Abstract
MicroRNAs (miRNAs), acting as oncogenes or tumor suppressors in humans, play a key role in regulating gene expression and are believed to be important for developing novel therapeutic treatments and clinical prognoses. Due to their short lengths (17–25 nucleotides) and extremely low concentrations (typically < pM) in biological samples, quantification of miRNAs has been challenging to conventional biochemical methods, such as Northern blotting, microarray, and quantitative polymerase chain reaction (qPCR). In this work, a biotinylated miRNA (biotin-miRNA) whose sequence is the same as that of a miRNA target is introduced into samples of interest and allowed to compete with the miRNA target for the oligonucleotide (ODN) probe preimmobilized onto an electrode. Voltammetric quantification of the miRNA target was accomplished after complexation of the biotin-miRNA with ferrocene (Fc)-capped gold nanoparticle/streptavidin conjugates. The Fc oxidation current was found to be inversely proportional to the concentration of target miRNA between 10 fM and 2.0 pM. The method is highly reproducible (RSD < 5%), regenerable (at least 8 regeneration/assay cycles without discernible signal decrease) and selective (with sequence specificity down to a single nucleotide mismatch). The low detection levels (10 fM or 0.1 attomoles of miRNA in a 10-HL solution) allow the direct quantification of miRNA-182, a marker correlated to the progression of glioma in patients, to be performed in serum samples without sample pretreatment and RNA extraction and enrichment. The concentration of miRNA-182 in glioma patients was found to be 3.1 times as high as that in healthy persons, a conclusion in excellent agreement with a separate qPCR measurement of the expression level. The obviations of the requirement of an internal reference in qPCR, simplicity, and cost-effectiveness are other additional advantages of this method for detection of nucleic acids in clinical samples.
INTRODUCTION
MicroRNAs (miRNAs) constitute a family of small non-protein coding RNAs (typically 17–25 nucleotides long) that serve as a regulator of gene expression.1,2 As oncogenes or tumor suppressors, miRNAs have been closely related to a variety of human cancers3,4 and their expression levels may provide useful information for clinical diagnosis as well as drug development.5,6 MiRNAs are characterized by their short lengths and high sequence similarity, which impose difficulty to hybridization-based detection methods.5,7,8 Northern blotting, arguably the most frequently used method for miRNA assay, provides information about the size and expression level of miRNAs.9,10 However, Northern blotting with radioactive labels is time- and sample-consuming and semi-quantitative at best. Without sample enrichment, its sensitivity (~ nM)9 is not sufficient for the miRNA assay in many biological samples (e.g., circulating miRNAs are present at femtomolar or even lower levels in blood11). Real time or quantitative PCR (qPCR) is a remedy for low sensitivity, as small amounts of miRNA in samples can be significantly amplified. However, the approach generally requires extraction of RNA from real samples, purification, and reverse transcription to cDNA prior to the amplification step.12,13 Furthermore, the expression of miRNA determined by qPCR is relative to that of an internal nucleic acid reference that does not undergo post-translational modification or remains at a constant concentration. Fluorescent microarray-based analysis has the capability of simultaneously determining multiple miRNAs,14,15 but is frequently subject to poor reproducibility and inaccuracy resulted from cross hybridization and non-specific adsorption.14 Although its sensitivity has been improved by the use of locked nucleic acid (LNA) probes, owing to the costs in chip manufacturing and availability of specialized read-out instruments, microarray analyses might be overly complex for the cases wherein only the concentration (or expression level) of a specific target miRNA is measured. Such cases are particularly relevant in point-of-care testing.
A great deal of effort, therefore, has been devoted to developing analytical methods for miRNA analysis with higher sensitivity and better reliability. For example, the expression levels of miRNA in lung cancer cells have been determined using fluorescent metal nanoshell probes.16 By fluorescence amplification via a cation exchange reaction between ionic crystals of CdSe and silver ions, miRNA targets from healthy breast tissues and diseased cells have also been successfully identified.17 Very recently, magnetic fluorescent nanoparticle-based molecular beacon has been constructed for monitoring intracellular miRNA during neuronal differentiation.18 However, this method has certain limitations, such as photobleaching and blinking.19 In addition, the strong fluorescence background from biological samples could affect the assay sensitivity. The bioluminescence-based method for miRNA detection offers certain advantages, such as no requirement for external excitation light and high signal-to-noise ratio.20 However, labeling of miRNA with Renilla luciferase specific to the bioluminescence reaction was required. Surface plasmon resonance (SPR) is an optical technique that has been widely utilized for label-free and real-time monitoring of biomolecular interactions.21–25 With imaging SPR, three miRNA sequences in a sample were determined with a detection limit of 10 fM.26 Though appealing for multiplexed miRNA detection, the procedure includes several steps involving labeling the miRNA targets with the poly-A polymerase and signal amplification with poly-T-tagged gold nanoparticles. Using an antibody specific to duplexes formed between a DNA probe and a miRNA target for signal amplification, Homola and coworkers recently achieved a detection level of 2 pM.27 The method did not require the use of LNAs for the probe and yields results comparable to qPCR assays. Gao’s group was the first to develop electrochemically or electronically based methods for miRNA analysis.28–31 A detection limit of 80 fM was achieved using electrocatalytic OsO2 nanoparticles tagged onto miRNA.28 A nanogap microelectrode-based biosensor array was also constructed by the same group for target miRNA quantification.29 Though sensitive, the abovementioned approaches tend to be complicated or require expensive reagents for signal amplification. For early detection of miRNAs, an innovative electrochemical approach based on the oxidation of guanine during the hybrid formation has also been reported.32
Several groups have reported sensitive detection of DNA by using Fc derivatives.33–37 We have performed voltammetric detection of oligonucleotide (ODN) targets via hybridization with ODN probes followed by the attachment of Fc-capped gold nanoparticle/streptavidin conjugates.38 A detection limit of 2.0 pM was achieved due to the signal amplification by the large number of Fc moieties on the conjugates. However, such a detection limit is still insufficient for reliable miRNA assays since miRNAs are present at concentrations ranging between sub-pM and fM levels. Moreover, our previous study did not explore the possibility of regenerating the electrode surface for continuous assays of real samples at a single electrode. For new detection schemes to become clinically relevant, they must be validated with data from analyses of many real samples by a separate method. In this work, by introducing into sample solutions a biotinylated, short-stranded RNA whose sequence is identical to that of a specific miRNA target, we show that the amplified voltammetric signal is inversely proportional to the target miRNA concentration in a range that is typical of miRNA in biological milieu. We also demonstrate that the resultant competitive hybridization possesses greater sequence specificity and a remarkable detection limit (down to 10 fM). The drastically decreased detection limit enabled us to carry out direct quantification of a miRNA target without the need of an internal reference in untreated sera of glioma patients and to assess the expression of this miRNA with respect to that from healthy persons.
EXPERIMENTAL SECTION
Chemicals and Materials
Potassium perchlorate, EDTA, KH2PO4, K2HPO4, tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl), 1-hexanethiol (HT), gold nanoparticle/streptavidin conjugate, 6-ferrocenyl-1-hexanethiol, and bovine serum album (BSA) were acquired from Sigma-Aldrich (St. Louis, MO). The oligonucleotide (ODN) probe with a sequence of 5′-HS (CH2)6-TTT TTA GTG TGA GTT CTA CCA TTG CCA AA-3′ whose 5′ end was modified with a hexylthiol group was purchased from Sangon Co., Ltd. (Shanghai, China). The target miRNA and its variant whose 5′ end is modified with a biotin tag were obtained from GenePharma Co., Ltd. (Shanghai, China). The biotinylated miRNA (biotin-miRNA) has a sequence of 5′-biotin-UUU GGC AAU GGU AGA ACU CAC ACU-3′. Target miRNAs used to hybridize with the ODN probe have the following sequences (mismatching sequences underlined): 5′-UUU GGC AAU GGU AGA ACU CAC ACU-3′ (fully complementary), 5′-UUU GGC AAU GGU CGA ACU CAC ACU-3′ (one base mismatch), 5′-GUU GGC AAU AGU AGA AAU CAC AAU-3′ (four-base mismatch), and 5′-GCG UAU GGC AAC CUC CAG AGA GAC-3′ (noncomplementary). The preparation and characterization of ferrocene (Fc)-capped gold nanoparticle/streptavidin conjugates have been reported by us previously.38 All miRNA stock solutions were prepared daily with diethylpyrocarbonate-treated water in a RNase-free environment, similar to an earlier report.39 Unless otherwise stated, all hybridization reactions were carried out at room temperature.
Serum samples were obtained from cancer patients (confirmed by pathological examinations) at Xiangya Hospital (Hunan, China). Signed informed consent was obtained from each patient participating in the study before surgery. All of the protocols were reviewed by the Joint Ethics Committee of the Central South University Health Authority and performed in accordance with national guidelines. Blood samples (10 mL) were collected from patients directly into serum collection tubes. The whole blood was allowed to stand for about 1 h before being centrifuged at 1000 g for 10 min at room temperature. The resultant serum was aliquoted into eppendorf tubes and stored at −80°C.
Procedures
Electrodes
Gold working electrodes with a diameter of 2 mm (CH Instruments, Austin, TX) were polished with alumina slurry down to 0.3 µm on a polishing cloth (Buehler, Lake Bluff, IL), followed by sonication in ethanol and water. A platinum wire and a Ag/AgCl electrode were used as the auxiliary and reference electrodes, respectively.
Immobilization of Thiolated ODN
Immobilization of the thiolated ODN probe onto Au electrodes was carried out by covering electrodes with 10 µL of TE (10 mM Tris-HCl and 1.0 mM EDTA) comprising 1.0 µM ODN overnight. The amount of probe for immobilization was chosen according to the well-established protocol reported by Steel et al.40 The immobilization step was followed by washing the electrode surface thoroughly with water and drying with nitrogen. The ODN-covered electrodes were then treated with 0.1 mM HT for 5 min and 1% BSA for 1 h.
Competitive Hybridization Followed by Attachment of the Nanoconjugates
The biotin-miRNA and target miRNAs were prepared in TNE buffer (TE + 0.1 M NaCl). The competitive hybridization between the biotin-miRNA and the target miRNA for the pre-immobilized ODN probe was performed either at room temperature or an elevated temperature (60 °C). For room-temperature hybridization, a 10-µL mixture comprising the biotin-miRNA of a fixed concentration and the miRNA target of varying concentrations was cast onto the ODN-covered electrode. The competitive hybridization was carried out for 4.5 h at room temperature. For hybridization performed at 60 °C, the ODN-covered electrode was inserted into the 100-µL solution in a centrifuge tube that was immersed in a 60 °C water bath. The electrode was then thoroughly rinsed with TNE buffer and water, and exposed to the Fc-capped gold nanoparticle/streptavidin conjugates for 1 h.
Electrochemical Characterization
Once the Fc-capped gold nanoparticle/streptavidin conjugate had been attached onto the electrode surface, the electrochemical detection was performed in 0.1 M KClO4 with a CHI 660 electrochemical workstation (CH Instruments, Austin, TX) via scanning the electrode potential within the range of 0.0–0.6 V.
Regeneration of the Electrode Surface
After each assay, the electrode surface can be regenerated with a mixture of 70% ethanol and 0.1 M NaOH for 5 min (i.e., desorbing the biotinylated miRNA, target miRNA and the Fc-capped gold nanoparticle/streptavidin conjugates). The ODN-covered electrodes were then immersed in a 1% BSA solution for 1 h. The hybridization between 5.0 nM biotin-miRNA or a mixture of 5.0 nM biotin-miRNA and an untagged target miRNA with the pre-immobilized ODN probe was carried out at room temperature for 0.5 h. After thoroughly rinsing with TNE buffer and water, the electrode was exposed to the Fc-capped gold nanoparticle/streptavidin conjugates for 20 min.
Quantitative Polymerase Chain Reaction (qPCR)
All qPCR experiments were performed on an IQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad, Irvine, CA). Total RNAs were extracted from glioma tissues with the TRIZOL® reagent (Invitrogen, Wuhan, China). Real time fluorescent detection was performed with SYBR-green-containing PCR kit (GenePharma, Shanghai, China). The primers for qPCR to detect miRNA were designed based on the miRNA sequences provided by the Sanger Center miRNA Registry and were synthesized and purified by Shanghai Gene-Pharma (Shanghai, China). Human U6 small nuclear (sn)-RNA was used for normalization of the expression level. The forward and reverse primers for PCR amplification of miRNA-182 are 5'-ACTTTTGGCAATGGTAGAACTCAC-3' and 5'-AATCCATGAGAGATCCCTAGCG-3', respectively, whereas those for U6 snRNA are 5'-ATTGGAACGATACAGAGAAGATT-3' and 5'-GGAACGCTTCACGAATTT G-3', respectively.
RESULTS AND DISCUSSION
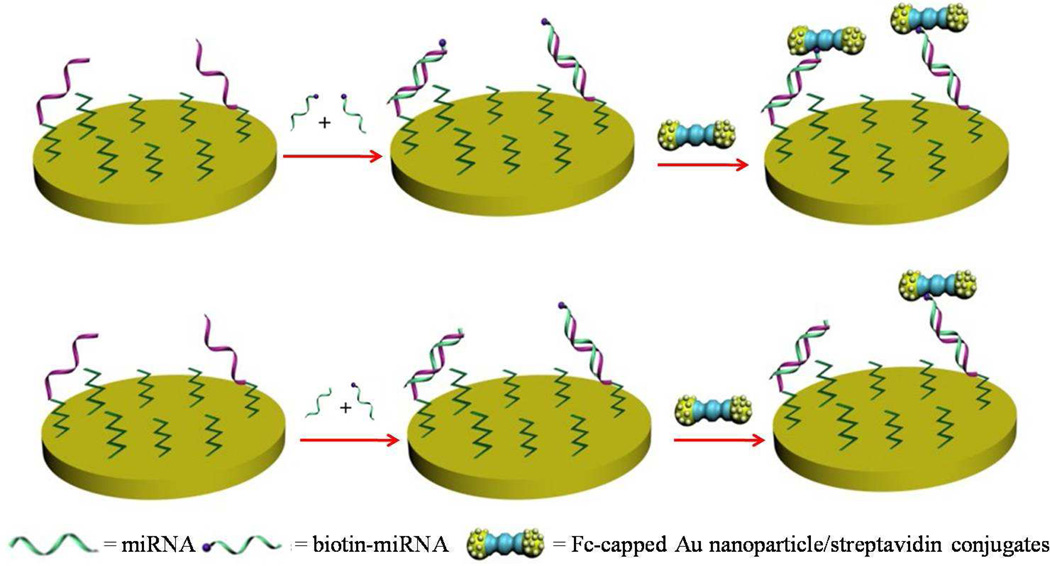
In Figure 1, formation of a mixed self-assembled monolayer (SAM) of thiolated ODN and HT helps tether the ODN probe onto the electrode for enhanced hybridization efficiency with the miRNA target.40–42 Because our goal was to detect miRNAs present in complex biological matrices, the electrode was further blocked with BSA to ensure that little nonspecific adsorption of other species from samples would occur prior to the voltammetric detection. In samples that do not contain the same sequence of the externally introduced biotinylated miRNA (biotin-miRNA), its hybridization with the ODN probe leads to subsequent attachment of a large number of Fc-capped gold nanoparticle/streptavidin conjugates, resulting in a large voltammetric signal (top scheme in Figure 1). For samples containing the miRNA of interest, competitive hybridization leads to a decrease of the biotinylated miRNA molecules at the electrode (bottom scheme), which in turn decreases the number of Fc-capped gold nanoparticle/streptavidin conjugates and renders a voltammetric signal smaller than that in the top scheme. Note that each gold nanoparticle was decorated with a large number of Fc tags (about 127 ± 10 tags per nanoparticle),38 the voltammetric signal in the absence of a specific miRNA target is thus significantly amplified. With the competitive hybridization, however, the peak currents are attenuated and become inversely proportional to the target miRNA concentration.
Figure 1.
Schematic representation of the miRNA detection. The absence of a miRNA with the same sequence as that of the externally added biotin-miRNA leads to more Fc-capped gold nanoparticle/streptavidin conjugates attached to the electrode and a large voltammetric signal (top). In the presence of the miRNA, a smaller number of the conjugates are attached to the electrode due to the competitive hybridization reaction and consequently a lower voltammetric signal is produced (bottom).
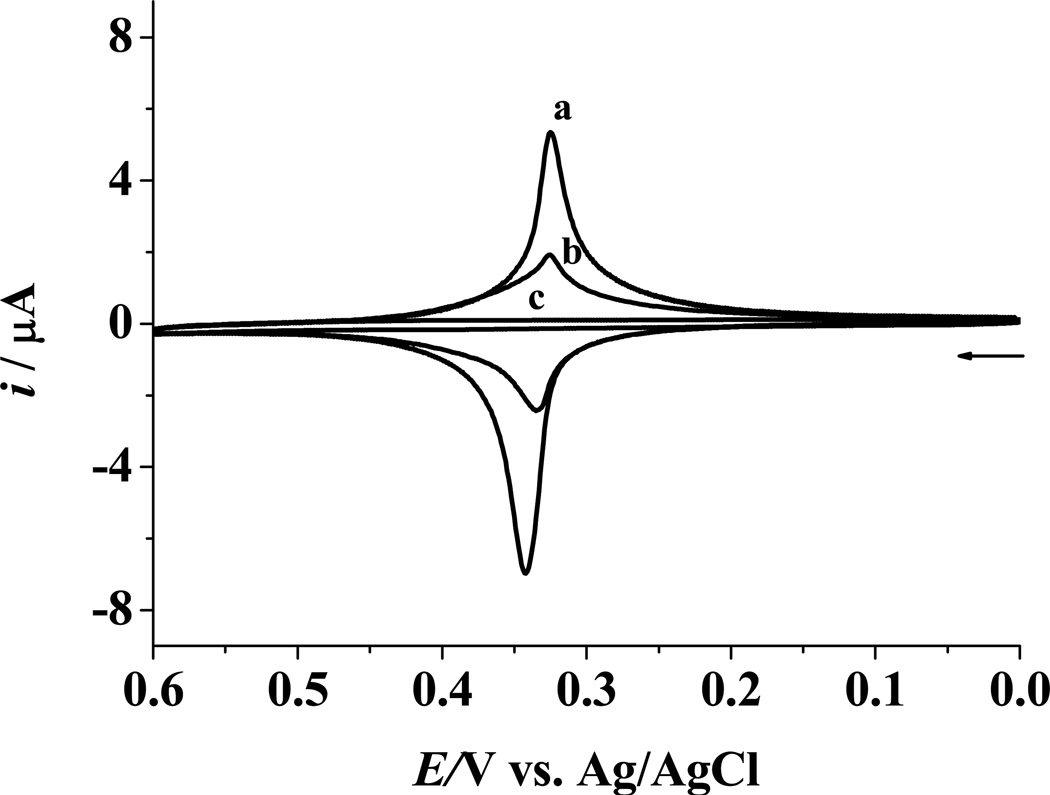
Figure 2a shows the voltammetric response acquired at an electrode after hybridization of the ODN probe with a biotin-miRNA and the follow-up attachment of the Fc-capped gold nanoparticle/streptavidin conjugates. A pair of well-defined redox waves was observed with the anodic and cathodic peak potentials at 0.342 and 0.323 V, respectively. In the presence of a target miRNA, the competitive hybridization results in a much attenuated peak current (curve b). For the experiment wherein biotin-miRNA was not introduced to the miRNA sample (curve c), no peaks were observed. This experiment indicates that formation of the DNA/miRNA duplex without a biotin label does not allow the Fc-capped Au nanoparticle/streptavidin conjugates to adsorb onto the electrode. Therefore, blockage of the mixed SAM surface with BSA is effective in eliminating nonspecific adsorption of the conjugates.
Figure 2.
Cyclic voltammograms (CVs) acquired at mixed SAMs of ODN probe/HT after hybridization with 10 nM biotin-miRNA (a), 10 nM biotin-miRNA + 10 nM target miRNA (b), or 10 nM target miRNA (c) in a TNE buffer at room temperature. All the hybridization reactions are followed by the attachment of Fc-capped gold nanoparticle/streptavidin conjugates. The scan rate was 0.1 V/s and the arrow indicates the scan direction.
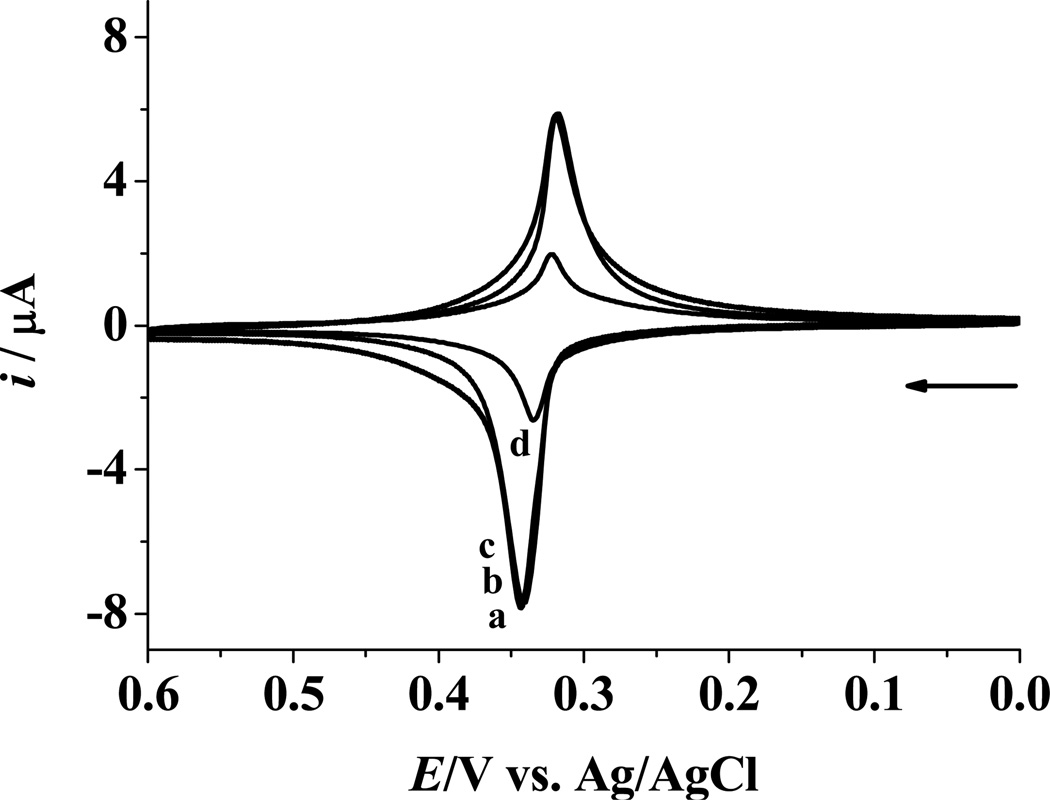
The selectivity of our method was assessed at an elevated temperature (Figure 3). For the mixture comprising the biotin-miRNA and a miRNA target with either a four-base (curve b) or a single (A–U) mismatch (curve c), the voltammetric peak currents are nearly identical to that of a solution containing the biotin-miRNA and a totally noncomplementary target miRNA (curve a). However, the anodic peak current (ipa) from the mixture of the biotin-miRNA and the complementary target miRNA (curve d) is 31% of those of curves a–c, indicating that, at an elevated temperature, only the complementary target can compete with the biotin-miRNA for hybridization with the ODN probe. The same result was obtained when a G–C mismatch was used to replace the A–U mismatch. The above results demonstrate that our method is capable of performing assays of single nucleotide polymorphoism. Due to the short lengths of miRNAs (17–25 nucleotides) and the numerous miRNA species in human genome, sequence similarity is frequently encountered in the miRNA family. As a result, methods that are capable of performing assays of single nucleotide polymorphoism are advantageous for identifying and quantifying miRNAs. This is a particularly attractive figure of merit, since the high sequence similarity of miRNAs has been known to cause uncertainty in both miRNA identification and quantification.43,44 Even at room temperature, differentiation of a target of a single base mismatch from a four-base mismatch is possible, as their ipa values decreased by 16 and 4.7% relative to that of a completely noncomplementary target miRNA, respectively.
Figure 3.
CVs acquired at ODN probe/HT mixed SAMs after hybridization at 60 °C in a mixture of 10 nM biotin-miRNA whose sequence complements that of the ODN probe and 10 nM target miRNA of different sequences: (a) a noncomplement, (b) a four-base mismatch, (c) a single mismatch, and (d) a full complement. All the hybridization reactions are followed by the attachment of the Fc-capped Au nanoparticle/streptavidin conjugates and the voltammetric detection was performed using the same experimental conditions as those in Figure 2.
We also examined the optimal biotin-miRNA concentration needed for an effective competitive hybridization reaction to achieve the highest sensitivity. We observed that the peak current increases with concentration of the biotin-miRNA between 0.1 pM and 50 nM. Beyond 10 nM, the peak currents begin to level off and the current-conentration relationship can be fitted with the Langmuir isotherm (data not shown), indicating that the electrode surface has been saturated with the biotin-miRNA molecules. Fixing the biotin-miRNA concentration to 10 nM, we can detect the miRNA concentration from 0.1 pM to 10 nM with a linear range of 0.1–10 pM. In this work, because of the extremely low concentration of miRNA in serum, we used 5.0 nM biotin-miRNA to obtain a lower detection limit (10 fM). Such a biotin-miRNA concentration produced the current value that was right in the middle of the Langmuir isotherm. Below 5.0 nM, the oxidation peak currents are much more attenuated and were not found to lead to a significant decrease of the detection limit.
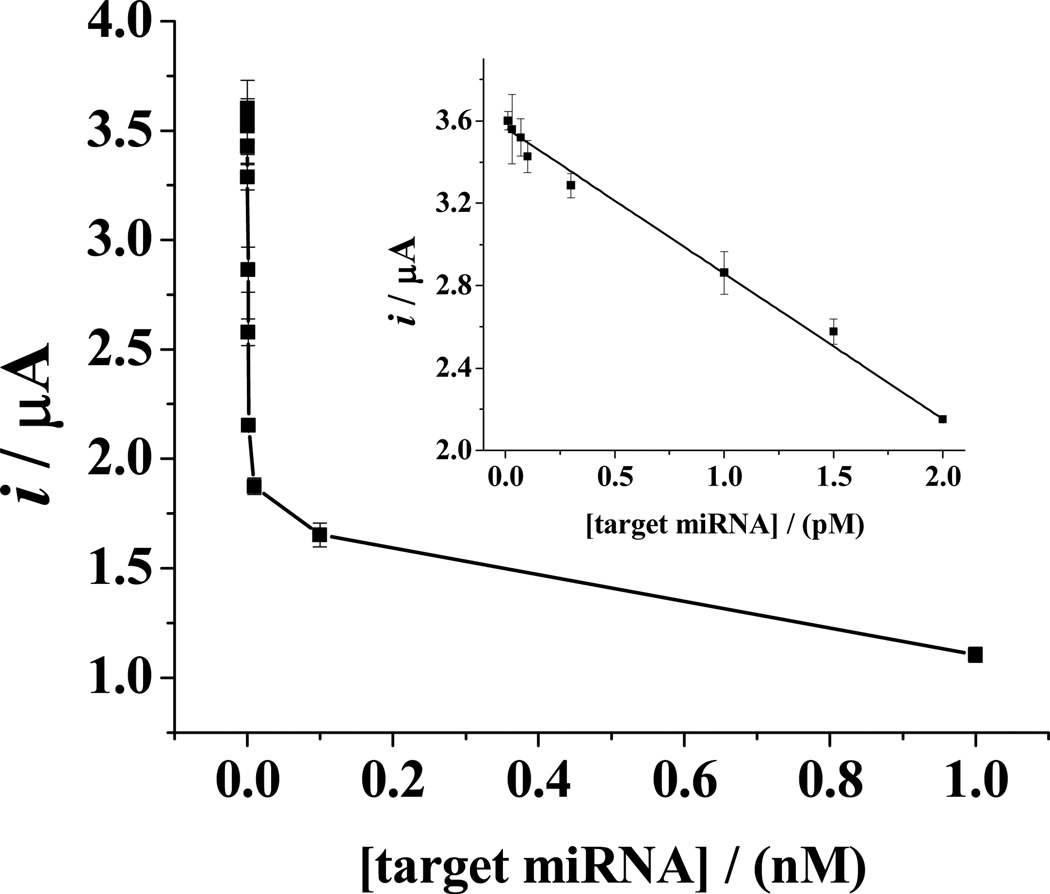
Figure 4 depicts the dependence of the ipa values on the concentration of the target miRNA. The inset is the linear portion of the calibration curve between 10 fM and 2.0 pM. The linear regression equation can be expressed as i(µA) = −0.706 [miRNA] (pM) + 3.57 (R2 =0.997). Our results are highly reproducible, as the RSD values from three replicate measurements are all below 5% for all the concentrations determined. The detection level of 10 fM is highly comparable with those achievable by nanoparticle-amplified SPR imaging26 and nanogap electrode-based biosensor array.29 However, our method is simpler and does not require sophisticated devices or instruments. It also obviates the use of expensive bioreagents (e.g., enzymes or peptide nucleic acids for signal amplification).26,29,39,45,46 Moreover, the detection level of our method is much lower than those achieved by conventional or modified biochemical techniques, such as fluorescent correlation spectroscopy,47 quantum dot-based miRNA profiling microarray assay,48 and Northern blotting assay.9,10
Figure 4.
Dependence of anodic peak currents on the concentrations of target miRNA. The absolute errors deduced from three replicate measurements are shown as the error bars. The inset shows the linear portion of the curve between 10 fM and 2.0 pM.
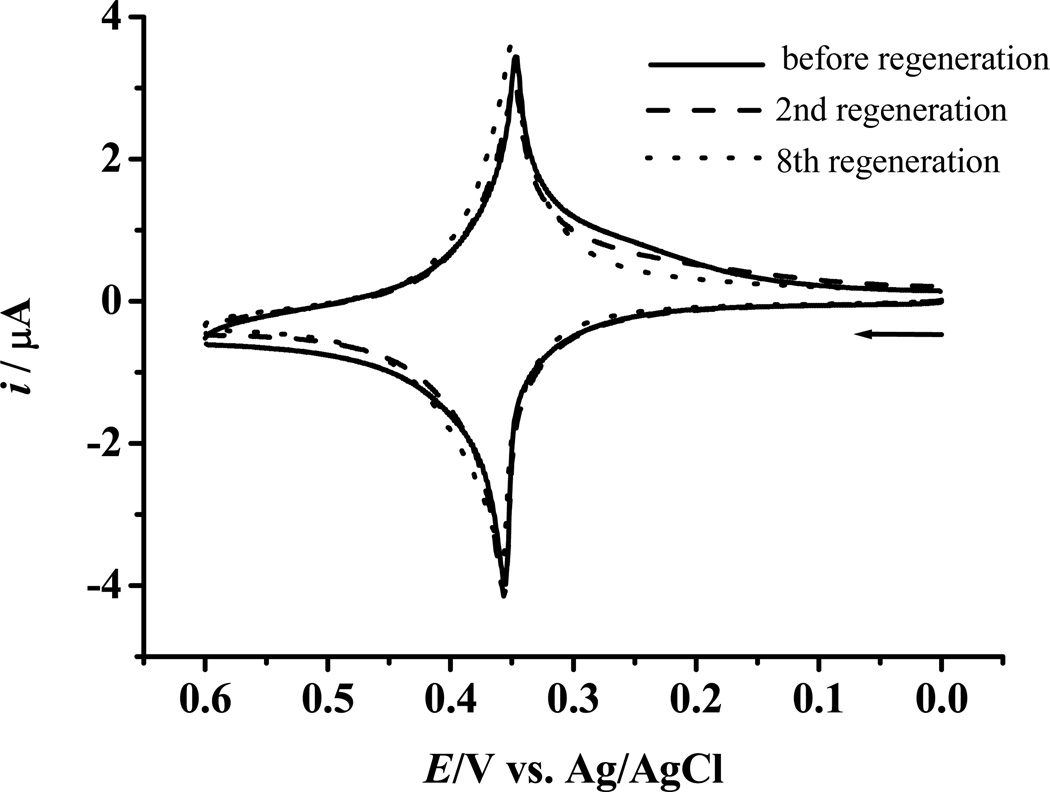
We found that the electrode surface can be conveniently regenerated by simply soaking the electrode in a mixture of 70% ethanol and 0.1 M NaOH to desorb the biotinylated miRNA and the Fc-capped gold nanoparticle/streptavidin conjugates. As can be seen from Figure 5, after 8 regeneration/assay cycles, the Fc redox peaks remain essentially unchanged. By conducting these regeneration/assay cycles at three different electrodes in parallel, we found that the %RSD ranges between 2.7–5.7%. These results suggest that repeated assays can be carried out at a single electrode for multiple samples, drastically increasing the sample throughput of our method and cutting down the cost and time for each assay. Since the surface regeneration is simple and mainly involves soaking an electrode in different solutions, multiple electrodes can be prepared concurrently for assays of many different samples. Such a feature also enhances the fidelity and repeatability of replicate sample measurements.
Figure 5.
CVs showing the regeneration of the electrode surface for subsequent miRNA assays. The regenerated surface was treated with 1% BSA and re-hybridized with 5.0 nM biotin-miRNA, followed by the attachment of Fc-capped gold nanoparticle/streptavidin conjugates. The solid, dashed, and dotted line curves correspond to CVs acquired at electrodes before regeneration and after 2nd and 8th regenerations, respectively. The RSD of 8 regenerations is 5.7%.
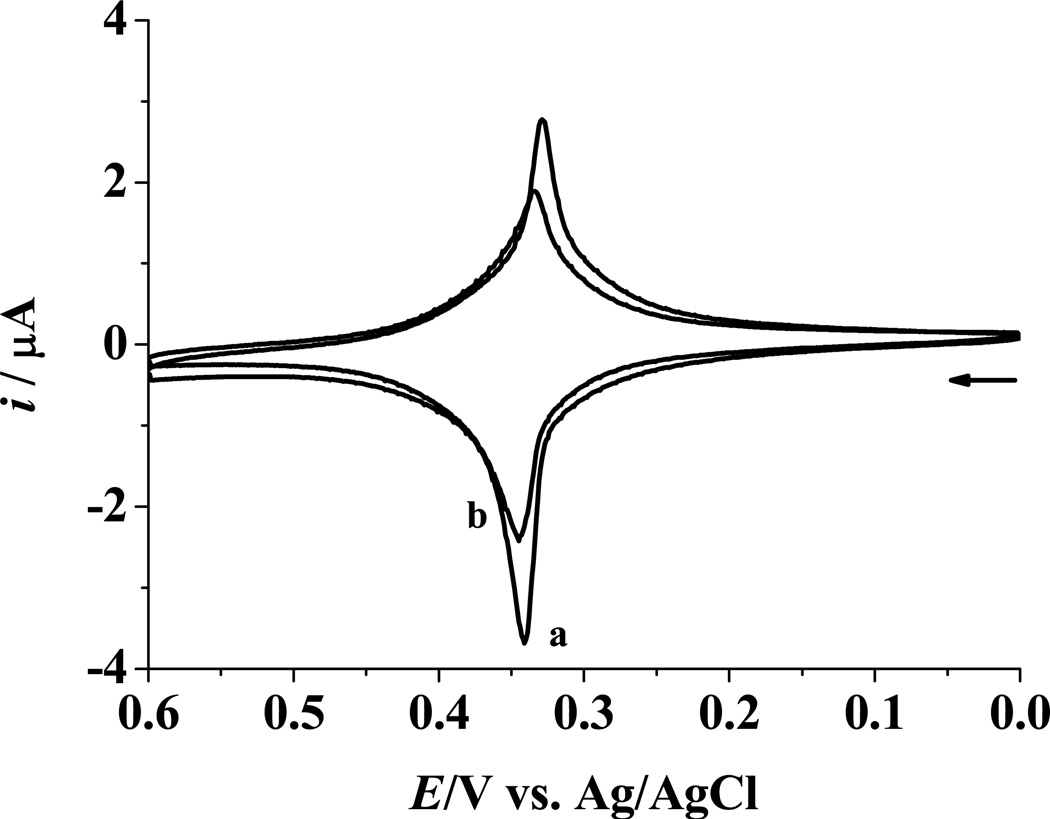
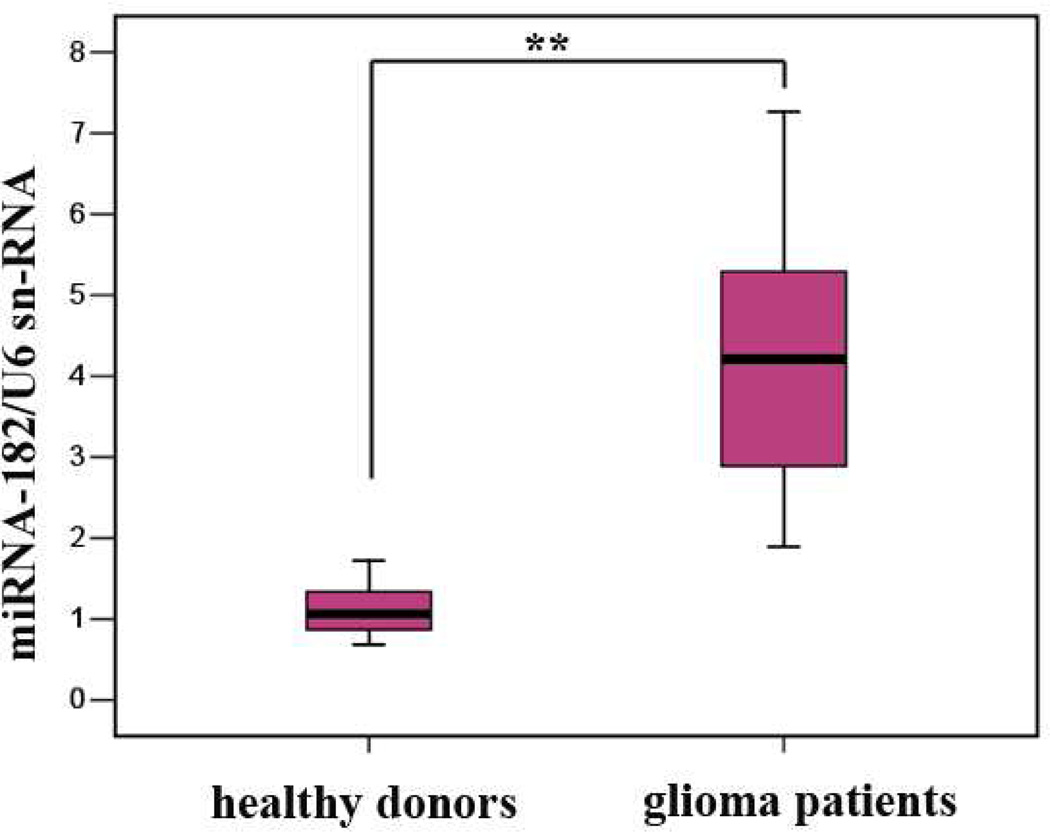
To demonstrate the amenability of our method to miRNA analysis in real samples, the expression levels of miRNA-182 in sera extracted from glioma patients and normal controls were determined. The association between miRNA-182 and gliomas has been clearly established,49 and the expression of miRNA-182 could be used as a prognostic marker of glioma progression and patient survival.50 In Figure 6, the peak current measured from serum sample of a glioma patient (curve b) is substantially lower than that from a healthy donor (curve a). Such a difference suggests that the expression level of miRNA-182 has been up-regulated in the glioma patient. To further demonstrate the clinical relevance of our method, we carried out assays of miRNA-182 in serum samples from multiple healthy donors and glioma patients. Three points are worth noting from Table 1. First, miRNA-182 in normal and glioma serum samples are at concentrations (low to sub-pM levels) not accessible by some of the developed methods. For example, without analyte enrichment, which is time- and sample-consuming, sub-pM concentrations are too low for Northern blotting to measure.10 Second, the average number of miRNA-182 copies per microliter of serum is well within the range of several miRNAs in sera from prostate cancer patients (103–106 copies/HL).11 Finally, the average miRNA-182 concentration in glioma patients is about 3.1 times as high as that in healthy donors. To verify our voltammetric results and conclusion, we performed a separate real time (RT)-PCR analysis of miRNA-182 in sera of both healthy donors and glioma patients (Figure 7). In the RT-PCR assay, the expression level of miRNA-182 was measured with respect to that of U6 small nuclear RNA (snRNA). Because U6 snRNA is the most highly conserved among the snRNAs involved in post-transcriptional modification, its concentration is considered to be invariant between glioma patients and healthy persons.51 From Figure 7, the expression levels of miRNA-182 pooled from 54 glioma patients and 22 healthy donors were estimated to be 4.21 ± 1.15 and 1.12 ± 0.27, respectively. Thus the miRNA-182 was elevated by about 3.7 times in glioma patients, in excellent agreement with our voltammetric assay. We should point out that, unlike in quantitative RT-PCR, the absolute quantities of miRNA are measured by our method. Thus, the need for an internal reference, which in many cases might not be available or reliable for nucleic acid research, can be circumvented. Furthermore, our method allows samples to be analyzed for their miRNAs without RNA extraction, reverse transcription, and concentration amplification, greatly simplifying the analytical procedure.
Figure 6.
CVs collected at electrodes after hybridization with 5.0 nM biotin-miRNA in the presence of serum samples from a healthy donor (a) and a glioma patient (b). Other experimental conditions are the same as those in Figure 2.
Table 1.
Voltammetric assays of serum samples for the miRNA-182 target
| Healthy donors (pM) | Patients (pM) | Healthy donors (copies/μL) |
Patients (copies/μL) |
|---|---|---|---|
| 0.67 ± 0.09 | 1.59 ± 0.09 | (0.41 ± 0.6)×106 | (0.99 ± 0.6)×106 |
| 0.55 ± 0.04 | 1.92 ± 0.07 | (0.34 ± 0.02)×106 | (1.20 ± 0.4)×106 |
| 0.35 ± 0.17 | 1.82 ± 0.08 | (0.22 ± 0.10)×106 | (1.13 ± 0.5)×106 |
| 0.76 ± 0.25 | 1.84 ± 0.02 | (0.48 ± 0.16)×106 | (1.14 ± 0.1)×106 |
| Average: | (0.36 ± 0.05)×106 | (1.12 ± 0.10)×106 | |
Figure 7.
Boxplot showing differential expressions of miRNA-182 in the sera of glioma patients and healthy donors by quantitative RT-PCR. Data are presented as mean ± SD. The RNA input was normalized to human U6 snRNA and the symbol “**” indicates that the p value from the Mann-Whitney test with respect to the normal donors is less than 0.01.
CONCLUSIONS
By adding into sample solutions a biotinylated short-stranded RNA (biotin-miRNA) whose sequence is the same as that of the miRNA of interest, competitive hybridization between the biotin-miRNA and the miRNA target for the ODN probe preimmobilized onto an electrode is initiated. The attachment of Fc-capped gold nanoparticle/streptavidin conjugates to biotin-miRNA produces an enhanced voltammetric signal. Owing to the competitive hybridization by miRNA that reduces the otherwise significantly elevated signals, trace levels of miRNA (as low as 10 fM or 0.1 attomole) can be determined with excellent reproducibility (RSD% < 5%). Moreover, our method obviates the need of miRNA sample extraction and enrichment as well as the use of complex devices and expensive reagents. In addition the electrode is regenerable and the assay does not require an internal reference in a sample for determining the level of expression. The remarkable detection level is much lower than those measurable by Northern blotting, fluorescent correlation spectroscopy and miRNA profiling microarray assay and compares well with those achieved by other optical and electrochemical sensors. Besides the reusability and high reproducibility, it is also more than two orders of magnitude lower than that we reported in the past for assays of much longer oligonucleotides.38 The method described herein was extended to the quantification of miRNA-182 present in serum samples from glioma patients. The elevated miRNA-182 concentrations with respect to those in healthy persons are consistent with the higher expression levels measured by quantitative RT-PCR. These features of our method should make it an attractive alternative for clinical assays of miRNA in glioma patients.
ACKNOWLEDGEMENT
Partial support of this work by the National Natural Science Foundation of China (Nos. 21175156 and 20975114 to JW and No. 81171932 to MW), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20100162110018 to JW), Program for New Century Excellent Talents in University (NCET-10-0796 to JW), Hunan Province Natural Sciences Foundation (11JJ1013 to MW), the NIH (SC1MS070155-01 to FZ), and an NSF grant (No. 1112105 to FZ) is gratefully acknowledged. FZ also acknowledges support from the NSF-CREST Program at California State University, Los Angeles (NSF HRD-0931421).
REFERENCES
- 1.Lee Y, Ahn C, Han JJ, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 2.Buchan JR, Parker R. Science. 2007;318:1877–1878. doi: 10.1126/science.1152623. [DOI] [PubMed] [Google Scholar]
- 3.Calin GA, Croce CM. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 4.Tricoli JV, Jacobson JW. Cancer Res. 2007;67:4553–4555. doi: 10.1158/0008-5472.CAN-07-0563. [DOI] [PubMed] [Google Scholar]
- 5.Cissell KA, Shrestha S, Deo SK. Anal.Chem. 2007;79:4754–4761. [Google Scholar]
- 6.Bartel DP. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Cissell KA, Deo SK. Anal. Bioanal. Chem. 2009;394:1109–1116. doi: 10.1007/s00216-009-2744-6. [DOI] [PubMed] [Google Scholar]
- 8.Driskell JD, Seto AG, Jones LP, Jokela S, Dluhy RA, Zhao YP, Tripp RA. Biosens. Bioelectron. 2008;24:917–922. doi: 10.1016/j.bios.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 9.Ramkissoon SH, Mainwaring LA, Sloand EM, Young NS, Kajigaya S. Mol. Cell. Probes. 2006;20:1–4. doi: 10.1016/j.mcp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Valoczi A, Hornyik C, Varga N, Burgyan J, Kauppinen S, Havelda Z. Nucleic Acids Res. . 2004;32:e175. doi: 10.1093/nar/gnh171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lao K, Xu NL, Yeung V, Chen C, Livak KJ, Straus NA. Biochem. Biophys. Res. Commun. 2006;343:85–89. doi: 10.1016/j.bbrc.2006.02.106. [DOI] [PubMed] [Google Scholar]
- 13.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson JM, Parker J, Perou CM, Hammond SM. Nat. Methods. 2004;1:47–53. doi: 10.1038/nmeth704. [DOI] [PubMed] [Google Scholar]
- 15.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Mol. Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Fu Y, Mei Y, Jiang F, Lakowicz JR. Anal. Chem. 2010;82:4464–4471. doi: 10.1021/ac100241f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JS, Schachermeyer S, Wang Y, Yin YD, Zhong WW. Anal. Chem. 2009;81:9723–9729. doi: 10.1021/ac901983s. [DOI] [PubMed] [Google Scholar]
- 18.Hwang DW, Song IC, Lee DS, Kim S. Small. 2010;6:81–88. doi: 10.1002/smll.200901262. [DOI] [PubMed] [Google Scholar]
- 19.Vogelsang J, Kasper R, Steinhauer C, Person B, Heilemann M, Sauer M, Tinnefeld P. Angew. Chem. Int. Ed. 2008;47:5465–5469. doi: 10.1002/anie.200801518. [DOI] [PubMed] [Google Scholar]
- 20.Cissell KA, Rahimi Y, Shrestha S, Hunt EA, Deo SK. Anal. Chem. 2008;80:2319–2325. doi: 10.1021/ac702577a. [DOI] [PubMed] [Google Scholar]
- 21.He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, Keating CD. J. Am. Chem. Soc. 2008;122:9071–9077. [Google Scholar]
- 22.Komolov KE, Senin II, Philippov PP, Koch KW. Anal. Chem. 2006;78:1228–1234. doi: 10.1021/ac051629t. [DOI] [PubMed] [Google Scholar]
- 23.Liang X, Nazarenus TJ, Stone JM. Biochemistry. 2008;47:3645–3653. doi: 10.1021/bi701431y. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Tian S, Tiefenauer L, Nielsen PE, Knoll W. Anal. Chem. 2005;77:2756–2761. doi: 10.1021/ac048088c. [DOI] [PubMed] [Google Scholar]
- 25.Lyon LA, Musick MD, Natan MJ. Anal. Chem. 1998;70:5177–5183. doi: 10.1021/ac9809940. [DOI] [PubMed] [Google Scholar]
- 26.Fang SP, Lee HJ, Wark AW, Corn RM. J. Am. Chem. Soc. 2006;128:14044–14046. doi: 10.1021/ja065223p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sipova H, Zhang S-L, Dudley AM, Galas D, Wang K, Homola J. Anal. Chem. 2010;82:10110–10115. doi: 10.1021/ac102131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao ZQ, Yang ZC. Anal. Chem. 2006;78:1470–1477. doi: 10.1021/ac051726m. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, Chen XT, Trigg AD, Tung CH, Kong JM, Gao ZQ. J. Am. Chem. Soc. 2007;129:5437–5443. doi: 10.1021/ja067477g. [DOI] [PubMed] [Google Scholar]
- 30.Gao ZQ, Yuan HY. Biosens. Bioelectron. 2007;22:933–940. doi: 10.1016/j.bios.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Gao ZQ, Yuan HY. Sens. Actuators B. 2007;121:552–559. [Google Scholar]
- 32.Lusi EA, Passamano M, Guarascio P, Scarpa A, Schiavo L. Anal. Chem. 2009;81:2819–2822. doi: 10.1021/ac8026788. [DOI] [PubMed] [Google Scholar]
- 33.Yang W, Lai RY. Electrochem. Commun. 2011;13:989–992. [Google Scholar]
- 34.Huang YC, Ge B, Sen D, Yu H-Z. J. Am. Chem. Soc. 2008;130:8023–8029. doi: 10.1021/ja8011066. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Lee JS, Kraatz H-B. Anal. Chem. 2006;78:6096–6101. doi: 10.1021/ac060533b. [DOI] [PubMed] [Google Scholar]
- 36.Fan C, Plaxco KW, Heeger AJ. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9134–9137. doi: 10.1073/pnas.1633515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu CJ, Wan Y, Yowanto H, Li J, Tao C, James MD, Tan CL, Blackburn GF, Meade TJ. J. Am. Chem. Soc. 2001;123:11155–11161. doi: 10.1021/ja010045f. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Li JH, Baca AJ, Hu JB, Zhou FM, Yan W, Pang DW. Anal. Chem. 2003;75:3941–3945. doi: 10.1021/ac0344079. [DOI] [PubMed] [Google Scholar]
- 39.Su X, Teh HF, Lieu XH, Gao ZQ. Anal. Chem. 2007;79:7192–7197. doi: 10.1021/ac0709403. [DOI] [PubMed] [Google Scholar]
- 40.Steel AB, Herne TM, Tarlov MJ. Anal. Chem. 1998;70:4670–4677. doi: 10.1021/ac980037q. [DOI] [PubMed] [Google Scholar]
- 41.Levicky R, Herne TM, Tarlov MJ, Satija SK. J. Am. Chem. Soc. 1998;120:9787–9792. [Google Scholar]
- 42.Satjapipat M, Sanedrin R, Zhou FM. Langmuir. 2001;17:7637–7644. [Google Scholar]
- 43.Wark AW, Lee HJ, Corn RM. Angew. Chem. Int. Ed. 2008;47:644–652. doi: 10.1002/anie.200702450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asadi-Moghaddam K, Chiocca EA, Lawler SE. Expert Rev. Anticancer Ther. 2010;10:1753–1762. doi: 10.1586/era.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou WJ, Chen YL, Corn RM. Anal. Chem. 2011;83:3897–3902. doi: 10.1021/ac200422u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang GJ, Chua JH, Chee RE, Agarwal A, Wong SM. Biosens. Bioelectron. 2009;24:2504–2508. doi: 10.1016/j.bios.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 47.Neely LA, Patel S, Garver J, Gallo M, Hackett M, McLaughlin S, Nadel M, Harris J, Gullans S, Rooke J. Nat. Methods. 2006;3:41–46. doi: 10.1038/nmeth825. [DOI] [PubMed] [Google Scholar]
- 48.Liang RQ, Li W, Li Y, Tan CY, Li JX, Jin YX, Ruan KC. Nucleic Acids Res. 2005;33:e17. doi: 10.1093/nar/gni019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavon I, Zrihan D, Granit A, Einstein O, Fainstein N, Cohen MA, Cohen MA, Zelikovitch B, Shoshan Y, Spektor S, Reubinoff BE, Felig Y, Gerlitz O, Ben-Hur T, Smith Y, Siegal T. Neuro-Oncology. 2010;12:422–433. doi: 10.1093/neuonc/nop061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang LL, Mao P, Song LB, Wu JH, Huang JT, Lin CY, Yuan J, Qu LH, Cheng SY, Li J. Am. J. Pathol. 2010;177:29–38. doi: 10.2353/ajpath.2010.090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frendewey D, Barta I, Gillespie M, Potashkin J. Nucleic Acids Res. 1990;18:2025–2032. doi: 10.1093/nar/18.8.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]