Abstract
At least some cancer stem cells (CSCs) display intrinsic drug resistance that may thwart eradication of a malignancy by chemotherapy. We have explored the genesis of such resistance by studying mouse models of liver cancer driven by either MYC or the combination of oncogenic forms of AKT and NRAS. A common manifestation of chemoresistance in CSCs is efflux of the DNA-binding dye Hoechst 33342. We found that only the MYC-driven tumors contained a subset of cells that efflux Hoechst 33342. This “side population” (SP) was enriched for CSCs when compared to non-SP tumor cells and exhibited markers of hepatic progenitor cells. The SP cells could differentiate into non-SP tumor cells, with coordinate loss of chemoresistance, progenitor markers and the enrichment for CSCs. In contrast, non-SP cells did not give rise to SP cells. Exclusion of Hoechst 33342 is mediated by ABC drug transporter proteins that also contribute to chemoresistance in cancer. We found that the MDR1 transporter was responsible for the efflux of Hoechst from SP cells in our MYC-driven model. Accordingly, SP cells and their tumor-initiating subset were more resistant than non-SP cells to chemotherapeutics that are effluxed by MDR1.
Conclusion
The oncogenotype of a tumor can promote a specific mechanism of chemoresistance that can contribute to the survival of hepatic CSCs. Under circumstances that promote differentiation of CSCs into more mature tumor cells, the chemoresistance can be quickly lost. Elucidation of the mechanisms that govern chemoresistance in these mouse models may illuminate the genesis of chemoresistance in human liver cancer.
Liver cancer is the fifth most common and third deadliest cancer in the world1. Primary liver cancer in adults is usually caused by viral infections or sustained chemical or alcohol exposure2. Chemotherapy is a standard method of treatment for unresectable tumors, but meta-analysis of chemotherapy has revealed little statistical benefit in 1-year survival rates3. Understanding how chemoresistance arises in hepatic tumors may lead to improvements in therapy.
A major mechanism of chemoresistance in cancers, including those of hepatic origin, is the aberrant expression of ATP Binding Cassette (ABC) transporters4. ABC transporter proteins are composed of a single or multiple sets of transmembrane domains and nucleotide binding domains5. Various substrates, including ions, sugars, proteins, metabolites and hydrophobic drugs are effluxed through the transmembrane domains, while the nucleotide binding domains hydrolyze ATP and power the efflux. ABC transporters prevent the accumulation of toxic compounds in normal cells. High expression of these proteins can also impair chemotherapy in cancer cells, including cancer stem cells (CSCs)6.
CSCs have been identified in a number of solid cancer models, including liver cancer7. CSCs are characterized as having enhanced tumor initiating capabilities compared to other tumor cells8. Furthermore, these cells are often described as maintaining markers and molecular properties similar to undifferentiated adult stem or progenitor cells for the tissue of origin. Notable surface markers for hepatic CSCs that were previously identified in hepatic progenitor cells include CD90, CD44, CD133 and EpCAM9–12. Metabolic markers such as aldehyde dehydrogenase (ALDH) activity have also been associated with hepatic CSCs13.
CSCs frequently display enhanced chemoresistance, which may contribute to the survival of tumor initiating cells and the recurrence of tumors following chemotherapy7. One approach to identify CSCs exploits enhanced chemoresistance through fractionation of cancer cells based on the efflux of Hoechst 333426. Subsets of cells that efflux Hoechst 33342 at a greater rate than the rest of the cell population can by identified by flow cytometry as a poorly stained side population (SP) of cells. SP cells were initially isolated in order to purify murine hematopoetic stem cells from bone marrow14. SP cell purification has since been utilized in the isolation of CSCs from both hematopoietic and solid malignancies, including hepatic carcinomas7, 15–17. Normal hepatic progenitors also display increased Hoechst 33342 efflux activity18. Most work on hepatic cancers with SP purification has involved established human or rat hepatic cancer cell lines and chemically-induced cancer models rather than oncogene-specific in vivo models7, 15, 16. The ABC transporter proteins P-glycoprotein (P-gp/MDR1), encoded by the multidrug resistance gene 1 (Mdr1), and the breast cancer resistance protein (BCRP), encoded by the Bcrp1 gene, have both been shown to contribute to SP formation in a variety of cell types19–21. The molecular mechanisms that determine which drug transporter protein mediates SP formation in different cancer samples and models remain unclear. At least one study with lung cancer in vivo, however, suggests that the initiating oncogene(s) may play a key role in dictating CSC properties22, 23. We have used mouse models for liver cancer to explore the possibility that the oncogenotype of a tumor can determine the nature of chemoresistance in SP cells.
MYC is a transcription factor that contributes to a number of cellular processes including proliferation, apoptosis and metabolism24. Chromosomal amplification of the MYC locus (8q24) has been found in 40–60% of early HCC samples25, 26. Activation of AKT, which affects cell survival, proliferation, metabolism and other cellular processes in tumor cells, is seen in up to 26.5% of recurrent HCC cases and is associated with poor prognosis27, 28. Aberrant activation of the RAS signaling pathway, which contributes to cell growth and survival processes, is also a common occurrence following the downregulation of RAS inhibitor proteins in HCC29. Here, we show that CSCs with increased Hoechst 33342 efflux activity could be isolated from a MYC-driven murine hepatic tumor model, but not from hepatic tumors elicited by the combination of AKT and RAS. SP cells isolated from MYC-driven tumors were enriched for both increased colony formation in vitro and tumor-initiating capability in NOD/Scidil2Rγ−/− (NSG) mice. Furthermore, these cells exhibited several properties of hepatic progenitor cells and could differentiate into more mature non-SP hepatic cancer cells. In contrast, non-SP hepatic cancer cells appeared to be terminally differentiated, as they did not revert to SP cancer cells following allograft.
Increased MDR1 expression has been found in primary and metastatic liver tumors taken from patients following chemotherapy30. While both MDR1 and BCRP1 have been implicated in SP cell formation in CSCs, we found that only MDR1 mediated the formation of SP cells in our murine liver tumor model. Tumor-initiating SP cells were also enriched in tumors following treatment with chemotherapeutics that can be efficiently effluxed by MDR1. We conclude that the nature of chemoresistance in CSCs may be determined by the particular oncogene(s) responsible for tumorigenesis. In addition, our work provides a model for isolating and studying primary hepatic CSCs driven by MYC.
Materials and Methods
Animals
The Tet-o-MYC/LAP-tTA (LT2-MYC) murine hepatoblastoma tumor model has been previously described31. For hydrodynamic transfection-induced MYC and AKT/RAS tumors, 20 μg of plasmids encoding MYC and transposon or 20 μg of two plasmids encoding oncogenic forms of AKT (myristylated AKT) and NRAS (NRASV12) and transposon were mixed with 2 μg of plasmids encoding the Sleeping Beauty transposase in 2.5 mls of PBS and injected into the lateral tail vein of 6- to 8-week old female wildtype FVB/N mice (Jackson Laboratory, Maine, USA). Allograft experiments were performed in NOD/Scidil2Rγ−/− (NSG) mice (Jackson Laboratory, Maine, USA). All animal studies were approved by the Committee for Animal Research at the University of California, San Francisco.
Isolation and Analysis of SP cells
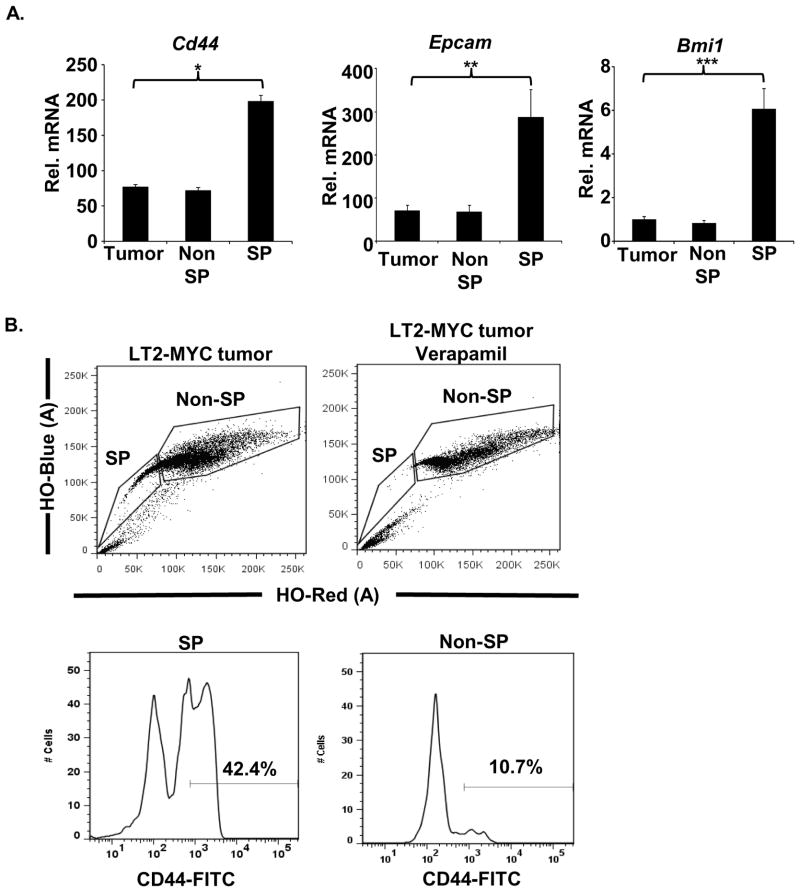
In order to obtain single cell suspensions, normal liver and liver tumors were isolated and diced into 2–5mm size pieces. Tumor pieces were treated with collagenase/dispase (1 mg/ml) (Roche, Indianapolis, IN, USA) for 10 minutes at 37° C with gentle rocking. Following treatment, cells were filtered through sterile gauze, 70 μm and then 40 μm cell strainers. Cell suspensions were treated with 1X Red Blood Cell (RBC) Lysis Buffer (eBioscience, San Diego, CA, USA) for 5 minutes on ice and washed 3 times with PBS. The average percentage of viable cells from normal cells was 67.15% ± 7.97% (n=4) and from tumor cells was 50.97% ± 7.65% (n=4). Aliquots of 106 cells from individual tumors were resuspended in 1 ml of DMEM+ (2% FBS and 10mM HEPES buffer) and treated with Hoechst 33342 at a final concentration of 5 μg/ml at 37° C for 50 minutes in the presence or absence of verapamil (50 μM). The length of incubation with Hoechst 33342 and verapamil was optimized as previously described32. Following treatment, cells were resuspended in HBSS+ (Hanks Balanced Salt Solution with 2% FBS and 10 mM HEPES buffer). CD44 expression was analyzed by staining cells with anti-CD44 (IM7) antibody (eBioscience, San Diego, CA, USA) for 30 minutes prior to analysis. Stained cells were analyzed by FACSAria II (BD Biosciences, San Jose, CA, USA) with a UV laser excitation of 350 nm and fluorescence was measured with a 450/50 filter. Propidium Iodide (PI) (0.2 μg/ml) was used to exclude dead or dying cells. The SP was defined as the fraction eliminated by the pump inhibitor verapamil.
Colony Formation Assay and Allograft Tumor Initiation Assay
SP, non-SP and live hepatic tumor cells were isolated by flow cytometry. 1×105 cells were seeded in a 24 well cell culture plate in supplemented ESP-Gro media (GigaCyte, Branford, CT, USA). Colony formation was counted following 12 days of growth. For allografts, cells were resuspended in supplemented serum-free media and mixed at 1:1 ratio with Growth Factor Reduced Matrigel (BD Biosciences, San Jose, CA, USA) and injected into the hindquarters of NOD/Scidil2γR−/− (NSG) mice.
Immunohistochemistry and Western Blot
Paraffin-embedded liver or tumor samples were stained with hemotoxylin and eosin (H&E) (UCSF Craniofacial Histology Core Facility). In situ fluorescent TUNEL staining was done according to the manufacturer’s protocol (Millipore, Bellirica, MA, USA). Stained samples were analyzed by fluorescent microscopy (Zeiss Axiophot) and apoptosis was quantified by ImageJ (NIH). Western blots were performed with the Criterion system (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol and probed with antibodies for MYC (Epitomics, Burlingame, CA, USA), AFP, C/EBPα, β-Actin (Cell Signaling, Beverly, MA) and MDR1, MRP1 and BCRP1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay
LT2-Myc tumor cells were isolated from primary tumors and seeded overnight in 96 well plates at 1×105 cells per well in RPMI media containing 10% FBS and penicillin (100 IU/ml)/streptomycin (100 μg/ml). Following treatment with drugs at IC50 dosages, cell growth was analyzed by TACStm MTT assay according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA). Treatments were performed in triplicate.
Real-time Quantitative PCR (Q-PCR) analysis
Following isolation of SP and non-SP cells, mRNA was isolated with the Arcturus PicoPure RNA Isolation Kit according to the manufacturer’s protocol (Applied Biosystems, Carlsbad, CA, USA). Following reverse transcription of RNA/sample (iScript, Invitrogen, Carlsbad, CA, USA), Q-PCR was performed with the SYBR Green PCR kit according to the manufacturer’s protocol (Applied Biosystems, Carlsbad, CA, USA).
Results
SP Cells Possess Greater Tumor-Initiating Potential than do Non-SP Cells
Hepatic overexpression of the human oncogene MYC in mice results in the formation of highly aggressive poorly differentiated tumors that resemble human hepatoblastomas31, 33. MYC-mediated hepatic tumorigenesis can be elicited by either induction of transgenic human MYC or hydrodynamic transfection of human MYC, with both methods resulting in histologically similar forms of tumors (Fig. 1A). Hydrodynamic co-transfection of plasmids that express oncogenic forms of human AKT1 and human NRAS promotes hepatic tumors (AKT/RAS tumors) resembling moderately differentiated hepatocellular carcinoma and cholangiocarcinoma (Fig. 1A)34. While AKT/RAS tumors have been demonstrated to express MYC in excess of the levels in normal liver tissue34, MYC-induced tumors have much higher levels of MYC (Supplementary Fig. 1A), which may augment expression of MYC-specific properties. We investigated whether the MYC or AKT/RAS-induced primary hepatic tumors contain an increased SP population when compared to normal livers. Normal livers and AKT/RAS-induced hepatic tumors contained few if any SP cells (Fig. 1B, C). In contrast, up to 10.43% of the cells in MYC-induced hepatic tumors fractionated as SP (Fig. 1B, C). The SP gate was determined by treating samples with Hoechst 33342 in the presence or absence of verapamil, which inhibits drug binding to drug-transporter proteins35.
Fig. 1. MYC-induced hepatic tumors have increased SP cells compared to normal liver.
(A) Upper row, gross morphology of livers and tumors from FVB/N Wt, LT2 and LT2-MYC transgenic mice and MYC-transfected mice 8 weeks after removal of doxycycline or hydrodynamic transfection. Lower row, microscopic images of H&E stained normal livers and MYC-induced tumors. Scale bar = 100 μm. (B) SP analysis of LT2 normal liver cells and LT2-MYC hepatic tumor cells. (C) SP analysis of FVB/N normal liver cells and hepatic tumor cells induced by hydrodynamic transfection of AKT/RAS and MYC.
Because CSCs have greater tumor-initiating potential than other subpopulations in tumors8, we compared the tumor-initiating potential of SP cells to non-SP cells to determine if CSCs are enriched in the SP. We first performed colony formation assays in supplemented serum-free media that promotes growth of hepatic progenitor cells36. While unsorted tumor cells formed colonies, sorting for SP cells resulted in a nearly 5-fold increase in colony forming units (CFUs) (Fig. 2A). Non-SP cells failed to form colonies, whereas large colonies were formed by SP cells (Fig. 2A, B). These in vitro experiments encouraged analysis of SP tumor-initiating potential in vivo.
Fig. 2. SP cells are enriched for tumor-initiating potential.
(A) Colony forming assay of unsorted LT2-MYC tumor cells (Total), sorted SP cells and non-SP cells performed in supplemented HpBGro serum-free media. (n=4), *p<0.00001 (B) Colony assays with SP and non-SP cells. (C) Primary allograft of LT2-MYC tumor cells. 10000 (n=4), 1000 (n=4) or 100 (n=4) unsorted LT2-Myc tumor cells (Total) and 10000 (n=4), 1000 (n=16) or 100 (n=12) sorted SP cells and 10000 (n=4), 1000 (n=16) or 100 (n=8) non-SP cells were seeded subcutaneously in NSG mice. Animals were monitored for tumors for 90 days post-injection. *p<0.02, **p<0.00001, ***p<0.003 (D) Secondary allograft of LT2-MYC tumors derived from SP cells. 100 (n=10) sorted SP cells and 100 (n=10) non-SP cells from LT2-Myc SP cell derived allograft tumors were subcutaneously seeded in NSG mice. Animals were monitored for tumors for 90 days post-injection. *p<0.000006 (E) Representative image of a NSG mouse seeded with 100 SP and 100 non-SP from LT2-Myc tumors in opposite flanks 60 days after injection.
Serial dilution allografts were performed to determine the tumor-initiating potential of SP cells in vivo. SP cells from MYC-induced tumors formed tumors in highly immunocompromised NSG mice following subcutaneous injections of 100 cells, whereas at least 1000 non-SP cells were required to produce any tumors (Fig. 2C, E). In contrast, SP and non-SP cells from AKT/RAS-induced tumors failed to form any tumors in NSG mice following subcutaneous injections of up to 1000 cells (Supplemental Fig. 1B). Tumors derived from allografts of MYC-driven SP cells contained SP and non-SP cells at percentages similar to those found in primary tumors (Supplemental Fig. 2). SP cells sorted from SP-derived tumors also formed tumors when seeded at 100 cells in secondary allograft experiments, whereas the same number of non-SP cells sorted from SP cell-derived tumors failed to initiate tumors (Fig. 2D). Additionally, SP cell allografts could give rise to non-SP tumor cells, whereas cells from non-SP allografts did not engender SP cells (Supplemental Fig. 2). We conclude that a subset of SP cells possesses the CSC-like property of tumor-initiation. SP cells also appear able to differentiate in vivo into a population of non-SP cells that does not display the enrichment for tumor-initiating potential found in the SP.
SP Cells Possess Properties of Hepatic Progenitor Cells
CSCs are thought to share properties with normal progenitor cells16. We examined the SP for evidence of such properties. CD44 has been characterized as a marker of CSCs and is expressed in hepatic progenitors9, 37. Cd44 mRNA was elevated in SP cells compared to non-SP cells when measured by quantitative PCR (Q-PCR) (Fig. 3A). Flow cytometry analysis revealed that CD44 protein expression was enriched on the surface of SP cells compared to non-SP cells (Fig. 3B). Additionally, other genes found to be upregulated in hepatic progenitors, including Epcam and Bmi1, were also upregulated in SP cells (Fig. 3A)38, 39. Since CSCs are a subpopulation of the SP, we conclude that they too may carry markers of normal progenitors.
Fig. 3. SP cells derived from liver tumors express markers of hepatic progenitor cells.
(A) Q-PCR analysis of markers for hepatic progenitor cells (Cd44, Epcam and Bmi1) in unsorted (Total), sorted SP and non-SP LT2-MYC hepatic tumor cells, *p-value<0.002, **p-value<0.02, ***p-value <0.01 (B) Flow cytometry analysis for CD44 in SP and non-SP LT2-Myc hepatic tumor cells. Gating determined by verapamil control.
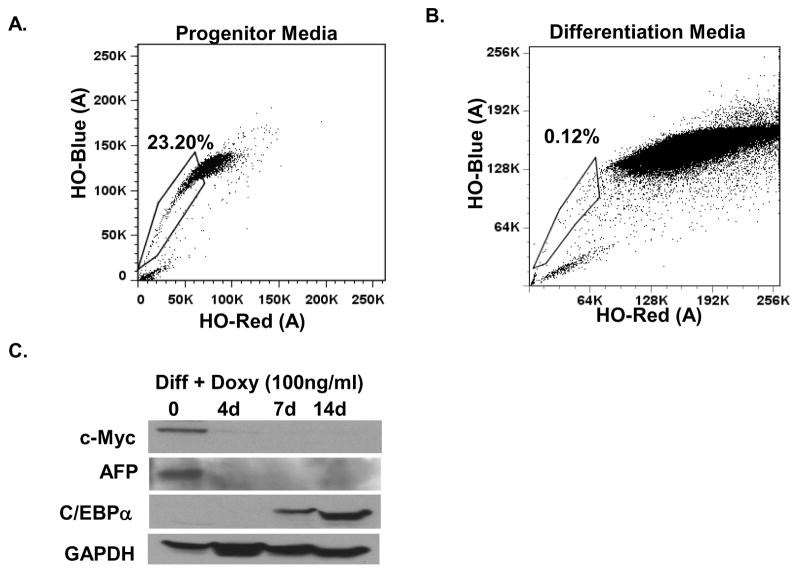
When unsorted tumor cells were exposed to media that favors the survival and growth of heptic progenitor cells, the percentage of SP cells increased (Fig. 4A). In contrast, non-SP cells failed to propagate or even survive in progenitor media (Supplemental Fig. 3), in accord with the view that they are more differentiated than SP cells. The SP population was reduced when tumor cells were incubated in media that elicits differentiation of hepatic progenitors into mature hepatocytes40 (Fig. 4B). Previous work has shown that MYC tumor cells can differentiate into mature hepatic cells upon the inactivation of MYC in vivo31. We found that concurrent repression of the MYC transgene by doxycycline enhanced the effect of differentiation media on the MYC-driven tumor cells, as manifested by complete loss of the progenitor marker AFP and an increase in C/EBPα, a marker for mature hepatocytes (Fig. 4C, Supplemental Fig. 4D). We conclude that SP cells from the MYC-driven hepatic tumors possess properties similar to normal progenitor cells, and that the same is likely to be true of the CSC subset of SP cells.
Fig. 4. SP cells derived from liver tumors have properties resembling those of hepatic progenitor cells.
(A) SP analysis of LT2-MYC tumor cells grown in hepatocyte progenitor media for 7 days. SP gate was determined by loss of SP in verapamil treated controls. (B) SP analysis of LT2-Myc tumor cells grown on collagen-coated plates in mature hepatocyte differentiation media for 7 days. (C) Western blot analysis of whole cell lysate collected from LT2-MYC tumor cells grown on collagen-coated plates in mature hepatocyte differentiation ESP-Diff media with doxycycline (Doxy) (100 ng/ml) for 0, 4, 7 and 14 days. AFP is a marker for hepatic progenitors and C/EBPα is a marker for mature hepatocytes.
MDR1 Mediates SP Formation and Chemoresistance in MYC-driven hepatic tumors
The ABC transporter proteins MDR1 and BCRP have been shown previously to efflux Hoechst 33342 dye19, 20. Sorted tumor cells were analyzed for the mRNA of ABC transporters as well as MRP1 (Abcc1a and Abcc1b). Only Mdr1a and Mdr1b mRNAs were more highly expressed in SP cells than in non-SP cells (Fig. 5A). These results were also confirmed by Western Blot analysis (Fig. 5B). Notably, expression of BCRP was not detected by either means. Exposure of LT2-MYC tumor cells to progenitor media enriched for MDR1 expression, whereas differentiation media did not (Supplemental Fig. 4D).
Fig. 5. MDR1 mediates formation of SP cells.
(A) Q-PCR analysis of ABC transporter pump genes in unsorted (Total), sorted SP and non-SP LT2-MYC hepatic tumor cells and LT2 normal liver cells (Normal). Data are presented as fold-change relative to normal samples. *p-value<0.03, **p-value<0.002 (B) Western blot analysis of whole cell lysate from LT2 normal liver (N) and unsorted (T), sorted SP, non-SP LT2-Myc hepatic tumor cells and FVB/N wildtype kidney tissue (MRP1 and BCRP positive control). (C) SP analysis in the absence (top row) and presence (bottom row) of verapamil (50μM) of tumor cells from FVB/N Wt, Bcrp−/− and Mdr1a/1b−/− mice following MYC hydrodynamic transfection.
Since MDR1 was more highly expressed than BCRP in SP cells, we used a functional analysis to determine whether it was MDR1 that mediated SP formation. To this end, we used hydrodynamic transfection of MYC to elicit hepatic tumors in mice that were deficient in either Mdr1a/1b or Bcrp and analyzed the resulting tumors for SP cells. Hydrodynamic transfection of MYC elicted hepatic tumors in mice of all genotypes by 90 days (Supplementary Fig. 4C). MYC induction of tumors in wildtype and Bcrp−/− mice resulted in the formation of a SP population, whereas hepatic tumors in Mdr1a/1b−/− mice did not have a SP population (Fig. 5C). The role of MDR1 in mediating the SP phenotype was further verified in vitro: overexpression of MDR1 enhanced the SP phenotype while partial knockdown reduced it (Supplemental Fig. 4A, B). These data demonstrate that, while MDR1 does not affect tumorigenesis, it is responsible for the SP phenotype seen in our tumor model.
MDR1 and BCRP efflux a number of similar chemotherapeutics, including doxorubicin (Dox), which is utilized in the treatment of primary hepatic tumors41, 42. MDR1 and BCRP can also efflux other chemotherapeutics with very different efficacy. For example, MDR1 effluxes paclitaxel (PTX), whereas BCRP does not. In contrast, BCRP is the preferential transporter for the drug SN3843, 44. Because MDR1 mediates SP formation, we investigated the role that MDR1 might play in chemoresistance in our hepatic tumor model. The efficacy of Dox and PTX treatment against LT2-MYC tumor cells was increased when combined with verapamil (Fig. 6A). SN38 treatment also inhibited cell growth (Fig. 6A), but the efficacy was not affected by verapamil. Unfractionated LT2-MYC tumor cells were analyzed for Hoechst 33342 efflux activity following treatment with Dox, PTX and SN38. The percentage of tumor cells in the SP increased following treatment with Dox or PTX, providing evidence that SP cells are resistant to chemotherapeutics effluxed by MDR1 (Fig. 6B).
Fig. 6. MDR1 confers chemoresistance to MDR1-effluxed drugs but not BCRP-effluxed drugs in LT2-MYC SP cells.
(A) MTT assay of LT2-MYC hepatic tumor cells following treatment for 72 hours with either Dox (10μM), PTX (0.1μM) or SN38 (0.1μM) (n=3–4). Cell viability is shown as a percentage of MTT conversion compared to 0.1% DMSO (PBS) treated samples. Parentheses denote drug transporters that preferentially efflux these drugs. (B) SP analysis of isolated LT2-MYC tumor cells treated overnight in vitro with 0.1% DMSO (PBS), PTX (2.5 nM) or Dox (2.5 nM). (C) TUNEL analysis of LT2-Myc tumors. LT2-MYC tumor bearing mice were treated with 0.1% DMSO (PBS) or PTX (200 μg) every 3 days and analyzed after 2 weeks of treatment. (D) SP analysis of LT2-MYC tumors following in vivo treatment with 0.1% DMSO (PBS) or PTX (200 μg). (E) LT2-MYC tumor cells were treated with either 0.1% DMSO (PBS) or PTX (200 μg). Following the treatment, 300 live cells from each sample (n=10) were subcutaneously seeded in NSG mice. Percentages of mice with tumors were evaluated up to 90 days post-injection.
Similar results were also seen in vivo. Treatment of LT2-MYC tumors with PTX elicited apoptosis (Fig. 6C, Supplemental Fig. 5) and increased the SP fraction in the surviving cells, when compared to the results of PBS treatment (Fig. 6D). Additionally, cells from PTX treated LT2-MYC tumors had enhanced tumor-initiating potential compared to cells from PBS treated LT2-MYC tumors when seeded at 300 cells per injection into NSG mice (Fig. 6E). Thus, PTX treatment selected for tumor-initiating cells that were resistant to MDR1-effluxed drugs.
Discussion
The chemoresistance of hepatic cancer stem cells
We have demonstrated that tumor-initiation by MYC creates a chemoresistant CSC population not seen following tumor initiation by AKT/RAS. Furthermore, this population can be enriched by isolating SP cells that exclude Hoechst 33342 dye. Previous studies have identified SP cells at very low percentages in developing and fully mature livers18. In these studies, hepatic progenitors represented a portion of the SP cells present in developing livers and the majority of SP cells present in mature livers. A portion of cells in MYC-induced hepatic tumors possess similar Hoechst 33342 efflux activity. These SP cells in our LT2-MYC hepatic tumor model were enriched for tumor-initiating cells, in comparison with non-SP cells, similar to CSCs identified as SP cells in other tumor models. The SP cells in the MYC-driven tumors were also capable of differentiating into more mature, non-SP cancer cells. This differentiation can occur fairly rapidly in vitro as evidenced by the loss of chemoresistance, hepatic progenitor markers and tumor-initiating capacity. Since MYC has been previously demonstrated to regulate global epigenetic states, the rapid differentiation could be a result of epigenetic reprogramming45. In mammary epithelial cells, neoplastic nonstem cells can spontaneously give rise to stem-like CSCs suggesting a bidirectional interconversion between stem and non-stem cell states46. This does not appear to be the case in our model as SP cells can differentiate into non-SP cells in vivo, but non-SP cells do not appear to spontaneously revert back to an SP state. While tumor-initiating CSCs typically represent only a subset of cells within the SP, enrichment by phenotypic markers such as chemoresistance can represent a reasonable first step in the purification of CSCs.
MDR1-mediated chemoresistance in hepatic CSCs
Intrinsic and acquired chemoresistance contribute to treatment failure in 90% of recurrent and metastatic tumors47. Consequently, understanding the mechanisms that may allow CSCs to escape chemotherapy and contribute to recurrence is important in improving the treatment of cancer. While BCRP and MDR1 have both been implicated in the chemoresistance of CSCs, the evidence until now had consisted mainly of increased expression of ABC transporters in CSCs compared to other subpopulations of tumor cells. We found an increase of MDR1 in SP cells compared to non-SP cells. In addition, however, we performed a functional analysis by using hydrodynamic transfection of MYC to elicit hepatic tumor formation in mice that were deficient in either BCRP or MDR1. The results demonstrated that the formation of MYC-induced SP cells is dependent upon MDR1.
There are at least two possible explanations for the expression of MDR1 in MYC-driven SP cells. First, MYC may directly regulate transcription from the Mdr1 genes. Previous studies have shown that MYCN can enhance MDR1 expression in human neuroblastoma cell lines and bind in vitro to E-box sequence oligonucleotides derived from putative MYC binding sites in the MDR1 proximal promoter48. MYC itself might play a similar role in the murine hepatic cancers that we have studied here. Alternatively, MYC may elicit hepatic tumors from precursor cells that are already chemoresistant due to intrinsic expression of MDR1. For example, MDR1 expression is increased during hepatic damage at reactive bile ductules, where proliferation of bipotential hepatic progenitor cells is thought to occur49. If hepatic progenitors are the precursor cells of CSCs, expression of MDR1 in the SP fraction (including CSCs) could represent a legacy from the normal precursor in which tumorigenesis originated. Whatever its genesis, expression of MDR1 is extinguished when SP cells differentiate into the non-SP cells that constitute the bulk of the MYC-driven tumors.
We conclude that the characteristics of CSCs can be determined by the oncogenotype responsible for tumorigenesis. We found that in MYC-driven hepatic tumors, MDR1 expression is required for formation of the SP and is responsible for the resistance of these cells to the chemotherapeutics that MDR1 can efflux. Chemoresistant CSCs that are enriched in the SP could survive initial rounds of chemotherapy and regenerate the bulk tumor following treatment withdrawal. We conclude that therapeutic inhibition of MDR1 might increase the efficacy of chemotherapeutics such as paclitaxel and doxorubicin against MYC-driven hepatic CSCs. This in turn might improve the therapeutic outcome. The models for primary hepatic cancer presented should be useful in exploring how different oncogenic events promote chemoresistance in CSCs. An understanding of how chemoresistance arises in CSCs is likely to be important in the personalization of cancer therapy.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by a postdoctoral fellowship to EKC from the American Cancer Society (PF-08-196-01-MGO), a NIH grant R01CA136606 to XC, China Scholarship Council (CSC) Contract No.2009616101 to LF and funds from the UCSF G.W. Hooper Research Foundation.
We thank Tara Rambaldo for her technical assistance with flow cytometry analysis, Linda Prentice for her technical assistance in histological processing of samples and Luda Urisman for her technical assistance with maintenance of mouse inventory. We would also like to thank Bishop and Chen lab members for helpful discussions.
List of Abbreviations
- CSC
cancer stem cells
- SP
Side population
- ABC
ATP Binding Cassette
- ALDH
aldehyde dehydrogenase
- P-gp/MDR1
P-glycoprotein (P-gp/MDR1)
- Mdr1
multidrug resistance gene 1
- BCRP
breast cancer resistance protein
- AKT
Activation of v-akt murine thymoma viral oncogene homolog
- NSG
NOD/Scidil2Rγ−/−
- LT2-MYC
Tet-o-MYC/LAP-tTA
- Doxy
doxycycline
- RBC
Red Blood Cell
- CFU
colony forming units
- Dox
doxorubicin
- PTX
paclitaxel
- FC
fold-change
Contributor Information
Edward Kai-Hua Chow, Email: Edward.chow@ucsf.edu.
Ling-ling Fan, Email: fine_323@hotmail.com.
Xin Chen, Email: Xin.chen@ucsf.edu.
J Michael Bishop, Email: bishop@cgl.ucsf.edu.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001 Oct 15;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000 Nov 27;160:3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 3.Mathurin P, Rixe O, Carbonell N, et al. Review article: Overview of medical treatments in unresectable hepatocellular carcinoma--an impossible meta-analysis? Aliment Pharmacol Ther. 1998 Feb;12:111–26. doi: 10.1046/j.1365-2036.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- 4.Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. 2009 Jul;109:2989–3011. doi: 10.1021/cr9000226. [DOI] [PubMed] [Google Scholar]
- 5.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008 Jan;9:105–27. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Haraguchi N, Utsunomiya T, Inoue H, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006 Mar;24:506–13. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 7.Chiba T, Kita K, Zheng YW, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006 Jul;44:240–51. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 8.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 9.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008 Feb;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Yang ZF, Ngai P, Ho DW, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008 Mar;47:919–28. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 11.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006 Dec 29;351:820–4. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009 Mar;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma S, Chan KW, Lee TK, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008 Jul;6:1146–53. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 14.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996 Apr 1;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquardt JU, Raggi C, Andersen JB, et al. Human hepatic cancer stem cells are characterized by common stemness traits and diverse oncogenic pathways. Hepatology. 2011 May 26; doi: 10.1002/hep.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu WH, Tao KS, You N, Liu ZC, Zhang HT, Dou KF. Differences in the properties and mirna expression profiles between side populations from hepatic cancer cells and normal liver cells. PLoS One. 2011;6:e23311. doi: 10.1371/journal.pone.0023311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshaver B, van Rhenen A, Kelder A, et al. Identification of a small subpopulation of candidate leukemia-initiating cells in the side population of patients with acute myeloid leukemia. Stem Cells. 2008 Dec;26:3059–67. doi: 10.1634/stemcells.2007-0861. [DOI] [PubMed] [Google Scholar]
- 18.Wulf GG, Luo KL, Jackson KA, Brenner MK, Goodell MA. Cells of the hepatic side population contribute to liver regeneration and can be replenished with bone marrow stem cells. Haematologica. 2003 Apr;88:368–78. [PubMed] [Google Scholar]
- 19.Bunting KD, Zhou S, Lu T, Sorrentino BP. Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood. 2000 Aug 1;96:902–9. [PubMed] [Google Scholar]
- 20.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002 Jan 15;99:507–12. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 21.Jonker JW, Freeman J, Bolscher E, et al. Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells. 2005 Sep;23:1059–65. doi: 10.1634/stemcells.2005-0150. [DOI] [PubMed] [Google Scholar]
- 22.Curtis SJ, Sinkevicius KW, Li D, et al. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell. 2010 Jul 2;7:127–33. doi: 10.1016/j.stem.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan JP, Minna JD. Tumor oncogenotypes and lung cancer stem cell identity. Cell Stem Cell. 2010 Jul 2;7:2–4. doi: 10.1016/j.stem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008 Oct 15;22:2755–66. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zondervan PE, Wink J, Alers JC, et al. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J Pathol. 2000 Oct;192:207–15. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH690>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Kaposi-Novak P, Libbrecht L, Woo HG, et al. Central role of c-Myc during malignant conversion in human hepatocarcinogenesis. Cancer Res. 2009 Apr 1;69:2775–82. doi: 10.1158/0008-5472.CAN-08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002 Jul;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer. 2005 Jan 15;103:307–12. doi: 10.1002/cncr.20774. [DOI] [PubMed] [Google Scholar]
- 29.Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006 Apr;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Zhao Z, Li G, et al. Relevance of two genes in the multidrug resistance of hepatocellular carcinoma: in vivo and clinical studies. Tumori. 2010 Jan-Feb;96:90–6. doi: 10.1177/030089161009600115. [DOI] [PubMed] [Google Scholar]
- 31.Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004 Oct 28;431:1112–7. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 32.Goodell MA. Stem cell identification and sorting using the Hoechst 33342 side population (SP) Curr Protoc Cytom. 2005 Nov;Chapter 9(Unit9):18. doi: 10.1002/0471142956.cy0918s34. [DOI] [PubMed] [Google Scholar]
- 33.Tward AD, Jones KD, Yant S, Kay MA, Wang R, Bishop JM. Genomic progression in mouse models for liver tumors. Cold Spring Harb Symp Quant Biol. 2005;70:217–24. doi: 10.1101/sqb.2005.70.058. [DOI] [PubMed] [Google Scholar]
- 34.Ho C, Wang C, Mattu S, et al. AKT and N-Ras co-activation in the mouse liver promotes rapid carcinogenesis via mTORC1, FOXM1/SKP2, and c-Myc pathways. Hepatology. 2011 Oct 12; doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornwell MM, Pastan I, Gottesman MM. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262:2166–70. [PubMed] [Google Scholar]
- 36.Wang J, Clark JB, Rhee GS, Fair JH, Reid LM, Gerber DA. Proliferation and hepatic differentiation of adult-derived progenitor cells. Cells Tissues Organs. 2003;173:193–203. doi: 10.1159/000070375. [DOI] [PubMed] [Google Scholar]
- 37.Kon J, Ooe H, Oshima H, Kikkawa Y, Mitaka T. Expression of CD44 in rat hepatic progenitor cells. J Hepatol. 2006 Jul;45:90–8. doi: 10.1016/j.jhep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008 Feb;47:636–47. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- 39.Chiba T, Zheng YW, Kita K, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology. 2007 Sep;133:937–50. doi: 10.1053/j.gastro.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Cui CB, Yamauchi M, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrixscaffolds. Hepatology. 2011 Jan;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 41.Litman T, Brangi M, Hudson E, et al. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000 Jun;113( Pt 11):2011–21. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 42.Malogolowkin MH, Katzenstein HM, Krailo M, et al. Redefining the role of doxorubicin for the treatment of children with hepatoblastoma. J Clin Oncol. 2008 May 10;26:2379–83. doi: 10.1200/JCO.2006.09.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Candeil L, Gourdier I, Peyron D, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004 May 10;109:848–54. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 44.Sorrentino BP, Brandt SJ, Bodine D, et al. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science. 1992 Jul 3;257:99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- 45.Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006 Jun 21;25:2723–34. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaffer CL, Brueckmann I, Scheel C, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011 May 10;108:7950–5. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005 Jan;205:275–92. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 48.Blanc E, Goldschneider D, Ferrandis E, et al. MYCN enhances P-gp/MDR1 gene expression in the human metastatic neuroblastoma IGR-N-91 model. Am J Pathol. 2003 Jul;163:321–31. doi: 10.1016/S0002-9440(10)63656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J Pathol. 2003 Aug;200:553–60. doi: 10.1002/path.1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.