Abstract
Axon degeneration is a common hallmark of many neurodegenerative diseases. There is now an abundance of spontaneous and genetically engineered mice available to study the mechanisms of axonal degeneration and to screen for axonal protective agents. However, many of these mouse models exhibit slow progressive axonal loss which can span over many months. Consequently, there is a pressing need to accelerate the pace of axonal loss over a short interval for high-throughput screening of pharmacological and genetic therapies. Here, we present a novel technique using acrylamide, an axonal neurotoxin, to provoke rapid axonal degeneration in murine models of neuropathies. The progressive axonal loss which typically occurs over 8 months was reproduced within 7 to 10 days of the acrylamide intoxication. This approach was successfully applied to Myelin Associated Glycoprotein knockout (MAG−/−) mouse and Trembler-J mouse, a popular murine model of Charcot-Marie-Tooth disease type 1 (CMT-1). Acrylamide intoxication in transgenic mouse models offers a novel experimental approach to accelerate the rate of axonal loss over short intervals for timely in vivo studies of nerve degeneration. This report also provides for the first time an animal model for medication or toxin induced exacerbation of pre-existing neuropathies, a phenomenon widely reported in patients with neuropathies.
Keywords: Acrylamide, axonal degeneration, Charcot-Marie-Tooth, Animal Model
INTRODUCTION
Axonal degeneration is a major feature of many disorders of the nervous system, including peripheral neuropathy, spinal cord and head injury, stroke, and even primary demyelinating diseases such as multiple sclerosis and Charcot-Marie-Tooth (CMT). Axonal degeneration accounts for much of the irreversible clinical deficits seen in these disorders (Krajewski et al., 2000; Trapp et al., 1998). Intensive research has focused on molecules and signaling pathways that promote or inhibit axonal degeneration. Widely used genetically engineered mice now offer new tools for detailed studies of axonal degeneration and protection. A limitation in many of these murine models is the slow rate of axonal loss over many months. For certain in vivo studies such as screening of pharmacological and genetic therapies, there is a need for a practical and reliable technique to increase the rate of axonal degeneration so that these mice can be studied at a relative young age and over short intervals.
As early as the 1950s, researchers realized that industrial workers exposed to acrylamide developed peripheral neuropathy characterized by a stocking-and-glove distribution of sensory, motor, and autonomic deficits (Auld and Bedwell, 1967; Garland and Patterson, 1967). More recent studies showed that pre-existing peripheral neuropathy is a widely accepted risk factor for increased susceptibility to neurotoxic agents (Chaudhry et al., 2003). A well-known example of this phenomenon is the aggravation of CMT by chemotherapy. Vincristine, a widely used vinca alkaloid employed as a first line chemotherapeutic agent with axonal toxic effect similar to acrylamide, has been very well documented to accelerate neuropathy in Charcot Marie tooth patients (CMT) (Dickerhoff et al., 1988; Griffiths et al., 1985; Hayakawa et al., 1998; Olek et al., 1999). In one recent study, the minimum threshold for vincristine induced sensory neuropathy was observed at 8 mg in the general population, while similar exacerbation of symptoms in CMT patients was seen as low as 2 mg (Weimer and Podwall, 2006). This vincristine induced increased neuropathy is often severe enough in CMT patients for further chemotherapy to be discontinued (Graf et al., 1996; Martino et al., 2005).
Similar to humans, laboratory animals also develop distal axonopathy of the “dying-back type” when they are exposed to acrylamide (Griffin et al., 1977; Ko et al., 1999; Nguyen et al., 2009; Schaumburg et al., 1974). To date, there is no published report on the effect of acrylamide or other neurotoxic agents on the pace of nerve degeneration in laboratory animals with pre-existing neuropathies (Weimer and Podwall, 2006). We hypothesize that neurotoxins, such as acrylamide, may quicken the pace of axonal loss in laboratory animals with pre-existing neuropathy in a manner similar to that seen in patients with neuropathies.
Here we developed a novel system where acrylamide is used to induce rapid axonal loss in genetically engineered mouse models of neuropathies. The ready availability of pure acrylamide and its ability to reliably reproduce a robust model of distal axonal degeneration in laboratory animals encouraged its use as an axonal toxic agent in this study (Griffin et al., 1977; Ko et al., 1999; Nguyen et al., 2009; Schaumburg et al., 1974). In applying this paradigm to transgenic mouse models of neuropathy, we observed a marked acceleration of distal axonal loss, whereby the axonal loss which typically occurs over 8–10 months was reproduced following 7 to 10 days of acrylamide intoxication. We have successfully applied this system to two transgenic mouse models, MAG knockout and Trembler-J mice, a murine model of CMT-1. Both represent commonly used mouse models characterized by slowly progressive axonal loss occurring over 8–12 months. The effectiveness of this system was demonstrated by a combination of behavioral, electrophysiological, and immuno-pathological studies. This method offers a feasible and unique in vivo system (i) to model toxin induced exacerbation of pre-existing neuropathy as seen in patients and (ii) to accelerate the axonal degeneration process in transgenic mouse models so that relative young mice can be used to study nerve degeneration over short interval for timely studies of axonal degeneration and screening of neuroprotective agents.
MATERIAL AND METHODS
Animals
Male Trembler J (TrJ) mice (6 weeks old) were obtained from the Jackson’s laboratory. Littermate wild-type mice were used as control animals for TrJ mice.
MAG −/− founder mice, kindly provided by Dr. John Roder, University of Toronto, Ontario, Canada, were constructed by disruption of exon 5 of the MAG gene as previously reported (Ng et al., 1996). The strain provided (identical to that available from the Jackson Laboratory, Bar Harbor, ME) was on a C57BL/6, 129 inbred strains and CD1 random bred strain. To enhance comparisons between mutant strains, mutant mice were repeatedly back-crossed onto a C57BL/6 background to >99% strain purity (Nguyen et al., 2009; Pan et al., 2005). All animal protocols were approved by Johns Hopkins University Animal Care and Use Committee (ACUC)
Acrylamide treatment
Groups of five 6-week-old male mice were treated with acrylamide ad libitum by adding acrylamide to the drinking water at 400mg/kg. Control mice drank regular water. Five mice were housed in each plastic cage throughout the experimental period. Experimental procedures followed the principles in the “Use of Animals in Toxicology” and NIH guidelines (“Guide for the Care and Use of Laboratory Animals,” NIH Publication No. 86–23, 1985).
Rotarod studies
Groups of five mice were trained one week prior to acrylamide treatment on 3 consecutive days by performing the Rotarod at a constant speed of 4 rotation per minute (rpm) on day 1 and 8 rpm on day 2 and 3 for 3 minutes (pre-training), followed by an accelerating protocol in which the speed of rotation was initially set at 4 rpm and accelerated an additional 4 rpm every 30 seconds to a maximum of 40 rpm (Rotamex 28, Columbus instruments, Columbus Ohio). Due to the severity of the pre-existing phenotypes all trembler mice experiments were initially set at 2rpm and accelerated every 30 seconds to a maximum of 40 rpms. Three trials were performed on each mouse on each of 3 consecutive days with approximately 30 minutes rest between trials. Following initiation of acrylamide treatment, the mice were further trained for another 3 consecutive days. Seven to ten days after acrylamide intoxication, mice were again pre-trained and tested for 3 days, 3 trials per day.
To analyze data, we recorded the speed and duration during which the mouse stayed on the rod. Results of all 3 trials of each test date pooled to generate a mean and SEM (n= 15). Results were compared by two-tailed Students t-test. Values of p<0.05 were considered significant. The tester was blinded to the treatment. No mice or values were excluded.
Electrophysiological studies
Nerve conduction studies were performed on groups of five mice at 6–8 weeks, 6 months, and 8 months of age. The mice were anesthetized with isofluorane using a nose cone. Body temperature was maintained on a warm blanket to keep the surface temperature at 32°C prior to the taking of measurements. All compound motor action potentials (CMAP) measurements were performed with a Power Lab signal acquisition setup (AD Instruments, Colorado Springs, CO, USA). The CMAPs were measured by stimulation with subdermal needle electrodes placed near the sciatic nerve at the sciatic notch at a 50 mV range. Recording electrodes were placed in the tibial nerve-innervated intrinsic foot muscles in the plantar surface. Recordings were made with supramaximal stimulation at a 500 mV range with a time base of 100 kHz over 10 ms and with 1250 samples. We determined the latencies, negative peak amplitudes, and durations of the sciatic compound muscle action potentials. The distance between stimulation sites, determined by calipers, was also used to calculate conduction velocities. CMAP measurements were obtained at the initiation of acrylamide intoxication and 7–10 days after intoxication. Results of all readings were pooled to generate a mean and SEM. (n= 8). Results were compared by the two-tailed Students t-test. Values of p<0.05 were considered significant. The tester was blinded to the treatment. No mice or values were excluded.
Grip strength
To train and acclimatize the animals, each mouse was guided for 5 min to pull slanted grid of the grip strength meter 3–4 times before any readings were recorded. Grip strength was measured using a grip strength meter (Almemo 2450 Berlin, Germany). The grip strength meter consists of a digital display coupled with a force transducer and a metal plate grid trapeze. Each mouse was placed on the metal grid and was pulled by its tail with increasing force until it was unable to grasp the trapeze and the grip was broken. The grip strength digitally captures and displays the peak pull-force achieved. Grip strength was defined as the peak weight (g) indicated on the display. Individual recordings were pooled to generate a mean and SEM. (n= 8). Results were compared by the two-tailed Students t-test. Values of p<0.05 were considered significant. The tester was blinded to the treatment. No mice or values were excluded.
Thermal latency
Timed latency to hind limb withdrawal was assessed at 32° C (IITC life sciences Model 400 heated base, woodlands Hills, C.A). Following brief radiant heat exposure aimed at the ventral aspect of the paw from a heated lamp applied through a temperature modulated Plexiglas, thermal latency was recorded (four trials, 2 minutes apart) on mice from each group; age timeline and acrylamide treatment for both MAG−/− and Trembler-J mice, (n=8). Results of all recorded latencies were pooled to generate a mean and SEM. (n= 8). Results were compared by two-tailed Students t-test. Values of p<0.05 were considered significant. The tester was blinded to the treatment. No mice or values were excluded.
Morphological analysis
Mice were anesthetized with chloral hydrate and perfused through the ascending aorta with freshly prepared 4% paraformaldehyde in 0.1 M sodium phosphate (pH-7.4). Eight mice were used in each group. The feet, skin, diaphragm, and sciatic and distal tibial nerves were harvested from groups of mice at 6, 12, and 15 months of age. Harvested tissues were further fixed in 4% paraformaldehyde / 3% glutaraldehyde in Sorenson’s buffer overnight at 4°C, post-fixed in OsO4, and embedded in Epon-Araldite resin.
Immunostaining
Specimen (i.e., footpad and gastrocnemius muscle) were permeabilized by immersion in −20°C acetone for 10 min, blocked at room temperature for at least 1 hr in 5% Normal goat serum, and 3% BSA containing 0.5% Triton X-100 and 0.1% DMSO in PBS, and incubated for 24–48 hours at 4°C with various primary antibodies diluted in blocking solution. After incubating with the primary antibodies; SMI 32R,1:1000 (Covance), slides and whole mounts were thoroughly washed and re-blocked with blocking buffer for 1 hr. Samples were then incubated with the appropriate fluorescein and rhodamine, conjugated cross-affinity-purified secondary antibodies; diluted 1:100; Jackson ImmunoResearch Laboratories, bungarotoxin, 1:500 (Sigma Aldrich, St. Louis, MO, USA) for 4 hrs, and mounted with Vectashield (Vector Laboratories, Burlingame, CA). All slides were examined with the EGFP 38 He filter and rhodamine filters using the Axiovision v.4.8 software on a Zeiss Axioimager. Z1 microscope (Zeiss, Munich, Germany).
RESULTS
Axonal Degeneration in Trembler-J Mice
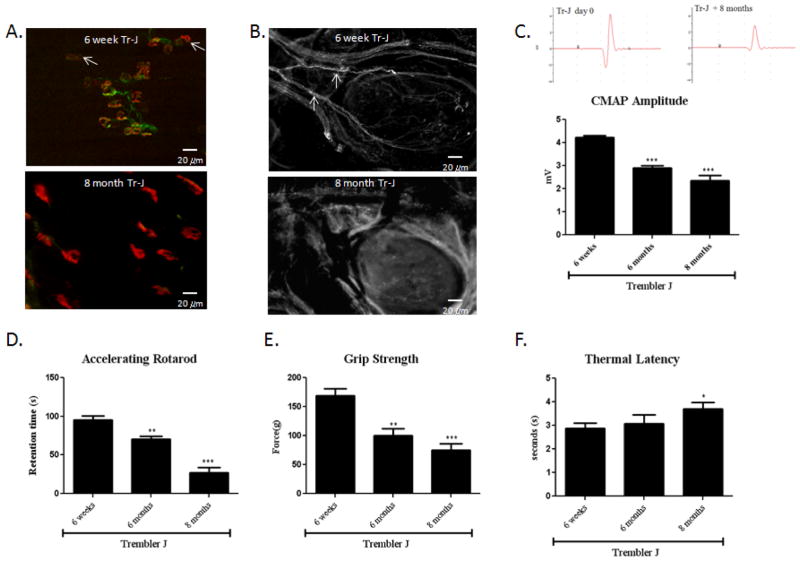
Trembler J (TrJ) mouse represents a classic model of Charcot-Marie-Tooth disease type 1, a common inherited human demyelinating disorder. TrJ mouse has a point mutation in PmP22 which results in myelin loss and slowly progressive distal axonal degeneration over many months (Valentijn et al., 1992). There were mild abnormalities in 6-week old TrJ mice consistent with current literature (Meekins et al., 2004; Street et al., 2002; Tanaka and Hirokawa, 2002). On gross observation, there was only mild gait unsteadiness. On immunostaining, some small distal nerve branches fragmentation was observed. There was also a mild characteristic swelling in the terminal branches in the foot pad and NMJ denervation in the gastrocnemius muscle (Fig. 1. A, B). To understand the functional significance of pathological abnormalities, we performed nerve conduction studies on motor nerves, which showed significantly delayed distal motor latencies (1.25 +/− 0.09 msec) in 6-week old TrJ mice compared to the latencies (2.04 +/− 0.12 msec) in normal littermates. This indicates a primary demyelinating process, an observation consistent with the existing literature (Meekins et al., 2004). However, chronic demyelination results in secondary progressive axonal loss (Henry et al., 1983, Heath et al., 1991, Nguyen et al., 2009). Trembler J mice showed significantly smaller CMAP amplitudes in comparison those in normal littermates (Fig. 1. C; Fig. 2. B). When challenged with the Rotarod test, 6-week old Trembler J mice had mild difficulty in maintaining their balance on the rotating rods and had a modest decline in retention time on the Rotarod (Fig. 1. D).
Figure 1. Distal axonal degeneration in aging Trembler-J mice.
(A) Gastrocnemius muscles were stained with SMI-32R (green) to detect axons and with α-bungarotoxin (red) to detect acetylcholine receptors at the muscle endplates. Muscle endplates that are positive for only α-bungarotoxin are not innervated (white arrows). NMJs in the 6-week old TrJ mice appear compact and well defined. Furthermore there are only a few scattered denervated NMJs. In contract, most NMJs are denervated and have a diffused, irregular and fragmented appearance in the 8 month old TrJ mice. (B) 6 week old TrJ mice show normal appearing nerve fibers (white arrows) with minimal axonal swelling and segmentation as shown by neurofilaments (NF–H 200) immunostaining of the foot pad. In contrast, the foot pad of 8 month old TrJ mice showed an absence of nerve fibers consistent with severe cutaneous axonal degeneration and loss. (C) This was accompanied by barely recordable CMAP responses around 1.8 mV. Examples of a typical CMAP tracing are shown in the left. (*6-week vs. 8-month old TrJ mice, n=8, p<0.01). (D) 8-month old TrJ mice showed severe decrease in retention time.(n=8, p<0.01). 8-month old TrJ mice also showed a marked decline in grip strength (E) and prolonged thermal latency (F). (n=8, p<0.01).
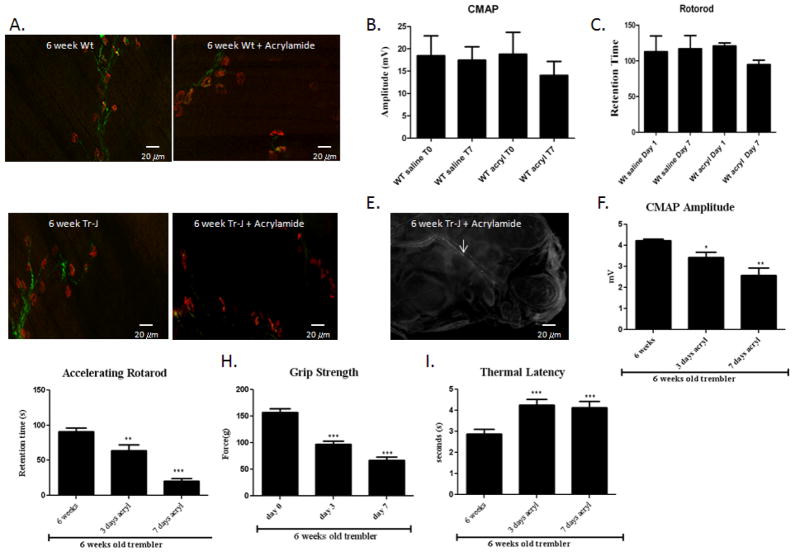
Figure 2. Acrylamide intoxication in wild-type and Trembler-J mice.
Wild-type and Trembler-J mice were exposed to 400 ppm acrylamide in drinking water for 7 days. (A) The gastrocnemius muscles were stained with SMI-32R (green) to detect axons and with α-bungarotoxin (red) to detect acetylcholine receptors at the muscle endplates. NMJs in the 6-week old wild-type (Wt) mice appear compact and well defined. There was no denervated NMJ. With acrylamide intoxication, there are a few scattered denervated NMJs. There was no significant statistically changes. (B) CMAP amplitude and (C) retention time of the Rotarod. (n=8, p<0.01) in the wild type mice even after acrylamide intoxication for 7 days. (D) In contract, in 6-week old TrJ mice treated with 400 ppm arylamide for 7 days, most NMJs were denervated and had a diffused, irregular and fragmented appearance. (E) There were also fragmented nerve fibers (white arrows) in the foot pad consistent with severe cutaneous axonal degeneration and loss. (F) This was associated with barely recordable CMAP responses around 2 mV. (n=8, p<0.01). (G) Retention time on the Rotarod was also severely decreased. (n=8, p<0.01). (H) The grip strength likewise declined. (I) Thermal latency was markedly prolonged. (n=8, p<0.01).
By 8 months of age, the TrJ mice showed markedly impaired gait with severe sensory ataxia and spreading of the toes of the hind limbs and dragging of the hind limbs. On immunostraining, many small distal nerve branches were fragmented or absent. There was severe characteristic swelling in the terminal branches in the foot pad and most NMJ were denervated in the gastrocnemius muscle (Fig. 1. A, B). This was accompanied by barely recordable CMAP responses around 1.6 mV (Fig. 1. C), a finding consistent with a secondary slowly progressive axonal loss. Note that 6 week old TrJ mice showed significantly smaller CMAP amplitudes in comparison those in normal littermates. This pre-existing axonal damage in TrJ mice may have masked an even higher statistical significant axonal loss associated with aging. When challenged with the Rotarod test, 8 month old TrJ mice showed severe motor impairment. Most 8 month old TrJ mice fell off the rod even during pre-training and had profound decline in the retention time on the Rotarod (Fig. 1. D). The severe motor impairment observed in 8 month old trembler J mice was reinforced by a significant decline in the grip strength as well as prolonged thermal latencies after stimulation with a focused heat source (Fig. 1. E, F). Consistent with previous studies, the morphological analysis showed only mild signs of axonal degeneration in the proximal sciatic nerve (data not shown), indicating that the pathological changes began from the most terminal parts of axons and evolved proximally.
Acrylamide Intoxication in Wild-type and Trembler-J Mice
We speculated that the TrJ mice have increased vulnerability and consequently accelerated axonal degeneration when they are exposed to axonal neurotoxins. To evaluate the utility of acrylamide in inducing an acceleration of the axonal degeneration in mice with pre-existing neuropathy, we administered acrylamide to 6-week old TrJ mice and age-matched C57BL/6J normal littermates ad libitum. All mice were exposed to 400 ppm acrylamide dissolved in the mice drinking water. When control mice at 6 weeks of age were exposed to 400 ppm acrylamide in drinking water for 7 days, there was scattered, distally predominant axonal degeneration in the tibial nerve (Fig. 2. A). On gross observation, there was only mild gait unsteadiness. When challenged with the Rotarod test, acrylamide intoxicated control mice had mild difficulty in maintaining their balance on the rotating rods and had a modest decline in retention time on the Rotarod in comparison with untreated mice (Fig. 2. C). This decrease was accompanied by a small (statistically insignificant) decrease in compound motor action potential (CMAP) amplitude on electrophysiological testing (Fig. 2. B).
In contrast, TrJ mice exposed to acrylamide showed markedly impaired gait with severe sensory ataxia, spreading of the toes of the hind limbs as well as dragging of hind limbs. At baseline, trembler mice had lower retention time on a Rotarod in comparison to control littermates. Acrylamide treated TrJ mice developed more rapid and severe motor impairment. Most TrJ mice treated with acrylamide fell off the rod even during pre-training and had profound decline in the retention time on the Rotarod (Fig. 2. G). The grip strength was similarly significantly decreased and the thermal latency was also prolonged, compared to the untreated TrJ mice (Fig. 2. H, I). Furthermore there were barely recordable CMAP responses in the acrylamide treated TrJ mice compared to the untreated TrJ mice (Fig. 2. F). However, the morphological analysis of the proximal sciatic nerve showed only minimal signs of axonal degeneration (data not shown), a finding consistent with rapid, progressive distally predominant axonal degeneration (Fig. 2. D, E). Taken together, the 6 week old TrJ mice exposed to 7 days of acrylamide showed comparable pathological, behavioral, and electrophysiogical findings as those seen in untreated 8 month old Trembler J mice.
Acrylamide intoxicated MAG−/− mice
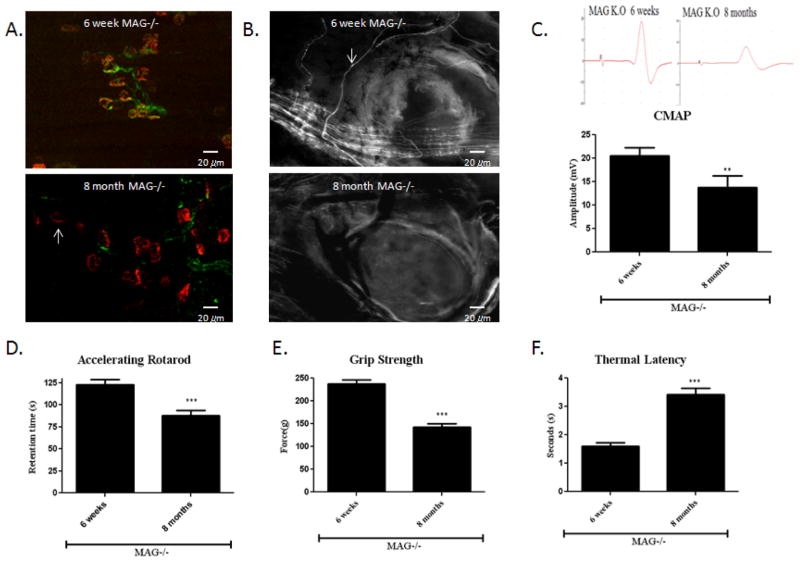
We next asked whether acrylamide likewise increases the pace of axonal degeneration in other transgenic mouse models of neuropathies. We have previously reported that MAG−/− mice underwent progressive axonal degeneration within the PNS over many months (Nguyen et al., 2009). The numbers of intact nerve fibers and those undergoing degeneration were enumerated in tibial nerve. We have also previously reported a distally predominant progressive decrease in axonal numbers, cumulating in a 22% reduction by 9 months in the tibial nerves of MAG−/− (Nguyen et al., 2009; Pan et al., 2005; Yin et al., 1998). Here we show characteristic denervated NMJs in the distal limbs, fragmentation and degenerated nerve terminals in the footpad (Fig. 3. A,B), reduced CMAP amplitude (Fig. 1. C), decreased retention time on a Rotarod (Fig. 3. D) as well as decreased grip strength in 8 month old MAG knockout mice. Thermal latency was also significantly elevated in 8 month old MAG knockout mice in comparison to untreated 6 week old MAG−/− mice (Fig. 3. E,F).
Figure 3. Axonal degeneration in aging MAG−/− mice.
(A) Gastrocnemius muscles were stained with SMI-32R (green) to detect axons and with α-bungarotoxin (red) to detect acetylcholine receptors at the muscle endplates. NMJs in the 6-week old MAG−/− mice appear compact and well defined. In the 8 month old MAG−/− mice, there are many denervated NMJs that have a diffused, irregular and fragmented appearance. (B) 6 week old MAG−/− mice showed normal appearing nerve fibers with minimal axonal swelling and segmentation as shown by neurofilament (NF–H 200) immunostaining (white arrows). In contrast, 8 month old MAG−/− mice showed many fragmented and enlarged nerve fibers consistent with cutaneous axonal degeneration and loss. (C) This was accompanied by a 27% reduction in CMAP responses b. representative CMAP tracing are shown in the left. (*6-week vs. 8-month old MAG−/−mice, n=8, p<0.01). (D) 8-month old AMG−/− mice also showed a decrease in retention time.(n=8, p<0.01) as well as decline in grip strength (E) and prolonged thermal latency (F). (n=8, p<0.01).
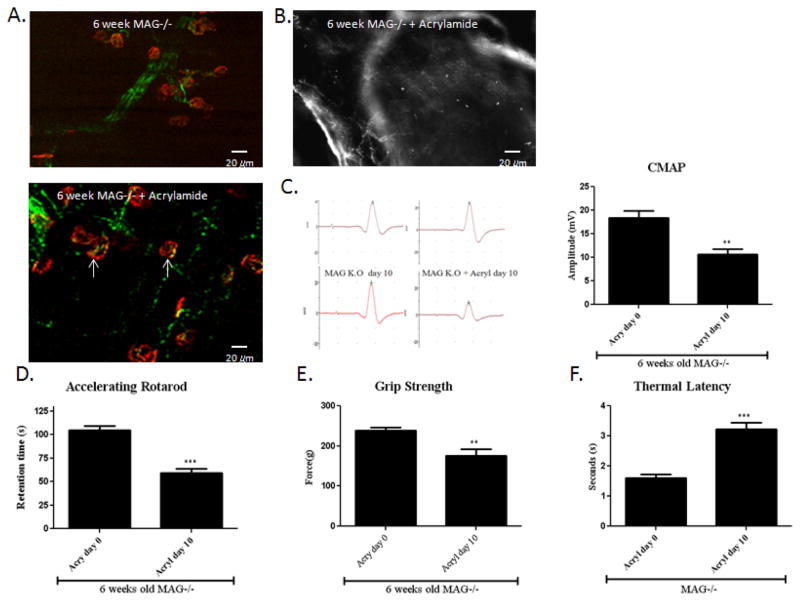
To evaluate whether acrylamide accelerates the axonal degeneration in MAG−/− mice, 6 weeks old MAG−/− mice were exposed to acrylamide for 10 days. We observed noticeably impaired gait with marked sensory ataxia, spreading of the toes of the hind limbs as well as dragging of the hind limbs by day 10. This observation was further reinforced by profound swelling in the nerve terminals in the foot pad and NMJs in the distal limbs (Fig. 4. A,B). At baseline, MAG knock-out mice had slightly lower retention time on the rotarod than wild-type controls. Like the TrJ mice following acrylamide intoxication, MAG knock-out mice developed severe motor impairment. There was a 45% decrease in amplitudes of CMAP after 10 days of acrylamide treatment (Fig. 4. C), and a profound decline in the retention time on the rotarod (Fig. 4. D). Similarly the grip strength was markedly reduced while the thermal latency was significantly prolonged in the MAG knockout mice treated with acrylamide in comparison comparison to the untreated littermates (Fig. 4. E, F). In summary, acrylamide intoxicated 6-week MAG−/− mice showed similar but more severe pathological, electrophysiogical, and behavioral changes in comparison to untreated MAG−/− mice at 8 months of age.
Figure 4. Acrylamide intoxication in MAG−/− mice.
MAG−/− mice were exposed to 400 ppm acrylamide in drinking water for 10 days. (A) The gastrocnemius muscles were stained with SMI-32R (green) to detect axons and with α-bungarotoxin (red) to detect acetylcholine receptors at the muscle endplates. Muscle endplates that are positive for only α-bungarotoxin are not innervated (white arrows). As shown in figure 3.B, NMJs in the 6-week old MAG−/− mice appear compact and well defined. With acrylamide intoxication, there are dernervated NMJs. There were associated with reduced (B) Acrylamide intoxicated MAG−/−mice showed severe cutaneous axonal degeneration and loss as shown by neurofilament (NF–H 200) immunostaining of the foot pad. (C) There was also significant decline in CMAP amplitude and (D) retention time of the Rotarod. (n=8, p<0.01). (H) The grip strength likewise declined. (I) Thermal latency was markedly prolonged. (n=8, p<0.01). The pathologic, behavioral and electrophysiological changes associated with acrylamide treatment paralleled those seen in MAG−/− at 8 months of age.
DISCUSSION
Acrylamide neuropathy has been a popular experimental animal model for studying the processes of axonal transport (Miller and Spencer 1984; Gold et al 1985), dying-back neuropathy (Schaumburg et al 1974), and axonal swelling. Acting as an electrophile, acrylamide interacts with neurophilic sulfhydryl groups in axonal proteins to form neurotoxic adducts which inactivate critical axonal proteins (Hashimoto and Aldridge, 1970, Miller and Spencer, 1985, LoPachin et al., 2007). Acrylamide induces changes in axonal transport proteins like neurofilaments and microtubules, resulting in a dysfunction of retrograde axonal transport (Miller et al., 1983, Miller and Spencer, 1984). The decreased availability of essential elements also is thought to play a major role in distal retrograde axonal degeneration (Hashimoto and Aldridge, 1970, Miller and Spencer, 1985). The ability of acrylamide to reliably reproduce a model for toxic neuropathy in laboratory animals and the ready availability of pure acrylamide has made it a particularly suitable model for drug development studies, particularly for this type of distal retrograde axonal degeneration. When acrylamide was applied to two transgenic mouse models of neuropathies, we observed that (i) the distal progressive axonal loss which typically occurs over 8 months was reproduced within 7 to 10 days of acrylamide intoxication and (ii) the pathological, behavioral, and electrophysiological changes in acrylamide treated transgenic mice at 7 days were the same as those observed in the untreated mice at 8 months of age.
Acrylamide intoxication accelerates axonal degeneration
In humans, a pre-existing neuropathy can be severely aggravated by exposure to toxic agents. The best example is exacerbation of CMT by chemotherapy (Chaudhry et al., 2003; Dickerhoff et al., 1988; Graf et al., 1996). Patients with pre-existing neuropathy such as Charcot-Marie-Tooth disease have been reported to develop a faster pace of nerve degeneration after toxin or drug exposure (Chaudhry et al., 2003; Weimer and Podwall, 2006). This represents a major clinical challenge in treating patients with neuropathies. However, research into this disease process has been hampered by the lack of a laboratory model.
This report clearly demonstrates the influence of existing neuropathy on increased susceptibility to axonal degeneration by employing acrylamide intoxication in two transgenic mouse models of neuropathies. The model presented here offers the first animal model for medication or toxin induced exacerbation of pre-existing neuropathy. Our data demonstrate that the progressive axonal loss which typically occurring over 8 to 9 months in transgenic mice with pre-existing neuropathy was reproduced within 7 to 10 days following exposure to acrylamide. In contrast, control wild-type mice did not show significant pathological, behavioral, or electrophysiological changes with the same acrylamide treatment and over the same duration.
This highlights the potential hazard of exposure to neurotoxic drugs in patients with pre-existing neuropathy. Dissecting the mechanism for medication or toxin induced exacerbation of neuropathy in previously existing clinical conditions like Charcot Marie Tooth (CMT) remains a desirable but elusive challenge (Weimer and Podwall, 2006). The animal model introduced here would make it possible to begin to detailed studies of the mechanism of toxin induced acceleration of neuropathy and drug development studies, particularly for distal retrograde axonal degeneration.
A model for rapid in vivo screening axonal protective agents
There is now intensive research on the molecules and signaling pathways that prevent axonal degeneration resulting in potentially new axonal protective agents (Nguyen et al., 2009). For efficient screening of these agents over a reasonable time frame, there is a pressing need to accelerate the pace of axonal loss over a short interval for high-throughput screening of pharmacological and genetic therapies. Our model offers a novel experimental approach to accelerate the rate of axonal loss over short intervals for timely in vivo studies of nerve degeneration.
We documented the sequence of pathological, behavioral, and electrophysiological changes associated with aging and exposure to acrylamide. Upon exposure to a low dose o acrylamide, we observed characteristic swelling in the nerve terminals and NMJs in the distal limbs within 7–10 days. This is accompanied by poor performance on the rotarod test and reduction in CMAP amplitude. The reduction in amplitude of muscle potentials is attributed to Wallerian-like degeneration and denervation at the nerve terminals. These findings closely resemble the pathological, behavioral and electrophysiological changes seen in untreated transgenic mice at 8–9 months of age.
Taken together, we demonstrate here a method to reliably and reproducibly accelerate the progression of nerve degeneration in transgenic models of neuropathies using acrylamide intoxication. Our protocol can serve as a useful technique for researchers using transgenic animal models to study cellular and biochemical mechanism of axonal degeneration. This approach can also allow in vivo high-throughput screening for pharmacological and genetic therapies to prevent nerve degeneration, in particular, distal axonopathy.
HIGHLIGHTS.
Acrylamide accelerates the rate of axonal loss in mice with neuropathy.
Existing neuropathy in transgenic mice enhances the susceptibility to acrylamide.
Progressive axonal loss due to aging was reproduced with acrylamide intoxication.
An animal model for medication induced exacerbation of pre-existing neuropathies.
An animal model for high-throughput screening of pharmacological therapies.
Acknowledgments
We thank Drs. Bao Han Pan and Mohamed Farah for technical assistance. We thank Dr. Wei-Chun Chin for helpful discussions and input during the preparation of the manuscript.
FUNDING
This work was supported by the National Institutes of Health Grants K08 NS055135, and the MDA 157388 grant.
Abbreviation(s)
- CMT-1
Charcot-Marie-Tooth disease type 1
Footnotes
AUTHOR CONTRIBUTIONS
T.N. and J.W.G. designed the study. O.E., M.T., and T.N. performed the experiments. O.E. and T.N. wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auld RB, Bedwell SF. Peripheral neuropathy with sympathetic overactivity from industrial contact with acrylamide. Can Med Assoc J. 1967;96:652–4. [PMC free article] [PubMed] [Google Scholar]
- Chaudhry V, Chaudhry M, Crawford TO, Simmons-O’Brien E, Griffin JW. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60:337–40. doi: 10.1212/01.wnl.0000043691.53710.53. [DOI] [PubMed] [Google Scholar]
- Dickerhoff R, Lindner W, Scheiber W. Severe vincristine neurotoxicity in a patient with Charcot-Marie-Tooth disease. Pediatr Hematol Oncol. 1988;5:61–4. doi: 10.3109/08880018809031253. [DOI] [PubMed] [Google Scholar]
- Garland TO, Patterson MW. Six cases of acrylamide poisoning. Br Med J. 1967;4:134–8. doi: 10.1136/bmj.4.5572.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf WD, Chance PF, Lensch MW, Eng LJ, Lipe HP, Bird TD. Severe vincristine neuropathy in Charcot-Marie-Tooth disease type 1A. Cancer. 1996;77:1356–62. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1356::AID-CNCR20>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Price DL, Drachman DB. Impaired axonal regeneration in acrylamide intoxication. J Neurobiol. 1977;8:355–70. doi: 10.1002/neu.480080407. [DOI] [PubMed] [Google Scholar]
- Griffiths JD, Stark RJ, Ding JC, Cooper IA. Vincristine neurotoxicity in Charcot-Marie-Tooth syndrome. Med J Aust. 1985;143:305–6. doi: 10.5694/j.1326-5377.1985.tb123018.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Aldridge WN. Biochemical studies on acrylamide, a neurotoxic agent. Biochem Pharmacol. 1970;19:2591–604. doi: 10.1016/0006-2952(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Itoh T, Niwa H, Mutoh T, Sobue G. NGF prevention of neurotoxicity induced by cisplatin, vincristine and taxol depends on toxicity of each drug and NGF treatment schedule: in vitro study of adult rat sympathetic ganglion explants. Brain Res. 1998;794:313–9. doi: 10.1016/s0006-8993(98)00305-9. [DOI] [PubMed] [Google Scholar]
- Heath JW, Inuzuka T, Quarles RH, Trapp BD. Distribution of P0 protein and the myelin-associated glycoprotein in peripheral nerves from Trembler mice. J Neurocytol. 1991;20:439–49. doi: 10.1007/BF01252272. [DOI] [PubMed] [Google Scholar]
- Henry EW, Cowen JS, Sidman RL. Comparison of Trembler and Trembler-J mouse phenotypes: varying severity of peripheral hypomyelination. J Neuropathol Exp Neurol. 1983;42:688–706. doi: 10.1097/00005072-198311000-00008. [DOI] [PubMed] [Google Scholar]
- Ko MH, Chen WP, Lin-Shiau SY, Hsieh ST. Age-dependent acrylamide neurotoxicity in mice: morphology, physiology, and function. Exp Neurol. 1999;158:37–46. doi: 10.1006/exnr.1999.7102. [DOI] [PubMed] [Google Scholar]
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, Kamholz J, Shy ME. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain. 2000;123 (Pt 7):1516–27. doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Geohagen BC, Das S. Neurotoxic mechanisms of electrophilic type-2 alkenes: soft soft interactions described by quantum mechanical parameters. Toxicol Sci. 2007;98:561–70. doi: 10.1093/toxsci/kfm127. [DOI] [PubMed] [Google Scholar]
- Martino MA, Miller E, Grendys EC., Jr The administration of chemotherapy in a patient with Charcot-Marie-Tooth and ovarian cancer. Gynecol Oncol. 2005;97:710–2. doi: 10.1016/j.ygyno.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Meekins GD, Emery MJ, Weiss MD. Nerve conduction abnormalities in the trembler-j mouse: a model for Charcot-Marie-Tooth disease type 1A? J Peripher Nerv Syst. 2004;9:177–82. doi: 10.1111/j.1085-9489.2004.09310.x. [DOI] [PubMed] [Google Scholar]
- Miller MS, Spencer PS. Single doses of acrylamide reduce retrograde transport velocity. J Neurochem. 1984;43:1401–8. doi: 10.1111/j.1471-4159.1984.tb05400.x. [DOI] [PubMed] [Google Scholar]
- Miller MS, Spencer PS. The mechanisms of acrylamide axonopathy. Annu Rev Pharmacol Toxicol. 1985;25:643–66. doi: 10.1146/annurev.pa.25.040185.003235. [DOI] [PubMed] [Google Scholar]
- Miller MS, Miller MJ, Burks TF, Sipes IG. Altered retrograde axonal transport of nerve growth factor after single and repeated doses of acrylamide in the rat. Toxicol Appl Pharmacol. 1983;69:96–101. doi: 10.1016/0041-008x(83)90124-2. [DOI] [PubMed] [Google Scholar]
- Ng WP, Cartel N, Li C, Roder J, Lozano A. Myelin from MAG-deficient mice is a strong inhibitor of neurite outgrowth. Neuroreport. 1996;7:861–4. doi: 10.1097/00001756-199603220-00005. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Mehta NR, Conant K, Kim KJ, Jones M, Calabresi PA, Melli G, Hoke A, Schnaar RL, Ming GL, Song H, Keswani SC, Griffin JW. Axonal protective effects of the myelin-associated glycoprotein. J Neurosci. 2009;29:630–7. doi: 10.1523/JNEUROSCI.5204-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olek MJ, Bordeaux B, Leshner RT. Charcot-Marie-Tooth disease type I diagnosed in a 5-year-old boy after vincristine neurotoxicity, resulting in maternal diagnosis. J Am Osteopath Assoc. 1999;99:165–7. doi: 10.7556/jaoa.1999.99.3.165. [DOI] [PubMed] [Google Scholar]
- Pan B, Fromholt SE, Hess EJ, Crawford TO, Griffin JW, Sheikh KA, Schnaar RL. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice. Exp Neurol. 2005;195:208–17. doi: 10.1016/j.expneurol.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumburg HH, Wisniewski HM, Spencer PS. Ultrastructural studies of the dying-back process. I. Peripheral nerve terminal and axon degeneration in systemic acrylamide intoxication. J Neuropathol Exp Neurol. 1974;33:260–84. doi: 10.1097/00005072-197404000-00006. [DOI] [PubMed] [Google Scholar]
- Street VA, Meekins G, Lipe HP, Seltzer WK, Carter GT, Kraft GH, Bird TD. Charcot-Marie-Tooth neuropathy: clinical phenotypes of four novel mutations in the MPZ and Cx 32 genes. Neuromuscul Disord. 2002;12:643–50. doi: 10.1016/s0960-8966(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Hirokawa N. Mouse models of Charcot-Marie-Tooth disease. Trends Genet. 2002;18:S39–44. doi: 10.1016/s0168-9525(02)02839-1. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Valentijn LJ, Bolhuis PA, Zorn I, Hoogendijk JE, van den Bosch N, Hensels GW, Stanton VP, Jr, Housman DE, Fischbeck KH, Ross DA, et al. The peripheral myelin gene PMP-22/GAS-3 is duplicated in Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992;1:166–70. doi: 10.1038/ng0692-166. [DOI] [PubMed] [Google Scholar]
- Weimer LH, Podwall D. Medication-induced exacerbation of neuropathy in Charcot Marie Tooth disease. J Neurol Sci. 2006;242:47–54. doi: 10.1016/j.jns.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, Roder J, Trapp BD. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci. 1998;18:1953–62. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]