECs and could be associated with disease non-progression. Overall, these data strongly implicate Foxo3a as a critical regulator of memory T and B cell survival as well as an integrator of aberrant signals during HIV infection.
The immune defects related to Foxo3a signaling observed in HIV infected subjects, in the presence or absence of HAART, are reversible even after many years of infection. Thus, identification of target molecules within the Foxo3a pathway could be used for the development of therapeutic and preventive HIV vaccines.
Biographies

Julien van Grevenynghe is currently a senior research associate in the Molecular Oncology Group at the Lady Davis Institute for Medical Research, Jewish General Hospital, McGill University. He earned his PhD degree in INSERM U456 (Toxicology and Oncology) at Rennes 1 University (France, 2005). As a post-doctoral fellow in Dr. R.P. Sekaly (Université de Montreal, 2005–2010), he identified the transcriptional factor Foxo3a as a key player in the maintenance of central memory CD4+ T cells during chronic HIV infection. He was a recipient of a fellowships from the Fond de la Recherche Medicale (2006–7) and from the Canadian Institutes of Health Research. In 2010, he moved to the Vaccine & Gene Therapy Institute of Florida as a staff scientist in the team of Dr. E. K. Haddad. Their research interests include: the maintenance of memory B cells in HIV-infected subjects and the establishment of humoral responses mediated by the CD4 follicular helper T cells.

Rafael Cubas: Dr. Rafael Cubas is a Postdoctoral Researcher in the Elias K. Haddad laboratory at VGTI-Florida since September of 2010. He obtained his PhD in Molecular Virology and Microbiology from Baylor College of Medicine in April 2010 where he studied the effects of a chimeric simian-immunodeficiency virus (SIV) virus-like particles (VLP) containing the murine Trop2 oncogene (mTrop2-VLP) for the treatment of murine pancreatic cancer. Since the initiation of his post doctoral research, he has been working extensively with follicular T helper (Tfh) cells and memory B cells in humans determining their phenotypic and functional characterization.
Dr. Sandrina Dafonseca has recently joined the Research Center at the Hospital Center of the University of Montreal (CR-CHUM) as a post-doctoral fellow in the laboratory of Dr. Petronella Ancuta. She previously worked as a post-doctoral fellow in the Vaccine & Gene Therapy Institute of Florida on the characterization of the HIV reservoir and HIV latency.

Talibah Metcaf A postdoctoral research fellow at Vaccine & Gene Therapy Institute Florida in Dr. Elias Haddad's Lab. Ph.D in Molecular Microbiology and Immunology obtained from Johns Hopkins University School of Public Health. Doctoral research was conducted in the Lab of Dr. Diane Griffin and focused on B cell trafficking and long-term maintenance in the CNS of alphavirus infected C57BL/6 mice. Also, MS in Microbiology from the University of Florida. B.S. was obtained from the University of South Florida. Before starting her PhD, worked for two years as a Research Assistant in Dr. Chris West's Lab at the University of Oklahoma Health Science Center.

Dr. Cecile Tremblay is currently the director of the Public Health Laboratories of Quebec (Canada). She is also a professor at the University of Montreal at the Department of Microbiology and Immunology. Dr. Tremblay has been actively involved in the establishment of a multiple HIV cohorts including the Canadian Cohort of HIV Infected Slow Progressors. Dr. Tremblay's research focused on studying CD4 response during HIV infection and understanding the correlates of natural protection to HIV infection

Rafick-Pierre Sekaly Dr. Sékaly has been for the past two years the Co-Director and Chief Scientific Officer of VGTI –Florida, an Institute focused on the development of better immune therapies and vaccines to chronic viral diseases and cancer. Dr Sékaly obtained his Ph.D. in Biochemistry at the Université of Lausanne in 1984. He has been involved in the areas of AIDS and AIDS pathogenesis for the past fifteen years. He is now fully focused on applying systems biology to unravelling defects in different cells (innate and adaptive) of the immune response. In addition to his scientific work and leadership at VGTI -FL, Dr. Sekaly is the Founder and Scientific Director for the National Laboratory of Immune Monitoring in Montreal, Canada.

Dr. John Schatzle: Dr. Schatzle is the Director of Scientific Affairs at VGTI-FL. He manages scientific staff, core facilities, information technology, facilities maintenance and environmental health and safety departments. His training includes a B.Sc. in Microbiology from the University of Louisiana, PhD training in gene regulation and developmental biology from LSU medical school and post-doctoral training in retrovirology and oncology from the U. of Texas. Prior to coming to VGTI-FL, he was an associate professor of Pathology and Immunology at the U. of Texas Southwestern Medical Center in Dallas, TX. He had an independent research program at UTSW for the past 13 years studying the innate immune response with an emphasis on Natural Killer Cell biology and recently was focused on murine models of lupus. His research focused on signal transduction pathways associated with cell surface receptors on lymphocytes with recent emphasis on the SLAM family of receptors.

Elias K. Haddad, Ph.D. Dr. Haddad is an associate member at the Vaccine & Gene Therapy Institute of Florida. Dr. Haddad is an expert on viral pathogenesis where his laboratory studies the innate and adaptive T cell and B cell host responses to chronic and emerging viruses. Dr. Haddad obtained his Ph.D. from McGill University and was trained at the National Institutes of Health and at University of Montreal. Dr. Haddad has identified novel mechanisms responsible for the generation and maintenance of memory T and memory B cells in homeostatic and during viral infection. Dr. Haddad research has led to the identification of novel negative regulators associated with the dysfunction of the innate and adaptive responses during viral infection. Currently, Dr. Haddad's research focuses on studying CD4+ differentiation programs in homeostatic and pathological conditions. In particular, Dr. Haddad is studying the biology of the interaction of T follicular helper cells with B cells in secondary lymphoid organs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breen EC. Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome. Pharmacol Ther. 2002;95:295–304. doi: 10.1016/s0163-7258(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 3.Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–259. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- 4.Herbeuval JP, Nilsson J, Boasso A, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 6.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes JD, Wietgrefe S, Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 8.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:e315–322. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 9.Iannello A, Boulassel MR, Samarani S, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 10.Titanji K, De Milito A., Cagigi A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 11.Van grevenynghe J, Cubas RA, Noto A, et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. The journal of Clinical Investigation. 2011 doi: 10.1172/JCI59211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Grevenynghe J, Procopio FA, He Z, et al. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med. 2008;14:266–274. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- 13.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 14.Elrefaei M, Burke CM, Baker CA, et al. HIV-specific TGF-beta-positive CD4+ T cells do not express regulatory surface markers and are regulated by CTLA-4. AIDS Res Hum Retroviruses. 2010;26:329–337. doi: 10.1089/aid.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porichis F, Kwon DS, Zupkosky J, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118:965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 18.Migliaccio M, Alves PM, Romero P, Rufer N. Distinct mechanisms control human naive and antigen-experienced CD8+ T lymphocyte proliferation. J Immunol. 2006;176:2173–2182. doi: 10.4049/jimmunol.176.4.2173. [DOI] [PubMed] [Google Scholar]
- 19.Riou C, Yassine-Diab B, Van grevenynghe J, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 21.Landires I, Bugault F, Lambotte O, et al. HIV infection perturbs IL-7 signaling at the step of STAT5 nuclear relocalization. Aids. 2011 doi: 10.1097/QAD.0b013e32834a3678. [DOI] [PubMed] [Google Scholar]
- 22.Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 25.Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 26.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 27.Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–297. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- 28.Kops GJ, Medema RH, Glassford J, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi J, Oh S, Lee D, et al. Mst1-FoxO signaling protects Naive T lymphocytes from cellular oxidative stress in mice. PLoS One. 2009;4:e8011. doi: 10.1371/journal.pone.0008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2008;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer EL, Oskoui PR, Banko MR, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 34.Birkenkamp KU, Coffer PJ. FOXO transcription factors as regulators of immune homeostasis: molecules to die for? J Immunol. 2003;171:1623–1629. doi: 10.4049/jimmunol.171.4.1623. [DOI] [PubMed] [Google Scholar]
- 35.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 36.Poonia B, Salvato MS, Yagita H, Maeda T, Okumura K, Pauza CD. Treatment with anti-FasL antibody preserves memory lymphocytes and virus-specific cellular immunity in macaques challenged with simian immunodeficiency virus. Blood. 2009;114:1196–1204. doi: 10.1182/blood-2009-02-202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. doi: 10.1038/nri1087. [DOI] [PubMed] [Google Scholar]
- 38.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T. FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood. 2008;112:4485–4493. doi: 10.1182/blood-2008-05-159848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Moller C, Alfredsson J, Engstrom M, et al. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood. 2005;106:1330–1336. doi: 10.1182/blood-2004-12-4792. [DOI] [PubMed] [Google Scholar]
- 42.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Mandal M, Crusio KM, Meng F, et al. Regulation of lymphocyte progenitor survival by the proapoptotic activities of Bim and Bid. Proc Natl Acad Sci U S A. 2008;105:20840–20845. doi: 10.1073/pnas.0807557106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakker WJ, Blazquez-Domingo M, Kolbus A, et al. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004;164:175–184. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, et al. Differential regulation of Foxo3a target genes in erythropoiesis. Mol Cell Biol. 2007;27:3839–3854. doi: 10.1128/MCB.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmud DL, M GA, Deb DK, Platanias LC, Uddin S, Wickrema A. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene. 2002;21:1556–1562. doi: 10.1038/sj.onc.1205230. [DOI] [PubMed] [Google Scholar]
- 47.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Mol Endocrinol. 2007;21:2503–2515. doi: 10.1210/me.2006-0498. [DOI] [PubMed] [Google Scholar]
- 48.Duy C, Yu JJ, Nahar R, et al. BCL6 is critical for the development of a diverse primary B cell repertoire. J Exp Med. 2010;207:1209–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herzog S, Hug E, Meixlsperger S, et al. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol. 2008;9:623–631. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- 50.Harada Y, Harada Y, Elly C, et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207:1347–1350. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dejean AS, Beisner DR, Ch'en IL, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huntington ND, Puthalakath H, Gunn P, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu MC, Lee DF, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 55.Kornblau SM, Singh N, Qiu Y, Chen W, Zhang N, Coombes KR. Highly phosphorylated FOXO3A is an adverse prognostic factor in acute myeloid leukemia. Clin Cancer Res. 2010;16:1865–1874. doi: 10.1158/1078-0432.CCR-09-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007;98:1949–1958. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong W, He L, Coppola M, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869–17879. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Sakoe Y, Sakoe K, Kirito K, Ozawa K, Komatsu N. FOXO3A as a key molecule for all-trans retinoic acid-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia. Blood. 2010;115:3787–3795. doi: 10.1182/blood-2009-05-222976. [DOI] [PubMed] [Google Scholar]
- 60.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 61.Wu W, Zou M, Brickley DR, Pew T, Conzen SD. Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol Endocrinol. 2006;20:2304–2314. doi: 10.1210/me.2006-0131. [DOI] [PubMed] [Google Scholar]
- 62.Essafi M, Baudot AD, Mouska X, Cassuto JP, Ticchioni M, Deckert M. Cell-penetrating TAT-FOXO3 fusion proteins induce apoptotic cell death in leukemic cells. Mol Cancer Ther. 2011;10:37–46. doi: 10.1158/1535-7163.MCT-10-0482. [DOI] [PubMed] [Google Scholar]
- 63.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 64.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 65.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Li D, Qu Y, Mao M, et al. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:1903–1913. doi: 10.1038/jcbfm.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: new paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin W, Zhao W, Ho L, et al. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer's disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Relling DP, Esberg LB, Fang CX, et al. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens. 2006;24:549–561. doi: 10.1097/01.hjh.0000203846.34314.94. [DOI] [PubMed] [Google Scholar]
- 70.Su B, Liu H, Wang X, et al. Ectopic localization of FOXO3a protein in Lewy bodies in Lewy body dementia and Parkinson's disease. Mol Neurodegener. 2009;4:32. doi: 10.1186/1750-1326-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cicala C, Arthos J, Rubbert A, et al. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc Natl Acad Sci U S A. 2000;97:1178–1183. doi: 10.1073/pnas.97.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacotot E, Ravagnan L, Loeffler M, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selliah N, Finkel TH. Biochemical mechanisms of HIV induced T cell apoptosis. Cell Death Differ. 2001;8:127–136. doi: 10.1038/sj.cdd.4400822. [DOI] [PubMed] [Google Scholar]
- 74.Dabrowska A, Kim N, Aldovini A. Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes. J Immunol. 2008;181:8460–8477. doi: 10.4049/jimmunol.181.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim N, Kukkonen S, Gupta S, Aldovini A. Association of Tat with promoters of PTEN and PP2A subunits is key to transcriptional activation of apoptotic pathways in HIV-infected CD4+ T cells. PLoS Pathog. 2010;6:e1001103. doi: 10.1371/journal.ppat.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients. Diabetes. 2005;54:23–31. doi: 10.2337/diabetes.54.1.23. [DOI] [PubMed] [Google Scholar]
- 77.Fichtenbaum CJ. Metabolic abnormalities associated with HIV infection and antiretroviral therapy. Curr Infect Dis Rep. 2009;11:84–92. doi: 10.1007/s11908-009-0012-8. [DOI] [PubMed] [Google Scholar]
- 78.Wilk A, Urbanska K, Yang S, et al. Insulin-like growth factor-I-forkhead box O transcription factor 3a counteracts high glucose/tumor necrosis factor-alpha-mediated neuronal damage: implications for human immunodeficiency virus encephalitis. J Neurosci Res. 2011;89:183–198. doi: 10.1002/jnr.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noursadeghi M, Tsang J, Miller RF, Katz DR. Comment on “Transcription factor FOXO3a mediates apoptosis in HIV-1-infected macrophages”. J Immunol. 2008;180:7783. doi: 10.4049/jimmunol.180.12.7783. author reply 7783-7784. [DOI] [PubMed] [Google Scholar]
- 80.Cui M, Huang Y, Zhao Y, Zheng J. Transcription factor FOXO3a mediates apoptosis in HIV-1-infected macrophages. J Immunol. 2008;180:898–906. doi: 10.4049/jimmunol.180.2.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y, Erdmann N, Peng H, et al. TRAIL-mediated apoptosis in HIV-1-infected macrophages is dependent on the inhibition of Akt-1 phosphorylation. J Immunol. 2006;177:2304–2313. doi: 10.4049/jimmunol.177.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Grevenynghe J, Halwani R, Chomont N, et al. Lymph node architecture collapse and consequent modulation of FOXO3a pathway on memory T- and B-cells during HIV infection. Semin Immunol. 2008;20:196–203. doi: 10.1016/j.smim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Younes SA, Yassine-Diab B, Dumont AR, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cerutti A. HIV infection: TRAILing the killers. Blood. 2009;114:3723–3724. doi: 10.1182/blood-2009-09-239889. [DOI] [PubMed] [Google Scholar]
- 85.Herbeuval JP, Boasso A, Grivel JC, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105:2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]
- 86.Kaplan D, Sieg S. Role of the Fas/Fas ligand apoptotic pathway in human immunodeficiency virus type 1 disease. J Virol. 1998;72:6279–6282. doi: 10.1128/jvi.72.8.6279-6282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poonia B, Pauza CD, Salvato MS. Role of the Fas/FasL pathway in HIV or SIV disease. Retrovirology. 2009;6:91. doi: 10.1186/1742-4690-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stary G, Klein I, Kohlhofer S, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 89.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Bello G, Velasco-de-Castro CA, Bongertz V, et al. Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. J Med Virol. 2009;81:1681–1690. doi: 10.1002/jmv.21565. [DOI] [PubMed] [Google Scholar]
- 91.Fonseca SG, Procopio FA, Goulet JP, Yassine-Diab B, Ancuta P, Sekaly RP. Unique features of memory T cells in HIV elite controllers: a systems biology perspective. Curr Opin HIV AIDS. 2011;6:188–196. doi: 10.1097/COH.0b013e32834589a1. [DOI] [PubMed] [Google Scholar]
- 92.Nakayama K, Nakamura H, Koga M, et al. Imbalanced Production of Cytokines by T Cells Associates with the Activation/Exhaustion Status of Memory T Cells in Chronic HIV Type 1 Infection. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0073. [DOI] [PubMed] [Google Scholar]
- 93.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamamoto T, Price DA, Casazza JP, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 2011;117:4805–4815. doi: 10.1182/blood-2010-11-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]