Abstract
From early in the HIV epidemic it was appreciated that many inflammatory markers such as neopterin and TNF-α were elevated in patients with AIDS. With the advent of modern technology able to measure a broad array of cytokines, we now know that from the earliest points of infection HIV induces a cytokine storm. This review will focus on how cytokines are disturbed in HIV infection and will explore potential therapeutic uses of cytokines. These factors can be used directly as therapy during HIV infection, either to suppress viral replication or prevent deleterious immune effects of infection, such as CD4+ T cell depletion. Cytokines also show great promise as adjuvants in the development of HIV vaccines, which would be critical for the eventual control of the epidemic.
Keywords: HIV, cytokine, chemokine, immune activation, vaccine
1. Acute HIV infection
1.1 Animal models of acute simian immunodeficiency virus (SIV)
1.1.1. Pathogenic infection models
The most common simian model of HIV infection is the macaque, with infected animals showing declines in CD4+ T cell counts and rapid progression to AIDS after infection [1]. The model has been used to test the effects of antiretroviral therapy and vaccination, and germane to the current review, as a model to understand HIV pathogenesis. The early period of SIV infection has been extensively studied in an attempt to understand how the virus gains a foothold in the host and alters the immune environment. Soon after SIV inoculation the virus disseminates and is found throughout the body of infected animals between 7 – 12 days after mucosal inoculation [2]. There is massive infection of CD4+ T cells, particularly within gut-associated lymphatic tissue [3]. For some time it has been known that acute SIV infection is associated with early elevations in both pro- and anti-inflammatory cytokines. In 1996 Benveniste et al. demonstrated that peripheral blood mononuclear cells (PBMCs) from acutely infected cynomolgous macaques show increased mRNA levels of IL-1β, IL-6, IL-10, and TNF-α, and that for most analytes these changes are transient, lasting only weeks[4]. In rhesus macaques (RMs) IFN-α is detectable as early as four days after intravenous (IV) inoculation of virus, though the presence of the cytokine does not prevent dissemination of the virus [5]. The peak in secretion of TNF-α and IFN-γ occurs by seven to eight days post IV inoculation of SIV[6], and β-chemokines as well as cytokines can be found in lymphoid tissues as early as a week after IV SIV inoculation [7]. Of note, cytokine responses in tissues such as lung mononuclear cells can precede those in peripheral blood by several days [8].
1.1.2. Non-pathogenic infection models
Shortly after the identification of SIV as the causative agent of immune deficiency in captive macaque populations, it became clear that African green monkeys (AGM) in the wild are also infected with this virus and do not appear to suffer immune deficiency [9]. It is now appreciated that “natural” hosts of SIV, including sooty mangabeys (SMs), have co-evolved with the virus, and in spite of high level viremia do not mount a vigorous immune response to the virus during the chronic phase of infection and do not suffer immune collapse [10]. It is hoped that study of these animals will reveal clues as to why they do not suffer the catastrophic effects of humans infected with HIV. While the chronic phase of infection in natural hosts is relatively immune quiescent, these animals mount an immune response to acute SIV infection, and several groups have compared the acute cytokine responses to SIV infection of pathogenic (RM) and non-pathogenic (SM and AGM) hosts. Acute AGM infection is characterized by early TGF-β elevation 1 day post-infection, followed by IL-10 elevation by 6–10 days. In contrast, RM infection shows lower TGF-β expression and delayed IL-10 elevation during acute infection, consistent with a more pronounced pro-inflammatory response in pathogenic infection [11]. In addition to blunted adaptive immune responses to SIV, SMs also show decreased innate immune activation. It was originally reported that plasmacytoid dendritic cells from SM produce markedly less IFN-α after stimulation with SIV than pDC from humans stimulated with HIV or RM stimulated with SIV [12]. This defect appears specific for SIV in SM, as the IFN-α response to influenza virus stimulation is normal. More recent work using immunohistochemical analysis of RM, SM, and AGM infected with SIV showed that IFN-α/β is produced in all hosts, but is truncated in the non-pathogenic infection [13]. Consistent with this notion, IFN-stimulated gene (ISG) expression rises during infection of these hosts, often to a level comparable to that of pathogenic infection in RMs, but the expression of ISGs is only transient in the natural host [14, 15]. In addition to comparing different animal models of infection, there have been several studies analyzing attenuated SIV infection of non-natural hosts. The most commonly used attenuated SIV contains deletion within the Nef gene, with infected monkeys not suffering immune dysfunction. Nef-deleted virus leads to decreased pro-inflammatory cytokine levels during acute infection in most studies [5, 7, 16].
In summary, non-pathogenic infection with SIV induces a slightly blunted immune response compared to SIV infection of non-natural hosts. Most markedly, the initial immune activation is rapidly curtailed in natural hosts of SIV, allowing avoidance of subsequent immune pathology. This finding has sparked interest in immune therapy of HIV designed to block the ongoing inflammation associated with chronic infection, with the hopes of avoiding CD4+ T cell exhaustion and depletion, as well as preventing accelerated aging and HIV-associated co-morbidities such as heart and kidney disease.
1.2. Studies of early infection in humans
The acute stage of HIV infection has been broken into sequential phases based on the appearance of HIV RNA and p24 protein and the evolution of anti-HIV antibody responses [17]. Study of the earliest events in primary HIV infection is difficult, as patients often do not present to health care providers until several weeks into their disease course. Furthermore, patients with symptomatic acute HIV infection are more likely to seek medical care, and symptomatic infection has been associated with higher set point viral load[18], further complicating interpretation of many studies of acute HIV infection. The earliest work examining cytokine responses of symptomatic acute HIV revealed elevated IFN-α, neopterin, β2-microglobulin, IL-1β, IFN-γ, and TNF-α levels in acute infection [19–22]. The magnitude of cytokine elevations in acute EBV infection or nonspecific viral syndrome is equal to or greater than that for acute HIV for many analytes [23, 24]. Observation of mRNA expression levels of selected cytokines in PBMCs shows early increases in IFN-γ secretion, coincident with oligoclonal expansion of CD8+ T cells [25]. Subsequent gene array analysis has revealed extensive activation of both pro- and anti-inflammatory genes in lymph node tissues of subjects with acute HIV infection, and this activation is more pronounced than at later stages of disease [26].
A major advance in understanding the natural history of cytokine responses in acute HIV infection came with through study of paid plasma donors [24]. These subjects give plasma regularly for a fee, and panels from these subjects have been identified with samples spanning pre-infection times through seroconversion. Notably, the subjects are documented to be afebrile at each plasma donation visit. TNF-related apoptosis-inducing ligand (TRAIL) and TNF receptor type 2 levels are elevated soon after the peak in viral replication, 7 and 12 days after the first detectable plasma virus, respectively [27]. Utilizing multiplex bead arrays, our group has been able to characterize at the protein level the timing of a broad array of cytokines that arise early in infection (Figure 1), revealing successive waves of cytokines that arise during acute infection [28]. The major weakness of the plasma donor studies is the lack of clinical outcome data associated with these subjects, precluding the ability to use cytokine levels to predict set point viral load or CD4+ T cell depletion rate. Nonetheless, it is now appreciated that the earliest stage of HIV infection induces a striking cascade of systemic cytokine levels greater than seen in other acute viral infections [28–30].
Figure 1.
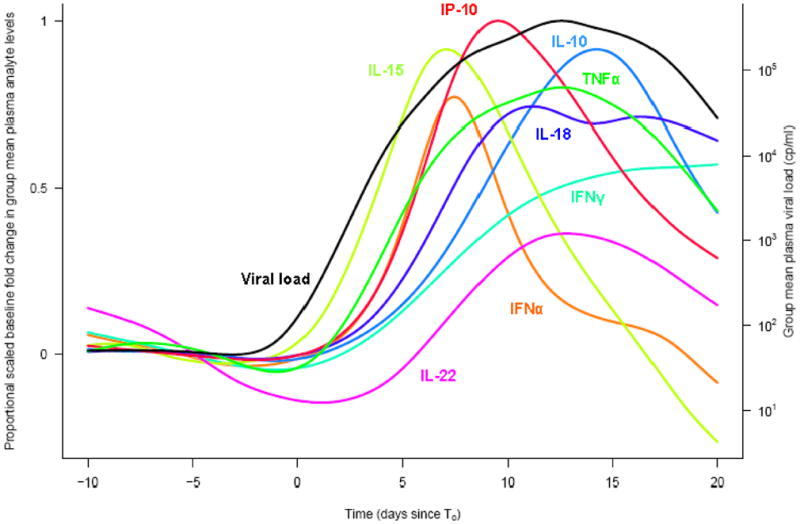
The proportional fold-change relative to baseline in plasma levels of selected analytes and viral load in 35 plasma donors with acute HIV infection is shown. Time is plotted in days relative to T0, the day of first detectable plasma HIV.
Figure reused with permission from the Journal of Virology, originally published in [28].
1.3. Mucosal cytokine responses
HIV is a blood-borne pathogen that can gain access to humans through a variety of routes, predominantly via the bloodstream directly in injection drug users or via mucosal surfaces during sexual transmission, which accounts for the vast majority of infections worldwide. Because of the importance of mucosal transmission, there has been much interest in the cytokine environment of the mucosa before and soon after HIV exposure. Intravaginal cytokine levels and inflammation appear to be important factors influencing the effectiveness of microbicides designed to block HIV transmission. Initial enthusiasm for the use of nonoxynol-9, a spermicide with in vitro activity against HIV [31], proved unfounded in subsequent clinical trials[32]. Of note, nonoxynol-9 causes genital tract inflammation, which may be a side-effect that could counterbalance any direct anti-HIV activity of the compound [33]. A more recent attempt in the RM model demonstrated that down-modulating pro-inflammatory responses in the vaginal tract can prevent SIV infection after challenge with multiple intra-vaginal exposures [34].
The mucosal environment directly after HIV inoculation could be important in either blocking subsequent viral expansion or in recruiting target cells to the area, allowing expansion of the infection. In a macaque vaginal infection model pro-inflammatory cytokines are up-regulated in the mucosa prior to appearance of detectable virus or induction of IFN-α or IFN–β [35]. A recent study of at-risk women in South Africa showed that increased inflammatory cytokines in cervico-vaginal lavage fluid is associated with the presence of sexually transmitted infection [36]. Genital tract inflammation is associated with higher systemic viral load and more rapid CD4+ T cell depletion, but remarkably does not change significantly between pre- and post-HIV infection samplesin this cohort of African women. In addition, cervico-vaginal IL-1β, IL-6, and IL-8 concentrations during acute HIV correlate with low CD4+ T cell counts during this period [37], again suggesting a role for mucosal pro-inflammatory cytokines in amplifying the initial infection and immune consequences.
1.4. Early cytokines and subsequent HIV clinical course
The massive immune activation associated with acute HIV infection raises the question of whether this profound early perturbation could affect subsequent disease course. Early depletion of CD4+ T cells from gut-associated lymphatic tissue occurs [38], and preservation of HIV-specific CD4+ T cell responses predicts subsequent control of HIV replication [39]. It is possible that early cytokine responses help determine the balance between detrimental immune activation and loss of viral control vs. efficient promotion of antiviral immune responses favoring control of replication. Early IFN-α and chemokine responses do not appear to predict rapid vs. slow progressors in SIV infection[40], though higher peak IFN-α is seen in some macaques that do not rapidly progress to AIDS [5]. IL-17 secreting natural killer T (NKT) cells peak in lymphatic tissue two weeks after IV infection of RM, and the levels are higher in macaques that subsequently do not control viral replication [41]. A study of women in South Africa measured levels of 30 cytokines during acute HIV and followed the women for one year. Remarkably, the levels of five cytokines predict two-thirds of the variation in viral load set point, with IL-12p40, IL-12p70, and IFN-γ associated with lower viral load and IL-7 and IL-15 associated with higher viral load [42]. These findings are exciting as they allow the prospect of manipulating the cytokine environment of early HIV to alter disease course. If successful, the major challenge in implementing such therapies would be in identifying subjects during early HIV infection.
2. Cytokine perturbations in chronic HIV infection
2.1. HIV effects on cytokine production
As the acute phase of HIV infection quickly induces anti-viral immunity and cytokine production, these immune responses continue through the early phase and into the chronic phase of infection [28, 43]. Although cytokines may be required for induction of immune responses to control viremia, HIV infection leads to chronic activation, persistent cytokine production, and increased inflammation, leading to loss of immune cell replicative capacity and eventual immune senescence [44–46]. These responses are believed to be caused not only by HIV but also by loss of immune control and co-infection with other viral, fungal or bacterial infections [47, 48]. During the chronic stage of HIV infection there is an increase in the inflammatory cytokine TNF-α and its receptor TNF-RII, indicating prolonged inflammation [43, 44]. IL-6 is elevated in late-stage AIDS [49] but not earlier in HIV infection [44], and elevated IL-6 levels are associated with increased risk of death [50]. It is known that successful therapy and eradication of circulating HIV virus does not completely normalize the inflammatory response [44].
2.2. Cytokine effects on HIV control or viral persistence
Cytokines may play a role in AIDS-associated pathologies; they are a product of an activated immune response engaged in trying to control viral infection. In early HIV infection induction of cytokines coincides with the oligoclonal expansion of antigen specific CD8+ T cells and decrease in viremia[25]. Cytokines produced, including IL-2, TGF-β, IL-4 and IFN-γ, are required in the support of expansion of antiviral T cells and antibody responses, but they may also play a role in activation of HIV-infected cells and increased viral replication [51].
Supernatants of HIV vaccine-induced cytotoxic T lymphocytes (CTLs) stimulated with gp120 show increased production of TNF-α and increased HIV-1 transcripts after co-culture [52]. This early paper raised the possibility that increased antiviral immune responses would be accompanied by deleterious virus enhancing cytokine responses. Studies have been done to investigate the effects of IL-12, IL-15 and IL-2 on in vitro p24 production in HIV infected cells [53]. These cytokines have differential effects on p24 and viral replication in cell lines and in vitro models of acute and latent HIV infection and allude to the potentially competing effects of cell activation and the ability of cytokines to suppress viral infection. However, development of poly-functional, multi-cytokine-producing T cell responses is associated with better immune control, especially in the mucosa[54]. Poor functional profiles of T cells from HIV progressors during chronic HIV infection include having lower production of IFN-γ, MIP-1β, TNF-α, and IL-2 compared to non-progressors; the quantity of these multi-functional cells inversely correlates with viral load while the quantity of CD8+ HIV-specific T cells does not [55].
Early HAART is important in preserving T cell function[56]; prolonged activation of CD8+ T cells and detection of elevated levels of IFN-α and IL-12 occur with successful therapy and this has been seen in longitudinal studies of acute HIV infection up to 6 months after HIV-positive diagnosis [57]. Long-term, chronic HIV infection is characterized by increases of inflammatory cytokine responses, including TNF-α, IL-1β, IL-6, and IL-10[49, 58]. Other pro-inflammatory cytokines are observed, including IL-18, potentially increasing viral replication in CD4+ T cells [59]. These and other cytokines have been found to be associated with cardiovascular disease and cognitive dysfunction even in individuals on antiretroviral therapy [60, 61]. HIV infection causes ongoing systemic inflammation, likely implicated in co-morbid complications during the course of chronic HIV infection.
2.3. Cytokine associations with HIV co-morbidities
After initial HIV infection, the subsequent disease course depends on factors such as early treatment interventions and good immune control of viral replication. Although systemic inflammation is reduced by HAART treatment, it is not eliminated, and the detection of inflammatory cytokines have been associated with increased morbidity [44, 62]. HIV-related immune dysfunction involving low CD4+ T cell counts, high viral HIV RNA, and co-infection complications worsen prognosis and drive increases in inflammatory cytokines [63]. These complications can lead to a range of co-morbidities including cardiovascular disease [64–66], loss of bone development [67], liver dysfunction [68], HIV-associated nephropathy (HIVAN) [69], and cognitive dysfunction [70] (Table 1A and B). These HIV-related co-morbidities are believed to be partly caused by dysregulated cytokine production[71].
Table 1A.
Cytokine effects on HIV co-morbidities
| HIV Co-Morbidities | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cytokines | Lymphoma | Cardiovascular disease | Neurocognitive dysfunction | Bone loss | Liver disease | Vasculopathy | Neuropathy | Nephropathy |
| Pathogenic | ||||||||
| TNF-α | X | X | X | |||||
| IL-1α/β | X | X | X | X | ||||
| IL-6 | X | X | X | X | X | X | ||
| IL-10 | X | |||||||
| IL-18 | X | |||||||
| MCP-1 | X | X | ||||||
| Protective | ||||||||
| TGF-β | X | X | X | |||||
| SDF-1 | X | X | ||||||
| IL-27 | X | |||||||
Table 1B.
Specific cytokine associations with co-morbidities
| Cytokines | Effect | Reference |
|---|---|---|
| TNF-α | Cardiovascular: Induction of inducible nitric oxide synthase (iNOS) in cardiomyocytes inducing apoptosis. | [179] |
| Neurocognitive: Predictor of time to HIV associated dementia. | [180] | |
| Bone loss: Triggers apoptosis in osteoblast in vitro. | [181] | |
| IL-1α/β | Cardiovascular: Increased in enhanced cardiac iNOS. | [179] |
| Neuropathology: Activation of glial cells leading to enhanced pain. | [182] | |
| Vasculopathy: Increased expression with leukocyte adhesion molecules in HIV infection leading to increased leukocyte adhesion, vascular permeability and leakiness. | [183] | |
| IL-6 | Lymphoma: Drives immune activation and elevated identified long before lymphoma diagnosis. | [184] |
| Neurocognitive: Elevated in HIV associated neurocognitive dysfunction. | [185] | |
| Bone Loss: Associated with lower osteocalcin levels. | [186] | |
| Vasculopathy: Causes endothelial activation. | [187] | |
| IL-10 | Lymphoma: Elevated levels in non-Hodgkin’s B cell lymphoma in HIV positive individuals. | [188] |
| IL-18 | Cardiovascular: Directly linked to the production of inflammatory cytokines and cell adhesion molecules in LPS-induced myocardial dysfunction in animal studies. | [189] |
| MCP-1 | Cardiovascular: Induced by IL-6 and associated with atherosclerotic lesions | [190] |
| Neurocognitive: Elevated in HIV associated neurocognitive dysfunction; Associated with increase in monocytes across the blood brain barrier. Correlates with brain injury in HIV infection. | [191] | |
| TGF-β | Lymphoma: Inhibits lymphoma development | [192] |
| Liver disease: Protects from fibrosis | [193] | |
| Nephropathy: Associated with glomerular disease and matrix accumulation | [194] | |
| SDF-1 | Cardiovascular: Protective effect on cardiomyocytes and motivation of cardiac progenitor cells. | [195] |
| Neurocognitive: Polymorphism in SDF-1 abrogates protective efficacy of wildtype and associated with development of neurocognitive impairment. | [196] | |
| IL-27 | Neuropathy: Protects against Oncostatin M-mediated TNF-α expression in microglia. | [197] |
Early in the AIDS epidemic it was noted during post-mortem studies that there is increased prevalence of cardiac disease in patients with AIDS. Hypotheses for this increase were attributed to viral or opportunistic co-infections[72, 73], autoimmune responses to cardiac proteins, or drug related toxicity [74]. The etiology of cardiac disease in AIDS patients is unclear; however, HIV gene transcripts have been identified in cardiac tissues and in vitro studies have shown that HIV-1 can infect cardiac myocytes[75]. Although it was hypothesized that HIV-infection itself could cause cardiac pathology, in vitro infection studies have shown that although HIV-1 infects cardiomyocytes, this infection does not yield in the production of new HIV-1 virions and therefore may not be the direct cause of pathology[76]. Instead, systemic immune activation and the inflammation of interstitial cardiac immune cells, including macrophages and T cells, could cause increases in cytokine production and cardiac related pathology[76]. With HIV infection and loss of immune function, systemic lipopolysaccharides (LPS) and free radicals increase, inducing the production of systemic inflammatory cytokines, including IL-1, IL-6, and TNF-α[77], and leading to increases in immune associated cardiac fibrosis and cardiac disease[76].
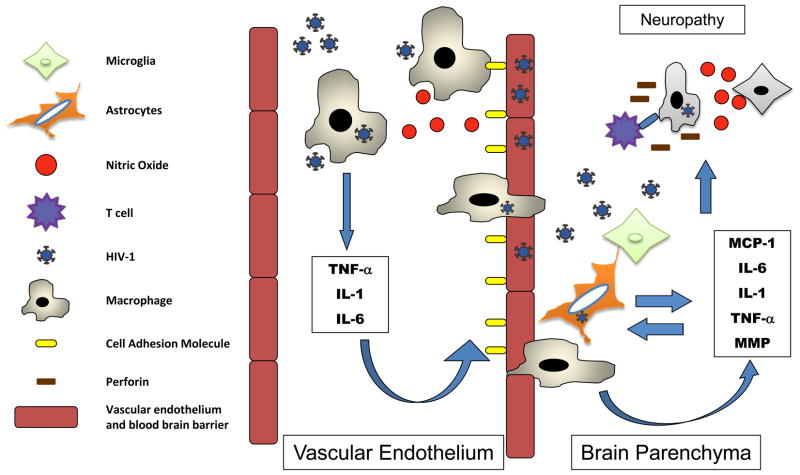
Establishment of cerebral effects during HIV infection may occur during the first weeks of infection, but it is not until late infection with the loss of immune control and increase in viral load that major neurological disorders occur. Viral RNA can be found in the central nervous system (CNS) of acutely infected individuals as early as 8 days after the estimated date of infection [78], suggesting that viral or host factors may play a role in the ability of virus to cross the blood brain barrier. Immune cells enter the cerebral spinal fluid (CSF) during acute infection, and elevated chemokines including monocyte chemotactic protein (MCP)-1 and IP-10, not only indicate monocyte activation[78], but also disruption of the regulating endocrine function in neuronal and glial cells[79]. This generalized activation can lead to increased production of matrix metalloproteinases (MMP-2 and MMP-9), resulting in the degradation of neuronal laminins and leading to neuronal damage and neurocognitive dysfunction [80]. While elevated expression of cytokines in the CSF is clearly associated with neurological inflammation, systemic levels of cytokines have also been implicated in neurocognitive dysfunction. Associations can be made, but mechanism of function is difficult to establish. It can be postulated that increases in the circulating inflammatory cytokines TNF-α, IL-6 and IL-1 by HIV-1 infected cells, increased plasma gp120, other viral co-infections, or increased LPS levels due to microbial translocation can activate vascular endothelium and up-regulate cell adhesion molecules. This would enable more efficient movement of activated macrophages through the blood brain barrier or could directly affect neuronal subpopulations [81](Figure 2). On the other hand, the immunomodulatory cytokines IL-10 and IL-4 or tissue inhibitors of MMP (TIMP) may help to reverse the inflammatory effects and repair immunopathology [80]. The mechanisms of cytokine action in HIV infection in neuropathology and neurocognitive dysfunction need to be further elucidated.
Figure 2.
Macrophage-produced cytokines and nitric oxide (NO) induce endothelium-associated cell adhesion molecules enabling macrophages to move to the brain parenchyma. Cytokines and NO drive continued activation, MMP disrupts neuronal survival-signals, and T cells kill infected cells leading to damage and dysfunction.
Systemic cytokines have been associated with the above and many other HIV related co-morbidities. For the most part, these studies have been conducted in the context of one disease or of one type of co-morbidity. The reality is that the inflammation of HIV infection can affect multiple disease pathways and one disease cannot be studied exclusively. Therapies that address not only the infection to improve overall immune responses and reduce viral burden but also modulate sources of inflammation and reduce pathology will enable a holistic treatment of multiple aspects of HIV-related diseases that occur late in HIV infection.
3. Potential therapeutic roles of cytokines in HIV
3.1. IFN-α Therapy
IFN-α is a well-known antiviral and pro-inflammatory cytokine with wide ranging effects on the immune system. From early in the AIDS epidemic, there has been evidence for dysfunction in the IFN system of HIV infected patients, yet there have been a considerable number of in vitro studies showing that IFN-α can block HIV replication [82, 83]. Thus, the known antiviral effects of IFN-α and knowledge of its dysfunction in HIV infection has led to studies investigating its use as a potential therapeutic. IFN-α has been used as therapy for HIV mono-infection, but most of the more recent studies utilizing IFN-α as a therapeutic involve patients who are HIV/HCV co-infected (reviewed in [84]). However, the first in vivo studies of IFN-α as a therapeutic were undertaken in the very early days of the pandemic, with treated Kaposi’s sarcoma (KS) patients showing tumor regression and reduced HIV p24 levels. These studies and others resulted in FDA approval in 1988 for IFN-α treatment of AIDS-associated KS [85–90].
Following these early studies, there have been multiple studies where IFN-α was used in combination with anti-retroviral drugs [91–99]. These studies showed mixed results, probably related to differing preparations of IFN-α or utilizing p24 levels (though some utilized HIV-1 RNA) as a biomarker for HIV replication. Additionally, the actual anti-retroviral effect of IFN-α HIV replication could not be determined due to the co-treatment with antiretroviral drugs. However, in general these studies were supportive of the potential efficacy of IFN-α therapy. There have been three studies where IFN-α was given alone where the antiretroviral effects of IFN-α therapy could be assessed [100–102]. These studies showed a dose dependent decrease in viral load, and the two more recent studies showed tolerable side effects.
As standard of care for HCV infection, IFN-α is currently administered as pegylated-IFN-α (pIFN-α, polyethylene glycol attached IFN-α). The earlier studies discussed above all utilized non-pegylated IFN-α. However, there have been three studies utilizing pIFN-α (summarized in Table 2). First, in a study by the Primoferon A study group, transient and early dosing of pIFN-α along with HAART was evaluated in 12 patients with primary HIV infection for tolerance and efficacy [103]. This study showed that pIFN-α was well tolerated and resulted in early control of infection as determined by HIV-1 RNA as well as by incorporation of HIV DNA in PBMC. Following this study, Portales et al. published a study where pIFN-α treatment resulted in restored NK cell lytic activity [104]. Although pIFN-α was not able to restore NK cell numbers to levels similar to uninfected subjects, perforin expression was restored. This increase was seen as both a percentage of NK cells expressing perforin as well as intracellular concentration of perforin. The authors concluded that there was potential for pIFN-α treatment in conjunction with other cytokines that are capable of reconstituting cell numbers to achieve both functional and numerical restoration of immune populations. Finally, a recently published safety and tolerability study by Asmuth et al. is the first to study pIFN-α monotherapy as a treatment for anti-retroviral naïve HIV infected patients without HCV co-infection or associated malignancy [105]. pIFN-α treatment resulted in a median decrease in HIV-1 RNA of 0.61 log10 and was well tolerated. Although these studies are preliminary, they show evidence for efficacy of pIFN-α as a front line therapy for HIV together with other anti-retroviral drugs or potentially with other cytokine therapies.
Table 2.
Human cytokine therapy trials
| Therapy | Enrolled Patients | Study | Clinical Effect to HIV Infected Patients | References |
|---|---|---|---|---|
| IFN-α | 12 | Primoferon A (ANRS 086) | Well tolerated and showed early control of infection as determined by HIV RNA and HIV DNA incorporation. | [103] |
| IFN-α | 63 | IFN-α effect on NK cells | No increase in NK cell numbers, but significant increase in lytic function of NK cells. | [104] |
| IFN-α | 11 | ACTG protocol 5192 | Treatment naïve patients displayed median decrease of HIV-1 RNA | [105] |
| IL-2 | 1695 | SILCAAT | Significant increase CD4+ T cell numbers that was accompanied by increased association with opportunistic infection. | [111, 112] |
| IL-2 | 4111 | ESPRIT | Significant increase CD4+ T cell numbers that was accompanied by increased association with opportunistic infection. | [111, 112] |
| IL-2 | 31 | SILCAAT and ESPRIT Follow-up | Increased CD4+ T cells were primarily CD4+CD25+ T regulatory cells. | [113] |
| IL-2 | 12 | IL2 therapy in Immunological Non Responders | Restored lympho-monocyte system ability to kill Myocbacteriumavium. | [118] |
| IL-2 | 56 | COSMIC Trial | Increased CD4+ T cell counts, but no effect on levels of integrated proviral DNA. | [122] |
| IL-2 | 267 | STALWART Trial | Increased CD4+ T cells was successful in delaying ART, but there was increased risk for OI. | [124] |
| IL-2 | 130 | ANRS 119-IL-2 before antiretroviral therapy | Increased CD4+ T cells allowed deferment of ART, with reduced rates of disease progression. | [125] |
| IL-2 | 86 | TILT: IL-2 and HAART interruption | Compared to patients interrupting HAART without IL-2, there was a significant increase in the time to restart HAART with IL-2 treatment. | [126] |
| IL-2 | 41 | ICARUS: IL-2 and HAART interruption | IL-2 alone was inferior to IL-2 with HAART in maintaining CD4+ T cells at least 90% of baseline. | [127] |
| IL-7 | 14 | IL-7: Repeated dose escalating safety study | Significant and sustained increase in functional naïve and central memory CD4+/8+ T cells. | [139] |
| IL-7 | 25 | ACTG 5214-Single Dose IL-7 safety study | Recruitment of CD4+/8+ T cells into cell cycle with increases in central memory and to a lesser extent naïve cells. | [198] |
ACTG=AIDS Clinical Trials Group; ANRS= AgenceNationale de Recherchessur le SIDA et les HepatitesVirales; HAART= Highly Active Antiretroviral Therapy
3.2. IL-2 Therapy
The common gamma chain cytokines, IL-2, -7, -15, and -21, are critically important to T cell biology, where they influence proliferation, function and cell homeostasis. In HIV infection there is dysregulated cytokine production, with reduced IL-2 and IL-15 and increased IL-7. Despite these cytokine perturbations, the use of HAART in treatment of HIV has drastically reduced mortality rates [106]. However, there are drawbacks to long-term HAART which could potentially be alleviated with cytokine therapy. Since IL-2 producing CD4+ T cells are the primary target in HIV infection, IL-2 therapy would seem to be a good candidate, and in fact has been explored the most. There have been multiple phase I studies utilizing IL-2 reaching as far back as mid 1980’s [107], as well as multiple phase II studies from the late 1990’s and early 2000’s (reviewed in [108]). These studies determined that intermittent IL-2 alone or in combination with anti-retroviral therapy results in increased CD4+ T cells. Also, two more recent studies in small patient populations demonstrated that intermittent IL-2 treatment leads to long-term increases in CD4+ T cell counts and that IL-2-induced prolongation of cell survival is critical to the observed increases [109, 110]. From the early phase I and II studies, multiple phase III studies have been undertaken exploring the use of IL-2 therapy in 4 different settings: 1) IL-2 with ART vs. ART alone, 2) The role of IL-2 in purging the viral reservoir, 3) the role of IL-2 in deferring the start of continuous ART, and 4) IL-2 and ART interruption.
3.2.1. IL-2 in conjunction with ART
The Subcutaneous Recombinant, Human Interleukin-2 in HIV-Infected Patient with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) study, and the Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT) study are the two most well-known trials to investigate IL-2 treatment in conjunction with ART [111, 112]. Both were randomized, endpoint trials investigating the clinical benefit of IL-2 treatment in conjunction with ART. Patients from both trials were randomized into two arms; IL-2 plus antiretroviral therapy, or antiretroviral therapy alone and followed for 7–8 years. Both trials showed significant and substantial increases in CD4+ T cell counts when compared to antiretroviral treatment alone that was in agreement with earlier phase II studies. However, both trials failed to demonstrate clinical benefits to patients treated with IL-2 and ART as measured by risk of opportunistic disease and death. Immediately following these two trials, two studies were undertaken to explain why the increase in CD4+ counts did not translate to a clinical benefit. Weiss and colleagues investigated how IL-2 treatment affected the phenotypic, functional, and molecular characteristics of CD4+ T cell populations [113]. They found that IL-2 therapy resulted in expansion of T cells expressing markers functional characteristics of regulatory T cells(Tregs). The expansion of the Treg compartment in IL-2 treated patients would leave them with an impaired T cell response despite having higher numbers of CD4+ T cells. This finding would support the hypothesis that IL-2 treatment resulted in an expanded population of CD4+ T cells that do not participate in host defense. The second study by Fontas et al. focused on subgroups of patients determined by their baseline CD4+T cell counts [114]. They found that only patients with a baseline CD4+ T cell count of <200 showed significant clinical benefit with IL-2 treatment. These data would support the importance of proper balance in the T cell compartment, where individuals with higher cell counts would see a greater expansion of the Treg compartment, resulting in a T cell compartment that was refractory to host defense. There were also two studies published at the same time as and shortly after the release of the SILCAAT/ESPRIT study results that supported another hypothesis for the failure of the studies. Porter et al. and Barbai et al. both demonstrated that IL-2 cycling results in increases in acute-phase proteins, specifically C-reactive protein (CRP) [115, 116]. CRP typically increases in the blood in response to inflammation and is associated with increased cardiovascular disease. Therefore, increases in CRP as a result of IL-2 cycling could potentially explain the increase in cardiovascular side effects observed it the IL-2 arm of the ESPRIT trial, and support the second hypothesis that negative effects of IL-2 neutralized any improvements in host defense. Despite the negative results of the SILCAAT/ESPRIT trials, cytokine therapy remains viable. In fact, Read et al. published that the T cell repertoire (including CD4+CD25+ Tregs) in the gut remains unchanged following IL-2 therapy, leaving room for future therapies that target gut or other mucosal T cell populations [117]. Additionally, as pointed out above, patients that had low baseline CD4+ T cell counts or were non-responsive to ART showed significant clinical benefit from IL-2 therapy. These data are supported by a study designed to look specifically at immunologically non-responder (INR) HIV-infected patients [118]. This study demonstrated that PBMC from INRs showed significant increases in cytokine function and killing of Mycobacterium avium following IL-2 treatment. Since the previous studies did not specifically target INRs, it remains to be determined if IL-2 therapy will be a beneficial treatment for those patients that do not respond or respond poorly to ART.
3.2.2. IL-2 and purging the viral reservoir
IL-2 is critically important to both T cell survival as well as T cell activation and is a strong activator of T cells that promotes viral replication in vitro[119]. The ability to activate T cells could allow for the reactivation of latently infected cells, thus leaving them susceptible to elimination while on ART. Three important studies were undertaken to determine if IL-2 treatment could aide in purging the viral reservoir [120–122]. Initial findings by Chun et al. showed lower levels of replication-competent virus in patients receiving intermittent IL-2 plus ART. However, IL-2 therapy had no effect on proviral DNA in peripheral blood [122], and patients showed a considerable rebound in HIV RNA following cessation of ART therapy [121].
3.2.3. IL-2 and deferring continuous ART
Because of the potential side effects of long term ART, it was hypothesized IL-2 therapy could potentially allow for the delay in ART commencement in patients with stable CD4+ T cell counts. An early study by Youle et al. demonstrated increased CD4+T cell counts in treatment naïve patients given IL-2 without increased plasma RNA levels [123]. This study and others provided the basis for the Study of aldesleukin with and without antiretroviral therapy (STALWART). STALWART was designed to see how CD4+T cell counts responded to IL-2 alone or antiretroviral therapy given around IL-2 treatments [124]. Patients who were treatment naïve or without treatment for at least a year were randomized to 3 arms: no treatment, IL-2 for 5 consecutive days every 8 weeks, or the same IL-2 regimen with 10 days of ART around each IL-2 cycle. Similar to other studies, IL-2 treatment provided a boost in CD4+T cell counts in both the IL-2 and IL-2 plus antiretroviral therapy arms that were of equal magnitude. The boost in CD4+T cell counts with IL-2 was successful in delaying ART, but there was also an increased risk for opportunistic disease. It is important to note that ANRS 119, another trial comparing IL-2 treatment prior to ART, achieved similar results, with increased CD4+T cell counts allowing deferment of ART initiation. However, there were reduced rates of progression in this study [125]. With these data in mind, the association of IL-2 treatment with increased opportunistic events may not necessarily preclude use of IL-2 for deferment of ART, especially since such a small number of IL-2 treated patients experienced opportunistic events (12/176). Therefore as long-term follow up has been extended for STALWART study participants, additional findings may encourage ART deferment with IL-2 treatment.
3.2.4. IL-2 and ART interruption
Due to high cost and toxicities, there is still the possibility that patients will voluntarily interrupt ART treatment. Despite the fact that the SMART trial showed increased incidence of clinical events with treatment interruption, there have been multiple studies investigating if IL-2 therapy in conjunction with ART interruption increased the time to restarting ART. The Therapy Interruption with and without use of Interleukin-2 (TILT) study was an early study conducted to determine if IL-2 therapy during ART would increase time before therapy restart [126]. The study results showed that after 105 weeks, patients with IL-2 therapy had a 34% chance of restarting ART compared to 66% for patients not receiving IL-2. The authors concluded that although treatment interruption is not recommended, the use of IL-2 could lengthen the time before ART would need to be restarted. However, contrary to this data, the ICARUS study showed treatment interruption with IL-2 was inferior to IL-2 treatment with continuous HAART in maintaining CD4+ counts[127]. More recently, two additional studies were conducted to evaluate IL-2 treatment with treatment interruption. Although the studies utilized different endpoints, the two studies had conflicting results. Bosch et al. published the results of the AIDS Clinical Trials Group A5132 study that looked at IL-2 treatment with repeated short-term treatment interruptions [128]. The study contained 2 arms, A) Two 4 week treatment interruptions with IL-2 during the last 2 weeks of treatment interruption and the first week back on therapy; B) Two 4 week treatment interruptions with IL-2 during the first 5 days of treatment re-initiation. Treatment was then interrupted for 12–48 weeks after the second 12 week treatment re-initiation. Study results showed no difference between the treatment groups as determined by viral load set-point during the third treatment interruption. However, Levy et al. concluded following the ILIADE trial that IL-2 therapy during HAART is useful in delaying ART due to the persistence of lower activated CD4+CD25+ T cells [129]. This study showed both a significant increase in CD4+T cells with IL-2 treatment while on HAART, as well as a slower decline CD4+ T cells in the treatment group. In addition, this study performed an immunological sub-study to assess the phenotype and level of activation of the CD4+T cells and found that lower activated cells, as measured by CD38 and HLA-DR expression, persisted longer and had a slower decline in the treatment group, resulting in the circulation of cells that were less prone to HIV infection. It is important to note that the evidence supporting IL-2 therapy with treatment interruption is contradictory, however, this study represents the need to find better biomarkers to measure efficacy and that CD4+ T cell count alone is not sufficient. Specifically, it is possible that in other studies both with treatment interruption as well as the other uses for IL-2 therapy discussed above may well have had better outcomes if more than CD4+T cell counts alone were used as an endpoint biomarker. Therefore, it may be premature to rule out the further use of IL-2 therapy in treatment for HIV, and further characterization both phenotypically and functionally of CD4+ T cells will be necessary to completely assess the effects of IL-2 therapy as well as other potential therapies including other γ-chain cytokines (IL-7, IL-15), and IFN-α therapy.
3.3 Other Potential Immunotherapies
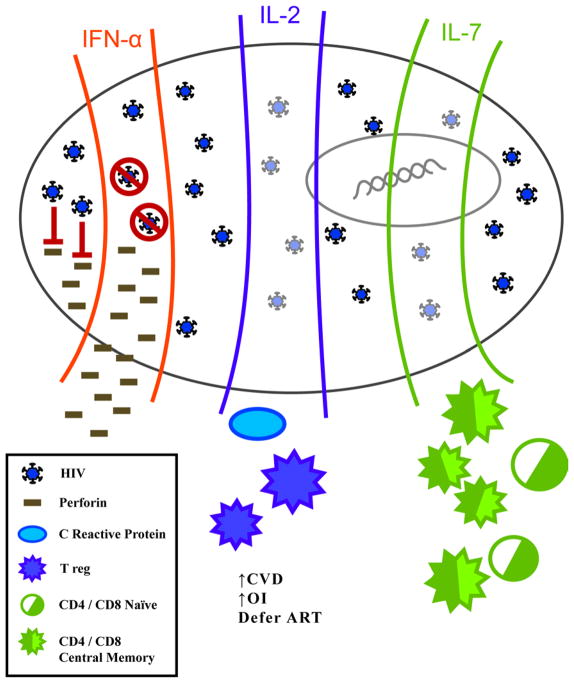
In addition to utilizing IL-2 as a potential cytokine therapy, other members of the common gamma chain cytokine family, IL-7 and IL-15, have also been considered for therapy due to their ability to regulate proliferation and survival T cell populations (Figure 3).
Figure 3.
pIFN-α treatment blocks viral replication with limited side effects and restores perforin production/secretion. IL-2 and IL-7 therapy have no direct effect on replication but result in increased CD4+ T cells, Tregs with IL-2 and naïve and central memory with IL-7.
3.3.1. IL-7
IL-7 is constitutively produced by lymphoid stromal tissue [130], is important in T cell homeostasis and survival [131], and was shown to be elevated in subjects with HIV-induced CD4+ T cell depletion [130]. Despite findings that IL-7 can increase HIV replication [132, 133], there have been several studies in the SIV macaque model showing the potential immune benefits of IL-7 therapy without adverse effects on plasma viremia [134, 135]. In addition, recent studies where cancer patients were treated with rhIL-7 showed beneficial results for immune reconstitution with limited side effects [136–138]. These early studies provided a rationale for two clinical trials investigating IL-7 therapy in HIV infection. The first study by Levy et al. was designed to assess safety and immunological effects of IL-7 therapy in HIV infected patients with low CD4+T cell counts despite suppressed viral load with ART[139]. This study found that IL-7 was well tolerated and resulted in sustained increases in functional CD4+ and CD8+ T cells, primarily of the naïve and central memory phenotype, which are prone to immunological exhaustion in chronic HIV infection. The second study by Sereti et al. published in the same year was also a dose escalating trial that evaluated safety and efficacy of a single dose of rhIL-7. Similar to the Levy study, this study also showed an increase in CD4+ and CD8+ T cells primarily of the central memory and less so of the naïve phenotype. They also found evidence of recruitment of CD4+ and CD8+ T cell recruitment into the cell cycle and that IL-7 treatment affected maturation of circulating B cells. In addition, there was no indication of increase Tregs upon treatment with IL-7, in contrast to IL-2 therapy, and could play a role in whether IL-7 is more effective than IL-2 as an immunotherapy in HIV infection. In both studies, there were small transient increases, or blips, in the viral load of some of the study cases, but no sustained increases in viral load. These episodic blips of viral load were investigated in a recent study by Imamichi and colleagues [140]. This study was designed to find the source of the HIV during viremic blips following IL-7 therapy. This study demonstrated that the plasma viruses detected during the viremic blips were both similar to viral quasi species present before and after IL-7 treatment, and genetically indistinguishable from provirus detected during sampling. The authors concluded that the burst in viral load probably resulted from amplification of virus already present, and not from previously silent latent reservoirs. Thus, although there are few in human studies of IL-7 therapy as a treatment for HIV, these early studies show the potential for targeted reconstitution of specific cell populations known to be depleted in HIV infection, resulting in immunological benefit without the side effects seen with IL-2 therapy. However, the ultimate success of IL-7 therapy clearly needs to be further investigated.
3.3.2 IL-15
As of yet there have been no human studies focusing on IL-15 treatment of HIV. However, as with IL-2 and IL-7, IL-15 is important for T cell homeostasis and in particular for memory CD8+ T cells ([141]and reviewed in [142]), and it is an important cytokine in development and maintenance of natural killer cells [143]. In addition, there is considerable evidence pointing to the importance of IL-15 in HIV infection. IL-15 was shown to enhance in vitro priming of CD8+ and CD4+ T cells for IFN-γ production and release of β-chemokine from T cells [144] and natural killer cells [145], and natural killer cell activity against HIV is augmented by IL-15 through a TRAIL-mediated mechanism [146]. More recently, IL-15 stimulated NK cells were shown to interfere with HIV replication [147]. The importance of IL-15 in modulating both T cell and NK cell immunity to HIV as well as recent trials with IL-15 to induce immune reconstitution in cancer (reviewed in [148]) has led to multiple in vivo studies with IL-15 in non-human primates designed to assess the immunological benefits of IL-15 therapy (reviewed in [149]). These studies along with the in vitro data provide encouraging insight into the usefulness of IL-15 as an immune therapeutic in HIV infection that will require future studies and human trials.
4. Cytokines as adjuvants in HIV vaccine development
4.1. Cytokine adjuvants for viral vaccines
Early research into immune correlates of protective efficacy against viruses has shown that a strong IFN response is required to control viral infections[150]. Factors required for protection against viral infections include both cell-mediated and humoral immune responses against the viral infection. In 1990 it was discovered that immune compromised individuals who were more susceptible to HBV infection and less likely to develop protective HBV responses after vaccination could achieve better immunity with the addition of IFN-α and IFN-γ during vaccination [151][152]. Vaccine development has evolved to utilize this mode of adjuvanting vaccines with cytokines, which has been shown to enhance anti-viral immune responses in other vaccine development models. Enhancement of anti-HSV immunity can be accomplished by incorporating IL-2 to target IFN-γ producing T cell responses. This induces stronger responses even with lower quantities of vaccine, albeit only inducing non-protective immunity [153, 154]. From the cancer vaccine field we have learned that at low dose GM-CSF can enhance vaccine responses, but at high dose can induce myeloid suppressor cells and impair vaccine responses [155].
4.2. Animal models for HIV vaccines
Although animal models have limitations in identifying HIV-protective immune responses in humans, animal studies are useful in identifying vaccine regimens that are capable of inducing and enhancing immune responses found to be essential in HIV viral control. Investigators commonly use murine models to show proof of concept in vaccine models. Many murine studies have incorporated cytokines as vaccine adjuvants and elucidated that incorporation of GM-CSF and IL-12 in peptide vaccines target the Th1 and suppress Th2 arms of the immune response [156, 157]. Timing the introduction of the cytokine adjuvant appears important in enhancing the antigen specific immune responses. Barouch et al. found that introduction of an IL-2 plasmid-Ig fusion protein only after priming with gp120 plasmid enhanced immune responses [158].
The bulk of HIV vaccine research has been performed in the non-human primate model. Early studies found that it was possible to induce protective immunity after vaccinating with inactive virus in adjuvant [159], although this protection was not long-lived. Enhancement of vaccination could be achieved by incorporating gene constructs for cytokines that support anti-viral immunity as shown in earlier murine studies. An IL-2 enhanced DNA vaccine induced augmented immune responses [160] and allowed control of viremia and prevention of AIDS in RMs [161]. IL-2 and IL-15 were compared in their ability to augment influenza and tetanus toxoid-specific vaccine induced responses in RMs. The highest primary vaccine responses were seen with IL-2 plus IL-15 co-administration, while the highest boosted responses were seen in the IL-15 plus vaccine alone group, implying that IL-15 may have advantages over IL-2 for enhancing vaccine responses [162]. In subsequent studies the effects of IL-15 on SIV vaccine augmentation have been mostly negative, showing no enhancement of SIV-specific immune responses or viral control [163, 164]. Combination of TLR agonists plus IL-15 was shown to lower viral load in treated RMs, and preserved CD4+ T cell counts were associated with higher levels of the innate SIV restriction factor APOBEC3G in mesenteric lymph node cells [165]. The other common gamma chain cytokine to be tested in combination SIV vaccines is IL-7, which was shown to expand SIV-specific CD4+ but not CD8+ T cells [164].
IL-12 and GM-CSF together as SIV vaccine adjuvants have shown mixed results, with one study showing vaccine impairment[166]and a second showing improved viral control with administration of the two cytokines followed by boosting with IL-12[167]. Immunizing with IL-12 alone as an adjuvant increases both T cell and antibody immune responses [168, 169]. Comparison of IL-12 to IL-15 as a vaccine adjuvant suggests that IL-12 is more effective in inducing immune responses and improving outcome after SIV challenge [163] and that IL-12 appears to boost the effector memory subset of SIV-specific CD8+ T cells [170]. Subsequent work has also shown that IL-12 as an adjuvant can boost SIV-specific CD4+ T cells [171], potentially important in early viral control [39].
As the mucosa is the site of infection for most people exposed to HIV, it is important to induce long lived antiviral antibody and T cell responses there. Vaccination with GM-CSF plasmid in addition to a DNA/MVA prime boost regimen has been shown to induce protective high avidity IgG and IgA responses in the mucosa[172], while vaccination with IL-15 plasmid enhances cellular immune responses and supports the persistence of mucosal memory CD4+ T cell responses [165, 173]. However, after infection increases in IL-15 production fail to impart any protective effect and has been associated with increased susceptibility of memory CD4+ T cells to infection [174]. The timing of the introduction of cytokines will be important in differential outcomes of cytokine enhanced immunity after vaccination.
4.3. Human trial data for HIV vaccines
One of the goals for any HIV vaccine is to induce an immune response that will prevent establishment of infection at the site of entry. The mucosa is the primary site for this immune response and a successful vaccine will likely need to target both humoral and cell mediated immunity to be localized in this area. A number of early clinical studies using protein antigens found that using pro-inflammatory cytokine adjuvants such as IL-1 intranasally effectively induces not only serum and vaginal IgG but also vaginal IgA [175].
Early safety and immunogenicity trials in humans allow for exploration of candidate vaccines. Using a plasmid delivery mode, Xin et al. incorporated an IL-15 plasmid and after intranasal vaccination was able to induce strong Th1 responses. In this study they also found that there was no enhancement of the immune response if IL-2 or IL-12 plasmids were added [176]. Although some animal work has been done to investigate the efficacy of cytokine adjuvanted vaccines in non-human primates, few of these HIV vaccine methods have moved into human clinical trials. A few safety and immunogenicity trials have been conducted. For example, GM-CSF was established as a good candidate for supporting protective immune responses. It was incorporated into a clinical protocol in the HIV Vaccine Trials Network [177] and was shown to be immunogenic and safe in a phase I trial. More recently, a phase I study of an IgG-IL-2 fusion protein has been conducted and shown to increase immune responses not when given during the antigenic vaccination, but only when given 48 hours after vaccination[178]. This shows that IL-2 is important in driving an ongoing immune responses rather than in supporting a new immune response. Although interesting as proof of concept, the staggered timing of the vaccine schedule would pose a difficulty in achieving successful vaccination regimens.
5. Conclusions
As the ability to measure multiple cytokines simultaneously has improved, it has become clear that HIV causes a profound disruption of the cytokine network from the earliest points of HIV infection, shortly after first detection of systemic virus. This dysregulation of cytokines can be partially but not completely reversed via suppression of viral replication through pharmacologic or immunologic control. While HIV likely exploits pro-inflammatory cytokines to promote viral replication, there are multiple potential avenues to use these factors to help control HIV replication or ameliorate the damage inflicted by the virus on CD4+ T cells. Examples of cytokines that have been explored in human studies include IFN-α, IL-2, IL-7, and IL-15. While none are used in current clinical practice, the therapeutic use of cytokines is a field still in its infancy. Another attractive prospect of cytokine therapy is as an adjuvant to vaccine approaches. HIV is notoriously difficult to prevent or even control via vaccines, and several cytokines have shown promise in augmenting virus-specific immune responses and aiding in control of viral replication in animal models. In summary, our knowledge of the depth and breadth of cytokine perturbation is increasing exponentially, and with this new knowledge comes the potential of harnessing the immune system to limit viral replication and damage to the host immune system.
Acknowledgments
We would like to thank Abigail Schrock for her work in graphic design for the figures and expert assistance in formatting the manuscript.
Biographies

Sheila Keating is a Scientist II and head of the Core Immunology Laboratory at Blood Systems Research Institute. Her research interests stem from her doctoral and post-doctoral work on the identification of vaccine induced immune responses and investigation of immunology memory. She is currently studying novel biomarkers of chronic and infectious diseases

Evan Jacobs is a Post-Doctoral fellow at Blood Systems Research Institute in the laboratory of Dr. Philip Norris. His research interests are in HIV pathogenesis and vaccine development, centering on cytokine responses to HIV infection.

Philip Norris is the Associate Director of Blood Systems Research Institute and an Associate Professor of Laboratory Medicine and Medicine at the University of California, San Francisco. He is an infectious diseases physician, and his research interests center on the interaction of the human immune system with viral infections and blood transfusion, with particular interest in T cell immune responses and cytokine network activation in acute viral infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, et al. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–3. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste O, Vaslin B, Le Grand R, Fouchet P, Omessa V, Theodoro F, et al. Interleukin 1 beta, interleukin 6, tumor necrosis factor alpha, and interleukin 10 responses in peripheral blood mononuclear cells of cynomolgus macaques during acute infection with SIVmac251. AIDS Res Hum Retroviruses. 1996;12:241–50. doi: 10.1089/aid.1996.12.241. [DOI] [PubMed] [Google Scholar]
- 5.Khatissian E, Tovey MG, Cumont MC, Monceaux V, Lebon P, Montagnier L, et al. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res Hum Retroviruses. 1996;12:1273–8. doi: 10.1089/aid.1996.12.1273. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg YJ, Cafaro A, Brennan T, Greenhouse JG, Villinger F, Ansari AA, et al. Virus-induced cytokines regulate circulating lymphocyte levels during primary SIV infections. Int Immunol. 1997;9:703–12. doi: 10.1093/intimm/9.5.703. [DOI] [PubMed] [Google Scholar]
- 7.Zou W, Lackner AA, Simon M, Durand-Gasselin I, Galanaud P, Desrosiers RC, et al. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–36. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheret A, Le Grand R, Caufour P, Neildez O, Matheux F, Theodoro F, et al. RANTES, IFN-gamma, CCR1, and CCR5 mRNA expression in peripheral blood, lymph node, and bronchoalveolar lavage mononuclear cells during primary simian immunodeficiency virus infection of macaques. Virology. 1999;255:285–93. doi: 10.1006/viro.1998.9558. [DOI] [PubMed] [Google Scholar]
- 9.Kanki PJ, Kurth R, Becker W, Dreesman G, McLane MF, Essex M. Antibodies to simian T-lymphotropic retrovirus type III in African green monkeys and recognition of STLV-III viral proteins by AIDS and related sera. Lancet. 1985;1:1330–2. doi: 10.1016/s0140-6736(85)92818-1. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 11.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–91. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–87. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 13.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–91. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benveniste O, Vaslin B, Le Grand R, Cheret A, Matheux F, Theodoro F, et al. Comparative interleukin (IL-2)/interferon IFN-gamma and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SICmac251 virus. Proc Natl Acad Sci U S A. 1996;93:3658–63. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J Acquir Immune Defic Syndr. 2007;45:445–8. doi: 10.1097/QAI.0b013e318074ef6e. [DOI] [PubMed] [Google Scholar]
- 19.Abb J, Zachoval R, Zachoval V, Deinhardt F. HIV antigen, HIV antibody and serum interferon in a patient with encephalopathy. Infection. 1987;15:425–6. doi: 10.1007/BF01647221. [DOI] [PubMed] [Google Scholar]
- 20.Gaines H, von Sydow MA, von Stedingk LV, Biberfeld G, Bottiger B, Hansson LO, et al. Immunological changes in primary HIV-1 infection. Aids. 1990;4:995–9. doi: 10.1097/00002030-199010000-00008. [DOI] [PubMed] [Google Scholar]
- 21.von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:375–80. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 22.Sinicco A, Biglino A, Sciandra M, Forno B, Pollono AM, Raiteri R, et al. Cytokine network and acute primary HIV-1 infection. Aids. 1993;7:1167–72. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Biglino A, Sinicco A, Forno B, Pollono AM, Sciandra M, Martini C, et al. Serum cytokine profiles in acute primary HIV-1 infection and in infectious mononucleosis. Clin Immunol Immunopathol. 1996;78:61–9. doi: 10.1006/clin.1996.0009. [DOI] [PubMed] [Google Scholar]
- 24.Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses. 2006;22:757–62. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graziosi C, Gantt KR, Vaccarezza M, Demarest JF, Daucher M, Saag MS, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A. 1996;93:4386–91. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Smith AJ, Schacker TW, Carlis JV, Duan L, Reilly CS, et al. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol. 2009;183:1975–82. doi: 10.4049/jimmunol.0803222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasper-Smith N, Crossman DM, Whitesides JF, Mensali N, Ottinger JS, Plonk SG, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–10. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobler LH, Cameron MJ, Lanteri MC, Prince HE, Danesh A, Persad D, et al. Interferon and interferon-induced chemokine expression is associated with control of acute viremia in West Nile virus-infected blood donors. J Infect Dis. 2008;198:979–83. doi: 10.1086/591466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanteri MC, O’Brien KM, Purtha WE, Cameron MJ, Lund JM, Owen RE, et al. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest. 2009;119:3266–77. doi: 10.1172/JCI39387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicks DR, Martin LS, Getchell JP, Heath JL, Francis DP, McDougal JS, et al. Inactivation of HTLV-III/LAV-infected cultures of normal human lymphocytes by nonoxynol-9 in vitro. Lancet. 1985;2:1422–3. doi: 10.1016/s0140-6736(85)92584-x. [DOI] [PubMed] [Google Scholar]
- 32.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 33.Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–31. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–72. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts L, Passmore JA, Mlisana K, Williamson C, Little F, Bebell LM, et al. Genital Tract Inflammation During Early HIV-1 Infection Predicts Higher Plasma Viral Load Set Point in Women. J Infect Dis. 2012;205:194–203. doi: 10.1093/infdis/jir715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bebell LM, Passmore JA, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–4. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 38.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T Cell Depletion during all Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, et al. HIV-Specific Cytolytic CD4 T Cell Responses During Acute HIV Infection Predict Disease Outcome. Science translational medicine. 2012;4:123ra25. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol. 2009;83:12229–40. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, Diop O, et al. AIDS progression is associated with the emergence of IL-17-producing cells early after simian immunodeficiency virus infection. J Immunol. 2010;184:984–92. doi: 10.4049/jimmunol.0902316. [DOI] [PubMed] [Google Scholar]
- 42.Roberts L, Passmore JA, Williamson C, Little F, Bebell LM, Mlisana K, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. Aids. 2010;24:819–31. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barcellini W, Rizzardi GP, Poli G, Tambussi G, Velati C, Meroni PL, et al. Cytokines and soluble receptor changes in the transition from primary to early chronic HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:325–31. doi: 10.1089/aid.1996.12.325. [DOI] [PubMed] [Google Scholar]
- 44.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS. 2011;25:1823–32. doi: 10.1097/QAD.0b013e3283489d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 47.Sheth PM, Sunderji S, Shin LY, Rebbapragada A, Huibner S, Kimani J, et al. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J Infect Dis. 2008;197:1394–401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 48.Cassone A, Cauda R. Candida and candidiasis in HIV-infected subjects. Where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS. 2012 doi: 10.1097/QAD.0b013e3283536ba8. [DOI] [PubMed] [Google Scholar]
- 49.Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–4. [PubMed] [Google Scholar]
- 50.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992;8:191–7. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 52.Bollinger RC, Quinn TC, Liu AY, Stanhope PE, Hammond SA, Viveen R, et al. Cytokines from vaccine-induced HIV-1 specific cytotoxic T lymphocytes: effects on viral replication. AIDS Res Hum Retroviruses. 1993;9:1067–77. doi: 10.1089/aid.1993.9.1067. [DOI] [PubMed] [Google Scholar]
- 53.Bayard-McNeeley M, Doo H, He S, Hafner A, Johnson WD, Jr, Ho JL. Differential effects of interleukin-12, interleukin-15, and interleukin-2 on human immunodeficiency virus type 1 replication in vitro. Clin Diagn Lab Immunol. 1996;3:547–53. doi: 10.1128/cdli.3.5.547-553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–89. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansen CA, De Cuyper IM, Steingrover R, Jurriaans S, Sankatsing SU, Prins JM, et al. Analysis of the effect of highly active antiretroviral therapy during acute HIV-1 infection on HIV-specific CD4 T cell functions. AIDS. 2005;19:1145–54. doi: 10.1097/01.aids.0000176214.17990.94. [DOI] [PubMed] [Google Scholar]
- 57.Byrnes AA, Harris DM, Atabani SF, Sabundayo BP, Langan SJ, Margolick JB, et al. Immune activation and IL-12 production during acute/early HIV infection in the absence and presence of highly active, antiretroviral therapy. J Leukoc Biol. 2008;84:1447–53. doi: 10.1189/jlb.0708438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navikas V, Link J, Persson C, Olsson T, Hojeberg B, Ljungdahl A, et al. Increased mRNA expression of IL-6, IL-10, TNF-alpha, and perforin in blood mononuclear cells in human HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:484–9. [PubMed] [Google Scholar]
- 59.Iannello A, Boulassel MR, Samarani S, Tremblay C, Toma E, Routy JP, et al. HIV-1 causes an imbalance in the production of interleukin-18 and its natural antagonist in HIV-infected individuals: implications for enhanced viral replication. J Infect Dis. 2010;201:608–17. doi: 10.1086/650314. [DOI] [PubMed] [Google Scholar]
- 60.Conaldi PG, Serra C, Dolei A, Basolo F, Falcone V, Mariani G, et al. Productive HIV-1 infection of human vascular endothelial cells requires cell proliferation and is stimulated by combined treatment with interleukin-1 beta plus tumor necrosis factor-alpha. J Med Virol. 1995;47:355–63. doi: 10.1002/jmv.1890470411. [DOI] [PubMed] [Google Scholar]
- 61.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antiviral therapy. 2008;13:177–87. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 62.Currie PF, Boon NA. Immunopathogenesis of HIV-related heart muscle disease: current perspectives. AIDS. 2003;17 (Suppl 1):S21–8. doi: 10.1097/00002030-200304001-00004. [DOI] [PubMed] [Google Scholar]
- 63.Longo DL, Steis RG, Lane HC, Lotze MT, Rosenberg SA, Preble O, et al. Malignancies in the AIDS patient: natural history, treatment strategies, and preliminary results. Ann N Y Acad Sci. 1984;437:421–30. doi: 10.1111/j.1749-6632.1984.tb37163.x. [DOI] [PubMed] [Google Scholar]
- 64.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–7. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 65.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 66.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malizia AP, Cotter E, Chew N, Powderly WG, Doran PP. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts. AIDS Res Hum Retroviruses. 2007;23:243–50. doi: 10.1089/aid.2006.0084. [DOI] [PubMed] [Google Scholar]
- 68.Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110:553–5. [PubMed] [Google Scholar]
- 69.O’Donnell MP, Chao CC, Gekker G, Modi KS, Kasiske BL, Keane WF. Renal cell cytokine production stimulates HIV-1 expression in chronically HIV-1-infected monocytes. Kidney international. 1998;53:593–7. doi: 10.1046/j.1523-1755.1998.00789.x. [DOI] [PubMed] [Google Scholar]
- 70.Shah A, Singh DP, Buch S, Kumar A. HIV-1 envelope protein gp120 up regulates CCL5 production in astrocytes which can be circumvented by inhibitors of NF-kappaB pathway. Biochem Biophys Res Commun. 2011;414:112–7. doi: 10.1016/j.bbrc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moskowitz L, Hensley GT, Chan JC, Adams K. Immediate causes of death in acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1985;109:735–8. [PubMed] [Google Scholar]
- 73.Fink L, Reichek N, Sutton MG. Cardiac abnormalities in acquired immune deficiency syndrome. The American journal of cardiology. 1984;54:1161–3. doi: 10.1016/s0002-9149(84)80178-2. [DOI] [PubMed] [Google Scholar]
- 74.Herskowitz A, Willoughby SB, Baughman KL, Schulman SP, Bartlett JD. Cardiomyopathy associated with antiretroviral therapy in patients with HIV infection: a report of six cases. Ann Intern Med. 1992;116:311–3. doi: 10.7326/0003-4819-116-4-311. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez ER, Nasim S, Hsia J, Sandin RL, Ferreira A, Hilliard BA, et al. Cardiac myocytes and dendritic cells harbor human immunodeficiency virus in infected patients with and without cardiac dysfunction: detection by multiplex, nested, polymerase chain reaction in individually microdissected cells from right ventricular endomyocardial biopsy tissue. The American journal of cardiology. 1991;68:1511–20. doi: 10.1016/0002-9149(91)90288-v. [DOI] [PubMed] [Google Scholar]
- 76.Twu C, Liu NQ, Popik W, Bukrinsky M, Sayre J, Roberts J, et al. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways. Proc Natl Acad Sci U S A. 2002;99:14386–91. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barbaro G, Di Lorenzo G, Soldini M, Giancaspro G, Grisorio B, Pellicelli A, et al. Intensity of myocardial expression of inducible nitric oxide synthase influences the clinical course of human immunodeficiency virus-associated cardiomyopathy. Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS (GISCA) Circulation. 1999;100:933–9. doi: 10.1161/01.cir.100.9.933. [DOI] [PubMed] [Google Scholar]
- 78.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. CNS viral invasion and inflammation during acute HIV. J Infect Dis. 2012 doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sei Y, Vitkovic L, Yokoyama MM. Cytokines in the central nervous system: regulatory roles in neuronal function, cell death and repair. Neuroimmunomodulation. 1995;2:121–33. doi: 10.1159/000096881. [DOI] [PubMed] [Google Scholar]
- 80.Leveque T, Le Pavec G, Boutet A, Tardieu M, Dormont D, Gras G. Differential regulation of gelatinase A and B and TIMP-1 and -2 by TNFalpha and HIV virions in astrocytes. Microbes and infection/Institut Pasteur. 2004;6:157–63. doi: 10.1016/j.micinf.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Pulliam L, Herndier BG, Tang NM, McGrath MS. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991;87:503–12. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pitha PM. Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antiviral research. 1994;24:205–19. doi: 10.1016/0166-3542(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 83.Shirazi Y, Pitha PM. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J Virol. 1992;66:1321–8. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhagani S. Current treatment for chronic hepatitis C virus/HIV-infected individuals: the role of pegylated interferon-alpha and ribavirin. Current opinion in HIV and AIDS. 2011;6:483–90. doi: 10.1097/COH.0b013e32834bd257. [DOI] [PubMed] [Google Scholar]
- 85.Abrams DI, Volberding PA. Alpha interferon therapy of AIDS-associated Kaposi’s sarcoma. Seminars in oncology. 1986;13:43–7. [PubMed] [Google Scholar]
- 86.de Wit R, Schattenkerk JK, Boucher CA, Bakker PJ, Veenhof KH, Danner SA. Clinical and virological effects of high-dose recombinant interferon-alpha in disseminated AIDS-related Kaposi’s sarcoma. Lancet. 1988;2:1214–7. doi: 10.1016/s0140-6736(88)90810-0. [DOI] [PubMed] [Google Scholar]
- 87.Krown SE, Real FX, Cunningham-Rundles S, Myskowski PL, Koziner B, Fein S, et al. Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi’s sarcoma. The New England journal of medicine. 1983;308:1071–6. doi: 10.1056/NEJM198305053081806. [DOI] [PubMed] [Google Scholar]
- 88.Krown SE, Real FX, Vadhan-Raj S, Cunningham-Rundles S, Krim M, Wong G, et al. Kaposi’s sarcoma and the acquired immune deficiency syndrome. Treatment with recombinant interferon alpha and analysis of prognostic factors. Cancer. 1986;57:1662–5. doi: 10.1002/1097-0142(19860415)57:8+<1662::aid-cncr2820571305>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 89.Lane HC, Kovacs JA, Feinberg J, Herpin B, Davey V, Walker R, et al. Anti-retroviral effects of interferon-alpha in AIDS-associated Kaposi’s sarcoma. Lancet. 1988;2:1218–22. doi: 10.1016/s0140-6736(88)90811-2. [DOI] [PubMed] [Google Scholar]
- 90.Volberding PA, Mitsuyasu R. Recombinant interferon alpha in the treatment of acquired immune deficiency syndrome-related Kaposi’s sarcoma. Seminars in oncology. 1985;12:2–6. [PubMed] [Google Scholar]
- 91.Adalid-Peralta L, Godot V, Colin C, Krzysiek R, Tran T, Poignard P, et al. Stimulation of the primary anti-HIV antibody response by IFN-alpha in patients with acute HIV-1 infection. Journal of leukocyte biology. 2008;83:1060–7. doi: 10.1189/jlb.1007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berglund O, Engman K, Ehrnst A, Andersson J, Lidman K, Akerlund B, et al. Combined treatment of symptomatic human immunodeficiency virus type 1 infection with native interferon-alpha and zidovudine. The Journal of infectious diseases. 1991;163:710–5. doi: 10.1093/infdis/163.4.710. [DOI] [PubMed] [Google Scholar]
- 93.Davey RT, Jr, Davey VJ, Metcalf JA, Zurlo JJ, Kovacs JA, Falloon J, et al. A phase I/II trial of zidovudine, interferon-alpha, and granulocyte-macrophage colony-stimulating factor in the treatment of human immunodeficiency virus type 1 infection. The Journal of infectious diseases. 1991;164:43–52. doi: 10.1093/infdis/164.1.43. [DOI] [PubMed] [Google Scholar]
- 94.Edlin BR, Weinstein RA, Whaling SM, Ou CY, Connolly PJ, Moore JL, et al. Zidovudine-interferon-alpha combination therapy in patients with advanced human immunodeficiency virus type 1 infection: biphasic response of p24 antigen and quantitative polymerase chain reaction. The Journal of infectious diseases. 1992;165:793–8. doi: 10.1093/infdis/165.5.793. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Cruz E, Lang JM, Frissen J, Furner V, Chateauvert M, Boucher CA, et al. Zidovudine plus interferon-alpha versus zidovudine alone in HIV-infected symptomatic or asymptomatic persons with CD4+ cell counts > 150 × 10(6)/L: results of the Zidon trial. Zidon Study Group. AIDS. 1995;9:1025–35. [PubMed] [Google Scholar]
- 96.Kovacs JA, Bechtel C, Davey RT, Jr, Falloon J, Polis MA, Walker RE, et al. Combination therapy with didanosine and interferon-alpha in human immunodeficiency virus-infected patients: results of a phase I/II trial. The Journal of infectious diseases. 1996;173:840–8. doi: 10.1093/infdis/173.4.840. [DOI] [PubMed] [Google Scholar]
- 97.Krown SE, Aeppli D, Balfour HH., Jr Phase II, randomized, open-label, community-based trial to compare the safety and activity of combination therapy with recombinant interferon-alpha2b and zidovudine versus zidovudine alone in patients with asymptomatic to mildly symptomatic HIV infection. HIV Protocol C91-253 Study Team. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1999;20:245–54. doi: 10.1097/00042560-199903010-00005. [DOI] [PubMed] [Google Scholar]
- 98.Orholm M, Pedersen C, Mathiesen L, Dowd P, Nielsen JO. Suppression of p24 antigen in sera from HIV-infected individuals with low-dose alpha-interferon and zidovudine: a pilot study. AIDS. 1989;3:97–100. doi: 10.1097/00002030-198902000-00008. [DOI] [PubMed] [Google Scholar]
- 99.Haas DW, Lavelle J, Nadler JP, Greenberg SB, Frame P, Mustafa N, et al. A randomized trial of interferon alpha therapy for HIV type 1 infection. AIDS research and human retroviruses. 2000;16:183–90. doi: 10.1089/088922200309278. [DOI] [PubMed] [Google Scholar]
- 100.Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, Herpin B, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Annals of internal medicine. 1990;112:805–11. doi: 10.7326/0003-4819-112-11-805. [DOI] [PubMed] [Google Scholar]
- 101.Skillman DR, Malone JL, Decker CF, Wagner KF, Mapou RL, Liao MJ, et al. Phase I trial of interferon alfa-n3 in early-stage human immunodeficiency virus type 1 disease: evidence for drug safety, tolerance, and antiviral activity. The Journal of infectious diseases. 1996;173:1107–14. doi: 10.1093/infdis/173.5.1107. [DOI] [PubMed] [Google Scholar]
- 102.Hatzakis A, Gargalianos P, Kiosses V, Lazanas M, Sypsa V, Anastassopoulou C, et al. Low-dose IFN-alpha monotherapy in treatment-naive individuals with HIV-1 infection: evidence of potent suppression of viral replication. J Interferon Cytokine Res. 2001;21:861–9. doi: 10.1089/107999001753238114. [DOI] [PubMed] [Google Scholar]
- 103.Emilie D, Burgard M, Lascoux-Combe C, Laughlin M, Krzysiek R, Pignon C, et al. Early control of HIV replication in primary HIV-1 infection treated with antiretroviral drugs and pegylated IFN alpha: results from the Primoferon A (ANRS 086) Study. AIDS. 2001;15:1435–7. doi: 10.1097/00002030-200107270-00014. [DOI] [PubMed] [Google Scholar]
- 104.Portales P, Reynes J, Pinet V, Rouzier-Panis R, Baillat V, Clot J, et al. Interferon-alpha restores HIV-induced alteration of natural killer cell perforin expression in vivo. AIDS. 2003;17:495–504. doi: 10.1097/00002030-200303070-00004. [DOI] [PubMed] [Google Scholar]
- 105.Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, et al. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. The Journal of infectious diseases. 2010;201:1686–96. doi: 10.1086/652420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–8. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 107.Rook AH, Hooks JJ, Quinnan GV, Lane HC, Manischewitz JF, Macher AM, et al. Interleukin 2 enhances the natural killer cell activity of acquired immunodeficiency syndrome patients through a gamma-interferon-independent mechanism. J Immunol. 1985;134:1503–7. [PubMed] [Google Scholar]
- 108.Pett SL, Kelleher AD, Emery S. Role of interleukin-2 in patients with HIV infection. Drugs. 2010;70:1115–30. doi: 10.2165/10898620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 109.Durier C, Capitant C, Lascaux AS, Goujard C, Oksenhendler E, Poizot-Martin I, et al. Long-term effects of intermittent interleukin-2 therapy in chronic HIV-infected patients (ANRS 048–079 Trials) AIDS. 2007;21:1887–97. doi: 10.1097/QAD.0b013e3282703825. [DOI] [PubMed] [Google Scholar]
- 110.Read SW, Lempicki RA, Di Mascio M, Srinivasula S, Burke R, Sachau W, et al. CD4 T cell survival after intermittent interleukin-2 therapy is predictive of an increase in the CD4 T cell count of HIV-infected patients. The Journal of infectious diseases. 2008;198:843–50. doi: 10.1086/591250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 therapy in patients with HIV infection. The New England journal of medicine. 2009;361:1548–59. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sellier P, Lafuente-Lafuente C, Bergmann JF. Interleukin-2 therapy in patients with HIV infection. The New England journal of medicine. 2010;362:270–1. doi: 10.1056/NEJMc0910951. author reply 1. [DOI] [PubMed] [Google Scholar]
- 113.Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10632–7. doi: 10.1073/pnas.1000027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fontas E, Kousignian I, Pradier C, Poizot-Martin I, Durier C, Weiss L, et al. IL-2 therapy: potential impact of the CD4 cell count at initiation on clinical efficacy--results from the ANRS CO4 cohort. The Journal of antimicrobial chemotherapy. 2010;65:2215–23. doi: 10.1093/jac/dkq296. [DOI] [PubMed] [Google Scholar]
- 115.Barbai VH, Ujhelyi E, Szlavik J, Vietorisz I, Varga L, Fey E, et al. Changes in the levels of some acute-phase proteins in human immunodeficiency virus-1 infected patients, following interleukin-2 treatment. Clinical and experimental immunology. 2010;161:134–41. doi: 10.1111/j.1365-2249.2010.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Porter BO, Shen J, Kovacs JA, Davey RT, Rehm C, Lozier J, et al. Interleukin-2 cycling causes transient increases in high-sensitivity C-reactive protein and D-dimer that are not associated with plasma HIV-RNA levels. AIDS. 2009;23:2015–9. doi: 10.1097/QAD.0b013e32832d72c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Read SW, Ciccone EJ, Mannon PJ, Yao MD, Chairez CL, Davey RT, et al. The effect of intermittent IL-2 therapy on CD4 T cells in the gut in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2011;56:340–3. doi: 10.1097/QAI.0b013e31820bf84c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabbatini F, Bandera A, Ferrario G, Trabattoni D, Marchetti G, Franzetti F, et al. Qualitative immune modulation by interleukin-2 (IL-2) adjuvant therapy in immunological non responder HIV-infected patients. PloS one. 2010;5:e14119. doi: 10.1371/journal.pone.0014119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kinter AL, Poli G, Fox L, Hardy E, Fauci AS. HIV replication in IL-2-stimulated peripheral blood mononuclear cells is driven in an autocrine/paracrine manner by endogenous cytokines. J Immunol. 1995;154:2448–59. [PubMed] [Google Scholar]
- 120.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nature medicine. 1999;5:651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 121.Kulkosky J, Nunnari G, Otero M, Calarota S, Dornadula G, Zhang H, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. The Journal of infectious diseases. 2002;186:1403–11. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 122.Stellbrink HJ, van Lunzen J, Westby M, O’Sullivan E, Schneider C, Adam A, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial) AIDS. 2002;16:1479–87. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- 123.Youle M, Emery S, Fisher M, Nelson M, Fosdick L, Janossy G, et al. A randomised trial of subcutaneous intermittent interleukin-2 without antiretroviral therapy in HIV-infected patients: the UK-Vanguard Study. PLoS clinical trials. 2006;1:e3. doi: 10.1371/journal.pctr.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tavel JA, Babiker A, Fox L, Gey D, Lopardo G, Markowitz N, et al. Effects of intermittent IL-2 alone or with peri-cycle antiretroviral therapy in early HIV infection: the STALWART study. PloS one. 2010;5:e9334. doi: 10.1371/journal.pone.0009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Molina JM, Levy Y, Fournier I, Hamonic S, Bentata M, Beck-Wirth G, et al. Interleukin-2 before antiretroviral therapy in patients with HIV infection: a randomized trial (ANRS 119) The Journal of infectious diseases. 2009;200:206–15. doi: 10.1086/599989. [DOI] [PubMed] [Google Scholar]
- 126.Angus B, Lampe F, Tambussi G, Duvivier C, Katlama C, Youle M, et al. TILT: a randomized controlled trial of interruption of antiretroviral therapy with or without interleukin-2 in HIV-1 infected individuals. AIDS. 2008;22:737–40. doi: 10.1097/QAD.0b013e3282f511f1. [DOI] [PubMed] [Google Scholar]
- 127.Porter BO, Anthony KB, Shen J, Hahn B, Keh CE, Maldarelli F, et al. Inferiority of IL-2 alone versus IL-2 with HAART in maintaining CD4 T cell counts during HAART interruption: a randomized controlled trial. AIDS. 2009;23:203–12. doi: 10.1097/QAD.0b013e32831cc114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bosch RJ, Pollard RB, Landay A, Aga E, Fox L, Mitsuyasu R. A randomized trial of interleukin-2 during withdrawal of antiretroviral treatment. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2011;31:481–3. doi: 10.1089/jir.2010.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levy Y, Thiebaut R, Gougeon ML, Molina JM, Weiss L, Girard PM, et al. Effect of Intermittent Interleukin-2 Therapy on CD4+ T cell counts following antiretroviral cessation in patients with HIV (ANRS 118/NIH 04-I-0018 ILIADE Trial) AIDS. 2012 doi: 10.1097/QAD.0b013e3283519214. [DOI] [PubMed] [Google Scholar]
- 130.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 131.Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 132.Managlia EZ, Landay A, Al-Harthi L. Interleukin-7 induces HIV replication in primary naive T cells through a nuclear factor of activated T cell (NFAT)-dependent pathway. Virology. 2006;350:443–52. doi: 10.1016/j.virol.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 133.Smithgall MD, Wong JG, Critchett KE, Haffar OK. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–30. [PubMed] [Google Scholar]
- 134.Beq S, Nugeyre MT, Ho Tsong Fang R, Gautier D, Legrand R, Schmitt N, et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006;176:914–22. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- 135.Nugeyre MT, Monceaux V, Beq S, Cumont MC, Ho Tsong Fang R, Chene L, et al. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J Immunol. 2003;171:4447–53. doi: 10.4049/jimmunol.171.8.4447. [DOI] [PubMed] [Google Scholar]
- 136.Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:727–35. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. The Journal of experimental medicine. 2008;205:1701–14. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang FX, Xu Y, Sullivan J, Souder E, Argyris EG, Acheampong EA, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. The Journal of clinical investigation. 2005;115:128–37. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. The Journal of clinical investigation. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Imamichi H, Degray G, Asmuth DM, Fischl MA, Landay AL, Lederman MM, et al. HIV-1 viruses detected during episodic blips following interleukin-7 administration are similar to the viruses present before and after interleukin-7 therapy. AIDS. 2011;25:159–64. doi: 10.1097/QAD.0b013e328340a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–26. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 142.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunological reviews. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 143.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. The Journal of experimental medicine. 2003;197:967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Forcina G, D’Ettorre G, Mastroianni CM, Carnevalini M, Scorzolini L, Ceccarelli G, et al. Interleukin-15 modulates interferon-gamma and beta-chemokine production in patients with HIV infection: implications for immune-based therapy. Cytokine. 2004;25:283–90. doi: 10.1016/j.cyto.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 145.d’Ettorre G, Andreotti M, Carnevalini M, Andreoni C, Zaffiri L, Vullo V, et al. Interleukin-15 enhances the secretion of IFN-gamma and CC chemokines by natural killer cells from HIV viremic and aviremic patients. Immunology letters. 2006;103:192–5. doi: 10.1016/j.imlet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 146.Lum JJ, Schnepple DJ, Nie Z, Sanchez-Dardon J, Mbisa GL, Mihowich J, et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. Journal of virology. 2004;78:6033–42. doi: 10.1128/JVI.78.11.6033-6042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.d’Ettorre G, Andreotti M, Ceccarelli G, Galluzzo CM, Mallano A, Massetti AP, et al. The role of IL-15 in challenging Acquired Immunodeficiency Syndrome. Cytokine. 2012;57:54–60. doi: 10.1016/j.cyto.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 148.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends in pharmacological sciences. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leone A, Picker LJ, Sodora DL. IL-2, IL-7 and IL-15 as immuno-modulators during SIV/HIV vaccination and treatment. Current HIV research. 2009;7:83–90. doi: 10.2174/157016209787048519. [DOI] [PubMed] [Google Scholar]
- 150.Hilleman MR. Immunologic, Chemotherapeutic and Interferon Approaches to Control of Viral Disease. Am J Med. 1965;38:751–66. doi: 10.1016/0002-9343(65)90195-6. [DOI] [PubMed] [Google Scholar]
- 151.Grob PJ, Joller-Jemelka HI, Binswanger U, Zaruba K, Descoeudres C, Fernex M. Interferon as an adjuvant for hepatitis B vaccination in non- and low-responder populations. European journal of clinical microbiology. 1984;3:195–8. doi: 10.1007/BF02014877. [DOI] [PubMed] [Google Scholar]
- 152.Quiroga JA, Castillo I, Porres JC, Casado S, Saez F, Gracia Martinez M, et al. Recombinant gamma-interferon as adjuvant to hepatitis B vaccine in hemodialysis patients. Hepatology. 1990;12:661–3. doi: 10.1002/hep.1840120407. [DOI] [PubMed] [Google Scholar]
- 153.Merigan TC. Is recombinant interleukin-2 the best way to deliver interferon-gamma in human disease? Journal of interferon research. 1987;7:635–9. doi: 10.1089/jir.1987.7.635. [DOI] [PubMed] [Google Scholar]
- 154.Wiryana P, Bui T, Faltynek CR, Ho RJ. Augmentation of cell-mediated immunotherapy against herpes simplex virus by interleukins: comparison of in vivo effects of IL-2 and IL-7 on adoptively transferred T cells. Vaccine. 1997;15:561–3. doi: 10.1016/s0264-410x(96)00212-5. [DOI] [PubMed] [Google Scholar]
- 155.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 156.Kusakabe K, Xin KQ, Katoh H, Sumino K, Hagiwara E, Kawamoto S, et al. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J Immunol. 2000;164:3102–11. doi: 10.4049/jimmunol.164.6.3102. [DOI] [PubMed] [Google Scholar]
- 157.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–58. [PubMed] [Google Scholar]
- 158.Barouch DH, Santra S, Steenbeke TD, Zheng XX, Perry HC, Davies ME, et al. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J Immunol. 1998;161:1875–82. [PubMed] [Google Scholar]
- 159.Desrosiers RC, Wyand MS, Kodama T, Ringler DJ, Arthur LO, Sehgal PK, et al. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989;86:6353–7. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barouch DH, Craiu A, Kuroda MJ, Schmitz JE, Zheng XX, Santra S, et al. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc Natl Acad Sci U S A. 2000;97:4192–7. doi: 10.1073/pnas.050417697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barouch D, Santra S, Schmitz J, Kuroda M, Fu T, Wagner W, et al. Control of viremia and prevention of clinical AIDS in Rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–92. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 162.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–21. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 163.Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. 2007;25:4967–82. doi: 10.1016/j.vaccine.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 164.Hryniewicz A, Price DA, Moniuszko M, Boasso A, Edghill-Spano Y, West SM, et al. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J Immunol. 2007;178:3492–504. doi: 10.4049/jimmunol.178.6.3492. [DOI] [PubMed] [Google Scholar]
- 165.Sui Y, Gagnon S, Dzutsev A, Zhu Q, Yu H, Hogg A, et al. TLR agonists and/or IL-15 adjuvanted mucosal SIV vaccine reduced gut CD4 memory T cell loss in SIVmac251-challenged rhesus macaques. Vaccine. 2011;30:59–68. doi: 10.1016/j.vaccine.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sykes KF, Lewis MG, Squires B, Johnston SA. Evaluation of SIV library vaccines with genetic cytokines in a macaque challenge. Vaccine. 2002;20:2382–95. doi: 10.1016/s0264-410x(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 167.O’Neill E, Martinez I, Villinger F, Rivera M, Gascot S, Colon C, et al. Protection by SIV VLP DNA prime/protein boost following mucosal SIV challenge is markedly enhanced by IL-12/GM-CSF co-administration. Journal of medical primatology. 2002;31:217–27. doi: 10.1034/j.1600-0684.2002.02008.x. [DOI] [PubMed] [Google Scholar]
- 168.Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. Journal of medical primatology. 2005;34:262–70. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 169.Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006;24:4677–87. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 170.Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol. 2008;180:7969–79. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- 171.Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011;85:9578–87. doi: 10.1128/JVI.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, et al. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–67. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dubie RA, Maksaereekul S, Shacklett BL, Lemongello D, Cole KS, Villinger F, et al. Co-immunization with IL-15 enhances cellular immune responses induced by a vif-deleted simian immunodeficiency virus proviral DNA vaccine and confers partial protection against vaginal challenge with SIVmac251. Virology. 2009;386:109–21. doi: 10.1016/j.virol.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eberly MD, Kader M, Hassan W, Rogers KA, Zhou J, Mueller YM, et al. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182:1439–48. doi: 10.4049/jimmunol.182.3.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Staats HF, Ennis FA., Jr IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J Immunol. 1999;162:6141–7. [PubMed] [Google Scholar]
- 176.Xin KQ, Hamajima K, Sasaki S, Tsuji T, Watabe S, Okada E, et al. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine. 1999;17:858–66. doi: 10.1016/s0264-410x(98)00271-0. [DOI] [PubMed] [Google Scholar]
- 177.Spearman P, Kalams S, Elizaga M, Metch B, Chiu YL, Allen M, et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine. 2009;27:243–9. doi: 10.1016/j.vaccine.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Baden LR, Blattner WA, Morgan C, Huang Y, Defawe OD, Sobieszczyk ME, et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J Infect Dis. 2011;204:1541–9. doi: 10.1093/infdis/jir615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Monsuez JJ, Escaut L, Teicher E, Charniot JC, Vittecoq D. Cytokines in HIV-associated cardiomyopathy. International journal of cardiology. 2007;120:150–7. doi: 10.1016/j.ijcard.2006.11.143. [DOI] [PubMed] [Google Scholar]
- 180.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–90. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 181.Gibellini D, De Crignis E, Ponti C, Cimatti L, Borderi M, Tschon M, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol. 2008;80:1507–14. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 182.Nolting T, Lindecke A, Hartung HP, Koutsilieri E, Maschke M, Husstedt IW, et al. Cytokine levels in CSF and neuropsychological performance in HIV patients. J Neurovirol. 2012 doi: 10.1007/s13365-012-0091-4. [DOI] [PubMed] [Google Scholar]
- 183.Zietz C, Hotz B, Sturzl M, Rauch E, Penning R, Lohrs U. Aortic endothelium in HIV-1 infection: chronic injury, activation, and increased leukocyte adherence. The American journal of pathology. 1996;149:1887–98. [PMC free article] [PubMed] [Google Scholar]
- 184.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1303–14. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Airoldi M, Bandera A, Trabattoni D, Tagliabue B, Arosio B, Soria A, et al. Neurocognitive Impairment in HIV-Infected Naive Patients with Advanced Disease: The Role of Virus and Intrathecal Immune Activation. Clinical & developmental immunology. 2012;2012:467154. doi: 10.1155/2012/467154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zamboni G, Antoniazzi F, Bertoldo F, Lauriola S, Antozzi L, Tato L. Altered bone metabolism in children infected with human immunodeficiency virus. Acta Paediatr. 2003;92:12–6. doi: 10.1111/j.1651-2227.2003.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 187.Ross MJ, Fan C, Ross MD, Chu TH, Shi Y, Kaufman L, et al. HIV-1 infection initiates an inflammatory cascade in human renal tubular epithelial cells. J Acquir Immune Defic Syndr. 2006;42:1–11. doi: 10.1097/01.qai.0000218353.60099.4f. [DOI] [PubMed] [Google Scholar]
- 188.Breen EC, Boscardin WJ, Detels R, Jacobson LP, Smith MW, O’Brien SJ, et al. Non-Hodgkin’s B cell lymphoma in persons with acquired immunodeficiency syndrome is associated with increased serum levels of IL10, or the IL10 promoter -592 C/C genotype. Clin Immunol. 2003;109:119–29. doi: 10.1016/s1521-6616(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 189.Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, Banerjee A, et al. ICAM-1 and VCAM-1 mediate endotoxemic myocardial dysfunction independent of neutrophil accumulation. American journal of physiology Regulatory, integrative and comparative physiology. 2002;283:R477–86. doi: 10.1152/ajpregu.00034.2002. [DOI] [PubMed] [Google Scholar]
- 190.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 191.Bernasconi S, Cinque P, Peri G, Sozzani S, Crociati A, Torri W, et al. Selective elevation of monocyte chemotactic protein-1 in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–101. doi: 10.1093/infdis/174.5.1098. [DOI] [PubMed] [Google Scholar]
- 192.Ruff KR, Puetter A, Levy LS. Growth regulation of simian and human AIDS-related non-Hodgkin’s lymphoma cell lines by TGF-beta1 and IL-6. BMC cancer. 2007;7:35. doi: 10.1186/1471-2407-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rallon NI, Barreiro P, Soriano V, Garcia-Samaniego J, Lopez M, Benito JM. Elevated TGF-beta1 levels might protect HCV/HIV-coinfected patients from liver fibrosis. Eur J Clin Invest. 2011;41:70–6. doi: 10.1111/j.1365-2362.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 194.Yamamoto T, Noble NA, Miller DE, Gold LI, Hishida A, Nagase M, et al. Increased levels of transforming growth factor-beta in HIV-associated nephropathy. Kidney international. 1999;55:579–92. doi: 10.1046/j.1523-1755.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 195.Veldkamp CT, Ziarek JJ, Su J, Basnet H, Lennertz R, Weiner JJ, et al. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009;18:1359–69. doi: 10.1002/pro.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singh KK, Barroga CF, Hughes MD, Chen J, Raskino C, McKinney RE, et al. Genetic influence of CCR5, CCR2, and SDF1 variants on human immunodeficiency virus 1 (HIV-1)-related disease progression and neurological impairment, in children with symptomatic HIV-1 infection. J Infect Dis. 2003;188:1461–72. doi: 10.1086/379038. [DOI] [PubMed] [Google Scholar]
- 197.Baker BJ, Park KW, Qin H, Ma X, Benveniste EN. IL-27 inhibits OSM-mediated TNF-alpha and iNOS gene expression in microglia. Glia. 2010;58:1082–93. doi: 10.1002/glia.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–14. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]