Abstract
Objective
Transforming growth factor-β-activated kinase (TAK1) is a member of the mitogen-activated protein kinase family that plays important roles in apoptosis and inflammatory signaling, both of which are critical components of stroke pathology. TAK1 has recently been identified as a major upstream kinase that phosphorylates and activates adenosine monophosphate-activated protein kinase (AMPK), a major mediator of neuronal injury after experimental cerebral ischemia. We studied the functional role of TAK1 and its mechanistic link with AMPK after stroke.
Methods
Male mice were subjected to transient middle cerebral artery occlusion (MCAO). The TAK1 inhibitor 5Z-7-oxozeaenol was injected either intracerebroventricularly or intraperitoneally at various doses and infarct size and functional outcome after long term survival was assessed. Mice with deletion of the AMPK α2 isoform were utilized to assess the contribution of downstream AMPK signaling to stroke outcomes. Levels of pTAK1, pAMPK, and other TAK1 targets including the pro-apoptotic molecule c-Jun-N-terminal kinase (JNK)/c-Jun and the pro-inflammatory protein cyclooxygenase-2 were also examined.
Results
TAK1 is critical in stroke pathology. Delayed treatment with a TAK1 inhibitor reduced infarct size and improved behavioral outcome even when given several hours after stroke onset. This protective effect may be independent of AMPK activation but was associated with a reduction in JNK and c-Jun signaling.
Conclusions
Enhanced TAK1 signaling, via activation of JNK, contributes to cell death in ischemic stroke. TAK1 inhibition is a novel therapeutic approach for stroke as it is neuroprotective with systemic administration, has a delayed therapeutic window, and demonstrates sustained neuroprotective effects.
Keywords: TAK1, AMPK, stroke, JNK
INTRODUCTION
Transforming-growth-factor-beta-activated kinase-1 (TAK1), also known as mitogen-activated protein kinase kinase kinase-7 (MAPKKK7), is a mitogen-activated protein kinase that can be activated by TGF-β, tumor necrosis factor-alpha (TNF-α) and other cytokines including interleukin-1 (IL-1) (Ninomiya-Tsuji, et al., 1999, Sakurai, et al., 2003, Yamaguchi, et al., 1995). Activation of TAK1 requires association with TAK1-binding protein 1 (TAB1) and phosphorylation at multiple sites including threonine-184 and threonine-187 (Sakurai, et al., 2000, Shibuya, et al., 1996). The function of TAK1 has been studied in a number of cell lines with conflicting results. Several studies have demonstrated an anti-apoptotic response to TAK1 activation (Omori, et al., 2008, Thiefes, et al., 2005), however others have suggested that TAK1 is pro-apoptotic via activation of downstream JNK signaling (Sorrentino, et al., 2008). TAK1 is also emerging as a possible contributing factor to pro-inflammatory signaling through induction of the transcription factor AP-1 with subsequent expression of inflammatory genes including COX-2 (Kumar, et al., 2009, Ninomiya-Tsuji, et al., 2003, Zhang, et al., 2010). Inflammation and apoptosis both contribute to ischemic neuronal cell death (Sims and Muyderman, 2010, Tuttolomondo, et al., 2009), and due to the delayed activation of these pathways after injury, may represent an attractive target for the development of neuroprotective agents.
TAK1 has been recently identified as one of the three upstream kinases that phosphorylates and activates adenosine monophosphate-activated protein kinase (AMPK), a key metabolic enzyme and sensor of cellular metabolic state (Li, et al., 2010). AMPK's activity is primarily regulated by the cellular AMP:ATP ratio (Omori, et al., 2006) but AMPK can also be activated by other cellular stressors including ischemia and hypoxia. AMPK is activated by phosphorylation at Threonine 172 via an upstream kinase triggering a cascade of events that reduces the activity of anabolic enzymes and increases catabolic pathways, thereby maintaining ATP levels. Although activation of AMPK appears to be a protective adaptive response to stress in peripheral tissues, in the brain, ischemia-induced AMPK activation exacerbates injury by enhancing metabolic failure and inducing lactic acidosis (Li, et al., 2010, Li and McCullough, 2010, Li, et al., 2007). Deletion of the catalytic isoform of AMPK responsible for phosphorylation and activation of AMPK is neuroprotective, as is pharmacological inhibition of AMPK. We hypothesized that inhibition of TAK1, due to its function as an upstream activator of AMPK (Li and McCullough, 2010), would lead to a reduction in stroke-induced AMPK phosphorylation and subsequent neuroprotection. TAK1 was recently shown to be activated in rodents after neonatal hypoxic-ischemic injury (Nijboer, et al., 2009), in a rodent model of middle cerebral artery occlusion (MCAO), and in vitro after oxygen glucose deprivation (Neubert, et al., 2011) but the signaling pathways induced by TAK and actions on AMPK signaling are unknown.. In the present study, the function of TAK1 was studied in a rodent model of ischemic stroke using the highly specific small molecule TAK1 inhibitor 5Z-7-oxozeaenol (Ninomiya-Tsuji, et al., 2003, Sicard, et al., 2009) and putative downstream targets of TAK1 were investigated.
MATERIALS AND METHODS
Stroke model
C57BL/6 mice were purchased from Charles River Laboratories (Willimantic, Connecticut, USA). Experiments were performed according to NIH guidelines for the care and use of animals in research and under protocols approved by the University of Connecticut Health Center Animal Care and Use Committee. Prior to experiments, animals were randomized into vehicle and drug groups. All treatments were performed by a blinded investigator. Focal transient cerebral ischemia was induced in male mice (20 to 25g) by 90 minutes of right middle cerebral artery occlusion (MCAO) under isoflurane anesthesia followed by reperfusion as described previously (Kilic, et al., 2002, Li, et al., 2007). In a separate cohort, physiological variables such as mean arterial blood pressure and cortical cerebral perfusion data were obtained as previously described (Li, et al., 2010). In this cohort, LDF was measured continuously from a baseline value pre-stroke (100%) through the ischemic period and for 30 minutes after reperfusion was initiated. The LDF data was calculated by averaging the LDF values taken at 15 minutes intervals, then provided as the percentage of the baseline value (the LDF value before the suture was inserted).
Drug treatment
The TAK1 inhibitor 5Z-7-oxozeaenol (Neubert, et al., 2011) (Tocris Bioscience) was dissolved in DMSO. 5Z-7-oxozeaenol was injected intracerebroventricularly (0.32 μg or 1.6 μg in 2 μL of DMSO). Additional cohorts were treated with intraperitoneal doses (0.5 mg/kg in 10% DMSO in PBS; pH = 7.4 or 5.0 mg/kg in DMSO/PBS). Control mice were injected with vehicle. Animals were randomly assigned to treatment cohorts. Separate cohorts were injected with 5 mg/kg IP of 5Z-7-oxozeaenol with delayed dosing, either 2 hours or 3.5 hours after onset of ischemia.
Functional outcome measurements
Please refer to supplemental materials.
Tissue staining and Infarct Quantification
24 hours after stroke, mice were euthanized and brains removed and cut into 5 2-mm coronal slices which were stained with a 1.5% 2,3,5-triphenyltetrazolium (TTC) solution at 37°C for 10 minutes. The stained slices were fixed with 4% formalin, images were digitalized, and the infarct volumes (corrected for edema) were analyzed using Sigmascan Pro5 as previously described (Li, et al., 2010). A separate cohort was subjected to the same procedure 7 days following stroke.
Western blotting
Brains were obtained 4 hours after stroke onset. Please refer to supplemental materials for methods (Li, et al., 2010, Li and McCullough, 2010, Li, et al., 2007).
Statistical analyses
Data from individual experiments are presented as mean ± SEM, except for neurological deficit scores, which are presented as mean ± interquartile range. Non-parametric data was log-transformed and compared using ANOVA (SPSS 17, SAS Corporation, Cary, NC). Parametric data was compared using ANOVA with Bonferroni correction for multiple comparisons where appropriate. When an effect of an intervention or characteristic was shown by ANOVA, the appropriate groups were compared with Student's t-test or Mann-Whitney U-test with Z approximation, as appropriate. p < 0.05 was considered statistically significant. All behavioral and histological assessments were performed by an investigator blinded to drug treatment.
RESULTS
Intracerbroventricular application of 5Z-7-oxozeaenol reduced stroke injury
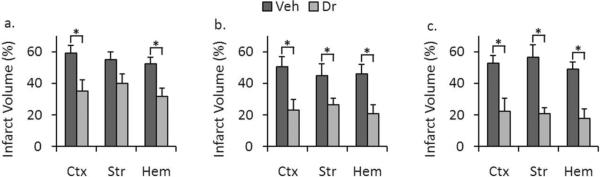
ICV administration of 0.32 ug of 5Z-7-oxozeaenol resulted in no significant differences in infarct volume (Cortex: 53.66 ± 5.72% for vehicle versus 39.01 ± 7.15% for drug, p = 0.151; Striatum, 48.89 ± 7.98% for vehicle versus 33.41 ± 7.01 for drug, p = 0.142; in whole-hemisphere, 44.58 ± 5.67% for vehicle versus 32.58 ± 5.93%, p = 0.149, n = 7 vehicle, 9 drug). However, injection of a higher dose (1.6 ug) significantly reduced cortical (Fig. 1a) (59.04 ± 4.98% for vehicle versus 35.00 ± 7.10% for drug, p = 0.014) and whole-hemisphere infarct volume (52.30 ± 4.12% for vehicle versus 31.79 ± 5.36% for drug, p = 0.008, n = 6 vehicle, 7 drug). Although there was a trend towards neuroprotection in the striatum, this did not reach statistical significance (54.89 ± 4.95% for vehicle versus 39.89 ± 6.03% for drug, (p = 0.066). ICV injection of low dose (0.32 ug) of 5Z-7-oxozeaenol had no effect on stroke-induced behavioral deficits (p = 0.081, n = 7 vehicle, 9 drug). However, at the higher dose (1.6 ug), there was a significant improvement in behavioral scores reflecting the neuroprotective effect of the drug (3(0) for vehicle-treated mice versus 2(0) for drug-treated mice, p = 0.038, n = 6 vehicle, 7 drug).
Figure 1.
Administration of 5Z-7-oxozeaenol decreased stroke severity in WT mice. a. ICV administration of 1.6 ug of 5Z-7-oxozeaenol 20 minutes prior to stroke led to a statistically significant reduction in stroke volume in cortex (p = 0.014) and total hemisphere (p = 0.008) but not in striatum (p = 0.066) (n = 6 vehicle, 7 drug). b. IP administration of 5Z-7-oxozeaenol (5 mg/kg) at stroke onset led to a statistically significant reduction in stroke volume in cortex (p = 0.011), striatum (p = 0.044), and total hemisphere (p = 0.010) (n = 7 vehicle, 8 drug). c. IP administration of 5Z-7-oxozeaenol (5 mg/kg) 2 hours after onset of stroke led to a statistically significant reduction in stroke volume in cortex (p = 0.007), striatum (p = 0.001), and total hemisphere (p = 0.001) (n = 7 vehicle, 8 drug). Data presented as mean ± s.e.m. * p < 0.05.
Systemic injection of 5Z-7-oxozeaenol reduced infarct volume
We then evaluated the effect of IP administration of 5Z-7-oxozeaenol. IP injection of 0.5 mg/kg resulted in no significant differences in infarct volume in any brain area (data not shown). However, injection of a higher 5 mg/kg dose showed statistically-significant differences in cortical (50.62 ± 6.27% for vehicle versus 23.12 ± 6.72% for drug, p = 0.011), striatal (44.91 ± 7.51% for vehicle versus 26.41 ± 4.22% for drug, p = 0.044), and whole-hemisphere infarct volume (Fig. 1b) (45.99 ± 6.16% for vehicle versus 20.95 ± 5.52% for drug, p = 0.010, n = 7 vehicle, 8 drug). Mice administered the 5 mg/kg dose showed an improvement in behavioral deficits; however this difference did not reach statistical significance (Table 3) (3(1) for vehicle-treated mice versus 2(0.5) for drug-treated mice, p = 0.073, n = 7 vehicle, 8 drug). No differences were seen in any physiological parameters (including pH, pCO2, pO2, glucose, mean arterial pressure) (Table 1) or local cerebral blood flow as measured by laser Doppler flowmetry (LDF) between drug- and vehicle-treated groups (Table 2).
Table 3.
TAK1 inhibitor treatment reduced neurological deficit score after stroke
| Route | ICV | IP | ||
|---|---|---|---|---|
| Treatment | Vehicle | Drug | Vehicle | Drug |
| Median (interquartile range) | 3 (0) | 2 (0) | 3 (1) | 2 (0.5) |
ICV injection of the drug (1.6μg) resulted in decreased neurological deficits (n = 7 vehicle, 9 drug, p = 0.038). IP injection of the drug (5.0 mg/kg) showed a similar trend but did not reach statistical significance (n = 7 vehicle, 8 drug, p = 0.073). Data was presented as median (interquartile range). The Mann-Whitney U test was used to detect any statistical significance between drug and vehicle groups.
Table 1.
There is no difference in physiological parameters between the vehicle and drug treated mice
| Group | Condition | pH | pCO2 | pO2 | Glucose | MABP (mmHg) |
|---|---|---|---|---|---|---|
| Vehicle | Pre-ischemic | 7.37 ± 0.01 | 29.3 ± 3.2 | 118 ± 2.9 | 153 ± 9.2 | 73 ± 5.6 |
| Intra-ischemic | 7.25 ± 0.01 | 31.8 ± 3.1 | 112 ± 10 | 159 ± 11 | 74 ± 2.5 | |
| Drug | Pre-ischemic | 7.39 ± 0.01 | 22.8 ± 2.2 | 119 ± 9.4 | 159 ± 23 | 72 ± 2.3 |
| Intra-ischemic | 7.29 ± 0.03 | 29.8 ± 1.9 | 129 ± 7.6 | 170 ± 40 | 78 ± 1.7 |
Blood samples were collected from the femoral artery at the onset of MCAO or 60 min from the onset of MCAO. Data are expressed as Mean±SEM. n = 4 per group. Student's t-test was used to compare the difference of each parameter between vehicle and drug treated groups.
Table 2.
There is no difference in cerebral blood flow (LDF) between the vehicle and drug treated mice
| LDF (% of baseline) | ||
|---|---|---|
| Group | Intra-ischemia | Reperfusion |
| Vehicle | 13.2 ± 1.3 | 82.3 ± 1.5 |
| Drug | 13.7 ± 3.1 | 83.4 ± 5.4 |
Cerebral blood flow was measured by using Laser Doppler Flow (LDF). Data are expressed as Mean±SEM. n = 4 per group. Student's t-test was used to compare the difference of each parameter between vehicle and drug treated groups.
Post stroke treatment of 5Z-7-oxozeaenol provided sustained neuroprotection
To determine if this agent could salvage ischemic brain when administered after stroke onset, IP administration of 5mg/kg of 5Z-7-oxozeaenol was delayed until after reperfusion. IP injection of 5.0 mg/kg 2 hours after the onset of MCAO resulted in a significant reduction in cortical (52.72 ± 5.08% for vehicle versus 22.29 ± 8.34% for drug, p = 0.007), striatal (56.63 ± 7.65% for vehicle versus 20.64 ± 3.83% for drug, p = 0.001), and whole-hemisphere infarct volume at 24 hours (Fig. 1c) (49.18 ± 4.18% for vehicle versus 17.81 ± 5.86% for drug, p = 0.001, n = 7 vehicle, 8 drug).
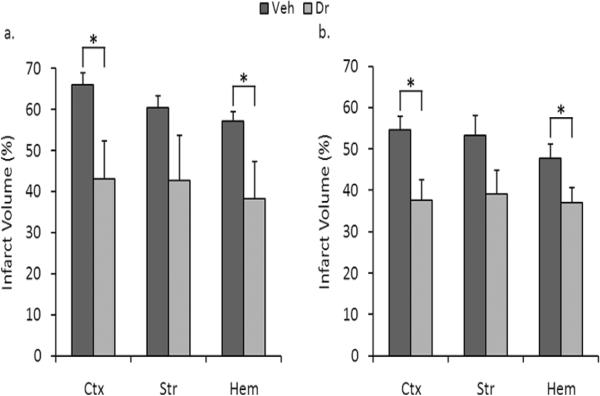
To determine whether the effect of this agent was durable and could lead to chronic neuroprotection, we subjected a separate cohort of mice to delayed treatment (3.5 hours after stroke onset) and determined infarct volumes 7 days later. Drug treatment decreased infarct volumes at 7 days, with significant decreases in cortical (Fig. 2a) (66.08 ± 2.73% for vehicle versus 43.15 ± 9.14% for drug, p = 0.028) and whole-hemisphere infarct volumes (57.16 ± 2.38% for vehicle versus 38.35 ± 8.97% for drug, p = 0.049) (n = 8 vehicle, 8 drug).
Figure 2.
Administration of 5Z-7-oxozeaenol decreased stroke severity when measured at 7 days following stroke in both WT and AMPK KO mice. a. IP administration of 5Z-7-oxozeaenol (5 mg/kg) to WT mice after stroke led to a statistically significant reduction in cortical (p = 0.028) and whole-hemisphere (p = 0.049), but not striatal (p = 0.117) stroke volume (n = 8 vehicle, 8 drug). b. IP administration of 5Z-7-oxozeaenol (5 mg/kg) to AMPK KO mice after stroke led to a statistically significant reduction in cortical (p = 0.008) and whole-hemisphere (p = 0.037) infarct volume (n = 11 vehicle, 9 drug), but not striatal (p = 0.066, n = 11 vehicle, 9 drug). Data presented as mean ± s.e.m. * p < 0.05.
TAK1 inhibition improved memory function in wild type (WT) mice subjected to stroke but had no effect on total locomotor activity
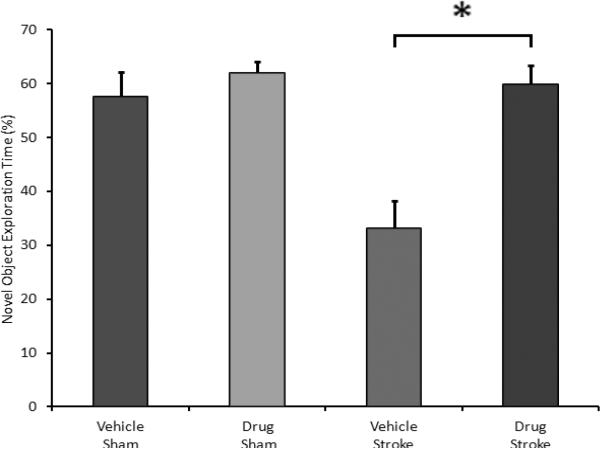
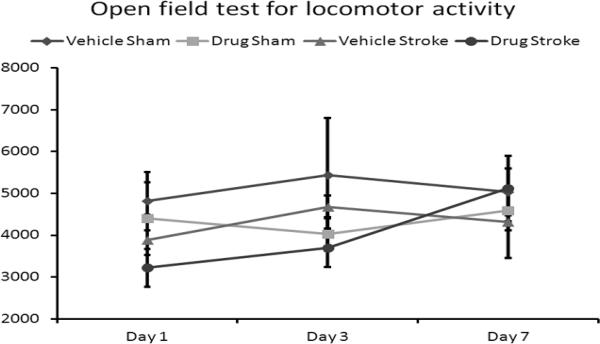
Delayed drug treatment also had a profound effect on memory/recognition tasks. Administration of the drug to WT mice 3.5 hours after onset of MCAO lead to a statistically-significant increase in the percentage of time that the mice spent exploring a novel object (Bevins, et al., 2006) (Fig. 3) (33.15 ± 5.04% for vehicle versus 59.85 ± 3.62% for drug, p = 0.001, n = 7 vehicle, 7 drug). This effect was not observed in mice subjected to sham surgery (57.51 ± 4.61% for vehicle versus 61.94 ± 2.17% for drug, p = 0.359, n = 5 vehicle, 5 drug) which showed the expected preference for a novel vs. a familiar object (Bevins, et al., 2006). Delayed administration of the drug had no effect on average locomotor activity as measured by total beam breaks by the mice 1 day after MCAO (Fig. 4) (3881 ± 604 for vehicle versus 3219 ± 451 for drug, p = 0.366), 3 days after MCAO (4676 ± 275 for vehicle versus 3694 ± 459 for drug, p = 0.07), or 7 days after MCAO (4310 ± 850 for vehicle versus 5114 ± 786 for drug, p = 0.488, n = 8 vehicle, 7 drug). The levels of activity were similar in sham groups when drug treated groups were compared with vehicle treated groups (Fig. 4). No differences were seen in a corner test between drug treated and vehicle treated stroke mice (day 1 vehicle 0.90 (n=9) versus drug 0.89 (n=9); day 3 vehicle 0.88 (n=9) versus drug 0.89 (n=9); day 7 vehicle 0.86 (n=8) versus drug 0.74 (n=7), p>0.05 on each day).
Figure 3.
Administration of the drug to WT mice 3.5 hours after onset of MCAO lead to a statistically-significant improvement in the novel object recognition test (33.15 ± 5.04% for vehicle versus 59.85 ± 3.62% for drug, p = 0.001, n = 7 vehicle, 7 drug). There was no difference between drug- and vehicle-treated mice subjected to sham surgery (p = 0.359, n = 5 vehicle, 5 drug). Data presented as mean ± s.e.m. * p < 0.05.
Figure 4.
Delayed administration of the drug had no effect on the average locomotor activity of stroke mice as measured by total beam breaks by the mice 1 day after MCAO (p = 0.366), 3 days after MCAO (p = 0.07), or 7 days after MCAO (p = 0.488, n = 8 vehicle, 7 drug). Drug administration had no effect in mice subjected to sham surgery 1 day after MCAO (p = 0.690), 3 days after MCAO (p = 0.298), or 7 days after MCAO (p = 0.523, n = 5 vehicle, 5 drug). Data presented as mean ± s.e.m. * p < 0.05.
5Z-7-oxozeaenol reduced the level of activated TAK1 in stroke
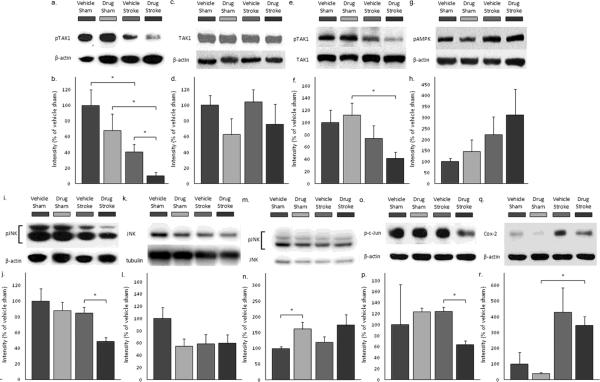
Experimental stroke reduced the level of phosphorylated TAK1 (the activated form) when measured 4 hours after ischemia (Figs. 5a and b) (p = 0.002, n = 5 sham, 6 stroke). Drug treatment significantly reduced levels of phosphorylated TAK1 compared to vehicle after MCAO (Figs. 5a and b) (p = 0.016, n = 6 vehicle, 5 drug), an effect not observed in sham mice (p = 0.203, n = 5 vehicle, 5 drug). Total TAK1 levels were not significantly affected by either stroke (Figs. 5c and d) (p = 0.128) or drug treatment (p = 0.672) (n = 5 vehicle shams, 6 vehicle strokes, 5 drug shams, 5 drug strokes). When pTAK1 levels were normalized to total TAK1, the ratio in the drug treated stroke group was not significantly reduced compared to that of the vehicle treated stroke groups, however a significant effect was still observed when compared to drug treated shams (Figs. 5e and f).
Figure 5.
Drug treatment reduced levels of activated TAK1, activated JNK, total JNK, and activated c-Jun, but not total TAK1, activated AMPK, or COX-2. a. Levels of phosphorylated TAK1 (activated form) were decreased by drug treatment during stroke (n = 5 vehicle shams, 6 vehicle strokes, 5 drug shams, 5 drug strokes). b. Quantitation of phospho-TAK1 western blots. c. Levels of total TAK1 were unchanged following stroke or drug administration (n = 5 vehicle shams, 6 vehicle strokes, 5 drug shams, 5 drug strokes). d. Quantitation of TAK1 western blots. e. Levels of pTAK1/TAK1 were decreased by stroke in drug-treated groups. Levels were unchanged by drug treatment (n = 8 vehicle shams, 10 vehicle strokes, 8 drug shams, 8 drug strokes). f. Quantitation of pTAK1/TAK1 western blots. g. Levels of phosphorylated AMPK (activated form) were unchanged by drug treatment before stroke (n = 7 vehicle shams, 9 vehicle strokes, 7 drug shams, 9 drug strokes). h. Quantitation of phospho-AMPK western blots. i. Levels of phosphorylated JNK (activated form) were decreased by drug treatment before stroke (n = 5 vehicle shams, 6 vehicle strokes, 5 drug shams, 5 drug strokes). j. Quantitation of phospho-JNK western blots. k. Levels of total JNK were not significantly changed by drug treatment or by stroke (n = 8 vehicle shams, 10 vehicle strokes, 8 drug shams, 8 drug strokes). l. Quantitation of JNK western blots. m Levels of pJNK/JNK were increased by drug treatment in sham groups (n = 8 vehicle shams, 10 vehicle strokes, 8 drug shams, 8 drug strokes). n. Quantitation of pJNK/JNK western blots. o. Levels of phosphorylated c-Jun (activated form) were decreased by drug treatment before stroke. (n = 2 vehicle shams, 2 drug shams, 3 vehicle strokes, 3 drug strokes). p. Quantitation of phospho-c-Jun western blots. q. Levels of COX-2 were increased following stroke, but were not affected by drug treatment (n = 2 vehicle shams, 2 drug shams, 3 vehicle strokes, 3 drug strokes). r. Quantitation of COX-2 western blots. Data presented as mean ± s.e.m. * p < 0.05.
Effect of 5Z-7-oxozeanol in stroke was independent of AMPK signaling
Treatment with 5Z-7-oxozeaenol reduced infarct volume, suggesting that TAK1 activation exacerbates ischemic injury. To explore possible underlying mechanisms, we first examined AMPK, a putative downstream target for TAK1 that plays an important role in stroke pathology. Consistent with previous reports (Li, et al., 2010, Li and McCullough, 2010), levels of pAMPK were elevated after stroke, but drug treatment had no effect on total or pAMPK levels (Figs. 5g and h) (p = 0.755, n =7 vehicle shams, 9 vehicle strokes, 7 drug shams, 9 drug strokes).
To further explore the possible contributions of AMPK to TAK1 inhibition-induced protection, we administered 5Z-7-oxozeaenol to AMPK α2 deficient mice 3.5 hours after the onset of MCAO and performed histological analysis 7 days after stroke. Administration of 5Z-7-oxozeaenol led to a significant decrease in cortical (Fig. 2b) (54.61 ± 3.29% for vehicle versus 37.60 ± 4.96% for drug, p = 0.008) and whole-hemisphere (47.81 ± 3.34% for vehicle versus 36.96 ± 3.65% for stroke, p = 0.037) infarct volume 7 days after stroke (n = 11 vehicle, 9 drug), suggesting that the neuroprotective effects of 5Z-7-oxozeaenol are independent of AMPK.
AMPK KO mice showed similar results to WT in open-field testing, with no difference in locomotor activity 1 day after MCAO (1550 ± 499 for vehicle versus 1609 ± 356 for drug, p = 0.92), 3 days after MCAO (2117 ± 318 for vehicle versus 3420 ± 663 for drug, p = 0.083), or 7 days after MCAO (4227 ± 725 for vehicle versus 3872 ± 430 for drug, p = 0.685, n = 11 vehicle, 10 drug).
Activated JNK levels were reduced by TAK1 inhibition after stroke
As the neuroprotective effects of TAK1 inhibition were not secondary to a reduction in stroke-induced pAMPK signaling, we investigated another major apoptotic pathway activated by TAK1, c-Jun N-terminal kinase (JNK) (Benakis, et al., 2010, Sanna, et al., 2002). Levels of phosphorylated JNK (the activated form of pJNK) were reduced following treatment with 5Z-7-oxozeaenol in stroke animals (Figs. 5i and j, p = 0.002, n = 5 vehicle shams, 6 vehicle strokes, 5 drug shams, 5 drug strokes). Total levels of JNK were not significantly affected by stroke (p = 0.213) or by 5Z-7-oxozeaenol treatment (Figs. 5k and l) (p = 0.132, (n = 8 vehicle shams, 10 vehicle strokes, 8 drug shams, 8 drug strokes). Drug treatment increased the ratio of pJNK/total JNK in sham animals (p = 0.009). However, the difference of the ratio of pJNK/total JNK in stroke animals between drug and vehicle treated mice was not significant (Figs. 5m and n) (p = 0.121). c-Jun, a component of the transcription factor complex AP-1 and a downstream target for phosphorylation by JNK, was also reduced with 5Z-7-oxozeaenol treatment (Figs. 5o and p) (p = 0.006, n = 2 vehicle sham, 3 vehicle stroke, 2 drug sham, 3 drug stroke).
COX-2 levels were increased by stroke but not effected by TAK1 inhibition
As TAK1 has recently been shown to have pro-inflammatory effects(Neubert, et al., 2011),(Kumar, et al., 2009),(Zeng, et al., 2010, Zhang, et al., 2010), levels of COX-2 were also evaluated. Levels of COX-2 were increased following stroke as has been reported previously (Candelario-Jalil and Fiebich, 2008) (Figs. 5q and r) (p = 0.014, n = 2 sham, 3 stroke). However, in stroke animals, TAK1 inhibition had no effect on COX-2 levels (p = 0.569, n = 3 drug, 3 vehicle).
DISCUSSION
TAK1 has recently been implicated as a contributor to cell death in several in vitro and in vivo models (Sicard, et al., 2009, Tang, et al., 2008). This study reveals several novel and important findings related to the role of TAK1 signaling in cerebral ischemia. First, levels of pTAK were significantly lower in the ischemic brain compared to sham. Second, direct intracerebroventricular delivery of 5Z-7-oxozeaenol, a TAK1 inhibitor, reduced the severity of experimental stroke as measured by both behavioral deficit scores and infarct volumes indicating a CNS-specific effect of this inhibitor. Third, systemic injection of 5Z-7-oxozeaenol also provided similar beneficial effects suggesting TAK1 inhibition is a potentially translatable therapy for stroke. Fourth, delayed treatment also reduced infarct size as 5Z-7-oxozeaenol, even when given 3.5 hours after stroke, provided sustained neuroprotection assessed 7 days following stroke, an effect that was also reflected in reduced neurological deficits as measured by the novel object recognition test. Fifth, treatment with 5Z-7-oxozeaenol specifically reduced pTAK1 levels after stroke, confirming drug specificity for this molecular pathway. TAK1 inhibition did not reduce stroke-induced AMPK activation, suggesting that TAK1's function as an upstream kinase of AMPK is not the mechanism by which TAK inhibition reduces ischemic injury. This was confirmed by demonstrating equivalent neuroprotective efficacy of TAK inhibition in both WT and AMPK α2 KO mice. Finally, treatment with 5Z-7-oxozeaenol selectively reduced levels of activated JNK, a known downstream target of TAK1, a finding that may shed light into the mechanism of neuroprotection of TAK1 inhibition.
5Z-7-oxozeaenol is a highly-selective inhibitor of TAK1 activity that has been used in several models to evaluate the role of TAK1 in apoptosis (Ninomiya-Tsuji, et al., 2003, Sicard, et al., 2009). 5Z-7-oxozeaenol selectively inhibits the catalytic activity of TAK1 but has minimal effects on other MAP kinases or JNK(Ninomiya-Tsuji, et al., 2003). As such, it is a powerful tool for examining TAK1 signaling. Most previous studies that have utilized 5Z-7-oxozeaenol have been performed in in vitro cell culture systems, in isolated organs, or utilized local administration of this agent. Our results indicate that TAK1 inhibition (as measured by significant decreases in p-TAK) can be obtained with either intracerebroventricular or systemic administration of 5Z-7-oxozeaenol. Interestingly, drug treatment did not affect levels of activated TAK1 in sham mice. However, after stroke, levels of activated TAK1 were significantly lower in drug-treated mice than in vehicle-treated mice. Ischemia may allow passage of the drug into the brain secondary to blood-brain barrier dysfunction, allowing it to exert its effects on TAK1. In contrast to our findings, previous work in a neonatal model of hypoxic-ischemic injury found higher levels of pTAK1 in the brain 3 hours after injury (Nijboer, et al., 2009). In that model, neonatal rats were subjected to 120 minutes of hypoxia, followed by 3 hours of permanent MCA occlusion. The differences in levels of TAK1 expression between these studies could be secondary to species, developmental and model difference. Protein phosphatase 6 (Broglie, et al., 2010, Kajino, et al., 2006) or protein phosphatase 2A (Kim, et al., 2008) have been shown to inactivate TAK1 in cell culture models, however their roles in regulating TAK1 levels in the stroke model remain to be investigated. As TAK1 inhibition by 5Z-7-oxozeaenol is neuroprotective, the observed decrease seen in vehicle-treated mice in pTAK1 in the reperfusion period may reflect an attempt at endogenous neuroprotection. We have previously found that inhibition of AMPK is protective after experimental stroke (Li, et al., 2010, Li, et al., 2007).The mechanisms by which acute AMPK activation enhances injury are unclear, but exacerbated lactate accumulation, enhanced autophagy, and increased glucose uptake due to unregulated glucose transporters in the reperfusion phase likely contribute to stroke damage (Li, et al., 2010).There are three known kinases (TAK1, LKB1(Hawley, et al., 1995, Sakamoto, et al., 2005) and CaMKKβ (Suter, et al., 2006)) that can activate AMPK by phosphorylation. The response of pAMPK to the manipulation of these upstream kinases has not been examined in the ischemic brain. We initially hypothesized that TAK1 was an important upstream regulator of AMPK in the ischemic brain (Li and McCullough, 2010) due to its effects on apoptosis and inflammatory signaling. We postulated that inhibition of TAK1 would lead to neuroprotection via a reduction in pAMPK levels. However, no decrease in stroke-induced pAMPK levels was seen after TAK1 inhibition, despite robust effects of the drug on pTAK1 levels. This suggests that stroke-induced AMPK phosphorylation is triggered by LKB1 or CaMKKβ rather than TAK1. This was further confirmed by studies utilizing mice deficient in AMPK α2, the isoform responsible for the detrimental effect of AMPK activation (Li, et al., 2007) in which TAK inhibition led to robust neuroprotection despite the loss of AMPK. These studies suggest that the effects of TAK1 in stroke are mediated through pathways independent of AMPK signaling.
Once it was recognized that AMPK may not be the signaling pathway by which TAK inhibition exerts its neuroprotective effects, we evaluated JNK, a major pro-apoptotic pathway recently found to be activated by TAK1. It is unclear whether activation of TAK1 is anti- or pro-apoptotic, and the overall effect appears to be tissue specific. Mice deficient in TAK1 are embryonic lethal due to apoptosis of hematopoietic cells and hepatocytes (Tang, et al., 2008). In the heart, unlike our findings in the brain, inhibition of TAK1 by 5Z-7-oxozeaenol led to increased cardiomyocyte death after cardiac ischemia (Sicard, et al., 2009), suggesting that TAK1 has anti-apoptotic effects. In contrast, several studies have shown that activation of TAK1 has enhanced apoptosis via activation of p38 mitogen-activated protein kinase (p38 MAPK) and JNK in fibroblasts (Matluk, et al., 2010, Resch, et al., 2009, Sorrentino, et al., 2008) and both JNK and its downstream target c-Jun, are well recognized pro-apoptotic factors in ischemic brain (Neubert, et al., 2011, Nijboer, et al., 2010) consistent with our results demonstrating neuroprotection and the significant decrease in stroke-induced pJNK levels with TAK inhibition. Therefore, the effects of TAK1 on apoptosis may be dependent on the duration of ischemia, reperfusion status, and the tissue examined, similar to what has been seen with AMPK activation (Weisova, et al., 2011).
TAK1 activation is also known to induce pro-inflammatory gene expression (Zeng, et al., 2010, Zhang, et al., 2010) leading to the production of pro-inflammatory cytokines and chemokines (Jang, et al., 2002, Ninomiya-Tsuji, et al., 2003) and activation of immune cells (Sato, et al., 2005). Accordingly, topical 5Z-7-oxozeaenol reduced picric acid induced inflammation in the mouse ear via an inhibitory action on TAK1 and subsequent COX-2 expression (Ninomiya-Tsuji, et al., 2003). As COX-2 is induced after stroke and leads to an exacerbation of ischemic damage by the promotion of inflammation (Candelario-Jalil and Fiebich, 2008, Iadecola and Ross, 1997), we also examined COX-2. Consistent with previous work, stroke induced a significant increase in COX-2 levels. However, there were no differences in COX-2 levels between vehicle and drug-treated stroke animals, suggesting that the anti-inflammatory properties of 5Z-7-oxozeaenol play a less significant role in neuroprotection. Importantly, the fact that COX-2 levels did not change, despite a significant reduction in infarct in drug-treated animals, suggests that the effects of 5Z-7-oxozeaenol on JNK and c-JUN signaling (which did decrease after treatment) are specific, and are not merely an epiphenomenon of changes in infarct size.
In addition to reducing infarct damage, TAK1 inhibition also led to improvements in behavioral deficits. Although no effect was seen on total locomotor activity or on forepaw usage between drug and vehicle treated mice, a significant improvement was seen on the novel object recognition test (NORT). In this test, the interest of an animal in a novel object versus a familiar one is measured and compared. If the exploration of the novel and the familiar object is equal (as normal uninjured animals prefer to explore the novel object), this is interpreted as a deficit in memory and cognition. Cognitive deficits have been well described in the MCAO model, and may be more of a “translatable” deficit to measure than pure motor deficits. In our experiment, sham mice had a preference for the novel object spending more than 50% of the test time examining the novel object, as expected. Drug treated MCAO mice were indistinguishable from shams, whereas vehicle-treated MCAO mice had a significant reduction in novel object preference. This deficit is not secondary to enhanced motor impairments in vehicle treated stroke mice, as their overall locomotor function was equivalent to sham. These results are consistent with a recent study (Dhawan, et al., 2011), that also demonstrated a preference for the familiar object in this task after stroke, although the exact underlying mechanisms and neuroanatomical substrates are unknown. Interestingly, we did not see an effect of drug treatment on stroke-induced motor deficits. This may be due to the mechanism of action of this agent, as we expect the effects of TAK1 inhibition to be greatest in the ischemic penumbra, where apoptosis predominates, rather than salvaging the ischemic core, which in the focal MCAO model primarily involves the striatum. The memory dysfunction created by MCAO may reflect diffuse cortical dysfunction which are reflected by the deficits seen in the NORT. Reversing these deficits may have greater relevance in clinical populations as memory and cognitive deficits lead to considerable disability and loss of independence for stroke patients.
There are several potential limitations of this work, and the data must be interpreted with these in mind. As with all pharmacological therapies, possible off-target effects of 5Z-7-oxozeaenol cannot be completely excluded. It is clear that the drug does act on TAK based on the significant reduction of pTAK1 with treatment, and currently other approaches are not feasible as TAK1 KO mice are embryonic lethal. Alternative approaches such as development of cell type selective or conditional KO mice could address some of these mechanistic questions. 5Z-7-oxozeaenol is one of the few pharmacological agents that appears to have efficacy on both histological and functional outcomes, has long-term durable effects, can be given systemically and most importantly, retains efficacy even when administered several hours after stroke onset. Interestingly, functional improvement was significant when the drug was given in the post-stroke treatment, but not when given immediately at stroke onset, despite robust neuroprotective effects in both paradigms. It is possible that later anti-inflammatory effects may mediate the behavioral recovery, whereas immediate anti-apoptotic effects are responsible for histological improvements. Although the half-life of the drug is yet unknown, later effects on inflammatory pathways remain to be investigated. Prior to moving forward in translational efforts, future studies to determine dose response curves, toxicity, to evaluate efficacy in both sexes and in models replicating the disease population (hypertensive, diabetic, aging etc.) need to be undertaken.
CONCLUSION
TAK1 inhibition is neuroprotective after induced cerebral ischemia and the mechanism may be independent of AMPK signaling. We demonstrated that 5Z-7-oxozeaenol has the potential to reduce the severity of ischemic stroke through its effects on TAK1 and downstream signaling via JNK. This work has uncovered a new, yet unexplored signaling pathway involved in ischemic cell death.
Supplementary Material
Highlights
Stroke injury propagation involves complex molecular processes.
Inhibiting transforming growth factor-β-activated kinase 1 (TAK1) reduced injury.
TAK1 is a critical molecule in deciding neuronal fate in stroke.
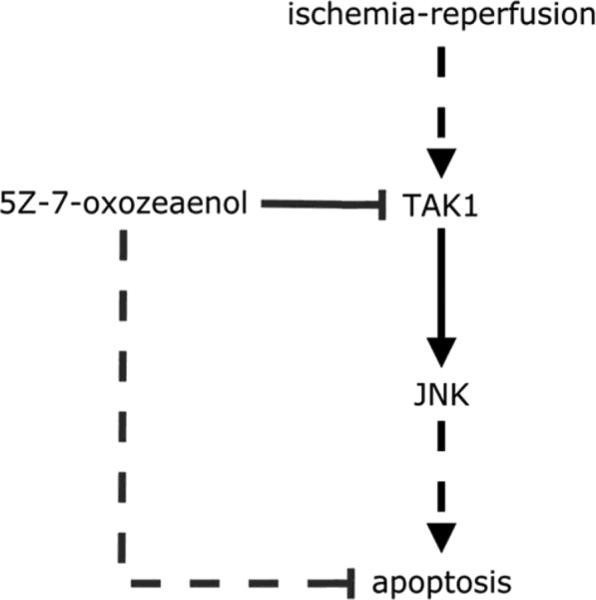
Figure 6.
Proposed mechanism for neuroprotection by TAK1 inhibition. Inhibition of TAK1 by 5Z-7-oxozeaenol reduces JNK activation, leading to reduced apoptosis following ischemic stroke.
Acknowledgement statement
This work was supported by National Institutes of Health grants R01 NS050505 and NS055215 (to L.D.M.) and American Heart Association grant 09SDG2261435 (to J.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: BJ White: nothing to declare; S Tarabishy: nothing to declare; VR Venna: nothing to declare; B Manwani: nothing to declare; S. Benashki: nothing to declare; LD McCullough: nothing to declare; J Li: nothing to declare.
REFERENCES
- 1.Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun. 2010;24:800–811. doi: 10.1016/j.bbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 3.Broglie P, Matsumoto K, Akira S, Brautigan DL, Ninomiya-Tsuji J. Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway. J Biol Chem. 2010;285:2333–2339. doi: 10.1074/jbc.M109.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candelario-Jalil E, Fiebich BL. Cyclooxygenase inhibition in ischemic brain injury. Curr Pharm Des. 2008;14:1401–1418. doi: 10.2174/138161208784480216. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan J, Benveniste H, Luo Z, Nawrocky M, Smith SD, Biegon A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol. 2011;6:823–834. doi: 10.2217/fnl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Ross ME. Molecular pathology of cerebral ischemia: delayed gene expression and strategies for neuroprotection. Ann N Y Acad Sci. 1997;835:203–217. doi: 10.1111/j.1749-6632.1997.tb48631.x. [DOI] [PubMed] [Google Scholar]
- 8.Jang SB, Won J, Kim H, Kim J, Lee KH, Han H, Rha HK, Choi CR. TAK1 mediates lipopolysaccharide-induced RANTES promoter activation in BV-2 microglial cells. Mol Cells. 2002;14:35–42. [PubMed] [Google Scholar]
- 9.Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL, Matsumoto K, Ninomiya-Tsuji J. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem. 2006;281:39891–39896. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilic E, Dietz GP, Hermann DM, Bahr M. Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann Neurol. 2002;52:617–622. doi: 10.1002/ana.10356. [DOI] [PubMed] [Google Scholar]
- 11.Kim SI, Kwak JH, Wang L, Choi ME. Protein phosphatase 2A is a negative regulator of transforming growth factor-beta1-induced TAK1 activation in mesangial cells. J Biol Chem. 2008;283:10753–10763. doi: 10.1074/jbc.M801263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M, Makonchuk DY, Li H, Mittal A, Kumar A. TNF-like weak inducer of apoptosis (TWEAK) activates proinflammatory signaling pathways and gene expression through the activation of TGF-beta-activated kinase 1. J Immunol. 2009;182:2439–2448. doi: 10.4049/jimmunol.0803357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Benashski SE, Siegel C, Liu F, McCullough LD. Adenosine monophosphate activated protein kinase inhibition is protective in both sexes after experimental stroke. Neurosci Lett. 2010;482:62–65. doi: 10.1016/j.neulet.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Benashski SE, Venna VR, McCullough LD. Effects of metformin in experimental stroke. Stroke. 2010;41:2645–2652. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matluk N, Rochira JA, Karaczyn A, Adams T, Verdi JM. A role for NRAGE in NF-kappaB activation through the non-canonical BMP pathway. BMC Biol. 2010;8:7. doi: 10.1186/1741-7007-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neubert M, Ridder DA, Bargiotas P, Akira S, Schwaninger M. Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ. 2011;18:1521–1530. doi: 10.1038/cdd.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijboer CH, Heijnen CJ, Groenendaal F, van Bel F, Kavelaars A. Alternate pathways preserve tumor necrosis factor-alpha production after nuclear factor-kappaB inhibition in neonatal cerebral hypoxia-ischemia. Stroke. 2009;40:3362–3368. doi: 10.1161/STROKEAHA.109.560250. [DOI] [PubMed] [Google Scholar]
- 21.Nijboer CH, van der Kooij MA, van Bel F, Ohl F, Heijnen CJ, Kavelaars A. Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav Immun. 2010;24:812–821. doi: 10.1016/j.bbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 23.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 24.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resch U, Schichl YM, Winsauer G, Gudi R, Prasad K, de Martin R. Siva1 is a XIAP-interacting protein that balances NFkappaB and JNK signalling to promote apoptosis. J Cell Sci. 2009;122:2651–2661. doi: 10.1242/jcs.049940. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. Embo J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai H, Miyoshi H, Mizukami J, Sugita T. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 2000;474:141–145. doi: 10.1016/s0014-5793(00)01588-x. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 30.Sanna MG, da Silva Correia J, Ducrey O, Lee J, Nomoto K, Schrantz N, Deveraux QL, Ulevitch RJ. IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol. 2002;22:1754–1766. doi: 10.1128/MCB.22.6.1754-1766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 33.Sicard P, Jacquet S, Kobayashi KS, Flavell RA, Marber MS. Pharmacological postconditioning effect of muramyl dipeptide is mediated through RIP2 and TAK1. Cardiovasc Res. 2009;83:277–284. doi: 10.1093/cvr/cvp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 36.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5'-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 37.Tang M, Wei X, Guo Y, Breslin P, Zhang S, Wei W, Xia Z, Diaz M, Akira S, Zhang J. TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med. 2008;205:1611–1619. doi: 10.1084/jem.20080297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiefes A, Wolter S, Mushinski JF, Hoffmann E, Dittrich-Breiholz O, Graue N, Dorrie A, Schneider H, Wirth D, Luckow B, Resch K, Kracht M. Simultaneous blockade of NFkappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor [corrected] target genes. J Biol Chem. 2005;280:27728–27741. doi: 10.1074/jbc.M411657200. [DOI] [PubMed] [Google Scholar]
- 39.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9:1240–1260. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- 40.Weisova P, Davila D, Tuffy LP, Ward MW, Concannon CG, Prehn JH. Role of 5'-adenosine monophosphate-activated protein kinase in cell survival and death responses in neurons. Antioxid Redox Signal. 2011;14:1863–1876. doi: 10.1089/ars.2010.3544. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 42.Zeng KW, Fu H, Liu GX, Wang XM. Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. Int Immunopharmacol. 2010;10:668–678. doi: 10.1016/j.intimp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Lei T, Chen X, Peng Y, Long H, Zhou L, Huang J, Chen Z, Long Q, Yang Z. Resistin up-regulates COX-2 expression via TAK1-IKK-NF-kappaB signaling pathway. Inflammation. 2010;33:25–33. doi: 10.1007/s10753-009-9155-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.