Abstract
Background
We aimed to identify combinations of biomarkers to enhance the definition of PGD for translational research.
Methods
Biomarkers reflecting lung epithelial injury (sRAGE and SP-D), coagulation cascade (PAI-1 and Protein C), and cell adhesion (ICAM-1) were measured in the plasma of 315 subjects derived from the LTOG cohort at 6 and 24 hours after transplantation. We assessed biomarker utility in two ways: first, we tested the discrimination of grade 3 PGD within 72 hours; second, we tested the predictive utility of plasma biomarkers for 90-day mortality.
Results
86/315 subjects (27%) developed PGD. 23 subjects (8%) died within 90 days of transplantation, of which 16 (70%) had PGD. Biomarkers measured at 24 hours had greater discrimination than at 6 hours. Individually, sRAGE (AUC 0.71) and PAI-1 (AUC 0.73) had the best discrimination of PGD. The combinations of sRAGE with PAI-1 (AUC 0.75), PAI-1 with ICAM-1 (AUC 0.75), and PAI-1 with SP-D (AUC 0.76) had the best discrimination. Combinations of greater than 2 biomarkers did not significantly enhance discrimination of PGD. ICAM-1 with PAI-1 (AUC 0.72) and ICAM-1 with sRAGE (AUC of 0.72) had the best prediction for 90-day mortality. The addition of ICAM-1, PAI-1, or sRAGE to the concurrent clinical PGD grade significantly improved prediction of 90-day mortality (p<0.001 each).
Conclusions
Measurement of the combination of a marker of impaired fibrinolysis with an epithelial injury or cell adhesion marker had the best discrimination for PGD and prediction for early mortality, and may provide an alternative outcome useful in future research.
Keywords: Primary Graft Dysfunction, Lung transplantation, Biomarkers, Acute Lung Injury
Introduction
Primary graft dysfunction (PGD) is a form of acute lung injury (ALI) that develops within 72 hours of lung transplantation. It is defined by the presence of hypoxemia and radiographic infiltrates [1] and is the major cause of death in the early post-transplant period [2]. PGD affects 10–30% of all patients receiving lung transplantation [3] and is associated with an increased risk of bronchiolitis obliterans syndrome (BOS), prolonged hospitalization, and increased short and long-term mortality [4, 5].
The ISHLT classification system, which grades PGD from 0–3 based on severity of hypoxemia and presence of radiographic infiltrates, is the currently accepted clinical definition of PGD [1]. The ISHLT grading system has demonstrated discriminant validity for survival and individual biomarker profiles [6]. However, the clinical definition of PGD is categorical and may not capture the full spectrum of PGD; potentially limiting its use in translational research [7]. Lung injury protein biomarkers may enhance PGD outcome definition for several reasons. First, they are objective measurements and can be standardized across centers. Second, they have a broad range and can be analyzed continuously, giving increased statistical power to smaller studies [8, 9]. Third, markers may identify biological processes for targeted therapies. Finally, biomarkers have the potential to strengthen the current clinical definition of PGD by allowing detection of subclinical lung injury not captured by the clinical grading scheme.
The aim of this study was to identify combinations of mechanism-specific acute lung injury biomarkers that may enhance the clinical definition of PGD and provide a continuous outcome to facilitate further research. We chose biomarkers representing three pathways that have been implicated in ALI and PGD: epithelial injury, endothelial injury, and the coagulation cascade. In previous studies, increased post-operative plasma levels of plasminogen activator inhibitor (PAI-1), soluble receptor for advanced glycation end products (sRAGE), intercellular adhesion molecule-1 (ICAM-1) and decreased levels of Protein C have been associated with PGD [10–13]. Plasma surfactant protein-D (SP-D) levels have been associated with PGD in patients with IPF receiving single lung transplants [14]. We determined the utility of biomarkers by assessing discrimination for clinically graded PGD, and evaluating predictive utility for 90-day mortality alone and when added to concurrent clinical PGD grade.
Methods
Study Population
The Lung Transplant Outcomes Group (LTOG) cohort is a multi-center, prospective study of lung transplant recipients that has been previously described [10–12]. In prior studies, our group studied the association between PAI-1, ICAM-1, and Protein C and PGD in a cohort of 128 subjects and between SP-D and PGD in a cohort of 104 subjects [10, 11, 14]. All subjects in the present study were enrolled in a prior study of sRAGE with PGD [12]. We included subjects transplanted between October 2002 and March 2006 from 6 centers with at least one biomarker measurement at 24 hours, allowing us to analyze all five biomarkers in an equivalent or larger population than our previous studies. Plasma samples were prospectively collected 6 and 24 hours after transplantation. Samples were centrifuged within 60 minutes and then stored at −80°C for subsequent analysis. Clinical data were collected prospectively for all subjects as described in detail elsewhere [15]. 90-day mortality information was collected from each center and supplemented with UNOS data. Mortality data is incomplete because one of our centers does not have institutional review board (IRB) approval for the collection of long-term outcomes. The IRB’s at each site approved our study. Informed consent was obtained from each subject enrolled in the cohort.
Determination of PGD Grade
PGD grade was determined using the consensus definition of the ISHLT [16]. Two blinded physicians examined chest radiographs to assess the presence of PGD. Radiographs and arterial blood gases were assessed at the time of admission to the ICU after transplantation (T0), and 24, 48, and 72 hours after transplantation. Radiographs qualified for PGD if the transplanted lung(s) had diffuse infiltrates. The severity of PGD was graded according to the PaO2/FiO2 ratio, with a PaO2/FiO2 ratio less than 200 defining grade 3 PGD [10, 15, 16].
Outcomes and Sensitivity Analyses
The primary outcome was the development of grade 3 PGD within 72 hours after transplantation. We used 90-day mortality as an additional end point to further evaluate the association of these biomarkers with early outcomes after transplantation. We performed sensitivity analyses varying the outcome to grade 3 PGD at 24 hours to reflect concurrent lung injury, and grade 3 PGD at 72 hours, to reflect persistent lung injury. We also evaluated PGD grade 2 or 3 within 72 hours and PGD grade 1, 2, or 3 within 72 hours as outcomes to analyze the full spectrum of PGD. We repeated the primary analysis using a restricted cohort of subjects with all 5 biomarker measurements to ensure that there was no bias from missing data. We performed a stratified analysis within our 2 largest diagnosis categories (IPF and COPD) and transplant type to determine whether there were significant differences in biomarker performance across key clinical co-variates [17]. In sensitivity analyses, we adjusted for center to evaluate potential variation in management practices.
Measurement of sRAGE, Protein C, SP-D, PAI-1, ICAM-1 levels
Biomarkers were chosen because of previously demonstrated association with ALI and PGD (Table 1). We measured plasma biomarkers at 6 and 24 hours to coincide with early PGD grading times. Protein C was measured using the Actichrome protein C assay (American Diagnostica, Greenwich, CT). PAI-1 antigen was measured with the Imubind PAI-1 ELISA (American Diagnostica); the intra-assay coefficient of variation was 6.8%. sRAGE was measured by sandwich ELISA (R&D, Minneapolis, MN). The intra-assay coefficient of variation was 6%. ICAM-1 levels were measured using ELISA (R&D systems, Minneapolis, MN); the intra-assay coefficient of variation was 3%. SP-D levels were measured using a commercially available ELISA kit (Yamasa Corporation, Tokyo, Japan). The intra-assay coefficient of variation was 5%. Samples were measured in duplicate and a standardized average value was used for analysis.
Table 1.
Biomarkers Selected for Analysis
| Biomarker | Function |
|---|---|
|
| |
| Intracellular Adhesion Molecule-1 (ICAM-1) | Adhesion molecule expressed on alveolar epithelial cells and endothelial cells [27]. Higher plasma levels associated with increased mortality and mechanical ventilation in ALI [28, 29]. |
| Surfactant Protein-D (SP-D) | Collectin secreted by type II alveolar cells, important in host defense. Mediates pathogen clearance and resolution of inflammation [30]. Elevated serum levels associated with increased mortality in ALI [30]. Also associated with PGD in IPF patients receiving single lung transplant [14]. |
| Receptor Advanced Glycation End Product (SRAGE) | Highly expressed in in type 1 epithelial cells, important in barrier function[31]. Plasma levels associated with longer ICU stay, duration of mechanical ventilation after lung transplant[32], and PGD [12]. |
| Plasminogen Activator Inhibitor-1 (PAI-1) | Inhibits fibrinolysis. Depletion of PAI-1 is protective against the development of IRI [33]. Plasma levels are associated with PGD [10]. |
| Protein C | Functions as an anticoagulant. Serum levels are decreased in ALI and PGD [10, 34], likely leading to intralveolar deposition of fibrin, and a pro-coagulant state. |
Statistical Analysis
Our goals were to identify easily measured plasma biomarkers that had the best discrimination for PGD and prediction for 90-day mortality. As many biomarkers have skewed distributions, we constructed standardized biomarkers by subtracting the mean from each value and dividing by the standard deviation. The standardized biomarkers were used for all analyses. We examined each biomarker individually and in combination at each timepoint. We compared areas under receiver operating characteristic (ROC) curves using methods suggested by Pepe and colleagues [8, 18, 19] starting with single biomarkers followed by two-, three-, four- and five-biomarker combinations. We have not used reclassification methods [20] for this analysis as the goal is to demonstrate utility of biomarkers alone as alternative outcomes to clinical PGD grading and mortality. We computed the predicted probability of grade 3 PGD or 90-day mortality for each individual by fitting regression models with 1–5 biomarkers, then created an ROC curve and calculated the area under the ROC curve (AUC) and corresponding 95% bootstrap confidence interval (CI). We tested for a significant improvement in AUC between models with different combinations of biomarkers. For 90-day mortality, we also included PGD grade as a categorical variable in the model. We present p-values for significant change in AUC between models. Analyses were performed using STATA versions 11.1 and 12.0 (STATA Corp., College Station, TX).
Results
Analysis of Grade 3 PGD within 72 hours
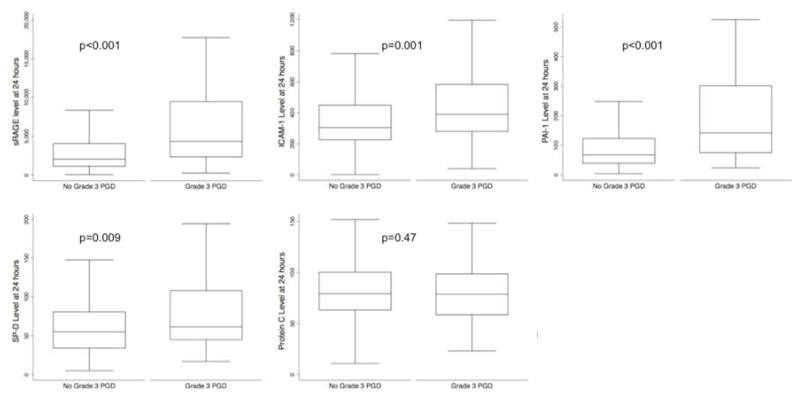
There were 315 subjects with at least one biomarker measurement. 86 (27%) developed grade 3 PGD within 72 hours. Recipient characteristics are described in Table 2. Median ICAM-1, sRAGE, SP-D, and PAI-1 levels were higher in the PGD group; however, Protein C levels were not significantly different between PGD and non-PGD groups (Figure 1).
Table 2.
Donor and recipient characteristics by primary graft dysfunction status. Comparisons are made with percentage of subjects or means (standard deviation). Chi square tests and t tests were used to compare co-variates between PGD and no PGD.
| PGD (n=86) | No PGD (n=229) | P Value | |
|---|---|---|---|
| Diagnosis | <0.01 | ||
| COPD | 26 (30%) | 127 (55%) | |
| CF | 5 (6%) | 29 (13%) | |
| IPF | 38 (44%) | 59 (26%) | |
| Other | 17 (20%) | 14 (6%) | |
| Recipient Race | <0.01 | ||
| Caucasian | 68 (79%) | 208 (75%) | |
| African American | 15 (17%) | 10 (4%) | |
| Hispanic | 1 (1%) | 6 (3%) | |
| Asian American | 1 (1%) | 1 (1%) | |
| Other | 1 (1%) | 4 (2%) | |
| Female Recipient | 42 (49%) | 108 (47%) | 0.07 |
| Recipient Age | 53 ± 12 | 53 ± 12 | 0.67 |
| Donor Race | 0.21 | ||
| Caucasian | 61 (73%) | 150 (66%) | |
| African American | 10 (12%) | 48 (21%) | |
| Hispanic | 10 (12%) | 24 (11%) | |
| Asian American | 1 (1%) | 4 (2%) | |
| Other | 2 (2%) | 1 (1%) | |
| Female Donor | 40 (47%) | 87 (38%) | 0.08 |
| Donor Age | 35 ± 14 | 33 ± 14 | 0.19 |
| Bilateral Transplant | 50 (58%) | 128 (72%) | 0.73 |
| Use of CBP | 49 (57%) | 59 (26%) | <0.01 |
| CBP TIME (min) | 239 ± 84 | 223 ± 74 | 0.31 |
| Mean PASP | 53± 26 | 38 ± 13 | <0.01 |
COPD=Chronic Obstructive Lung Disease, IPF=Idiopathic Pulmonary Fibrosis, CF= cystic fibrosis, CBP=cardiopulmonary bypass.
Figure 1.
Comparison of median levels of plasma biomarkers between subjects with grade 3 PGD within 72 hours and those without
Biomarkers measured at 24 hours had significantly better discrimination than biomarkers measured at 6 hours (Table 3). Therefore, we present our analyses using the measurements from the 24-hour time point. Individually, a marker of epithelial injury, sRAGE (AUC 0.71, 95% CI: 64, 78) and a marker of impaired fibrinolysis, PAI-1 (AUC 0.73, 95% CI: 66, 79) had the greatest discrimination for PGD. Protein C (AUC 0.53, 95% CI: 45, 61), SP-D (AUC 0.60, 95% CI: 53, 67) and ICAM-1 (AUC 0.63, 95% CI: 56, 70) did not perform as well when measured individually (Table 3).
Table 3.
Association of individual biomarkers measured at 6 and 24 hours with Grade 3 PGD within 72 hours
| Biomarker | AUC (95% CI) measured at 6 hours | AUC (95% CI) measured at 24 hours |
|---|---|---|
| ICAM-1 | 0.58 (0.51, 0.65) | 0.63 (0.56, 0.70) |
| sRAGE | 0.63 (0.55, 0.70) | 0.71 (0.64, 0.78) |
| SP-D | 0.62 (0.55, 0.69) | 0.60 (0.53, 0.67) |
| Protein C | 0.55 (0.47, 0.62) | 0.53 (0.45, 0.61) |
| PAI-1 | 0.64 (0.58, 0.71) | 0.73 (0.66, 0.79) |
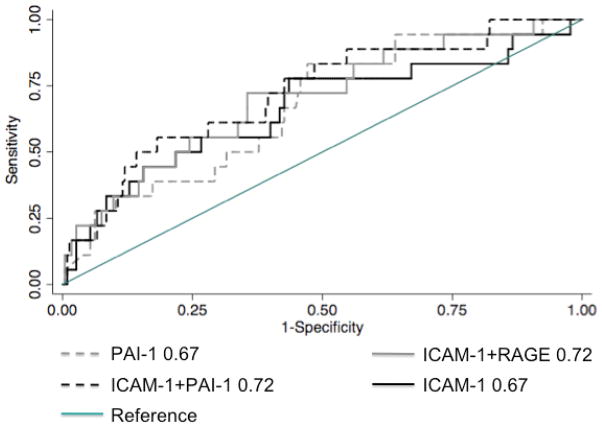
Qualitatively, the addition of PAI-1 to ICAM-1 (PAI-1+ICAM-1 AUC: 0.75, 95% CI: 0.69, 0.82) or to SP-D (PAI-1+SP-D AUC for 0.76 95% CI: 0.70, 0.82) improved the overall AUC, although, quantitatively there was not a significant difference in discrimination over PAI-1 alone, the addition of PAI-1 increased the discrimination of the other biomarkers. The combination of our 2 strongest individual biomarkers, sRAGE +PAI-1 had an AUC of 0.75 (95% CI: 0.68, 0.81), with no significant improvement over sRAGE alone (p=0.79) or PAI-1 alone (p=0.38) (Figure 2).
Figure 2.
Top performing biomarkers measured at 24 hours for any grade 3 PGD within 72 hours
Combinations of three biomarkers revealed small but non-significant improvements in discrimination from the analysis of two biomarkers (Table S1). Combinations of 4 and 5 biomarkers also did not have a significant improvement over the top performing combinations of 2 biomarkers (Table S2). There were no differences in our primary analysis when analyzing within transplant type. Both RAGE and ICAM-1 had a significant interaction with diagnosis (p=0.012 and p=0.037), with ICAM-1 performing better in subjects with COPD and sRAGE performing better in subjects with IPF (Table S3). There were no differences in the analysis after adjustment by center (Table S4a).
Sensitivity Analyses with Alternate PGD Definitions
Varying the outcome to a more severe phenotype, grade 3 PGD at 72 hours did not significantly change the results. There were 47 (15%) subjects with grade 3 PGD at 72 hours. Given the smaller number of cases, AUC’s were slightly lower, however, top-performing biomarkers did not change (Table S4b). We also analyzed the ability of the lung injury biomarkers to discriminate concurrent lung injury, by using grade 3 PGD at 24 hours as the outcome. There were 66 (21%) subjects with grade 3 PGD at 24 hours. The results of this analysis were similar to the primary analysis (Table S4b). There were 108 (34%) subjects with PGD grade 2 or 3 within 72 hours. Using grade 2 or 3 as an outcome, the biomarkers continued to have good discrimination, and our top-performing biomarkers did not change. 250 (80%) of subjects had PGD grades 1, 2, or 3 within 72 hours. Biomarkers did not perform as well using this outcome (Table S4c). There were 246 subjects with measurements of all five biomarkers, 71 (29%, 95% CI: 23%, 35%) developed grade 3 PGD within 72 hours of transplantation. The results using this sub-group were not different from the primary analysis (Table S4d).
Biomarker Prediction of 90-day Mortality
There were 304 subjects with information on 90-day mortality, of which 23 died (8%, 95% CI: 5%, 11%). 16 of the 23 had grade 3 PGD within 72 hours (70%, 95% CI: 47%, 87%). ICAM-1 and PAI-1 measured at 24 hours had the best individual prediction of 90-day mortality, with ICAM-1 with an AUC of 0.67 (95% CI: 0.54, 0.81) and PAI-1 with an AUC of 0.67 (95% CI 0.55, 0.80) (Figure 3). The addition of ICAM-1, a marker of cellular adhesion, to a marker of epithelial injury, sRAGE (AUC: 0.72, 95% CI: 0.60, 0.83) significantly improved prediction over ICAM-1 alone (p=0.05). In combination, ICAM-1+PAI-1 had an AUC (0.72, 95% CI: 0.60, 0.84) although it was not a significant improvement over ICAM-1 alone (p=0.39). Combinations of greater than two biomarkers did not add significant predictive ability.
Figure 3.
Top performing biomarkers measured at 24 hours for prediction of 90-day mortality
We evaluated incremental predictive utility of top performing biomarkers for 90-day mortality when added to a base model of concurrent PGD. PGD grade at 24 hours, as a categorical variable, had an AUC of 0.70 (95% CI: 0.59, 0.81) for 90-day mortality. Adding any of the individual biomarkers significantly increased prediction over PGD alone (p≤0.001), and the addition of our top performing combinations to PGD, ICAM-1+sRAGE+PGD had an AUC of 0.76 (95% CI: 0.65, 0.87), and ICAM-1+PAI-1+PGD had an AUC of 0.75 (95% CI: 0.63, 0.87) (Table 4). Therefore, the addition of biomarkers to clinical PGD grade added significant predictive value over PGD grade alone for 90-day mortality, demonstrating validity of the use of these biomarkers for prediction of early mortality.
Table 4.
Addition of biomarkers to PGD grade measured at 24 hours in predicting 90-day mortality.
| Biomarker | No. of subjects | AUC (95% CI) | P value |
|---|---|---|---|
| Grade of PGD at 24 hours (Base Model) | 304 | 0.70 (0.59, 0.81) | |
| Biomarker+Base Model | |||
| ICAM-1 | 267 | 0.75 (0.64, 0.86) | <0.001 |
| RAGE | 291 | 0.72 (0.61, 0.82) | <0.001 |
| SP-D | 264 | 0.72 (0.60, 0.83) | 0.001 |
| Protein C | 263 | 0.72 (0.59, 0.85) | <0.001 |
| PAI-1 | 263 | 0.73 (0.61, 0.86) | <0.001 |
| ICAM-1+PAI-1 | 253 | 0.75 (0.63, 0.87) | <0.001 |
| ICAM-1+sRAGE | 257 | 0.76 (0.65, 0.87) | <0.001 |
p value is for improvement over the base model
Discussion
We found that markers of impaired fibrinolysis (PAI-1) and epithelial injury (sRAGE) had the greatest individual discriminant ability for PGD, and the addition of PAI-1 improved the performance of markers of epithelial injury (SP-D) and cell adhesion (ICAM-1). Biomarkers measured at 24 hours had greater utility than 6 hours. Results were similar when evaluating a more severe phenotype, grade 3 PGD at 72 hours, as well as concurrent lung injury-grade 3 PGD at 24 hours. Furthermore, a marker of cell adhesion (ICAM-1) and a marker of impaired fibrinolysis (PAI-1) had the greatest individual predictive utility for 90-day mortality, and combinations of ICAM-1 with a marker of epithelial injury (sRAGE) or impaired fibrinolysis (PAI-1) had the greatest combined predictive utility for 90-day mortality after lung transplantation, above and beyond concurrent clinical PGD grading. These results suggest that the use of these lung injury biomarkers may enhance the definition of grade 3 PGD or may be used as an alternative to clinical PGD grading, particularly for use in translational research studies.
Our findings demonstrate that lung injury biomarkers reflect the clinical grading of PGD by discriminating lung injury within 72 hours of transplantation, as well as concurrent (at 24 hours) and persistent lung injury (at 72 hours). Biomarkers do not seem to perform as well with PGD grade 1. This may be because lower grades of PGD reflect a component of cardiogenic pulmonary edema, and the biomarkers are best suited for concurrent or persistent severe lung injury. Measurement of these biomarkers may provide a useful alternative to clinical PGD grading in small studies or early stage translational research where use of a continuous variable may provide more power. Discrimination of biomarkers was unchanged after adjustment by center, indicating biomarkers may be useful in a multi-center study, as they are not affected by practice variation across centers. Furthermore, as these markers may reflect specific lung injury mechanisms, early phase clinical trials aimed at reducing either epithelial injury or impaired fibrinolysis may choose to employ sRAGE or PAI-1 as useful measures for suggestion of treatment effect.
PGD is a known risk factor for early mortality after lung transplantation [15, 21–23]. We have demonstrated that biomarkers measured at 24 hours improve discrimination of concurrent clinical PGD grade for 90-day mortality. Therefore, these markers of lung injury may more fully capture a broader spectrum of lung injury than the categorical clinical PGD grade. We found that a marker of cell adhesion, ICAM-1, had the best individual prediction for mortality, but combinations with markers of epithelial injury (sRAGE), and impaired fibrinolysis (PAI-1), improved the ability to predict mortality. Therefore, measurement at 24 hours of the combination of a marker of cell adhesion with impaired fibrinolysis or epithelial injury could be used as an alternative outcome in translational research and early phase clinical trials aimed at reducing early mortality.
The biomarkers we have chosen represent epithelial injury, cell adhesion, and coagulation abnormalities. All of the chosen biomarkers have a previously demonstrated association with both PGD and non-transplant ALI [10–12, 14]. Our results indicate that sRAGE and PAI-1 can be used individually to provide continuous measures indicative of PGD. Combing PAI-1 with sRAGE or ICAM-1 seemed to improve the discrimination over that of the individual markers, although we were unable to demonstrate strict statistical improvement. Additionally, there appeared to be an interaction between ICAM-1 and RAGE with pre-transplantation diagnosis, and future studies may focus on identifying combinations of biomarkers within different sub-groups. Combinations of ICAM-1 with sRAGE or PAI-1 were useful in discriminating 90-day mortality. Although there is incremental gain as a greater number of biomarkers were analyzed, we were not able to demonstrate consistent statistical differences with more biomarkers, and the qualitative gains did not seem large enough to justify the use of more than two biomarkers as an alternate outcome. Currently, these biomarkers are best used as alternative or supplemental endpoints in early translational studies and small clinical trials. Perhaps after validation in an external population, biomarkers measured at 24 hours may be clinically useful in predicting early mortality [24].
There are several limitations to our study. Although our clinical data and plasma samples were collected prospectively, our analyses were limited by availability of sample volume for all assays. However, when analyses were limited to subjects with complete data on all biomarkers, there were no differences in the results. The dichotomous definition of grade 3 PGD is subject to misclassification bias. However, we have previously demonstrated construct validity to support use of grade 3 PGD as an outcome [6]; and sensitivity analyses did not demonstrate differences with measurement of PGD at different time points. Alternate analyses of PGD prediction were not possible as there were insufficient numbers of subjects with no clinical PGD at early timepoints who subsequently developed PGD at later timepoints. Future work may focus on using these biomarkers pre-operatively with clinical covariates to develop a predictive model for PGD. Our analyses were limited to five biomarkers with prior evidence of PGD association. In the future, other studies may explore addition of other biomarkers, including CRP, that have demonstrated predictive utility in other lung diseases [25, 26]. Cause of death was not available in this cohort, therefore, we cannot comment on the relationship between PGD and early mortality in this cohort. This should be addressed in a validation study. Finally, although this is a multicenter study using previously identified biomarkers, these results should be validated in another population, in particular, to address the possibility of a reduction in discriminant ability upon replication [24].
Our results demonstrate the utility of plasma biomarkers measured 24 hours after lung transplantation as alternative outcomes for translational PGD research. Combinations of PAI-1, sRAGE, and ICAM-1 had good discrimination for 90-day mortality, and added predictive utility to the clinical PGD definition, perhaps indicating a capture of subclinical lung injury. Future research utilizing these biomarkers as endpoints in translational research or early clinical trials is justified.
Supplementary Material
ABBREVIATIONS
- PGD
Primary graft dysfunction
- sRAGE
Soluble Receptor for Advance Glycation End Products
- ICAM-1
Intracellular Adhesion Molecule-1
- SP-D
Surfactant Protein-D
- PAI-1
Plasminogen Activator Inhibitor-1
- ALI
Acute Lung Injury
- LTOG
Lung Transplant Outcomes Group
- IPF
Idiopathic Pulmonary Fibrosis
- COPD
Chronic Obstructive Pulmonary Disease
- CF
Cystic Fibrosis
- PASP
Pulmonary Arterial Systolic Pressure
- IPAH
Idiopathic Pulmonary Arterial Hypertension
- ABG
Arterial Blood Gas
Footnotes
DISCLOSURES:
Drs. R. Shah, Diamond, Bellamy, Localio, Weinacker, Lama, Bhorade, Belperio, Crespo, Wille, Lee, Palmer, Orens, Reynolds, A. Shah, and Ware have nothing to disclose. Ms. Demissie and Ms. Wickersham have nothing to disclose. Dr. Lederer is on the steering committee for a clinical trial sponsored by Intermune, has received institutional research funding from Boehringer-Ingelheim and Gilead, has served on an advisory board for Gilead, and as a consultant for Gilead and ImmuneWorks, has pending institutional research funding from ImmuneWorks, and has research funding from the NIH. Dr. Wilkes is a co-founder of ImmuneWorks.
This study was funded by NIH grants HL087115, HL0861619, HL081332 and HL088263.
AUTHOR CONTRIBUTIONS:
Dr. R. Shah conducted statistical analyses and primarily wrote the manuscript. Drs. Bellamy and Localio conducted statistical analyses and edited the manuscript. Drs. Diamond, Kawut, Lederer, Weinacker, Lama, Bhorade, Belperio, Crespo, Wille, Lee, Palmer, Orens, Reynolds, A. Shah, Wilkes, and Ware assisted with subject recruitment and supervision of clinical assessments, sample collection and editing the manuscript. Ms. Demissie assisted with data collection and coordination across study centers. Dr. Christie supervised the data collection and the statistical analyses, assisted with interpretation of results, provided funding and edited the manuscript. Dr. Ware and Ms. Wickersham coordinated the performance of all plasma biomarker assays.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010;29(10):1104–18. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Christie JD, Keshavjee S. Primary Graft Dysfunction: Definition, Risk Factors, Short- and Long-Term Outcomes. Semin Respir Crit Care Med. 2010;31(02):161, 171. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 4.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of Immediate Primary Lung Allograft Dysfunction on Bronchiolitis Obliterans Syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, Robinson N, Localio AR, Wille K, Lama V, Palmer S, Orens J, Weinacker A, Crespo M, Demissie E, Kimmel SE, Kawut SM. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29(11):1231–9. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oto T, Griffiths AP, Levvey BJ, Pilcher DV, Williams TJ, Snell GI. Definitions of primary graft dysfunction after lung transplantation: differences between bilateral and single lung transplantation. J Thorac Cardiovasc Surg. 2006;132(1):140–7. doi: 10.1016/j.jtcvs.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Pepe MS, Cai T, Longton G. Combining predictors for classification using the area under the receiver operating characteristic curve. Biometrics. 2006;62(1):221–9. doi: 10.1111/j.1541-0420.2005.00420.x. [DOI] [PubMed] [Google Scholar]
- 9.Nahid P, Saukkonen J, Mac Kenzie W, Johnson JL, Phillips PJ, Anderson J, Bliven E, Belisle J, Boom H, Luetkemeyer A, Campbell T, Eisenach K, Hafner R, Lennox J, Makhene M, Swindells S, Villarino E, Weiner M, Benson C, Burman W. Tuberculosis Biomarker and Surrogate Endpoint Research Roadmap. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201105-0827WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, Milstone A, Orens J, Weinacker A, Demissie E, Bellamy S, Kawut SM. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, Orens J, Lama V, Wille K, Bellamy S, Shah C, Demissie E, Christie JD. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–8. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 12.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, Shah PD, Shah A, Weinacker A, Deutschman CS, Kohl BA, Demissie E, Bellamy S, Ware LB. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010–5. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelaez A, Force SD, Gal AA, Neujahr DC, Ramirez AM, Naik PM, Quintero DA, Pileggi AV, Easley KA, Echeverry R, Lawrence EC, Guidot DM, Mitchell PO. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant. 2010;10(4):900–7. doi: 10.1111/j.1600-6143.2009.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims MW, Beers MF, Ahya VN, Kawut SM, Sims KD, Lederer DJ, Palmer SM, Wille K, Lama V, Shah PD, Orens JB, Bhorade S, Crespo M, Weinacker A, Demissie E, Bellamy S, Christie JD, Ware LB. Effect of Single versus Bilateral Lung Transplantation on Plasma Surfactant Protein D Levels in Idiopathic Pulmonary Fibrosis. Chest. 2011 doi: 10.1378/chest.10-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, Kimmel SE. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–41. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 16.Christie JD, Van Raemdonck D, de Perrot M, Barr M, Keshavjee S, Arcasoy S, Orens J. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24(10):1451–3. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med. 2010;31(2):161–71. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 18.Janes H, Pepe MS. Adjusting for covariates in studies of diagnostic, screening, or prognostic markers: an old concept in a new setting. Am J Epidemiol. 2008;168(1):89–97. doi: 10.1093/aje/kwn099. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh MW, Pepe MS. Combining several screening tests: optimality of the risk score. Biometrics. 2002;58(3):657–64. doi: 10.1111/j.0006-341x.2002.00657.x. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 21.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, Kotloff RM. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127(1):161–5. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–6. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton CM, Iversen M, Milman N, Zemtsovski M, Carlsen J, Steinbruchel D, Mortensen J, Andersen CB. Outcome of lung transplanted patients with primary graft dysfunction. Eur J Cardiothorac Surg. 2007;31(1):75–82. doi: 10.1016/j.ejcts.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515–24. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 25.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–81. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 26.Krenn K, Klepetko W, Taghavi S, Lang G, Schneider B, Aharinejad S. Recipient vascular endothelial growth factor serum levels predict primary lung graft dysfunction. Am J Transplant. 2007;7(3):700–6. doi: 10.1111/j.1600-6143.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- 27.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med (Berl) 1996;74(1):13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 28.Flori HR, Ware LB, Glidden D, Matthay MA. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2003;4(3):315–21. doi: 10.1097/01.PCC.0000074583.27727.8E. [DOI] [PubMed] [Google Scholar]
- 29.Calfee CS, Eisner MD, Parsons PE, Thompson BT, Conner ER, Jr, Matthay MA, Ware LB. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009;35(2):248–57. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest. 2006;36(6):423–35. doi: 10.1111/j.1365-2362.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 31.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83(11):876–86. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 32.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, Arroliga AC. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant. 2007;26(7):675–80. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau CL, Zhao Y, Kim J, Kron IL, Sharma A, Yang Z, Laubach VE, Linden J, Ailawadi G, Pinsky DJ. Enhanced fibrinolysis protects against lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137(5):1241–8. doi: 10.1016/j.jtcvs.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L514–21. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.