Abstract
The nuclear factor-E2-related factor 2 (NRF2) serves as a master regulator in cellular defense against oxidative stress and chemical detoxification. However, persistent activation of NRF2 resulting from mutations of NRF2 and/or downregulation or mutations of its suppressor Kelch-like ECH-associated protein 1 (KEAP1) are associated with tumorigenicity and chemoresistance of non-small-cell lung carcinomas (NSCLCs). Thus, inhibiting NRF2-mediated adaptive antioxidant response is widely considered a promising strategy to prevent tumor growth and reverse chemoresistance in NSCLCs. Unexpectedly, stable knockdown of KEAP1 by lentiviral shRNA sensitized three independent NSCLC cell lines (A549, HTB-178 and HTB-182) to multiple chemotherapeutic agents, including arsenic trioxide (As2O3), etoposide and doxorubicin, despite moderately increased NRF2 levels. In lung adenocarcinoma epithelial A549 cells, silencing of KEAP1 augmented the expression of peroxisome proliferator-activated receptor γ (PPARγ) and genes associated with cell differentiation, including E-Cadherin and Gelsolin. In addition, KEAP1-knockdown A549 cells displayed attenuated expression of proto-oncogene Cyclin D1 and markers for cancer stem cells (CSCs), and reduced non-adherent sphere formation. Moreover, deficiency of KEAP1 led to elevated induction of PPARγ in response to As2O3. Pretreatment of A549 cells with PPARγ agonists activated PPARγ and augmented the cytotoxicity of As2O3. A mathematical model was formulated to advance a hypothesis that differential regulation of PPARγ and detoxification enzymes by KEAP1 and NRF2 may underpin the observed landscape changes in chemo-sensitivity. Collectively, suppression of KEAP1 expression in human NSCLC cells resulted in sensitization to chemotherapeutic agents, which may be attributed to activation of PPARγ and subsequent alterations in cell differentiation and CSC abundance.
Introduction
Nuclear factor-E2-related factor 2 (NRF2) is a master regulator of the transcription of many antioxidant and phase II detoxification enzymes [1]. Under normal homeostatic conditions, the low constitutive amount of NRF2 protein is mainly controlled by Kelch-like ECH-associated protein 1 (KEAP1)-mediated ubiquitination and the proteasomal degradation system [2]. Upon oxidative and/or electrophilic stress, the enzymatic activity of the KEAP1-Cullin3 E3 ubiquitin ligase is compromised, resulting in NRF2 stabilization and nuclear accumulation. Partnered with small Maf proteins, NRF2 binds to the antioxidant response elements (AREs) of target cytoprotective genes and augments their transcription [2, 3]. Thus, NRF2-mediated adaptive antioxidant response plays pivotal roles against oxidative/electrophilic stress and in chemical detoxification. As a result, activation of NRF2 has been demonstrated as an effective approach for cancer chemoprevention [4]. Paradoxically, a deleterious role of NRF2 activation in cancer progression has emerged, with evidence showing that the stress-response program is turned on in early tumour development and oncogene activity is coupled with NRF2 activation [5–7]. NRF2 and its downstream genes are overactivated/overexpressed in many cancer cells, thereby providing them a survival and growth advantage [8–10]. Most recently, DeNicola et al. reported that oncogene-induced NRF2 activation promotes reactive oxygen species (ROS) detoxification and tumorigenesis [6]. Since tumor cells may exploit the NRF2 pathway for their survival by deactivating chemotherapeutic agents [11], KEAP1 and NRF2 have been intensively investigated as a promising target to combat chemoresistance [2, 3, 12, 13].
Non-small-cell lung carcinoma (NSCLC) is the most common type of lung cancer, which is subdivided into squamous carcinoma, adenocarcinoma and large cell carcinoma. Currently, surgery, radiation and platinum-based chemotherapy are the standard treatment for NSCLCs. Compared to small cell carcinoma, NSCLCs are relatively insensitive to chemotherapy. Although the mechanism for the chemoresistance of NSCLC is poorly understood, low expression of KEAP1 and/or its inactivation due to mutations and attendant activation of NRF2 are common in NSCLC cells, suggesting persistent induction of cytoprotective and phase II enzymes by NRF2 underlie the enhanced resistance of NSCLC cells to chemotherapeutic agents [3, 11, 12, 14] and radiation [15]. Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors [16–19]. The expression of PPARγ was shown to correlate with the degree of differentiation and survival rate in lung cancer patients [20, 21]. In addition to adipogenic and anti-inflammatory effects, PPARγ activation was shown to modulate various hallmarks of cancer through its pleiotropic effects on different cell types in the tumor microenvironment. An overwhelming number of preclinical studies demonstrate the efficacy of PPARγ agonists in the control of tumor progression through their effects on various cellular processes, including differentiation, proliferation, apoptosis, angiogenesis, inflammation and metastasis [22]. Many PPARγ agonists, such as ciglitazone, troglitazone (Tro), pioglitazone and rosiglitazone (Rosi), were shown to inhibit tumor growth and progression in preclinical models of lung cancer, by influencing various signaling pathways in a PPARγ-dependent and independent manner [23–25]. We have recently demonstrated that NRF2 is an important nuclear factor regulating PPARγ expression in adipogenic differentiation [26]. It is intriguing to ascertain whether NRF2 is also involved in the regulation of PPARγ expression in other cell types, in particular NSCLC cells.
Arsenic trioxide (As2O3) is an anti-cancer drug approved by the U.S. Food and Drug Administration to specifically treat acute promyleocytic leukemia. As2O3 slows cancer cell growth by influencing multiple signaling pathways, including cell-cycle progression, differentiation and apoptosis [27]. As2O3 has also shown efficacy in treating other malignancies, particularly multiple myeloma and myelodysplastic syndromes [28]. Although As2O3 modulates DNA synthesis and apoptosis in lung carcinoma cells [29], NSCLCs are relatively resistant to As2O3 therapy, which may be associated with their enhanced NRF2 activity [30]. Inhibition of the NRF2-dependent antioxidant response through overexpression of KEAP1, knockdown of NRF2 or chemical inhibitors has been reported to render lung cancer cells more susceptible to chemotherapeutic agents [3, 13, 31, 32]. Our study found that silencing NRF2 in three independent NSCLC-derived cell lines – adenocarcinoma epithelial cell line A549, adenosquamous carcinoma cell line HTB-178 and squamous cell carcinoma cell line HTB-182 – sensitized them to multiple chemotherapeutic agents, including As2O3, etoposide and doxorubicin. Surprisingly stable knockdown of KEAP1 by lentiviral shRNA also sensitized the NSCLC cell lines to chemotherapeutic drugs, despite increased NRF2 activity attained. Suppression of KEAP1 in A549 cells resulted in increased expression of PPARγ, which was accompanied by induction of cell differentiation markers, attenuated expression of cancer stem cell (CSC) markers and reduced non-adherent sphere formation. A mathematical model was formulated to support the hypothesis that activation of PPARγ by KEAP1 knockdown could overcome the increase in chemoresistance resulting from NRF2 activation in these cancer cells, leading to enhanced chemo-sensitivity. Our studies provide new information about the mechanisms for chemoresistance of NSCLC, and raise new questions regarding the distinct roles of the KEAP1-NRF2 pathway and PPARγ in the process.
Materials and Methods
Cell culture and reagents
Human lung carcinoma A549 (#CCL-185), HTB-178 (#NCI-H596) and HTB-182 (#NCI-H520) cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). A549 cells were cultured with high-glucose DMEM (Invitrogen, Carlsbad, CA), whereas HTB-178 and HTB-182 cells were grown in the RPMI 1640 (ATCC) ; both media were supplemented with 10% fetal bovine serum (FBS), 100 U penicillin/ml, and 100 μg streptomycin/ml. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. Phosphate buffered saline (PBS, pH 7.4) and supplements for cell culture were purchased from Invitrogen. Rosiglitazone maleate (Rosi) was from SmithKline Beecham Pharmaceuticals (London, UK). All other reagents, including As2O3 and troglitazone (Tro), were purchased from Sigma-Aldrich (St. Louis, MO).
Lentiviral-based shRNA transduction
MISSION shRNA lentiviral particles were obtained from Sigma. Lentiviral transduction of A549, HTB-178 and HTB-182 lung cancer cell lines with particles for shRNAs targeting NRF2 (SHVRS-NM_010902), KEAP1 (SHVRS-NM_016679) or scrambled non-target negative control (sh-Scr/SCR, SHC002V) was performed as described previously [33]. The selection media for A549, HTB-178 and HTB-182 cells contained 3.0, 2.0 and 1.5 μg/ml of puromycin (Invitrogen), respectively. Stable cell lines were continuously grown in the media containing the same concentration of puromycin.
Non-adherent sphere formation (NASF) assay
NASF assay was performed as described previously [34]. In brief, KEAP1-KD A549 and SCR cells were plated at density of 1 X 104 viable cells/cm2 in 90-mm dishes. Following 48 h culture, the floating cells in medium were harvested by centrifuge. The resulted cell pellets were made into single-cell resuspensions with 4 ml complete medium with 10% FBS and seeded onto 30-mm Ultra low cluster plates (Corning Inc., Corning, NY). Cells were cultured for 14 days and 1 ml fresh complete medium was gently added every three days. On day 14, formed spheres were counted under a microscope and normalized by the number of attached cells.
Cell cycle analysis
Cell cycle analysis by flow cytometry was performed as described previously [25]. In brief, proliferating cells were trypsinized and washed two times with PBS and fixed in 70% ethanol-PBS. After 30 min of incubation at 4 °C, cells were centrifuged at 500 g for 10 min. The resulting cell pellet was resuspended and incubated at 37 °C for 30 min in 0.04 mg/ml propidium iodide (PI) and 1mg/ml RNAse A in PBS. The suspension was then analyzed on a flow cytometry (FACS CantoII with HTS option, BD Biosciences, San Jose, CA). The percentage of cells in the G0/G1, S and G2/M phases of cell cycle were determined by their DNA content using the FCS Express 4 Plus (De Novo Software, Los Angeles, CA).
Real-time RT-PCR analysis
Total RNAs were isolated with TRIzol reagent (Invitrogen). Quantitative real-time RT-PCR was performed as described previously [33]. The primers described in Online Table S1 were designed by using Primer Express 4 (Applied Biosystems) and synthesized by Bioneer Inc. (Alameda, CA). Real-time fluorescence detection was carried out by using an ABI PRISM 7900 Sequence Detector (Applied Biosystems). 18S ribosomal RNA (18S) was used for normalization.
Western blot analysis
Isolation of cell fractions and Western blotting were performed as detailed previously [33, 35]. Antibodies recognizing NRF2 (sc-13032; 1:500), KEAP1 (sc-15246; 1:500), heme oxygenase 1 (HO-1, sc-136902; 1:500), E-Cadherin (E-CAD; sc-8426; 1:500), OCT-4 (sc-102051; 1:500) and WNT3 (sc-5213; 1:500) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against PPARγ (2435S; 1:500) and β-ACTIN (A1978; 1:2000) were purchased from Cell Signaling Technology (Danvers, MA) and Sigma, respectively.
Immunostaining
Fluorescence immunostaining was performed as described previously [35]. Briefly, cells were grown on glass coverslips in six-well plate for 48 h. Then the cells were washed with PBS and fixed for 10 min at room temperature in 2% (v/v) formaldehyde. After washing in PBS, cells were permeabilized in 1% (v/v) Triton X-100 in PBS, washed, and incubated with 10% goat serum (sc-2043, Santa Cruz) in PBS for 1 h at room temperature. The cells were first treated with monoclonal mouse anti-E-CAD (sc-8426; 1:50, Santa Cruz) for 16 h at 4°C and subsequently with Rhodamine-linked goat-anti-mouse IgG1 (sc-2092,1:50, Santa Cruz) for 45 min at room temperature. After PBS washing, coverslips were mounted with the Prolong Gold antifade reagent with DAPI (P36931, Invitrogen) on microscope slides and examined using an Axio Observer Z1 fluorescence microscope (Carl Zeiss, Inc. Oberkochen, Germany).
Acute cytotoxicity assay
In vitro drug sensitivity to chemotherapeutic agents was assessed with 3-(4,5-dimethylthiazol-2-yl)-5-(3-Carboxymethoxyphenyl)-2-(sulfophenyl)-2H-tetrazolium (MTS) by using Cell Titer 96-Aqueous Assay Kit (Promega, Madison, WI) as detailed previously [36]. The cells were exposed to various concentrations of As2O3, etoposide or doxorubicin for 48 h. Measurements were expressed as percentage change from untreated control (Vehicle) of appropriate cells. Each data point represents a mean ± SD. The concentrations that were lethal to 50% of cells (LC50) were determined from analysis of the log-linear phase of the curves.
Cell death assessment by flow cytometry
A549 cells were seeded in a six-well plate and grown to approximately 70% confluence. After the cells were treated with As2O3 for 48 h, the floating and attached cells were harvested for apoptosis analysis. Apoptotic and necrotic cells were analyzed by flow cytometry (FACS CantoII with HTS option, BD Biosciences) using the TACS Annexin V-FITC Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) detailed previously [37]. For each sample, 10,000 cells were examined. The percent of apoptotic and necrotic cells was determined by statistical analysis of the various dot plots using CellQuest software (Diva 6.0, BD Biosciences).
Statistical analyses
All statistical analyses were performed by using Graphpad Prism 4 (GraphPad Software, San Diego, CA), with p < 0.05 taken as significant. For comparisons between two groups, t-test was performed. Statistical analyses to evaluate the effects of rosiglitazone or troglitazone on gene expression were carried out by using one-way ANOVA with Tukey’s Multiple Comparison Test. Statistical analyses to evaluate the time- and concentration-dependent effects of As2O3, etoposide and doxorubicin on gene expression and cell viability were performed by using two-way ANOVA with Bonferroni post hoc test.
Mathematical modeling
The mathematical model was constructed and simulated in MatLab (The Mathworks, Inc., Natick, MA, USA). It provided a provisional description of how the chemo-sensitivity landscape changes with varying KEAP1 and NRF2 levels, which are assumed to regulate the canonical antioxidant/phase II gene pathway and PPARγ pathway with different sensitivities. The two pathways have opposite effects on chemo-sensitivity. See online supporting material for model details.
Results
Stable knockdown of either NRF2 or KEAP1 in NSCLC cells results in sensitization to chemotherapeutic drugs
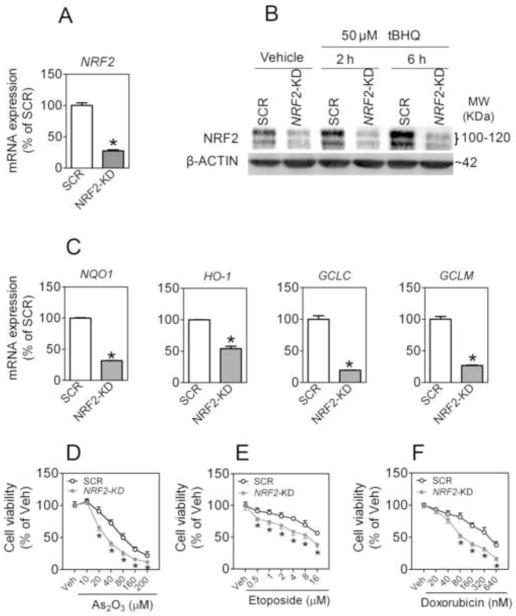
To study the role of the KEAP1-NRF2 pathway in chemoresistance of NSCLC cells, we investigated the effect of stable knockdown of NRF2 or KEAP1 on the susceptibility of various NSCLC cell lines to chemotherapeutic agents-induced cytotoxicity. In A549 cells, transduction of lentiviral shRNA against human NRF2 (termed NRF2-KD) significantly attenuated the mRNA level of NRF2 (Fig. 1A). The effectiveness of knockdown was confirmed by notably diminished protein accumulation of NRF2 in cells challenged with tBHQ, a potent NRF2 activator [38], as well as in control cells not treated with tBHQ (Fig. 1B). In addition, the expression of ARE-dependent genes, including NAD(P)H quinone oxidoreductase 1 (NQO1), HO-1, glutamate-cysteine ligase catalytic (GCLC) and regulatory subunit (GCLM), was significantly attenuated indicating that NRF2-mediated transcription was suppressed in NRF2-KD cells (Fig. 1C). Importantly, NRF2-KD cells displayed enhanced cytotoxicity in response to various chemotherapeutic agents, including As2O3, etoposide and doxorubicin (Fig. 1D, E and F).
Fig. 1.
Stable knockdown of NRF2 increases the susceptibility of A549 lung carcinoma cells to chemotherapeutic agents. (A) mRNA expression of NRF2 in A549 cells transduced with shRNA lentivirus targeted against human NRF2 or Scrambled non-target negative control (SCR). n = 3; *p < 0.05 vs. SCR. (B) Reduced protein expression of NRF2 in NRF2-KD cells under basal and tBHQ-treated conditions. Whole cell lysates were used for analysis and β-ACTIN was used as a loading control. Vehicle, medium. (C) Lack of NRF2 significantly reduces the expression of ARE-dependent genes. The expression of NQO1, HO-1, GCLC and CLCM was measured by real-time RT-PCR. (D–F) NRF2-KD cells are more sensitive to chemotherapeutic agents–induced cytotoxicity. Cell viability was assessed by MTT assay following 48 h treatment with the indicated concentrations of As2O3 (D), etoposide (E) or doxorubicin (F). Values are means ± SEM from 4 independent experiments, *p < 0.05 vs. SCR with the same treatment.
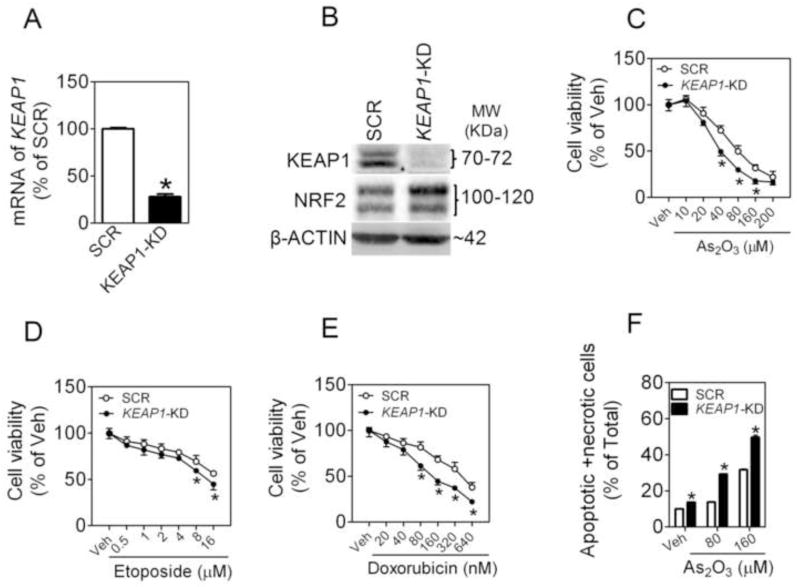
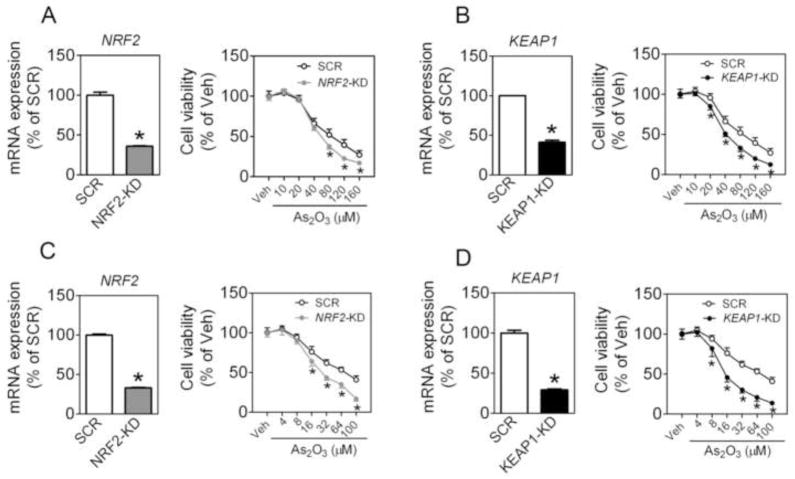
A549 cells transduced with lentiviral shRNA against human KEAP1 (termed KEAP1-KD) showed efficient knockdown of KEAP1 expression (Fig. 2A). Supporting the silencing effect of KEAP1, markedly reduced protein levels of KEAP1 (Fig. 2B), moderately increased protein levels of NRF2 (Fig. 2B), and induction of ARE-dependent genes (Online Figure S1) were observed in KEAP1-KD cells. Surprisingly, silencing KEAP1 rendered the cells somewhat more susceptible, rather than resistant, to cytotoxicity induced by As2O3, doxorubicin or etoposide (Fig. 2C, D and E). This unexpected sensitization to cytotoxicity was further confirmed by measurement of apoptosis and necrosis by using flow cytometry with Annexin V-FITC and PI double staining (Fig. 2F). To ascertain whether the finding is a common phenomenon in NSCLC cells, we investigated the effects of silencing NRF2 or KEAP1 on As2O3-induced cytotoxicity in lung adenosquamous carcinoma cell line HTB-178 cells and squamous cell carcinoma cell line HTB-182 cells. As with A549 cells, knockdown of either NRF2 or KEAP1 in both cell lines resulted in significantly increased sensitivity to As2O3 toxicity (Fig. 3).
Fig. 2.
Stable knockdown of KEAP1 expression by lentiviral shRNA sensitizes A549 cells to chemotherapeutic agents-induced cytotoxicity. (A) Reduced mRNA expression of KEAP1 in KEAP1-KD cells. SCR, Scrambled non-target negative control; KEAP1-KD, KEAP1-knockdown. n = 3; *p < 0.05 vs. SCR. (B) Protein levels of KEAP1 and NRF2 in SCR and KEAP1-KD cells. (C–E) KEAP1-KD cells are more sensitive to chemotherapeutic agents–induced cytotoxicity. Cell viability was assessed by MTT assay following 48 h treatment with the indicated doses of As2O3 (C), etoposide (D), or doxorubicin (E). (F) Apoptotic and necrotic cell death in response to As2O3 treatment was measured using flow cytometry. Cells were treated with As2O3 for 48 h. n = 4. *p <0.05 vs. SCR with the same treatment.
Fig. 3.
Effect of stable knockdown of NRF2 or KEAP1 on As2O3-induced cytotoxicity in HTB-178 and HTB-182 lung carcinoma cells. (A and C) Knockdown of NRF2 sensitizes HTB-178 (A) and HTB-182 cells (C) cells to As2O3-induced cytotoxicity. Left panel, mRNA expression of NRF2; Right panel, Cell viability was assessed by MTT assay following 48 h treatment with the indicated concentrations of As2O3. n = 3. *p < 0.05 vs. SCR with the same treatment. (B and D) Silence of KEAP1 in HTB-178 (B) and HTB-182 cells (D) increases their sensitivity to As2O3-induced cytotoxicity. Left panel, mRNA expression of KEAP1; Right panel, Cell viability was assessed by the MTT assay following 48 h treatment with the indicated concentrations of As2O3.
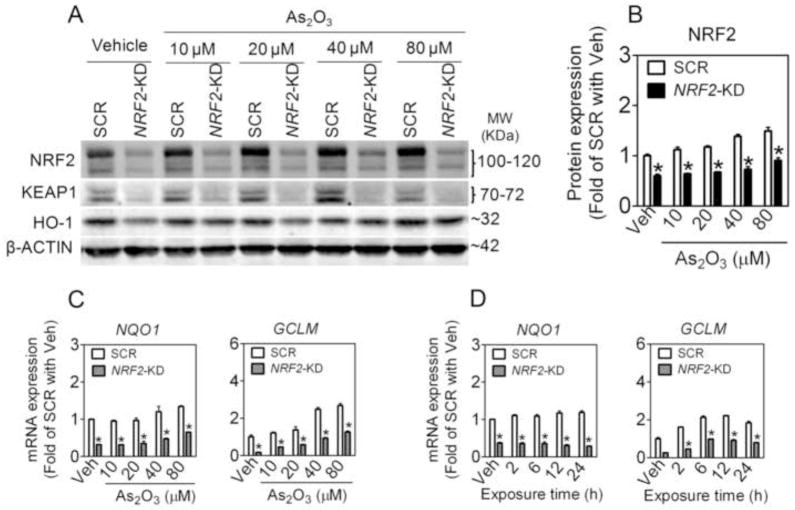
Effects of silencing NRF2 or KEAP1 on As2O3-induced antioxidant response in A549 cells
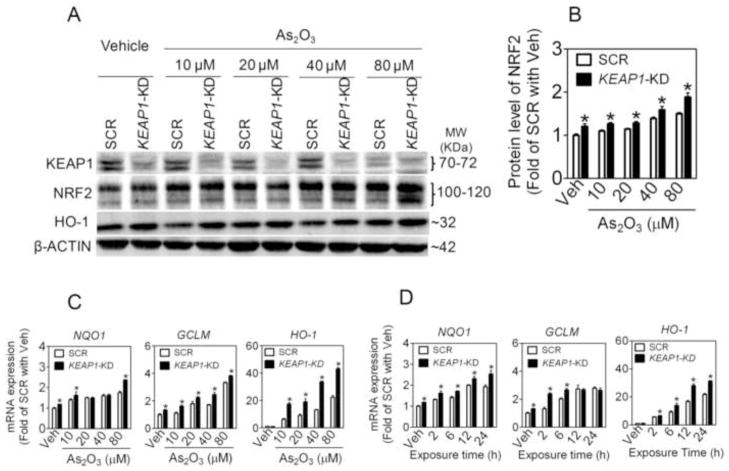
To define the role of NRF2-mediated antioxidant response in the chemoresistance of NSCLC cells, the expression of NRF2 and some ARE-dependent genes, including NQO1, GCLM and HO-1, were determined in SCR, NRF2-KD, and KEAP1-KD A549 cells following As2O3 exposure. In SCR cells NRF2 protein accumulation and ARE-dependent gene expression increased in a time- and concentration-dependent manner (Fig. 4 and 5), confirming our previous findings that arsenite is a potent NRF2 activator [35, 38]. In contrast, NRF2-KD cells exhibited dramatically attenuated expression of NRF2 and ARE-dependent genes under both basal and As2O3-treated conditions (Fig. 4). In addition, deficiency of NRF2 resulted in attenuated mRNA (not shown) and protein expression of KEAP1 under basal and As2O3-treated conditions (Fig. 4), which is consistent with the finding by Lee et al. [39] who reported that NRF2 regulates the transcription of KEAP1 through a specific ARE. As expected, in KEAP1-KD cells, enhanced NRF2 activity was observed, as measured by its protein accumulation and downstream gene and protein expression under both basal and As2O3-treated conditions (Fig. 5). The immunoblotting of HO-1 confirmed its gene expression in SCR, NRF2-KD and KEAP1-KD cells (Fig. 4 and 5).
Fig. 4.
Lack of NRF2 reduces NRF2-mediated antioxidant response induced by As2O3 in A549 cells. (A) Representative images of immunoblottings of NRF2, KEAP1 and HO-1. 80% confluent cells were treated with Vehicle (medium) or indicated concentrations of As2O3 for 6 h. Whole cell lysates were used for analysis and β-ACTIN was used as a loading control. (B) Quantification of NRF2 bands of three independent immunoblottings. Veh, Vehicle. n = 3; *p < 0.05 vs. SCR with the same treatment. (C) Concentration-response of As2O3-induced ARE-dependent gene expression. Cells were treated with As2O3 for 6 h. n = 3; *p < 0.05 vs. SCR with the same treatment. (D) Time-course of ARE-dependent gene expression induced by As2O3. Cells were treated with 40 μM of As2O3 for indicated time.
Fig. 5.
Silencing of KEAP1 in A549 cells augments NRF2-mediated antioxidant response in response to As2O3 treatment. (A) Representative images of immunoblottings of KEAP1, NRF2 and HO-1. 80% confluent cells were treated with Vehicle (Veh, medium) or indicated concentrations of As2O3 for 6 h. Whole cell lysates were used for immunoblotting. (B) Quantification of NRF2 bands of three independent immunoblottings. n = 3; *p < 0.05 vs. SCR with the same treatment. (C) Concentration-response of As2O3-induced ARE-dependent gene expression. Cells were treated with As2O3 for 6 h. n = 3; *p < 0.05 vs. SCR with the same treatment. (D) Time-course of ARE-dependent gene expression induced by As2O3. Cells were treated with 40 μM of As2O3 for indicated time.
Effects of silencing NRF2 or KEAP1 in A549 cells on expression of PPARγ and markers associated with cell differentiation and CSC attributes
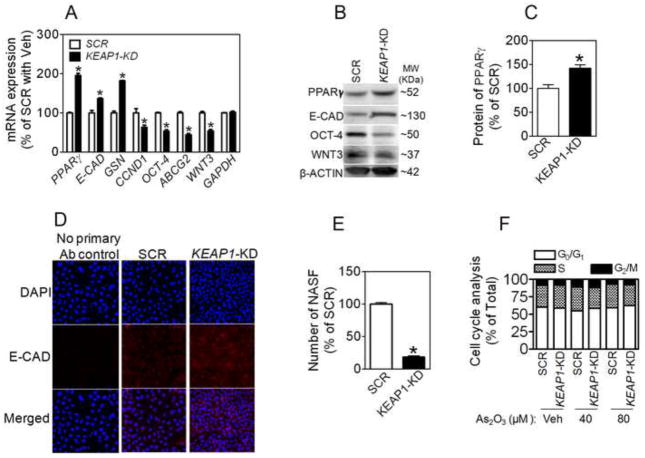
NRF2 is a key transcription factor regulating PPARγ gene expression [40, 41]. In A549 cells, we found that knockdown of NRF2 reduced the expression of PPARγ at mRNA and protein levels (Online Figure S2). In contrast, KEAP1 silencing resulted in a significantly increased expression of PPARγ at mRNA and protein level (Fig. 6A-C). Since NRF2 level is only moderately increased in KEAP1-KD cells (Fig. 2B and Fig. 5A and B), it is highly likely that the significantly increased PPARγ expression by KEAP1 silencing requires an NRF2-independent mechanism as well. Consistent with the pleiotropic roles of PPARγ in cell differentiation and proliferation, KEAP1-KD cells displayed significantly increased expression of E-CAD and GELSOLIN (GSN) and attenuated expression of proto-oncogene CYCLIN D1 (CCND1) (Fig. 6A, B and D). In addition, KEAP1-KD cells expressed reduced levels of several CSC markers, including OCT-4, ABCG2 and WNT3 (Fig. 6A and B), followed by decreased non-adherent sphere formation (Fig. 6E), suggesting silencing KEAP1 in A549 cells promotes differentiation of CSC cells. However, cell cycle analysis revealed that knockdown of KEAP1 did not significantly affect cell cycle, even under high concentrations of As2O3-treated conditions (Fig. 6F)
Fig. 6.
Effect of stable knockdown of KEAP1 on expression of PPARγ and markers of differentiation and CSC features at mRNA (A) and protein (B) levels in A549 cells. n = 3; *p < 0.05 vs. SCR. (C) Quantification of PPARγ expression in (B). n = 3; *p < 0.05 vs. SCR. (D) Immunostaining of E-CAD in SCR and KEAP1-KD cells. (E) Quantification of NASF formed in SCR and KEAP1-KD cells. (F) Cell cycle analysis of SCR and KEAP1-KD cells. Cells were treated with As2O3 or Vehicle (Veh, medium) for 24 hours.
As2O3 increases PPARγ expression in A549 cells
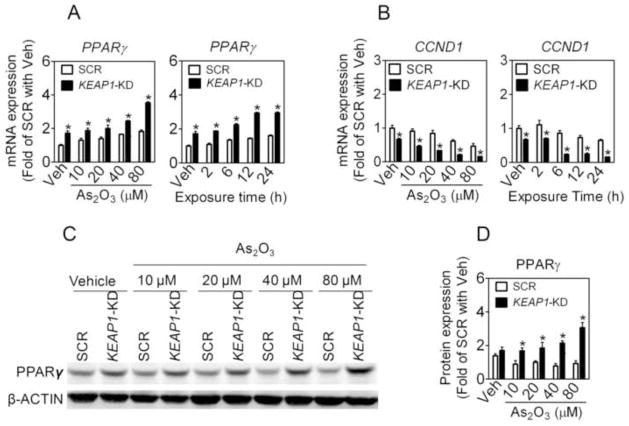
As shown in Online Figure S3 and Fig. 7A, As2O3 treatment caused a concentration- and time-dependent increase in the mRNA expression of PPARγ in SCR A549 cells. Knockdown of NRF2 significantly lowered As2O3-induced PPARγ induction (Online Figure S3). In contrast, deficiency of KEAP1 led to elevated mRNA expression of PPARγ, under both basal and As2O3-treated conditions (Fig. 7A). In addition, As2O3 concentration-dependently increased the protein level of PPARγ in KEAP1-KD A549 cells (Fig. 7C and D). Furthermore, KEAP1-KD cells exhibited a dramatic reduction in CCND1 expression under both basal and As2O3-treated conditions (Fig. 7B).
Fig. 7.
Knockdown of KEAP1 in A549 cells augments the expression of PPARγ under basal and As2O3-treated conditions. (A) mRNA expression of PPARγ. Cells were treated with As2O3 for 6 h at indicated concentrations (left panel) or 40 μM of As2O3 for indicated time (right panel). *, p < 0.05 vs SCR with the same treatment. (B) KEAP1-KD cells show reduced mRNA expression of CCND1 in response to As2O3 treatment. Cells were treated as (A). (C) Protein level of PPARγ. Cells were treated with As2O3 for 6 h at indicated concentrations. (D) Quantification of (C). n = 3; *p < 0.05 vs. SCR.
PPARγ agonists potentiate the toxic effect of As2O3 in A549 cells
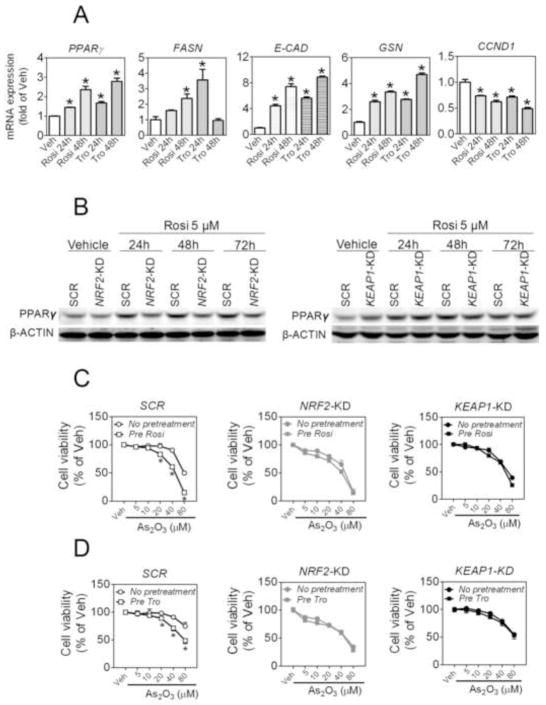
To verify that activation of PPARγ resulting from KEAP1 silencing is involved in the sensitization to chemotheraputic agents, we investigated the effect of PPARγ agonists on As2O3-induced cytotoxicity in A549 cells. As shown in Fig. 8A, non-cytotoxic levels of PPARγ agonists Rosi (5 μM) and Tro (5 μM) (Online Figure S4) time-dependently increased PPARγ mRNA expression in A549 cells. Consistent with the regulatory role of PPARγ in cell differentiation, increased mRNA expression of fatty acid synthsse (FASN), E-CAD and GSN and reduced expression of CCND1 were observed in both Rosi- and Tro-treated cells. In NRF2-KD cells, a substantially reduced expression of PPARγ was observed under Rosi-treated conditions (Fig. 8B). Knockdown of KEAP1 enhanced the basal expression of PPARγ, however, no additional induction by Rosi was observed in KEAP1-KD A549 cells. Interestingly, pretreatment of SCR A549 cells with 5 μM Rosi or Tro for 48 h significantly sensitized the cells to As2O3-induced cytotoxicity (Fig. 8C and D). However, the same pretreatments with Rosi or Tro exhibited no tangible sensitization to As2O3-induced cytotoxicity in NRF2-KD and KEAP1-KD cells (Fig. 8C and D).
Fig. 8.
PPARγ agonists activate PPARγ in A549 cells and sensitize the cells to As2O3–induced cytotoxicity in a NRF2-dependent fashion. (A) Effects of rosiglitazone and troglitazone on mRNA expression of PPARγ, FASN, E-CAD, GSN and CCND1 in A549 cells. A549 cells were treated with rosiglitazone (5 μM), troglitazone (10 μM) or Vehicle (Veh, medium) for 24 h and 48 h. *p < 0.05 vs. Veh. (B) Effects of rosiglitazone on protein expression of PPARγ in NRF2-KD (left panel) and KEAP1-KD cells (right panel). PPARγ expression was determined using immunoblotting. (C and D) Effects of rosiglitazone (C) and troglitazone (D) on As2O3–induced cytotoxicity in SCR (left), NRF2-KD (middle) and KEAP1-KD (right) A549 cells. Cells were pretreated with rosiglitazone (5 μM) or troglitazone (10 μM) for 48 h followed by subsequent 48 h As2O3 treatment. NRF2-KD, NRF2-knockdown; KEAP1-KD, KEAP1-knockdown; SCR, Scramble. *p < 0.05 vs. SCR with the same treatment.
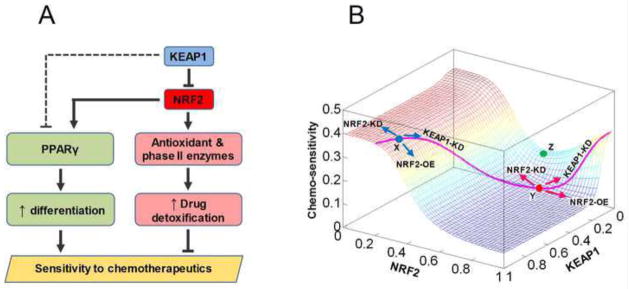
Mathematical model of modulation of chemo-sensitivity by KEAP1 and NRF2
Based on the above observations, we propose that KEAP1 and NRF2 regulate two opposing pathways with differential sensitivities to modulate the susceptibility of NSCLC cells to the killing effect of chemotherapeutic agents (Fig. 9A). With the canonical ARE pathway, an increase in NRF2 upregulates endogenous antioxidant and phase II enzyme expression. By enhancing detoxification of ROS and xenobiotics, elevated antioxidant and phase II enzyme levels provide cells survival advantage against cytotoxicity of ROS derived from immune cells and against chemotherapeutic agents (Fig. 9A, pink blocks). The current study suggests KEAP1 and NRF2 could also regulate a second, novel pathway involved in cancer sensitivity to chemotherapeutic agents. PPARγ can be directly activated by NRF2 and indirectly repressed by KEAP1 in an NRF2-dependent manner as well as in a yet-to-be-characterized NRF2-independent manner (Fig. 9A dashed line). By inhibiting cell proliferation and promoting differentiation, PPARγ reduces the self-renewing potential of cancer cells, leading to enhanced sensitivity to the killing effect of chemotherapeutic agents (Fig. 9A, green blocks). However, activation of this PPARγ-mediated pathway requires lower KEAP1 and higher NRF2 levels than would be required to activate the antioxidant/phase II enzyme pathway. Therefore a moderate decrease in KEAP1 and increase in NRF2 would first upregulate phase II enzymes, decreasing sensitivity to the toxicity of therapeutic agents. Only upon further decrease in KEAP1 and increase in NRF2 levels would PPARγ be significantly activated to promote cell differentiation and inhibit proliferation, leading to increases in the sensitivity to chemotherapeutics.
Fig. 9.
KEAP1 and NRF2 may modulate sensitivity of cells to chemotherapeutic agents through differentially regulating two opposing pathways. (A) In NSCLC cells, moderate downregulation of KEAP1 and activation of the canonical NRF2-ARE pathway due to mutations upregulate antioxidant and phase II enzymes without appreciably activating the PPARγ pathway. Augmented drug detoxification capacity reduces the sensitivity of cancer cells to chemotherapeutics. Further knockdown of KEAP1, which is already lower than in normal cells, would lift its inhibition on PPAR through an unknown mechanism (dashed line), permitting activation of PPARγ by NRF2. Increased PPARγ promotes cancer cell differentiation and inhibits proliferation, thus enhancing the killing effect of chemotherapeutic agents. (B) The mathematical model recapitulated the differential responses of normal cells and cancer cells to knockdown of KEAP1. The landscape describes how chemo-sensitivity changes as KEAP1 and NRF2 levels are independently varied. In normal cells (represented by location X, blue dot) containing high KEAP1 and low NRF2, knockdown of KEAP1 results in reduced sensitivity of cells to the toxicity of chemotherapeutic agents. In some cancer cells (represented by location Y, red dot) containing relatively lower KEAP1 and higher NRF2, further knockdown of KEAP1 would result in increased sensitivity to chemotherapeutics. In other cases, combined mutations in KEAP1 and NRF2 may push cells to a location such as Z (green dot), where both increasing and decreasing NRF2 would increase chemo-sensitivity. The pink curve delineates the changes in NRF2 and chemo-sensitivity as the KEAP1 level is independently varied in the model.
To investigate whether the above proposed mechanism is plausible, we formulated a simple mathematical model to capture the differential regulation of the antioxidant/phase II enzyme and PPARγ pathways by KEAP1 and NRF2 as illustrated in Fig. 9A (see online supporting material for model details). The model (Fig. 9B) produced a nonmonotonic landscape that describes how chemo-sensitivity might be altered as KEAP1 and NRF2 levels vary. Here chemo-sensitivity is defined as a function that correlates positively to PPARγ and negatively to antioxidant/phase II enzymes. A normal cell situated at location X on the landscape (blue dot) has high KEAP1 and low NRF2 levels. From this point, overexpression of NRF2 (NRF2-OE) decreases chemo-sensitivity by upregulating antioxidant/phase II enzymes (Online Figure S5A). Knockdown of NRF2 has an opposite effect on chemo-sensitivity by further decreasing the basal antioxidant/phase II enzyme level. KEAP1-KD cells, which acquire increased NRF2 activity, follow a trajectory delineated by the pink curve and exhibit decreased chemo-sensitivity due to increased expression of antioxidant/phase II enzymes (Online Figure S5A). Thus, the model recapitulates the effects of genetically manipulating NRF2 and KEAP1 in normal cells. In certain cells, including NSCLC cells, mutations in KEAP1 alone move the cellular state from location X to a state of less chemo-sensitivity (Location Y; Fig. 9B, red dot) with lower KEAP1 and higher NRF2. This state confers cells higher resistance (lower chemo-sensitivity) to chemotherapeutic agents through upregulation of antioxidant/phase II enzymes, yet the PPARγ level is only marginally increased due to its lower sensitivity to changes in KEAP1 and NRF2 levels (Online Figure S5). As expected, knockdown of NRF2 in these cells still leads to increased chemo-sensitivity. But more importantly, knockdown of KEAP1 also increases the sensitivity to chemotherapeutics (Fig. 9B). The latter occurs because knockdown of KEAP1 – which reduces KEAP1 to further lower levels – would lift its inhibition on PPARγ expression and allow the activation of PPARγ by NRF2 fully manifested (Online Figure S5B). The resulting significant upregulation of PPARγ would lead to an increase in chemo-sensitivity. In the absence of KEAP1 knockdown, overexpression of NRF2 alone either has no effect or only marginally increases chemo-sensitivity.
Discussion
Despite advances in developing effective therapeutic agents, chemoresistance remains the top obstacle in the treatment of NSCLCs. In keeping with the literature our current study supports the notion that inhibiting NRF2 sensitizes NSCLC cells to cytotoxicity induced by a variety of chemotherapeutic drugs, including As2O3, etoposide and doxorubicin. Unexpectedly, stable knockdown of KEAP1 in three independent NSCLC cell lines - A549, HTB-178 and HTB-182 cells - also resulted in sensitization to these drugs, despite moderately increased NRF2 activity attained with KEAP1 silencing. Induction of PPARγ and subsequent alterations in cell differentiation and CSC markers and/or abundance in A549 cells are associated with the phenotype of KEAP1-KD cells in response to chemotherapeutic drugs. The new finding that KEAP1-KD NSCLC cells exhibited enhanced sensitivity to multiple chemotherapeutic agents is different from that seen in normal human cells, such as HaCaT keratinocytes, where stable knockdown of KEAP1 resulted in NRF2 activation and resistance to As2O3 toxicity [42]. The distinctive phenotype of knockdown of KEAP1 in NSCLC cells indicates that the landscape of chemoresistance with respect to KEAP1 and NRF2 in these cells is altered.
Persistent activation of NRF2 resulting from missense mutations, insertions or deletions in KEAP1 and/or NRF2 genes or downregulation of KEAP1 are associated with enhanced resistance of NSCLCs to chemotherapeutic agents and radiation therapy [2, 5, 15]. The A549 cells have a mutation in the Kelch domain of KEAP1 (Gly333 to Cys) [11]. In these cells, reduced mRNA expression of KEAP1 has been attributed to hypermethylation of CpG sites in the KEAP1 (47) and these cells express high basal levels of NRF2, and many ARE-dependent antioxidant and detoxification enzymes. Stable knockdown of the mutated KEAP1 in A549 cells resulted in an additional increase in the basal expression level of NRF2 and its target genes, suggesting the mutated KEAP1 retains some residual activity to suppress NRF2. This interpretation is supported by the work of Tirumalai et al. [43] who demonstrates that acrolein stabilizes NRF2, increases ARE-DNA binding activity, induces ARE-mediated reporter activity, and induces GCLC and NQO1 in A549 cells. In addition, As2O3 caused an enhanced NRF2-mediated antioxidant response in KEAP1-KD cells. Thus, changes in expression of detoxification enzymes and antioxidants, all of which increase in KEAP1-KD cells, cannot explain the increased sensitivity to chemotherapeutic agents by KEAP1 silencing.
The frequent expression of PPARγ in various cancer tissues and cells, including NSCLC cells and tumor samples [25], and the ability of PPARγ agonists to inhibit cell proliferation and angiogenesis, promote differentiation and induce apoptosis suggest that PPARγ may play an important role in chemotherapy. Indeed, PPARγ agonists are efficacious in control of tumor progression in a PPARγ-dependent manner [22–25, 44, 45]. NRF2 is an important nuclear factor regulating PPARγ expression during adipogenesis [26]. Stable knockdown of Keap1 in 3T3-L1 cells results in increased expression of PPARγ and accelerated adipogenic differentiation [26]. Our studies here show that the sensitization to chemotherapeutic agents observed in KEAP1-KD cells is associated with induction of PPARγ expression and altered cell differentiation. Of note, NRF2 activation alone, such as As2O3 treatment in SCR A549 cells (Fig. 7A and C), had no significant effect on PPARγ expression. In addition, both Rosi and Tro had no effect on NRF2 protein accumulation in A549 cells (data not shown). These findings suggest that NRF2 is necessary, but not sufficient, for PPARγ induction in A549 cells.
As expected, KEAP1-KD A549 cells had augmented expression of PPARγ, and enhanced expression of genes associated with cell differentiation, including E-CAD and GSN, and downregulation of proto-oncogene CCND1. E-CAD is a marker of cell–cell junctions, which is important in the control of invasion and migration of cancer cells. GSN is a general differentiation marker induced during PPARγ-mediated differentiation [20]. PPARγ agonists promote cell cycle arrest by downregulating CCND1 in several tumour cell lines, including NSCLC cells [20, 46–48]. In the current study, a reduced expression of CCND1 accompanied with PPARγ upregulation occurred in KEAP1-KD cells. However, KEAP1 silencing did not significantly affect cell cycle, even under high concentrations of As2O3-treated conditions, suggesting that the alteration in cell differentiation is the major contributor to their increased sensitivity to chemotherapeutic agents.
A primary mechanism for As2O3’s effectiveness in treating cancers, in particular acute promyelocytic leukemia, is induction of apoptosis and differentiation and inhibition of proliferation [27]. In A549 cells, As2O3 dose-dependently enhanced the expression of PPARγ, suggesting As2O3 may stimulate NSCLC cell differentiation through induction of PPARγ. We saw a dramatic induction of PPARγ in response to As2O3 treatment followed by induction of E-CAD in KEAP1-KD A549 cells. Interestingly, non-cytotoxic levels of PPARγ agonists Rosi and Tro significantly enhanced the inhibitory effect of As2O3 on cell viability in SCR A549 cells. In contrast, no further inhibition was observed by Rosi and Tro pretreatment in KEAP1-KD cells. In these cells, PPARγ may already be close to maximal activation and non-responsive to further alteration by the agonists. Rosi and Tro pretreatment did not potentiate the toxicity of As2O3 in NRF2-KD cells, likely because silencing NRF2 already diminished the cellular detoxification capacity and substantially enhanced the sensitivity to toxicity of As2O3. These findings suggest that combination of PPARγ agoinsts with classical anti-tumor drugs may be a novel approach for combating chemoresistance in NSCLC treatment. Despite the fact that the induction of PPARγ expression resulting from KEAP1 silencing, might require NRF2, activation of NRF2 alone is not sufficient to significantly induce PPARγ. For instance, chemical activators of NRF2, such as As2O3 and tBHQ, have no significant effect on PPARγ expression in SCR A549 cells even though they both markedly increase NRF2. These results suggest that downregulation of KEAP1 is also necessary for maximal induction of PPARγ in NSCLC cells, potentially through an NRF2-independent mechanism.
CSCs are a small reservoir of self-sustaining cells with exclusive ability of self-renewal and tumor maintenance [49]. Cancer is most likely a disease of stem cells. More and more studies suggest that CSCs play a role in the formation and progression of tumours, such as chemoresistance, metastasis, and recurrence [50]. It is known that there is a differential distribution of progenitor cell markers among different histological types of lung cancer and that poorly differentiated tumors have the highest expression of stem cell markers [51]. Given that stem-like cells often display higher tolerance to cytotoxins [52], the sensitization of NSCLC cells by KEAP1 silencing to chemotherapeutic agents is likely to decrease the CSC population. We did find decreased expression of CSC markers, including ABCG2, WNT3 and OCT-4, and reduced NASF along with increased PPARγ expression in KEAP1-KD A549 cells. ABCG2 is molecular determinant of the side population (SP) phenotype [53, 54], and its expression is high in SP from A549 cells. Canonical WNT signaling plays an important role in lung CSCs [52]. OCT-4 is a marker of embryonic stem cells and a biological marker of lung CSCs. Thus, reduction of CSCs or cells with CSC attributes may explain some of the sensitization of KEAP1-KD cells to chemotherapeutic drugs.
In summary, our study highlights that the KEAP1-NRF2 pathway may be a double-edged sword in combating chemoresistance in NSCLC treatment. When KEAP1 and NRF2 are considered as a target to enhance chemo-sensitivity, the effect on differentiation and proliferation of CSCs may prove a serious liability for using this mode of action in chemotherapeutics. Combined mutations of KEAP1 and NRF2 may situate cancer cells in various locations on the landscape of chemo-sensitivity, leading to non-monotonic responses to manipulation of KEAP1 or NRF2 activities. If this hypothesis is true, an effective approach to increase cancer cell sensitivity to chemotherapeutic drugs would be simultaneous to silence both NRF2 and KEAP1, a proposal that sounds counter-intuitive. PPAR3 may become a new target to overcome chemoresistance in NSCLC treatment. Clearly, the physiological and pathophysiological role(s) of KEAP1, NRF2 and PPARγ will benefit from further investigations.
Supplementary Material
Highlights.
Silencing KEAP1 in NSCLC cells results in sensitization to chemotherapeutic agents.
Knockdown of KEAP1 augments PPARγ expression and cell differentiation-related genes.
Deficiency of KEAP1 leads to elevated induction of PPARγ in response to As2O3.
PPARγ agonists augment As2O3–induced cytotoxicity in NSCLC cells.
NRF2 and KEAP1 are involved in the regulation of PPARγ expression.
Acknowledgments
This research was supported in part by the NIH grant ES016005 (to J.P.), the DOW Chemical Company (to M.E.A.) and Unilever (to M.E.A.). We are grateful for Drs. Bin Sun and Joe Trask for their technical assistance.
List of Abbreviation
- ARE
antioxidant response element
- As2O3
arsenic trioxide
- CCND1
CYCLIN D1
- CSC
cancer stem cell
- E-CAD
E-CADHERIN
- FBS
fetal bovine serum
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase regulatory subunit
- GSN
GELSOLIN
- HO1
heme oxygenase 1
- KD
knockdown
- KEAP1
Kelch-like ECH-associated protein 1
- NASF
non-adherent sphere formation
- NQO1
NAD(P)H quinone oxidoreductase 1
- NRF2
nuclear factor-E2-related factor 2
- NSCLC
non-small-cell lung carcinoma
- OE
overexpression
- PPARγ
peroxisome proliferator-activated receptor γ
- Rosi
rosiglitazone
- SCR
scramble
- SP
side population
- tBHQ
tert-Butylhydroquinone
- Tro
troglitazone
Footnotes
Author Disclosure Statement
The content is solely the responsibility of the authors. All authors have agreed to its content. MEA received some funding from DOW Chemical Company and Unilever. All of the other authors declare no competing financial interests.
L.Z., H.Z., Q.Z., C.G.W, P.X., Y.H., J.F., K.Y., M.E.A. and J.P. are employees of The Hamner Institutes for Health Sciences. The Hamner is a 501(c)3 not-for-profit organization that has a diverse research portfolio that includes funding from the American Chemical Council, a trade association that represents chemical manufacturers. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), NIH, however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maher J, Yamamoto M. The rise of antioxidant signaling -- the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera RM, Bardeesy N. Cancer: when antioxidants are bad. Nature. 475:43–44. doi: 10.1038/475043a. [DOI] [PubMed] [Google Scholar]
- 8.Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, Lloyd B, Copple IM, Williams S, Owen A, Neoptolemos JP, Goldring CE, Park BK. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer. 10:37. doi: 10.1186/1476-4598-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinopoulos PA, Spentzos D, Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, Hecht JL, Cannistra SA. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- 10.Rushworth SA, Bowles KM, MacEwan DJ. High basal nuclear levels of Nrf2 in acute myeloid leukemia reduces sensitivity to proteasome inhibitors. Cancer Res. 71:1999–2009. doi: 10.1158/0008-5472.CAN-10-3018. [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1 NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 13.Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solis LM, Behrens C, Dong W, Suraokar M, Ozburn NC, Moran CA, Corvalan AH, Biswal S, Swisher SG, Bekele BN, Minna JD, Stewart DJ, Wistuba II. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal. 2010;13:1627–1637. doi: 10.1089/ars.2010.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 18.Heaney AP, Fernando M, Yong WH, Melmed S. Functional PPAR-gamma receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med. 2002;8:1281–1287. doi: 10.1038/nm784. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RA, Sarraf P, Mueller E, Brockman JA, Prusakiewicz JJ, Eng C, Willson TM, DuBois RN. Peroxisome proliferator-activated receptor gamma-mediated differentiation: a mutation in colon cancer cells reveals divergent and cell type-specific mechanisms. J Biol Chem. 2003;278:22669–22677. doi: 10.1074/jbc.M300637200. [DOI] [PubMed] [Google Scholar]
- 20.Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60:1129–1138. [PubMed] [Google Scholar]
- 21.Inoue K, Kawahito Y, Tsubouchi Y, Yamada R, Kohno M, Hosokawa Y, Katoh D, Bishop-Bailey D, Hla T, Sano H. Expression of peroxisome proliferator-activated receptor (PPAR)-gamma in human lung cancer. Anticancer Res. 2001;21:2471–2476. [PubMed] [Google Scholar]
- 22.Reka AK, Goswami MT, Krishnapuram R, Standiford TJ, Keshamouni VG. Molecular cross-regulation between PPAR-gamma and other signaling pathways: implications for lung cancer therapy. Lung Cancer. 72:154–159. doi: 10.1016/j.lungcan.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, Sidell N, Fisher PB, Roman J. Up-regulation of p21 gene expression by peroxisome proliferator-activated receptor gamma in human lung carcinoma cells. Clin Cancer Res. 2004;10:1911–1919. doi: 10.1158/1078-0432.ccr-03-0985. [DOI] [PubMed] [Google Scholar]
- 24.Satoh T, Toyoda M, Hoshino H, Monden T, Yamada M, Shimizu H, Miyamoto K, Mori M. Activation of peroxisome proliferator-activated receptor-gamma stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene. 2002;21:2171–2180. doi: 10.1038/sj.onc.1205279. [DOI] [PubMed] [Google Scholar]
- 25.Keshamouni VG, Reddy RC, Arenberg DA, Joel B, Thannickal VJ, Kalemkerian GP, Standiford TJ. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004;23:100–108. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 26.Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem. 285:9292–9300. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 28.Verstovsek S, Giles F, Quintas-Cardama A, Perez N, Ravandi-Kashani F, Beran M, Freireich E, Kantarjian H. Arsenic derivatives in hematologic malignancies: a role beyond acute promyelocytic leukemia? Hematol Oncol. 2006;24:181–188. doi: 10.1002/hon.787. [DOI] [PubMed] [Google Scholar]
- 29.Walker AM, Stevens JJ, Ndebele K, Tchounwou PB. Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells. Int J Environ Res Public Health. 7:1996–2007. doi: 10.3390/ijerph7051996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Zhang H, Smeester L, Zou F, Kesic M, Jaspers I, Pi J, Fry RC. The NRF2-mediated oxidative stress response pathway is associated with tumor cell resistance to arsenic trioxide across the NCI-60 panel. BMC Med Genomics. 3:37. doi: 10.1186/1755-8794-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci U S A. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Woods CG, Fu J, Xue P, Hou Y, Pluta LJ, Yang L, Zhang Q, Thomas RS, Andersen ME, Pi J. Dose-dependent transitions in Nrf2-mediated adaptive response and related stress responses to hypochlorous acid in mouse macrophages. Toxicol Appl Pharmacol. 2009;238:27–36. doi: 10.1016/j.taap.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokar EJ, Qu W, Liu J, Liu W, Webber MM, Phang JM, Waalkes MP. Arsenic-specific stem cell selection during malignant transformation. J Natl Cancer Inst. 2010;102:638–649. doi: 10.1093/jnci/djq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 36.Zhao R, Hou Y, Xue P, Woods CG, Fu J, Feng B, Guan D, Sun G, Chan JY, Waalkes MP, Andersen ME, Pi J. Long isoforms of NRF1 contribute to arsenic-induced antioxidant response in human keratinocytes. Environ Health Perspect. 119:56–62. doi: 10.1289/ehp.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pi J, He Y, Bortner C, Huang J, Liu J, Zhou T, Qu W, North SL, Kasprzak KS, Diwan BA, Chignell CF, Waalkes MP. Low level, long-term inorganic arsenite exposure causes generalized resistance to apoptosis in cultured human keratinocytes: potential role in skin co-carcinogenesis. Int J Cancer. 2005;116:20–26. doi: 10.1002/ijc.20990. [DOI] [PubMed] [Google Scholar]
- 38.Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Collins S, Waalkes MP. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic Biol Med. 2007;42:1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007;282:36412–36420. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- 40.Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem. 2010;285:9292–9300. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao R, Hou Y, Zhang Q, Woods CG, Xue P, Fu J, Yarborough K, Guan D, Andersen ME, Pi J. Cross-regulations among NRFs and KEAP1 and effects of their silencing on arsenic induced antioxidant response and cytotoxicity in human keratinocytes. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104580. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tirumalai R, Rajesh Kumar T, Mai KH, Biswal S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol Lett. 2002;132:27–36. doi: 10.1016/s0378-4274(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 44.Lee SY, Hur GY, Jung KH, Jung HC, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. PPAR-gamma agonist increase gefitinib’s antitumor activity through PTEN expression. Lung Cancer. 2006;51:297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther. 2006;5:430–437. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 46.Itami A, Watanabe G, Shimada Y, Hashimoto Y, Kawamura J, Kato M, Hosotani R, Imamura M. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth of pancreatic cancers both in vitro and in vivo. Int J Cancer. 2001;94:370–376. doi: 10.1002/ijc.1488. [DOI] [PubMed] [Google Scholar]
- 47.Yin F, Wakino S, Liu Z, Kim S, Hsueh WA, Collins AR, Van Herle AJ, Law RE. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochem Biophys Res Commun. 2001;286:916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- 48.Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E, Kawaguchi T, Sasatomi K, Kimura R, Hashimoto O, Ueno T, Yano H, Kojiro M, Sata M. Involvement of p21(WAF1/Cip1), p27(Kip1), and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33:1087–1097. doi: 10.1053/jhep.2001.24024. [DOI] [PubMed] [Google Scholar]
- 49.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells -- perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 50.Sabisz M, Skladanowski A. Cancer stem cells and escape from drug induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models. Cell Cycle. 2009;8:3208–3217. doi: 10.4161/cc.8.19.9758. [DOI] [PubMed] [Google Scholar]
- 51.Moreira AL, Gonen M, Rekhtman N, Downey RJ. Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol. 23:889–895. doi: 10.1038/modpathol.2010.68. [DOI] [PubMed] [Google Scholar]
- 52.Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Singh A, Wu H, Zhang P, Happel C, Ma J, Biswal S. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol Cancer Ther. 9:2365–2376. doi: 10.1158/1535-7163.MCT-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.