Abstract
Obstructive sleep apnea (OSA) is a common and progressive disorder accompanied by severe cardiovascular and neuropsychological sequelae, presumably induced by the brain injury resulting from the intermittent hypoxia and cardiovascular processes accompanying the syndrome. However, whether the predominant brain tissue pathology is acute or chronic in newly-diagnosed, untreated OSA subjects is unclear, an assessment essential to reveal pathological processes. Diffusion tensor imaging (DTI)-based mean diffusivity (MD) procedures can detect and differentiate acute from chronic pathology, and may be useful to reveal processes in the condition. We collected four DTI series from 23 newly-diagnosed, treatment-naïve OSA and 23 control subjects, using a 3.0-Tesla magnetic resonance imaging scanner. Mean diffusivity maps were calculated from each series, realigned, averaged, normalized to a common space, and smoothed. Global brain MD values of each subject were calculated using normalized MD maps and a global brain mask. Mean global brain MD values and smoothed MD maps were compared between groups using analysis of covariance (covariate: age). Mean global brain MD values were significantly reduced in OSA over controls (p=0.01). Multiple brain sites in OSA, including medullary, cerebellar, basal-ganglia, prefrontal and frontal, limbic, insular, cingulum bundle, external capsule, corpus callosum, temporal, occipital, and corona radiata regions showed reduced regional MD values over controls. The results suggest that global brain MD values are significantly reduced in OSA, with certain regional sites especially affected, presumably a consequence of axonal, glial, and other cell changes in those areas. The findings likely represent acute pathological processes in the newly-diagnosed OSA subjects.
Keywords: Diffusion tensor imaging, Acute condition, Cytotoxic edema, Vasogenic edema, Sleep-disordered breathing
INTRODUCTION
Obstructive sleep apnea (OSA) is a common and progressive condition associated with serious co-morbidities (Arzt et al. 2005; Bradley and Floras 2009; Elmasry et al. 2001; Gami et al. 2004; Peppard et al. 2000; Punjabi et al. 2004; Shahar et al. 2001), and affects about 10% of the adult population in the United States (Lee et al. 2008; Young et al. 2002). Significant brain injury accompanies the syndrome, principally in cerebellar and limbic regions and interconnecting fibers, as well as limbic cortex (Cross et al. 2008; Joo et al. 2010; Kumar et al. 2009; Macey et al. 2002; Macey et al. 2008; Morrell et al. 2010; Yaouhi et al. 2009; Zhang et al. 2009). The injury likely contributes to the exaggerated sympathetic tone and high incidence of hypertension and cardiac arrhythmia (Peppard et al. 2000), as well as short-term memory and planning deficits, and increased mood and anxiety symptoms found in the condition (Felver-Gant et al. 2007; Ferini-Strambi et al. 2003; Lis et al. 2008; Naegele et al. 1998; Saunamaki et al. 2009; Saunamaki et al. 2010). However, it is unclear whether the predominant pathology is acute or chronic in newly-diagnosed OSA subjects. Determining the pathological nature of injury in those subjects is essential, since it is unknown, for example, whether the principal “Gold Standard” for OSA intervention, continuous positive airway pressure, can recover brain tissue from chronic damage, or whether other interventions must be considered, even for acute brain changes in OSA patients. Such description of injury nature will assist interpretation of the processes contributing to the tissue damage.
Diffusion tensor imaging (DTI)-based fractional anisotropy (FA) and mean diffusivity (MD) procedures are commonly-used measures to examine tissue changes (Basser and Pierpaoli 1998; Le Bihan et al. 2001). Fractional anisotropy measures tissue organization, and can detect pathological tissue (Basser and Pierpaoli 1996); the procedures do not differentiate the stage of tissue injury (Basser and Jones 2002; Beaulieu 2002; Neil et al. 1998). Axonal loss and demyelination are reflected by decreased FA values in chronic stages of tissue injury (Li et al. 2011; Trip et al. 2006), and both acute (Shereen et al. 2011; Ward et al. 2006) and sub-acute (between acute and chronic) stages after hypoxia are also associated with reduced FA values (Ward et al. 2006). However, MD procedures distinguish acute from chronic stages after hypoxia/ischemia, with decreased values in acute stages, values similar to those of normal conditions in sub-acute stages, and increased values in chronic stages (Ahlhelm et al. 2002; Matsumoto et al. 1995). Thus, MD procedures are more sensitive than FA in differentiating pathological stages, and may be useful in determining the pathological nature of brain injury in OSA subjects.
Our aim was to assess global mean and regional brain MD values in newly-diagnosed, treatment-naïve OSA over age- and gender-matched control subjects using DTI procedures. We hypothesized that global mean MD values will be reduced in OSA patients compared to control subjects, and localized declines in MD values will appear in multiple brain areas, indicative of acute injury in those sites.
MATERIALS AND METHODS
Subjects
We studied 23 OSA subjects (age, 44.4±9.3 years; body-mass-index, 30.1±5.4 kg/m2; apnea-hypopnea-index, 34.9±24.1 events/hour; 20 males) and 23 age- and gender-matched control subjects (45.3±11.0 years; 26.2±3.7 kg/m2; 20 males). These subjects were part of a large study, and OSA and control subjects included here were selected to match for similar age-range, gender, and exact magnetic resonance imaging (MRI) scanning parameters, resulting in 23 OSA and 23 control subjects. All OSA subjects were newly-diagnosed via overnight polysomnography with at least moderate severity (apnea-hypopnea-index ≥ 15), treatment-naïve for breathing condition, and recruited from an accredited sleep disorders laboratory at the University of California at Los Angeles (UCLA) Medical Center. All OSA subjects were not taking any cardiovascular-altering medications, such as β-blockers, α-agonists, angiotension-converting enzyme inhibitors, and vasodilators, or mood altering drugs, such as serotonin reuptake inhibitors. Subjects were also without any history of stroke, heart failure, diagnosed brain conditions, metallic implants, or body weight more than 125 kg (the last issue was a scanner limitation). All control subjects were interviewed, with their co-sleeper when possible, to rule out any undetermined OSA condition; subjects were referred for an overnight polysomnography if such a condition was suspected. Control subjects were without any medications that may alter brain tissue, with no contradictions to the MRI scanner environment, and were recruited through the UCLA campus and West Los Angeles area. All procedures were carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at UCLA, and subjects provided written informed consent prior to the study.
Magnetic Resonance Imaging
Brain imaging studies were performed using a 3.0-Tesla MRI scanner (Siemens, Magnetom Tim-Trio, Erlangen, Germany), with a receive-only 8-channel phased-array head coil and a whole-body transmitter coil. We placed foam pads on both sides of the head to minimize head motion. High-resolution T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient-echo pulse sequence [repetition-time (TR) = 2200 ms; echo-time (TE) = 2.2 ms; inversion-time = 900 ms; flip-angle = 9° matrix-size = 256×256; field-of-view (FOV) = 230×230 mm; slice-thickness = 1.0 mm]. Proton-density (PD) and T2-weighted images were collected simultaneously in the axial plane, using a dual-echo turbo spin-echo pulse sequence (TR = 10,000 ms; TE1, 2 = 17, 134 ms; flip-angle = 130° matrix-size = 256×256; FOV = 230×230 mm; slice-thickness = 4.0 mm). Diffusion tensor imaging data were acquired using a single-shot echo-planar imaging with twice-refocused spin-echo pulse sequence (TR = 10,000 ms; TE = 87 ms; flip-angle = 90° band-width = 1346 Hz/pixel; matrix-size = 128×128; FOV = 230×230 mm; slice-thickness = 2.0 mm, b = 0 and 700 s/mm2, diffusion directions = 12). We collected four separate DTI series with the same imaging protocol for subsequent averaging. We used the parallel imaging technique, generalized autocalibrating partially parallel acquisition, with an acceleration factor of two in all MRI data acquisition.
Data Processing and Analysis
The statistical parametric mapping package SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), DTI-Studio (v3.0.1) (Jiang et al. 2006), MRIcroN (Rorden et al. 2007), and MATLAB-based (http://www.mathworks.com/) custom software were used for data processing and analyses.
We used high-resolution T1-weighted, PD- and T2-weighted images of OSA and control subjects to examine for any visible brain tissue pathology such as tumors, cysts, or any other major mass lesion. Diffusion and non-diffusion weighted images of all OSA and control subjects were also assessed for any head-motion related or other imaging artifacts before MD calculation. No subjects (OSA or controls), included in this study showed any major visible brain pathology, head-motion, or other imaging artifacts.
Mean diffusivity calculation
We calculated the average background noise level from outside the brain parenchyma using non-diffusion and diffusion-weighted images, and this noise threshold was used in all OSA and control subjects to suppress non-brain regions (only those regions outside the brain parenchyma) during MD calculations. We used diffusion (b = 700 s/mm2)-weighted images, collected from 12 diffusion directions, and non-diffusion (b = 0 s/mm2) images to compute diffusion tensor matrices using DTI-Studio software (Jiang et al. 2006). The diffusion tensor matrices were diagonalized, and principal eigenvalues (λ1, λ2, and λ3) were calculated (Basser and Pierpaoli 1998; Pierpaoli et al. 1996). Using the principal eigenvalues, MD values [(λ1+λ2+λ3)/3] were calculated at each voxel (Le Bihan et al. 2001; Pierpaoli et al. 1996), with voxel intensities on the MD maps representing the corresponding MD values.
Realignment, averaging, normalization, and smoothing of MD maps
We realigned the four MD maps, computed from each DTI series, to remove any potential differences in alignment due to head-motion, and averaged to create one MD map per subject. Non-diffusion weighted images (b0 images) were also realigned and averaged.
The averaged MD maps were normalized to Montreal Neurological Institute (MNI) space. For normalization of MD maps, averaged non-diffusion weighted images were normalized to MNI space, based on a priori-defined distributions of gray, white, and cerebrospinal fluid tissue types (Ashburner and Friston 2005), and the resulting normalization parameters were applied to corresponding MD maps and non-diffusion weighted images. The normalized MD maps were smoothed with an isotropic Gaussian filter (10 mm kernel). High-resolution T1-weighted images of OSA and control subjects were also normalized to MNI space. T1-weighted images were partitioned into gray, white, and cerebrospinal fluid tissue types, based on unified segmentation approach (Ashburner and Friston 2005), and normalization parameters were applied to corresponding T1-weighted images. The normalized images of all OSA and control subjects were averaged to create a whole-brain mean image (background image), which was used for structural identification.
Global brain mask
The normalized white matter probability maps from OSA and control subjects were averaged, and similarly, gray matter probability maps from all subjects were averaged. The averaged gray and white matter probability maps were thresholded (white matter probability >0.3; gray matter probability >0.3) and combined to create a global brain mask.
Calculation of global brain MD values
Using a global brain mask, the normalized MD maps of all OSA and control individuals were masked to remove cerebrospinal fluid and other non-brain regions. The masked MD maps were used to calculate mean global brain MD values using MATLAB-based custom software.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS, V 18.0, IBM, Chicago, IL) software was used to assess demographic and mean global brain MD data. We used independent samples t-tests and Chi-square to examine the demographic data. The mean global brain MD values were assessed for significant differences between groups using analysis of covariance (ANCOVA), with age included as covariate. Significance levels were set at p < 0.05 for all the statistical tests.
We compared the normalized and smoothed MD maps voxel-by-voxel between OSA and control groups using ANCOVA, with age as covariate (SPM8, uncorrected threshold, p < 0.005; minimum extended cluster size, 5 voxels). The extended cluster size was arbitrary, and was used to avoid brain sites showing significant differences between groups with a cluster size fewer than 5 voxels, which may not represent reliable findings (Kumar et al. 2011). The brain clusters with significant differences between OSA and control groups were overlaid onto background image for structural identification.
Region-of-Interest Analyses
We also performed region-of-interest (ROI) analyses to determine average MD values in those areas which show significant differences between OSA and control subjects based on whole-brain voxel-by-voxel comparisons. Region-of-interest masks were outlined for various brain areas using clusters determined by voxel-by-voxel analysis procedures, and used to calculate average MD values of those sites from individual OSA and control subjects using normalized and smoothed MD maps. The average MD values of those areas were compared between groups with multivariate ANCOVA (covariate, age) using SPSS software.
RESULTS
Demographics
No significant differences in age (p = 0.77) or gender (p = 1.0) appeared between the groups. However, body-mass-index showed significant group differences (p = 0.007); OSA subjects had significantly increased body-mass-index over controls.
Global MD Changes
The mean global brain MD value in control subjects was 1.045±0.072 × 10−3 mm2/s, and in OSA subjects was 1.003±0.062 × 10−3 mm2/s. The mean global brain MD value was significantly reduced in OSA over control subjects (p = 0.012).
Regional Voxel-by-Voxel MD Changes
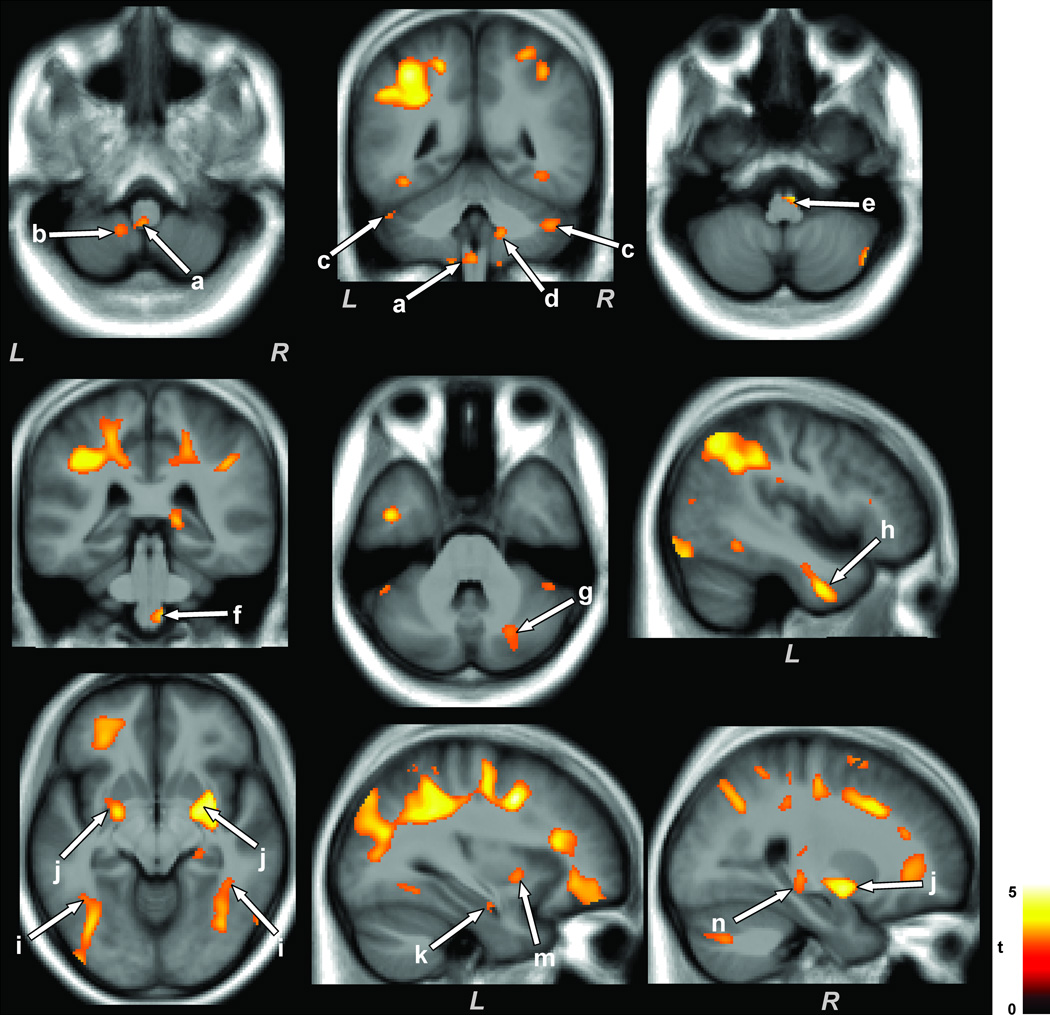
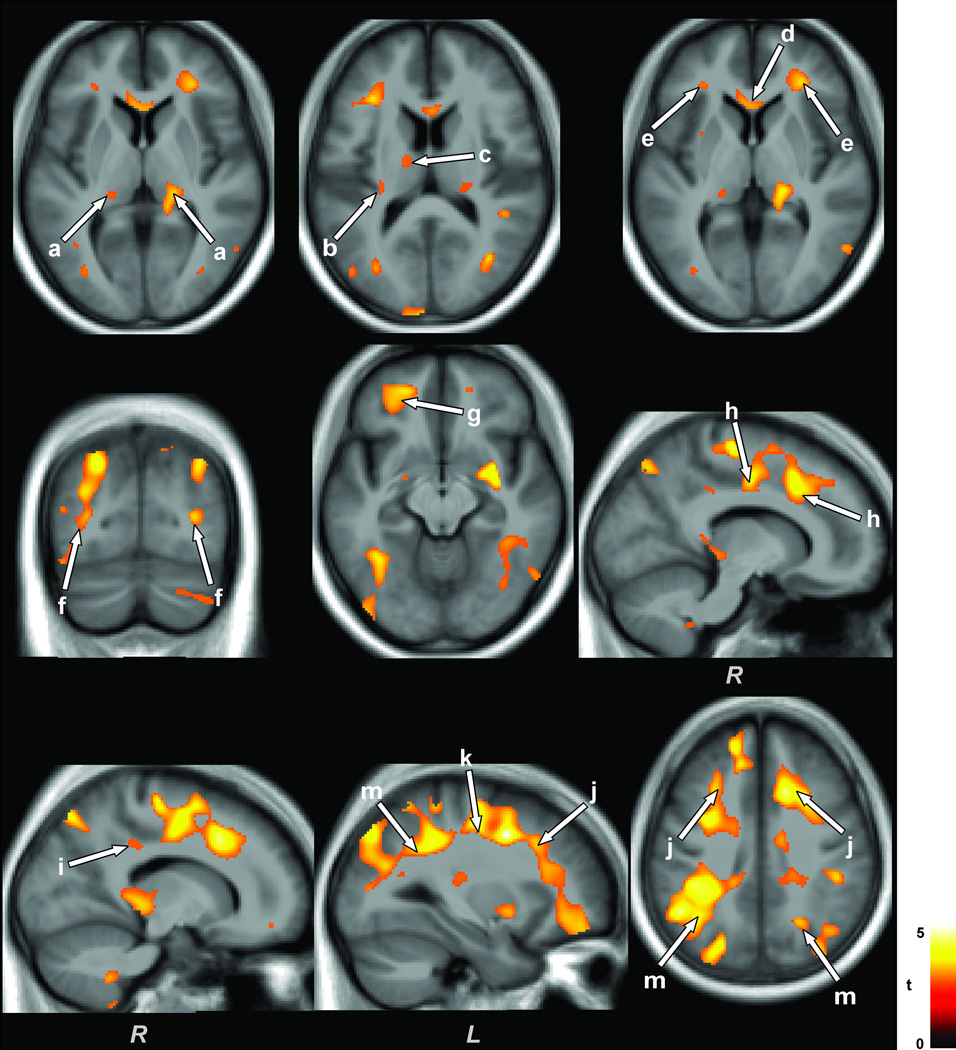
Multiple brain areas in OSA subjects showed reduced MD values compared to control subjects, with age-related changes controlled. No brain sites showed increased MD values in OSA over control subjects. Brain sites in OSA that showed reduced MD values included the dorsal (Fig. 1a), ventral (Fig. 1e), and ventrolateral (Fig. 1f) medulla, left cerebellar uvula (Fig. 1b), bilateral cerebellar crus I (Fig. 1c), right cerebellar crus II, extending to the middle cerebellar peduncle (Fig. 1g), right medial/inferior cerebellar peduncle (Fig. 1d), left ventrolateral temporal lobe (Fig. 1h), bilateral dorsal temporal white matter extending to occipital cortex (Fig. 1i), left ventral (Fig. 1k) and right mid hippocampus (Fig. 1n), bilateral putamen (Fig. 1j), right insular cortex (Fig. 1m), bilateral posterior thalamus (Fig. 2a), left anterior thalamus (Fig. 2c), left caudal external capsule (Fig. 2b), anterior corpus callosum (Fig. 2d), bilateral frontal (Fig. 2e) and occipital (Fig. 2f) white matter, left prefrontal cortex (Fig. 2g), right mid and posterior cingulate and cingulum bundle (Fig. 2h,i), and bilateral anterior (Fig. 2j), mid (Fig. 2k), and posterior (Fig. 2m) corona radiata.
Figure 1.
Medullary, cerebellar, temporal, basal-ganglia, and limbic brain areas with reduced MD values in OSA over control subjects. Multiple brain areas, including the dorsal (a), ventral (e), and ventrolateral medulla (f), cerebellar uvula (b), cerebellar crus I and II (c, g), middle and inferior cerebellar peduncles (d), ventrolateral temporal lobe (h), dorsal temporal white matter and occipital cortex (i), ventral (k) and mid hippocampus (n), putamen (j), and insular cortex (m), showed reduced MD values in OSA compared to control subjects. All brain images are shown in neurological convention, with the left side of the brain on left side of the axial and coronal images (L = Left, R = Right), and color bar represents t-statistic values.
Figure 2.
Thalamic, corpus callosum, frontal, occipital, cingulate, and corona radiata regions with decreased MD values in OSA over control subjects. Brain regions with decreased MD values in OSA emerged in thalamic areas (a, c), external capsule (b), anterior corpus callosum (d), frontal (e) and occipital (f) white matter, prefrontal cortex (g), mid and posterior cingulate cortices and cingulum bundle (h, i), and anterior (j), mid (k), and posterior (m) corona radiata. Figure conventions are the same as in Figure 1.
ROI Analyses
The regional MD values calculated from various brain sites from OSA and control groups are summarized in Table 1. Significantly reduced MD values emerged in all brain regions in OSA over control subjects, consistent with whole-brain voxel-based analysis findings.
Table 1.
Regional brain mean diffusivity (×10−3 mm2/s) differences between OSA and control subjects.
| Brain Sites | OSA (n = 23) | Controls (n = 23) | P values |
|---|---|---|---|
| Mean Diffusivity [A] |
Mean Diffusivity [B] |
[A] vs [B] | |
| Ventral Medulla | 2.00±0.22 | 2.18±0.15 | 0.002 |
| Ventrolateral Medulla | 2.02±0.22 | 2.19±0.15 | 0.003 |
| L Cerebellar Uvula | 1.59±0.23 | 1.79±0.18 | 0.003 |
| L Cerebellar Crus I | 1.37±0.24 | 1.57±0.30 | 0.007 |
| R Cerebellar Crus I | 1.23±0.16 | 1.40±0.25 | 0.004 |
| R Middle Cerebellar Peduncle | 0.80±0.05 | 0.86±0.09 | 0.002 |
| R Medial Inferior Cerebellar Peduncle | 1.00±0.09 | 1.08±0.07 | 0.003 |
| L Ventrolateral Temporal Lobe | 0.89±0.05 | 0.95±0.05 | <0.001 |
| L Dorsal Temporal White Matter | 0.81±0.04 | 0.85±0.04 | <0.001 |
| R Dorsal Temporal White Matter | 0.80±0.04 | 0.84±0.04 | 0.001 |
| L Ventral Hippocampus | 1.05±0.06 | 1.13±0.12 | 0.006 |
| R Mid Hippocampus | 0.99±0.08 | 1.07±0.10 | 0.003 |
| L Putamen | 0.84±0.04 | 0.88±0.04 | 0.001 |
| R Putamen | 0.87±0.03 | 0.92±0.05 | <0.001 |
| L Insular Cortex | 0.85±0.03 | 0.88±0.04 | 0.002 |
| R Insular Cortex | 0.89±0.03 | 0.93±0.06 | <0.001 |
| L Posterior Thalamus | 0.91±0.10 | 1.00±0.10 | 0.008 |
| R Posterior Thalamus | 1.11±0.10 | 1.21±0.11 | 0.002 |
| L Anterior Thalamus | 1.18±0.14 | 1.31±0.19 | 0.007 |
| L Caudal External Capsule | 0.87±0.05 | 0.92±0.06 | 0.007 |
| Anterior Corpus Callosum | 1.45±0.13 | 1.59±0.16 | 0.003 |
| L Frontal White Matter | 0.86±0.04 | 0.90±0.06 | 0.006 |
| R Frontal White Matter | 0.83±0.03 | 0.86±0.05 | 0.003 |
| L Occipital White Matter | 0.80±0.03 | 0.83±0.04 | 0.003 |
| Right Occipital White Matter | 0.82±0.02 | 0.85±0.04 | 0.002 |
| L Prefrontal Cortex | 0.95±0.05 | 1.02±0.10 | 0.001 |
| R Mid Cingulate | 0.88±0.04 | 0.94±0.09 | <0.001 |
| R Posterior Cingulate | 0.93±0.05 | 0.97±0.05 | 0.005 |
| L Anterior Corona Radiata | 0.81±0.03 | 0.86±0.06 | <0.001 |
| R Anterior Corona Radiata | 0.82±0.03 | 0.87±0.06 | <0.001 |
| L Mid Corona Radiata | 0.95±0.05 | 1.04±0.09 | <0.001 |
| R Mid Corona Radiata | 0.93±0.04 | 1.00±0.07 | <0.001 |
| L Posterior Corona Radiata | 1.00±0.11 | 1.12±0.12 | <0.001 |
| R Posterior Corona Radiata | 1.12±0.11 | 1.27±0.17 | 0.001 |
Table legend: OSA, Obstructive sleep apnea; L, Left; R, Right.
DISCUSSION
Overview
Newly-diagnosed, treatment-naïve OSA subjects showed significantly reduced global brain MD values, and these changes were localized in various brain regions including medullary and brainstem areas critical for cardiovascular and respiratory regulation, cerebellar, basal-ganglia, hippocampal and other limbic sites, and the corona radiata. The nature of MD changes in OSA (decreased values), indicates that the alterations are of a predominantly acute stage.
OSA and brain injury
Regional gray matter volume loss and fiber injury, as well as brain metabolic abnormalities appear in multiple brain areas in OSA subjects. Gray matter tissue loss appears in the cingulate and insular cortices, mammillary bodies, hippocampus, caudate and thalamus, cerebellar areas, and frontal, parietal, and temporal regions (Joo et al. 2010; Kumar et al. 2008; Macey et al. 2002; Yaouhi et al. 2009), and metabolic abnormalities emerge in the hippocampus, parietal-occipital cortex, centrum semiovale, and frontal and mid-temporal areas (Kamba et al. 2003; Morrell et al. 2003; Sarchielli et al. 2008; Tonon et al. 2007). Fiber changes in newly-diagnosed OSA subjects, assessed by DTI-based FA procedures, appear in the corpus callosum, cingulum bundle, fornix, internal capsule, cerebellum and peduncles, and cortico-spinal tract (Macey et al. 2008; Zhang et al. 2009). These brain areas with FA changes are similar to those found here with MD procedures, although MD changes are more wide-spread than FA changes. The current studies address the issue of whether OSA subjects show acute tissue changes from initial detection of the syndrome, or whether chronic injury appears at that time. Metabolic abnormalities and fiber changes, assessed with MR spectroscopy and FA procedures, can appear in early, as well as chronic stages of tissue changes, and gray matter volume losses indicate chronic pathological changes. However, earlier studies may have had variations between scanning evaluation and time of diagnosis in OSA subjects (Joo et al. 2010; Macey et al. 2002; Morrell et al. 2010), possibly leading to discrepancies between MD and gray matter findings, since increased MD values would be expected in those sites with gray matter volume loss. The findings from MD procedures provide insights into those issues.
Reduced MD values and source of tissue injury
The injury characteristics provide clues to the source of injury; the MD changes are heavily lateralized, suggesting consequences of perfusion, oxygenation, or reperfusion from the normally asymmetrical vascular supply to the brain to the intermittent hypoxia aspects of OSA (Joo et al. 2007), affecting both glia and neurons. Neurons in certain structures are especially vulnerable from excitotoxic processes during intermittent hypoxia or impaired perfusion in OSA. These areas include hippocampal CA1 regions, cerebellar Purkinje cells, the anterior cingulate, and the mammillary bodies, sites which are recipients of long-projecting axons from Schaeffer’s collaterals, inferior olive climbing fibers, cingulum bundle, and fornix, respectively (Aggleton et al. 2005; Ang et al. 2006; Shibata 1992; Welsh et al. 2002); the targeted structures show lower MD values. Thus, we speculate that the lateralized regional brain damage develops from preferential vascular lateralization, which is compromised in OSA through the extreme cardiovascular changes accompanying each apneic event leading to ischemia. In addition, hypoxia from apnea events leads to excessive neural discharge and excitoxic injury. Frontal, cerebellar, medullary, and other limbic areas show tissue injury in animal studies to intermittent hypoxic exposure, following protocols attempting to simulate OSA (Gozal et al. 2001; Pae et al. 2005; Veasey et al. 2004; Zhang et al. 2010).
Patients with stroke resulting from transient ischemia with acute diffusion changes show tissue and function recovery, if the ischemic condition is restored quickly and forcefully (Kidwell et al. 1999; Lecouvet et al. 1999). As in stroke treatment strategies, acute tissue changes in newly-diagnosed OSA subjects suggest a possibility for recovery of abnormal tissue and function with use of anti-inflammatory and myelin-repair agents, together with aggressive intervention for the breathing condition.
Effect of hypoxemia on brain tissue
Hypoxia and ischemia both decrease O2 supply to brain tissue. During acute cerebral hypoxemia, due to a mismatch between O2 demand and supply, the failure of energy delivery results in cell depolarization which modifies sodium and calcium ion movement into the cell, and potassium ions into extracellular space (Dirnagl et al. 1999; Hossmann 1971; Lowry et al. 1964; O'Dell et al. 1994). Such ionic changes osmotically obligate water movement from extracellular to intracellular space, causing cell swelling (Mintorovitch et al. 1994), which in extreme hypoxia, results in cytotoxic edema. Excessive glutamate, released during cell depolarization, is neurotoxic (Benveniste et al. 1984; Hagberg et al. 1985); additional injury can develop from calcium ion influx (Edwards et al. 1998). The affected neurons and glia cells, especially myelin-supporting oligodendrocytes cells, which play critical roles in maintaining axonal function and survival (Kassmann and Nave 2008), and are highly susceptible to hypoxemia, can lead to myelin separation from axons, and myelin and axonal swelling (Nukada and Dyck 1987; Shereen et al. 2011), with a decrease in the extracellular volume fraction. The increased cellular cytotoxic edema and intra-axonal water, reflected as reduced extra-cellular/extra-axonal volume, as well as extra-axonal water, are readily detected with MD measures.
Cytotoxic edema can be accompanied by brain swelling with consequent vascular compression, resulting in a reduction in cerebral perfusion and blood brain barrier alterations (Baethmann 2000; Xavier et al. 2003). The condition will allow water to leak from vascular space to extra-cellular/extra-axonal space, and thus, the sub-acute stage would be a mixture of cytotoxic and vasogenic edema (Xavier et al. 2003), i.e., cell/axonal swelling together with more water in extra-cellular/extra-axonal space (Matsumoto et al. 1995).
Since vasogenic edema itself works as an ischemic condition (Marmarou et al. 2000), more water in extra-cellular/extra-axonal space in the sub-acute stage will accelerate tissue degenerative processes, leading to demyelination, axonal, and cell loss in chronic stages of hypoxemia (Matsumoto et al. 1995).
OSA subjects undergo intermittent hypoxia during sleep; some axons and cells may recover functions during waking, in a similar fashion as occurs in transient ischemia (Kidwell et al. 1999; Lecouvet et al. 1999). However, most axons and cells, depending upon the severity of hypoxemia, may progress to a chronic pathological state over the long-term. Animal studies simulating intermittent hypoxia in human conditions show apoptosis and injury in brain areas after as little as 5 hrs of 12% O2 intermittent exposure (Pae et al. 2005). Thus, OSA with repeated nocturnal apneic events, likely leads to progressive pathological changes.
MD changes in different stages of pathology
Mean diffusivity of water within tissue is influenced by various factors, including tissue barriers (Le Bihan et al. 1991) and extra-cellular/extra-axonal fluid and space. In the case of acute stages of pathology after hypoxemia, cytotoxic edematous actions decrease extracellular water and lead to cell and axonal swelling, reducing extracellular space, resulting in restricted water diffusion, and thus, reduced MD values. Multiple studies with hypoxia-ischemia induced injury in acute stages show reduced MD values (Ahlhelm et al. 2002; Matsumoto et al. 1995). Sub-acute stages of hypoxemia are accompanied by cytotoxic and vasogenic edema, along with axonal and myelin swelling. Cytotoxic edema reduces MD values as a consequence of increased tissue barriers, and vasogenic edema helps to increase MD values; thus, sub-acute stages of hypoxemia will show either little-changed or similar MD values as those of control conditions (Ahlhelm et al. 2002; Matsumoto et al. 1995). However, demyelination or axonal loss in chronic stages of hypoxemia will reduce tissue barriers, increase extracellular volume, and escalate vasogenic edema; all factors will contribute to increased MD values (Ahlhelm et al. 2002; Matsumoto et al. 1995).
Sites of injury and autonomic, respiratory, and neuropsychologic deficits
A remarkable aspect of the MD findings was the specific sites of injury. Significant injury appeared in medullary sites, essential areas for regulation of blood pressure, chemosensation, and integration of baroreceptor and chemoreceptor afferents (Caverson et al. 1984; Stocker et al. 2007). Earlier studies did not use techniques that would address the issue of acute vs chronic injury, and the very first studies of regional volume reduction in OSA (Macey et al. 2002) used procedures that could not adequately resolve volume changes in the small medulla. The MD procedures also found injury in numerous other autonomic areas, including the insular cortex, a major influence on hypothalamic regulation of both sympathetic and parasympathetic outflow (Zhang et al. 1999). In addition, portions of the cingulate cortex (Critchley et al. 2003) and ventromedial frontal cortex (King et al. 1999), which play significant roles in cardiovascular regulation, showed injury. Neurocognitive deficits associated with OSA depend on the hippocampus, putamen, cerebellum and cingulate cortex, as well as integrity of the corpus callosum; all of those structures show injury as indicated by MD procedures. The findings confirm the now-long list of studies indicating neural changes that accompany the syndrome (Joo et al. 2010; Kamba et al. 2003; Kumar et al. 2008; Macey et al. 2002; Macey et al. 2008; Morrell et al. 2010; Morrell et al. 2003; Sarchielli et al. 2008; Tonon et al. 2007; Yaouhi et al. 2009; Zhang et al. 2009), and additionally point to specific medullary areas that are affected in the syndrome.
Limitations
Limitations of this study include the unavailability of precise OSA disease durations in individual subjects and the absence of follow-up studies after respiratory support intervention. Although all OSA subjects were newly-diagnosed, OSA duration in these subjects may be variable, depending on numerous issues that can delay recognition or assessment. However, since brain sites showed reduced MD values, indicating that the changes reflected an acute condition (Ahlhelm et al. 2002; Matsumoto et al. 1995), we believe that the greatest proportion of the subjects were in early-stages of chronic neural changes. We have insufficient data from follow-up studies post breathing-intervention to support conclusions for reversibility of brain tissue and function in OSA. However, such studies are in progress to answer these questions
CONCLUSIONS
Mean global brain MD values are significantly reduced in newly-diagnosed, treatment-naïve OSA over control subjects, indicating an acute stage of tissue changes. These brain changes appeared regionally in critical cardiovascular and respiratory areas of the medulla, as well as the cerebellum, basal-ganglia, limbic regions, corpus callosum, and multiple regions in the corona radiata. The pathological mechanisms of tissue injury likely include ischemia- and hypoxia-induced processes, leading to acute tissue changes in the condition. The findings raise the possibility that the initial brain tissue injury and resulting impaired function in OSA subjects can be recovered to some extent, by adopting strategies analogous to those found useful in acute stroke treatment.
ACKNOWLEDGEMENTS
Authors thank Ms. Rebecca Harper and Mr. Edwin Valladares for assistance with data collection, and Drs. Jennifer A. Ogren and Heidi L. Richardson for editing assistance.
Grant support: This research was supported by NR011230 to PMM and HD-22695 to RMH.
Footnotes
CONFLICTS OF INTEREST: All authors have no conflicts of interest to declare.
REFERENCES
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22(10):2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Ahlhelm F, Schneider G, Backens M, Reith W, Hagen T. Time course of the apparent diffusion coefficient after cerebral infarction. Eur Radiol. 2002;12(9):2322–2329. doi: 10.1007/s00330-001-1291-0. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26(46):11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baethmann A. Brain edema and leukocyte-endothelial interactions in cerebral ischemia. In: Oehmichen M, editor. Brain hypoxia and ischemia. Research in legal medicine. Vol. 24. Lübeck: Schmidt-Römhild; 2000. pp. 85–99. [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39(6):928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J, Calaresu FR. Chemoreceptor and baroreceptor inputs to ventrolateral medullary neurons. Am J Physiol. 1984;247(5 Pt 2):R872–R879. doi: 10.1152/ajpregu.1984.247.5.R872. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Cross RL, Kumar R, Macey PM, Doering LV, Alger JR, Yan-Go FL, Harper RM. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31(8):1103–1109. [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Edwards M, Kent TA, Rea HC, Wei J, Quast M, Izumi T, Mitra S, Perez-Polo JR. APE/Ref-1 responses to ischemia in rat brain. Neuroreport. 1998;9(18):4015–4018. doi: 10.1097/00001756-199812210-00005. [DOI] [PubMed] [Google Scholar]
- Elmasry A, Lindberg E, Berne C, Janson C, Gislason T, Awad Tageldin M, Boman G. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001;249(2):153–161. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- Felver-Gant JC, Bruce AS, Zimmerman M, Sweet LH, Millman RP, Aloia MS. Working memory in obstructive sleep apnea: construct validity and treatment effects. J Clin Sleep Med. 2007;3(6):589–594. [PMC free article] [PubMed] [Google Scholar]
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, Cappa SF. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61(1):87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21(7):2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab. 1985;5(3):413–419. doi: 10.1038/jcbfm.1985.56. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Cortical steady potential, impedance and excitability changes during and after total ischemia of cat brain. Exp Neurol. 1971;32(2):163–175. doi: 10.1016/0014-4886(71)90060-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Joo EY, Tae WS, Han SJ, Cho JW, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30(11):1515–1520. doi: 10.1093/sleep/30.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo EY, Tae WS, Lee MJ, Kang JW, Park HS, Lee JY, Suh M, Hong SB. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamba M, Inoue Y, Higami S, Suto Y. Age-related changes in cerebral lactate metabolism in sleep-disordered breathing. Neurobiol Aging. 2003;24(5):753–760. doi: 10.1016/s0197-4580(02)00191-4. [DOI] [PubMed] [Google Scholar]
- Kassmann CM, Nave KA. Oligodendroglial impact on axonal function and survival - a hypothesis. Curr Opin Neurol. 2008;21(3):235–241. doi: 10.1097/WCO.0b013e328300c71f. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, Saver JL. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999;30(6):1174–1180. doi: 10.1161/01.str.30.6.1174. [DOI] [PubMed] [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. J Comp Neurol. 1999;413(4):572–582. [PubMed] [Google Scholar]
- Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008;438(3):330–334. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26(5):480–491. doi: 10.1002/da.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307(1–2):106–113. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Moonen CT, van Zijl PC, Pekar J, DesPres D. Measuring random microscopic motion of water in tissues with MR imaging: a cat brain study. J Comput Assist Tomogr. 1991;15(1):19–25. doi: 10.1097/00004728-199101000-00002. [DOI] [PubMed] [Google Scholar]
- Lecouvet FE, Duprez TP, Raymackers JM, Peeters A, Cosnard G. Resolution of early diffusion-weighted and FLAIR MRI abnormalities in a patient with TIA. Neurology. 1999;52(5):1085–1087. doi: 10.1212/wnl.52.5.1085. [DOI] [PubMed] [Google Scholar]
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li J, He H, Wang Z, Lv B, Li W, Hailla N, Yan F, Xian J, Ai L. Directional diffusivity changes in the optic nerve and optic radiation in optic neuritis. Br J Radiol. 2011;84(1000):304–314. doi: 10.1259/bjr/93494520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis S, Krieger S, Hennig D, Roder C, Kirsch P, Seeger W, Gallhofer B, Schulz R. Executive functions and cognitive subprocesses in patients with obstructive sleep apnoea. J Sleep Res. 2008;17(3):271–280. doi: 10.1111/j.1365-2869.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(10):1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. [PMC free article] [PubMed] [Google Scholar]
- Marmarou A, Fatouros PP, Barzo P, Portella G, Yoshihara M, Tsuji O, Yamamoto T, Laine F, Signoretti S, Ward JD, Bullock MR, Young HF. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J Neurosurg. 2000;93(2):183–193. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Lo EH, Pierce AR, Wei H, Garrido L, Kowall NW. Role of vasogenic edema and tissue cavitation in ischemic evolution on diffusion-weighted imaging: comparison with multiparameter MR and immunohistochemistry. Am J Neuroradiol. 1995;16(5):1107–1115. [PMC free article] [PubMed] [Google Scholar]
- Mintorovitch J, Yang GY, Shimizu H, Kucharczyk J, Chan PH, Weinstein PR. Diffusion-weighted magnetic resonance imaging of acute focal cerebral ischemia: comparison of signal intensity with changes in brain water and Na+,K(+)-ATPase activity. J Cereb Blood Flow Metab. 1994;14(2):332–336. doi: 10.1038/jcbfm.1994.40. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, Pardoe H, Pell GS, Abbott DF, Rochford PD, Jackson GD, Pierce RJ, O'Donoghue FJ, Corfield DR. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65(10):908–914. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4(5):451–454. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Naegele B, Pepin JL, Levy P, Bonnet C, Pellat J, Feuerstein C. Cognitive executive dysfunction in patients with obstructive sleep apnea syndrome (OSAS) after CPAP treatment. Sleep. 1998;21(4):392–397. doi: 10.1093/sleep/21.4.392. [DOI] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209(1):57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Nukada H, Dyck PJ. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann Neurol. 1987;22(3):311–318. doi: 10.1002/ana.410220306. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994;265(5171):542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375(2):123–128. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the sleep heart health study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Presciutti O, Alberti A, Tarducci R, Gobbi G, Galletti F, Costa C, Eusebi P, Calabresi P. A 1H magnetic resonance spectroscopy study in patients with obstructive sleep apnea. Eur J Neurol. 2008;15(10):1058–1064. doi: 10.1111/j.1468-1331.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- Saunamaki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction in patients with obstructive sleep apnea syndrome. Eur Neurol. 2009;62(4):237–242. doi: 10.1159/000232156. [DOI] [PubMed] [Google Scholar]
- Saunamaki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction and learning effect after continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Eur Neurol. 2010;63(4):215–220. doi: 10.1159/000278301. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Shereen A, Nemkul N, Yang D, Adhami F, Dunn RS, Hazen ML, Nakafuku M, Ning G, Lindquist DM, Kuan CY. Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia-ischemia-induced thrombotic stroke. J Cereb Blood Flow Metab. 2011;31(4):1155–1169. doi: 10.1038/jcbfm.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H. Topographic organization of subcortical projections to the anterior thalamic nuclei in the rat. J Comp Neurol. 1992;323(1):117–127. doi: 10.1002/cne.903230110. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49(3):640–646. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- Tonon C, Vetrugno R, Lodi R, Gallassi R, Provini F, Iotti S, Plazzi G, Montagna P, Lugaresi E, Barbiroli B. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30(3):305–311. doi: 10.1093/sleep/30.3.305. [DOI] [PubMed] [Google Scholar]
- Trip SA, Wheeler-Kingshott C, Jones SJ, Li WY, Barker GJ, Thompson AJ, Plant GT, Miller DH. Optic nerve diffusion tensor imaging in optic neuritis. NeuroImage. 2006;30(2):498–505. doi: 10.1016/j.neuroimage.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27(2):194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Ward P, Counsell S, Allsop J, Cowan F, Shen Y, Edwards D, Rutherford M. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics. 2006;117(4):e619–e630. doi: 10.1542/peds.2005-0545. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O'Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- Xavier AR, Qureshi AI, Kirmani JF, Yahia AM, Bakshi R. Neuroimaging of stroke: a review. South Med J. 2003;96(4):367–379. doi: 10.1097/01.SMJ.0000063468.11503.C1. [DOI] [PubMed] [Google Scholar]
- Yaouhi K, Bertran F, Clochon P, Mezenge F, Denise P, Foret J, Eustache F, Desgranges B. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18(1):36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Fung SJ, Xi M, Sampogna S, Chase MH. Apnea produces neuronal degeneration in the pons and medulla of guinea pigs. Neurobiol Dis. 2010;40(1):251–264. doi: 10.1016/j.nbd.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Lu BX, Li TP. Correlation between white matter lesion and memory impairment in patients with obstructive sleep apnea syndrome. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(4):825–829. [PubMed] [Google Scholar]
- Zhang ZH, Dougherty PM, Oppenheimer SM. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience. 1999;94(2):351–360. doi: 10.1016/s0306-4522(99)00339-5. [DOI] [PubMed] [Google Scholar]