Abstract
Purpose of review
In immunodeficiency, an increased sarcoma risk is confirmed for Kaposi’s sarcoma. Whether rates of other sarcoma subtypes are elevated in the setting of immunodeficiency is not known. We therefore reviewed published case reports on HIV/AIDS patients and organ transplant recipients with sarcomas. For comparison, we assessed sarcomas in the U.S. general population using Surveillance Epidemiology End Results (SEER) data.
Findings
One hundred seventy-six non-KS sarcomas were identified, 75 in people with HIV/AIDS and 101 in transplant recipients. Leiomyosarcomas (n=101) were the most frequently reported sarcomas, followed by angiosarcomas (n=23) and fibrohistiocytic tumors (n=17). Leiomyosarcomas were reported with two age peaks, in children and young adults. Epstein-Barr virus (EBV) was detected in the tumor cells in 85% and 88% of leiomyosarcomas in HIV-infected people and transplant recipients, respectively. Angiosaromas and fibrohistiocytic tumors were most frequently reported in males. Among kidney transplant recipients, 20% of sarcomas arose at the site of an arteriovenous fistula. In comparison, leiomyoscarcomas, angiosarcomas, and fibrohistiocytic tumors comprised 16.9%, 3.8%, and 18.7% of sarcomas in the U.S. general population.
Summary
Leiomyosarcoma and angiosarcoma may occur disproportionately in immunodeficiency. Leiomyosarcomas appear etiologically linked to EBV while angiosarcomas might be correlated with an arteriovenous fistula. Additional studies are necessary to understand the contribution of immunodeficiency to the etiology of these sarcomas.
Keywords: HIV, AIDS, organ transplant, cancer, EBV, HIV, sarcoma. HHV-8
Introduction
A dramatic eruption of an otherwise rare cancer, Kaposi sarcoma (KS), in young men in San Francisco heralded the HIV epidemic in the US. The incidence of KS rose from approximately 0.5 per 100 000 people in 1975 to a peak of 33.3 in the late 1980s to the early 1990s [1]. Reports describing KS in solid organ transplant recipients also linked this sarcoma with immunosuppression [2]. Later, systematic analyses of population-based registry data clearly demonstrated a several thousand-fold increased KS risk in people with AIDS compared to the general population [3].
The incidence of as many as twenty distinct cancers is elevated in people with HIV infection and in solid organ transplant recipients [4], and the commonality in the spectrum of malignancies in these two immunosuppressed conditions is striking. Risk is most increased for cancers with an infectious etiology, including those caused by oncogenic viruses such as human herpesvirus 8 (HHV-8, linked to KS), human papillomavirus (HPV, linked to anogenital cancers), Epstein-Barr virus (EBV, linked to non-Hodgkin and Hodgkin lymphomas), hepatitis B and hepatitis C viruses (HBV and HCV, linked to liver cancer), and Merkel cell polyomavirus (MCPyV, linked to Merkel cell carcinoma) [4].
Sarcomas are diverse and include more than fifty distinct histological subtypes originating from connective tissues, including fibrous tissue, muscle, bone, cartilage, fat, blood vessels, and nerves [5]. However, the entire group of sarcomas together account for less than 1% of all malignancies. While sarcomas occur across all ages, there are age-associated peaks in incidence specific for distinct entities. Rhabdomyosarcoma and Ewing sarcoma are both pediatric cancers, the former occurring in the first decade of life and the latter in the second decade. In contrast, liposarcomas and leiomyosarcomas most often occur in adults [6].
The rarity, diversity, and age-dependency of specific sarcomas make it difficult to single out histological subcategories that are increased in people with immunosuppression, particularly if these increases are much smaller than observed for KS. In a prior analysis of population-based HIV/AIDS and cancer registry data, Biggar and colleagues demonstrated an excess risk of leiomyosarcomas in children with AIDS [7]. Over the past decade, other reports have described an excess of leiomyosarcomas in children with HIV or following organ transplantation [8–10].
Whether other sarcoma subtypes are affected by immunosuppression is unclear. Even with large population-based registries [11], the sensitivity of identifying an elevated risk of uncommon cancers depends upon the magnitude of the association, the incidence of these cancers in the general population, and the categorization of cancer types. Linkage-based studies of HIV and cancer have typically categorized cancers by site. As sarcomas can arise within multiple organs, this approach could have masked an elevated risk of sarcomas.
For these reasons, case reports remain an important source for understanding the spectrum of cancers in immunocompromised populations and ascertaining clues into cancer etiology. To identify sarcomas that occur frequently in immunosuppressed populations and describe the characteristics of these sarcomas, we systematically reviewed published case reports and case series to catalog sarcomas reported in people with HIV infection and recipients of solid organ transplants.
Literature review approach
We conducted a literature review using PubMed and references cited within published articles to compile case reports of all sarcomas, excluding KS, in people with HIV or a solid organ transplant. Our PubMed search strategy included combinations of the following keywords: organ transplantation, malignancy, de-novo malignancy, immunosuppression, immunodeficiency, HIV, AIDS, and sarcoma. We also searched with names of specific sarcoma subtypes (e.g., leiomyosarcoma, osteosarcoma). We excluded studies of recipients of bone marrow transplants or congenital immunodeficiency syndromes, and articles not published in English.
Sarcomas were categorized into widely accepted histologic subtypes. To identify over- or under-representation of specific sarcoma subtypes in people with immunodeficiency, we compared the relative proportions of these subtypes reported in people with HIV or solid organ transplants with the distribution in the general population. General population counts for sarcoma subtypes were obtained from the U.S. Surveillance, Epidemiology, and End Results database (SEER 17 regions, 1974–2008) [12] using International Classification of Diseases for Oncology morphology codes [13].
In cataloging leiomyosarcoma cases, case reports with a diagnosis of EBV-associated smooth muscle tumor were categorized as leiomyosarcomas. We also compared the age distribution of case reports of leiomyosarcomas in people with immunodeficiency with leiomyosarcomas in SEER (histology code 8890, regardless of site). Finally, for comparison, we also captured case reports of leiomyomas in HIV-infected people and transplant recipients, which were analyzed separately.
Sarcomas in Immunodeficiency
A total of 176 cases of sarcoma (other than KS) were reported in people with HIV (n=75) or recipients of solid organ transplants (n=101) (Table 1). In both immunodeficient populations, more than half of the reported sarcomas were leiomyosarcoma. Fifty-four leiomyosarcomas occurred in solid organ transplant recipients [10, 14–57], and 47 occurred in people with HIV [58–86]. Thus, 57.8% of all sarcomas reported in immunodeficient hosts were leiomyosarcomas. In comparison, in the U.S. general population (SEER data), only 16.9% of non-KS sarcomas were leiomyosarcomas (Table 1).
Table 1.
Distribution of sarcoma subtypes in cases reported among HIV-infected individuals, transplant recipients, and the general U.S. population
| HIV | Transplant | SEER | ||||
|---|---|---|---|---|---|---|
| Sarcoma (ICD-O-3 codes) | N | % | N | % | N | % |
| Osteosarcomas(9180-9187, 9191-9195, 9200) | 1 | 1.3 | 0 | 0 | 4,510 | 5.1 |
| Chondrosarcomas(9210, 9220-9221, 9230, 9240-9243) | 0 | 0 | 0 | 0 | 3,707 | 4.2 |
| Ewing tumor and related sarcomas of bone(9260, 9363-9365) | 1 | 1.3 | 1 | 1.0 | 2,456 | 2.8 |
| Malignant chordomas(9370-9372) | 0 | 0 | 0 | 0 | 1,191 | 1.3 |
| Rhabdomyosarcomas(8900-8905, 8910, 8912, 8920, 8991) | 6 | 8.0 | 2 | 2.0 | 3,283 | 3.7 |
| Fibrosarcomas(8810, 8811, 8813-8815, 8820-8827, 8834-8835, 9150, 9160) | 0 | 0 | 3 | 3.0 | 3,693 | 4.2 |
| Nerve sheath tumor(9540-9571) | 1 | 1.3 | 1 | 1.0 | 2,516 | 2.9 |
| Other fibromatous neoplasia (9491-9580) | 0 | 0 | 0 | 0 | 103 | 0.1 |
| Extrarenal rhabdoid tumor(8963) | 0 | 0 | 0 | 0 | 210 | 0.2 |
| Liposarcomas(8850-8858, 8860-8862, 8870, 8880, 8881) | 2 | 2.7 | 2 | 2.0 | 8,831 | 10.0 |
| Fibrohistiocytic tumors(8830-8833, 8836, 9251, 9252) | 4 | 5.3 | 13 | 12.8 | 16,481 | 18.7 |
| Leiomyosarcoma(8890-8898) | 47 | 62.7 | 54 | 53.5 | 14,943 | 16.9 |
| Synovial sarcomas(9040-9044) | 1 | 1.3 | 0 | 0 | 2,183 | 2.5 |
| Alveolar sort parts sarcoma(9581) | 0 | 0 | 0 | 0 | 182 | 0.2 |
| Angiosarcomas and other blood vessel tumors (9120-9125, 9130-9133, 9135, 9136, 9141, 9142, 9161, 9170-9175) | 5 | 6.7 | 18 | 17.8 | 3,320 | 3.8 |
| Gastrointestinal stromal tumor(8936) | 2 | 2.7 | 4 | 0 | 4,624 | 5.2 |
| Interdigitating dendritic cell sarcoma(9757) | 2 | 2.7 | 1 | 1.0 | 20 | 0 |
| Carcinosarcoma, NOS(8980-8982) | 0 | 0 | 1 | 1.0 | 5,369 | 6.1 |
| Miscellaneous sarcomas(8587, 8710-8713, 8806, 8840-8842, 8921, 8990, 9373) | 2 | 2.7 | 0 | 0 | 701 | 0.8 |
| Unspecified sarcomas(8800-8805) | 1 | 1.3 | 1 | 1.0 | 9,928 | 11.2 |
Abbreviations: ICD-O-3 International Classification of Diseases for Oncology third edition, NOS not otherwise specified, SEER Survival, Epidemiology, and End Results
The sex distribution of leiomyosarcoma cases was similar in people with HIV and transplant recipients. Of the 81 leiomyosarcomas for which EBV status was reported, EBV was detected in the tumor cells in 85% and 88% of cases in people with HIV and transplant recipients, respectively. EBV positive leiomyosarcomas were detected across all age groups. Among leiomyosarcoma cases with HIV infection, 94% had a prior AIDS diagnosis. Sixty-three percent of transplant-related leiomyosarcomas were reported among kidney recipients [22–47].
Figure 1a is a representation of the age distribution of case reports of leiomyosarcomas in HIV-infected individuals, transplant recipients, and the U.S. general population. In the general population, the number of leiomyosarcoma cases rises steeply with age, with 72% of cases occurring in people aged 50 years and older. In contrast, in people with HIV or transplant-associated immunodeficiency, leiomyosarcomas were reported across all age groups. Thirty-six percent of reports in HIV-infected individuals and 24% in transplant recipients were in children aged 0–9 years. In HIV-infected cases this proportion diminished between the ages 10–29 years and peaked again, with 34% of cases occurring in 30–39 year olds. In transplant recipients, this second peak (19%) occurred in people aged 50 years or older.
Figure 1.
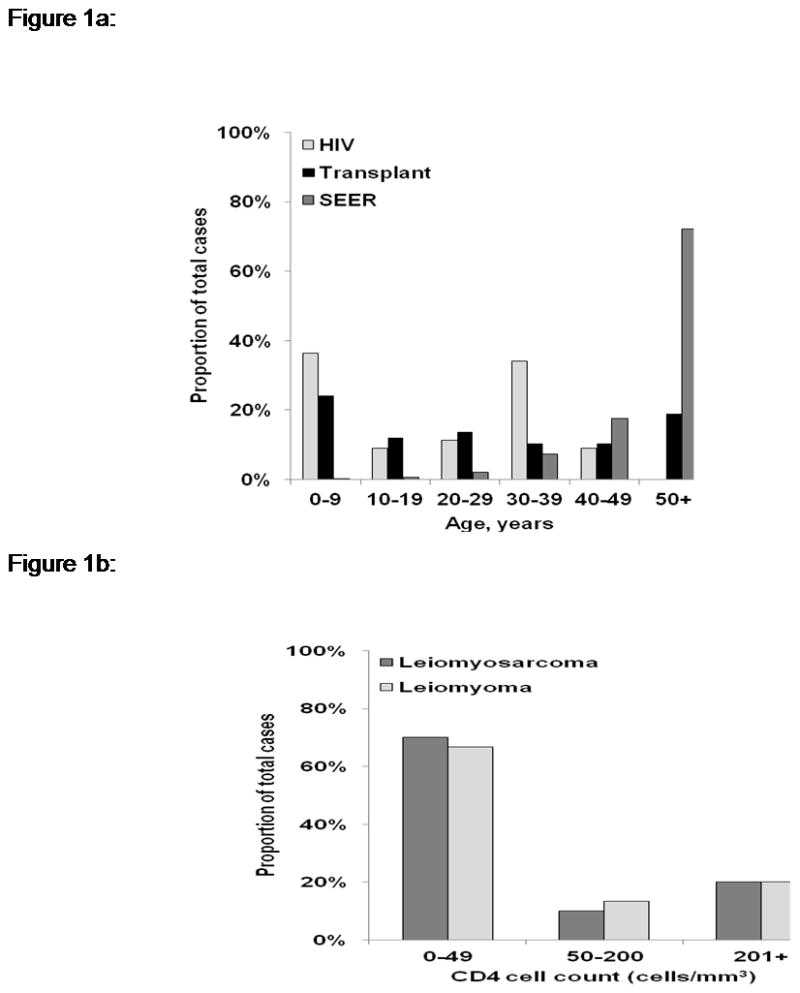
Figure 1a: Age distribution of leiomyosarcoma cases reported in HIV-infected individuals and transplant recipients, and in the US general population
Figure 1b: Distribution of CD4 counts in HIV-infected individuals with leiomyosarcoma (data based upon 20 cases) or leiomyoma (data based upon 15 cases)
Angiosarcomas also comprised a higher proportion of sarcomas in immunocompromised populations compared to the general population (6.7% of sarcomas in HIV-infected people and 18.0% in transplant recipients, vs. 3.8% in SEER; Table 1). We identified 23 cases of angiosarcomas, of which 5 cases were in HIV-infected adults (4 males and 1 female) [87–90]. The remaining 18 cases were reported in kidney recipients [91–106], and the vast majority were in males (n=16) (Table 2). The median age at diagnosis of angiosarcomas in transplant recipients was 43 years (range 24–71 years). The time to diagnosis following transplant ranged from 2–18 years.
Table 2.
Characteristics of angiosarcoma cases occurring in kidney transplant recipients.
| Characteristic | N (%) or median (range) |
|---|---|
| Total | 18 (100) |
| Sex , n (%) | |
| Female | 2 (11) |
| Male | 16 (89) |
| Age in years, median (range) | 43 (24–71) |
| Geographic location of study, n (%) | |
| North America | 6 (33) |
| Europe | 9 (50) |
| Australia | 1 (6) |
| Asia | 2 (11) |
| Located at the arteriovenous fistula, n (%) | |
| Yes | 11 (61) |
| No | 7 (39) |
| Months from transplant to angiosarcoma, median (range) | 84 (24–216) |
Eleven angiosarcomas in kidney transplant recipients (61%) arose at the site of an arteriovenous fistula. Of interest, an arteriovenous fistula was also the site of dermatofibrosarcoma protuberans (a subtype of fibrohistiocytic tumor) in three kidney recipients [107–109]. Thus, 14 sarcomas reported in kidney recipients occurred at an arteriovenous fistula, representing 20% of sarcomas in kidney recipients and 14% of sarcomas in transplant recipients overall.
Seventeen cases of fibrohistiocytic tumors were reported in immunodeficient individuals (Table 1) [107–120]. These include one case of angiomatoid fibrous histiocytoma in an HIV-infected person [107]; 5 cases of dermatofibrosarcoma protuberans [108–111]; 2 cases of atypical fibroxanthoma [112, 113]; and 9 cases of malignant fibrohistiocytoma [113–120], 8 of which were in transplant recipients. Fourteen of these seventeen cases occurred in males. In comparison, 18.7% of sarcomas in the U.S. general population were fibrohistiocytic tumors.
Eight cases of rhabdomyosarcoma were reported (Table 1), of which the majority (n=6) were in people with HIV [84; 121–124]. Three of these rhabdomyosarcoma cases occurred in children, but both cases in transplant recipients were adults, one a 21 year-old female liver recipient [125] and the other a 67 year-old male kidney recipient [126]. Only a limited number of other sarcomas were described in the case literature (Table 1) [127–152].
Fifty leiomyomas were reported in HIV-infected individuals [9, 60, 70, 73–75, 153–182] or transplant recipients [183–192], of which 80% were in HIV-infected individuals. Among HIV-infected people, 93% of leiomyomas occurred after an AIDS diagnosis. The male-to-female ratio of leiomyoma cases in HIV-infected individuals (2:1) differed from that in transplant recipients (1:1.5), and also differed from the ratio for leiomyosarcomas in HIV-infected individuals (1:1.5). Among transplant recipients, 80% of leiomyomas were reported in kidney recipients [183–190]. Among the 24 leiomyomas for which EBV status was reported, EBV was detected in 100% of transplant-related leiomyomas but only 53% of HIV-related leiomyomas (Table 3). We queried leiomyoma and leiomyosarcoma reports for CD4 counts as a measure of immunosuppression. As shown in Figure 1b, most cases were in people with CD4 counts below 50 cells/mm3.
Table 3.
Characteristics of leiomyoma cases occurring in HIV-infected individuals and transplant recipients.
| HIV/AIDS | Transplant | |
|---|---|---|
| N (%) | N (%) | |
| Total | 40 (100) | 10 (100) |
| Sex | ||
| Female | 13 (33) | 6 (60) |
| Male | 26 (67) | 4 (40) |
| Missing | 1 | 0 |
| Age, years | ||
| 0–9 | 15 (38) | 1 (10) |
| 10–19 | 8 (20) | 0 (0) |
| 20–29 | 3 (8) | 0 (0) |
| 30–39 | 12 (30) | 2 (20) |
| 40–49 | 1 (3) | 3 (30) |
| 50+ | 1 (3) | 4 (40) |
| EBV status of tumor | ||
| Positive | 9 (53) | 7 (100) |
| Negative | 8 (47) | 0 (0) |
| Missing | 23 | 3 |
| AIDS status | ||
| Present | 28 (93) | - |
| Absent | 2 (7) | - |
| Missing | 10 | - |
| Organ transplanted | ||
| Liver | - | 0 (0) |
| Lung | - | 1 (10) |
| Kidney | - | 8 (80) |
| Heart | - | 1 (10) |
| Multiple | - | 0 (0) |
Interpretations from case literature review
The objective of the current study was to identify subtypes of sarcomas other than KS that occur in immune suppressed populations. Given the rarity and broad diversity of sarcomas, our hypothesis was that modestly elevated risks for rare sarcomas might have been missed in large registry-based studies of HIV-infected people and solid organ transplant recipients. However, unusual individual cases or series of cases occurring in such individuals might have prompted clinicians to publish descriptive reports. Our literature review identified three sarcoma subtypes that are often reported in people with immunosupression: leiomyosarcoma, angiosarcoma, and fibrohistiocytic tumors. Of these, only leiomyosarcoma and angiosarcoma were disproportionately represented in both HIV and transplant compared to the general population (Table 1).
The most commonly reported sarcoma in HIV-infected people and transplant recipients was leiomyosarcoma, with distinct age peaks in very young children, in young adults (for HIV), and older individuals >50 (for transplant). EBV is not required for transformation, since leiomyomas and leiomyosarcomas that arise in immunocompetent individuals are not associated with EBV [193], but our review suggests that immunosuppression-related leiomyosarcomas are highly associated with EBV across all ages. Of interest, EBV is also detected in muscle tumors in individuals with congenital immunodeficiency syndromes [194]. When present in leiomyosarcoma, EBV infection occurs prior to the clonal proliferation of tumor cells [15], but most case reports indicate that EBV LMP-1, an important oncoprotein [195], is not detectable [15,5,192,]. EBV infection requires the expression of the cell surface receptor CD21. While some studies have demonstrated CD21 expression in smooth muscle tumors [9, 60], other studies have not [188]. EBV infection of rare mesenchymal cells has been reported in immunocompetent hosts [196], and high circulating levels of EBV during immunosuppression may more readily allow such infections.
The vast majority of HIV-infected individuals reported with leiomyosarcoma had AIDS, and CD4 cell counts were typically low among both leiomyosarcoma and leiomyoma patients. These observations highlight the importance of immunosuppression in facilitating transformation of mesenchymal cells. EBV appears to be less often detected in leiomyomas than in leiomyosarcomas in the setting of HIV infection. While the proportion of leiomyomas with missing data for EBV was large, the available reports point to a need for a systematic study of the molecular features of leiomyomas and leiomyosarcomas arising in immunosuppressed people.
Angiosarcomas are also frequently reported in people with immunodeficiency, raising the possibility of a viral etiology. Early studies, based solely upon amplification of DNA by polymerase chain reaction PCR, suggested the presence of HHV-8 genome in angiosarcomas [197], but more recent studies that have utilized in situ methods to detect HHV-8 RNA or proteins have been mixed [87, 198–200]. Our review found a male predominance of angiosarcomas (20 male vs. 3 female reported cases). These sarcomas were also more frequently reported following kidney transplant than other organ transplants. While this predominance may merely reflect that kidneys are the most commonly transplanted organ, it may instead be a clue to intrinsic factors that affect the development of angiosarcoma.
Along these lines, an interesting feature of the angiosarcomas reported in kidney recipients is their apparent predilection to occur at the site of an arteriovenous fistula used for hemodialysis. Angiosarcomas have also been reported in hemodialysis patients who have not received a transplant [201*]. Inert foreign material can induce inflammation that might lead to the development of sarcomas, including angiosarcomas [202]. The milieu around an arteriovenous fistula is characterized by chronic venous stasis, altered capillary pressures, and tissue hypoxia, which may induce neovascularisation and cellular proliferation. Angiosarcomas have also been documented within areas of lymphedema, as occur in the Stewart-Treves syndrome [203], and they are a known complication of radiation exposure [204].
Fibrohistocytic sarcomas reported in case literature in people with HIV and organ transplants included dermatosfibrosarcoma protuberans, atypical fibroxanthomas and malignant fibrous histocytomas. Our review identified 17 fibrohistocytic sarcomas, the majority (n=14) of which occurred in males. However, these sarcomas represented only 5% and 13% of all sarcomas in people with HIV and in transplant recipients, respectively (Table 1).
An important limitation of our study is that it is solely based upon case reports. There is an inherent publication bias in these reports, in that investigators are more likely to report novel cases, such as those with unusual features, involvement of EBV, or occurrence in children. Therefore, our results should not be interpreted as a direct measure of risk associated with immunodeficiency or a complete characterization of the sarcomas that arise in immunosuppressed people. Nonetheless, the disproportionate representation of leiomyosarcomas that we observed is consistent with an increased risk for this sarcoma subtype observed in previous studies of immunosuppressed populations [7,10]. Case report literature reviews also cannot incorporate a central pathology review or common standards for pathology diagnosis, which might introduce biases in the classification of sarcomas. The major strength of this report is our systematic review of the published literature, which provided a unique approach to assess rare sarcoma subtypes occurring in people with HIV and transplant recipients.
Conclusions
In summary, leiomyosarcomas and leiomyomas were frequently reported in HIV-infected people and organ transplant recipients, supporting an increased risk of this sarcoma in immunosuppressed people and highlighting the importance of EBV. The disproportionate occurrence of angiosarcomas may likewise reflect increased risks associated with immunodeficiency. Furthermore, among transplant recipients, angiosarcomas are most commonly reported in people with kidney transplants and might be correlated with an arteriovenous fistula. Additional studies are necessary to understand the contribution of immunodeficiency to the etiology of these sarcomas.
Key Points.
Other than Kaposi sarcoma, a limited number of other sarcomas, including leiomyosarcoma and angiosarcoma, may occur disproportionately in immunodeficient populations.
EBV is commonly associated with leiomyosarcoma in people with HIV and transplant recipients. EBV is also frequently detected in leiomyomas from these populations. In HIV-infected people, leiomyosarcomas are reported in two age peaks, for children and young adults.
Among transplant recipients, angiosarcomas are reported exclusively in kidney transplants and have a high predilection for the site of an arteriovenous fistula.
Angiosarcomas and fibrohistocytic tumors in transplant patients show a notable male predominance.
Acknowledgments
This study was funded by the Intramural Research Program of the National Cancer Institute.
Footnotes
Disclosure: The authors have declared no conflicts of interest.
Data from this manuscript were presented at the 13th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies in Bethesda, November 2011.
References
- 1.Eltom MA, Jemal A, Mbuliateye SM, Devasa SS, Biggar RJ. Trends in Kaposis sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 2.Penn I. Sarcomas in Organ Allograft Recipients. Transplantation. 1995;60:1485–1491. doi: 10.1097/00007890-199560120-00020. [DOI] [PubMed] [Google Scholar]
- 3.Biggar RJ, Rosenberg PS, Coté T. Kaposis sarcoma and Non Hodgkins lymphoma following the diagnosis of AIDS. Multistate AIDS Cancer Match study group. Int J Cancer. 1996;68(6):754–8. doi: 10.1002/(SICI)1097-0215(19961211)68:6<754::AID-IJC11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 5.Brennan MF. Soft tissue sarcoma: advances in understanding and management. Surgeon. 2005;3:216–223. doi: 10.1016/s1479-666x(05)80044-7. [DOI] [PubMed] [Google Scholar]
- 6.Wolden SL, Alektiar KM. Sarcomas across the age spectrum. Semin Radiat Oncol. 2010;20(1):45–51. doi: 10.1016/j.semradonc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Biggar RJ, Frisch M, Goedert JJ. Risk of cancer in children with AIDS. AIDS-Cancer Match Registry Study Group. JAMA. 2000;284(2):205–209. doi: 10.1001/jama.284.2.205. [DOI] [PubMed] [Google Scholar]
- 8.Pollock BH, Jenson HB, Leach CT, et al. Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA. 2003;289(18):2393–2399. doi: 10.1001/jama.289.18.2393. [DOI] [PubMed] [Google Scholar]
- 9.Jenson HB, Leach CT, McClain KL, et al. Benign and malignant smooth muscle tumors containing Epstein-Barr virus in children with AIDS. Leuk Lymphoma. 1997;27(3–4):303–314. doi: 10.3109/10428199709059684. [DOI] [PubMed] [Google Scholar]
- 10.Ha C, Haller JO, Rollins NK. Smooth muscle tumors in immuno compromised (HIV negative) children. Pediatr Radiol. 1993;23(5):413–414. doi: 10.1007/BF02011979. [DOI] [PubMed] [Google Scholar]
- 11.Grulich AE, Wan X, Law MG, Coates M, Kaldor JM. Risk of Cancer in people with AIDS. 1999 May 7;13(7):839–843. doi: 10.1097/00002030-199905070-00014. [DOI] [PubMed] [Google Scholar]
- 12.SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; 2009. [cited 2011 Oct 1]; Available from: http://seer.cancer.gov/csr/1975_2002/ [Google Scholar]
- 13.World Health Organization. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 14.Timmons CF, Dawson DB, Richards CS, Andrews WS, Katz JA. Epstein-Barr virus-associated leiomyosarcomas in liver transplantation recipients. Origin from either donor or recipient tissue. Cancer. 1995;76(8):1481–1489. doi: 10.1002/1097-0142(19951015)76:8<1481::aid-cncr2820760828>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Lee ES, Locker J, Nalesnik M, et al. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med. 1995;332(1):19–25. doi: 10.1056/NEJM199501053320104. [DOI] [PubMed] [Google Scholar]
- 16.Rougemont AL, Alfieri C, Fabre M, et al. Atypical Epstein-Barr virus (EBV) latent protein expression in EBV-associated smooth muscle tumours occurring in paediatric transplant recipients. Histopathology. 2008;53(3):363–367. doi: 10.1111/j.1365-2559.2008.03086.x. [DOI] [PubMed] [Google Scholar]
- 17.Brichard B, Smets F, Sokal E, et al. Unusual evolution of an Epstein-Barr virus- associated leiomyosarcoma occurring after liver transplantation. Pediatr Transplant. 2001;5(5):365–369. doi: 10.1034/j.1399-3046.2001.00022.x. [DOI] [PubMed] [Google Scholar]
- 18.Doyle H, Tzakis AG, Yunis E, Starzl TE. Smooth muscle tumor arising de novo in a liver allograft: A case report. Clin Transplant. 1991;5(1):60–62. [PMC free article] [PubMed] [Google Scholar]
- 19.Sunde J, Chetty-John S, Shlobin OA, Boice CR. Epstein-Barr virus-associated uterine leiomyosarcoma in an adult lung transplant patient. Obstet Gynecol. 2010;115(2 Pt 2):434–436. doi: 10.1097/AOG.0b013e3181c51ed0. [DOI] [PubMed] [Google Scholar]
- 20.Medlicott SA, Devlin S, Helmersen DS, Yilmaz A, Mansoor A. Early post-transplant smooth muscle neoplasia of the colon presenting as diminutive polyps: a case complicating post-transplant lymphoproliferative disorder. Int J Surg Pathol. 2006;14(2):155–61. doi: 10.1177/106689690601400212. [DOI] [PubMed] [Google Scholar]
- 21.Flint A, Lynch JP, 3rd, Martinez FJ, Whyte RI. Pulmonary smooth muscle proliferation occurring after lung transplantation. Chest. 1997;112(1):283–284. doi: 10.1378/chest.112.1.283. [DOI] [PubMed] [Google Scholar]
- 22.Chaves NJ, Kotsimbos TC, Warren MA, et al. Cranial leiomyosarcoma in an Epstein-Barr virus (EBV)-mismatched lung transplant recipient. J Heart Lung Transplant. 2007;26(7):753–755. doi: 10.1016/j.healun.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Sadahira Y, Moriaya T, Shirabe T, et al. Epstein Barr virus-associated post-transplant primary smooth muscle tumor of the liver: report of an autopsy case. Pathol Int. 1996;46:601. doi: 10.1111/j.1440-1827.1996.tb03660.x. [DOI] [PubMed] [Google Scholar]
- 24.Morel D, Mérville P, Le Bail B, et al. Epstein Barr virus (EBV)-associated hepatic and splenic smooth muscle tumours after kidney transplantation. Nephrol Dial Transplant. 1996;11:1864. [PubMed] [Google Scholar]
- 25.Ashfaq A, Haller JE, Mossey R, et al. Recurrent membranous nephropathy and leiomyosarcoma in the renal allograft of alupus patient. J Nephrol. 2004;17(1):134–138. [PubMed] [Google Scholar]
- 26.Sprangers B, Smets S, Sagaert X, et al. Posttransplant Epstein-Barr virus-associated myogenic tumors: case report and review of the literature. Am J Transplant. 2008;8(1):253–258. doi: 10.1111/j.1600-6143.2007.02054.x. [DOI] [PubMed] [Google Scholar]
- 27.Deyrup AT, Lee VK, Hill CE, et al. Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. Am J Surg Pathol. 2006;30(1):75–82. doi: 10.1097/01.pas.0000178088.69394.7b. [DOI] [PubMed] [Google Scholar]
- 28.Boudjemaa S, Boman F, Guigonis V, Boccon-Gibod L. Brain involvement in multicentric Epstein-Barr virus-associated smooth muscle tumours in a child after kidney transplantation. Virchows Arch. 2004;444(4):387–91. doi: 10.1007/s00428-004-0975-7. [DOI] [PubMed] [Google Scholar]
- 29.Rougemont AL, Alfieri C, Fabre M, Gorska-Flipot I, et al. Atypical Epstein-Barr virus (EBV) latent protein expression in EBV-associated smooth muscle tumours occurring in paediatric transplant recipients. Histopathology. 2008;53(3):363–367. doi: 10.1111/j.1365-2559.2008.03086.x. [DOI] [PubMed] [Google Scholar]
- 30.Moore Dalal K, Antonescu CR, Dematteo RP, Maki RG. EBV-Associated Smooth Muscle Neoplasms: Solid Tumors Arising in the Presence of Immunosuppression and Autoimmune Diseases. Sarcoma. 2008 doi: 10.1155/2008/859407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debiec-Rychter M, Croes R, De Vos R, et al. Complex genomic rearrangement of ALK loci associated with integrated human Epstein-Barr virus in a post-transplant myogenic liver tumor. Am J Pathol. 2003;163(3):913–922. doi: 10.1016/S0002-9440(10)63451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To KF, Lai FM, Wang AY, et al. Posttransplant Epstein-Barr virus-associated myogenic tumors involving bone. Cancer. 2000;89(2):467–72. [PubMed] [Google Scholar]
- 33.Walker D, Gill TJ, 3rd, Corson JM. Leiomyosarcoma in a renal allograft recipient treated with immunosuppressive drugs. JAMA. 1971;215(13):2084–2086. [PubMed] [Google Scholar]
- 34.van Gelder T, Vuzevski VD, Weimar W. Epstein-Barr virus in smooth-muscle tumors. N Engl J Med. 1995;332(25):1719. doi: 10.1056/nejm199506223322516. [DOI] [PubMed] [Google Scholar]
- 35.Fujita H, Kiriyama M, Kawamura T, et al. Primary hepatic leiomyosarcoma in a woman after renal transplantation: report of a case. Surg Today. 2002;32(5):446–449. doi: 10.1007/s005950200073. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Urushihara N, Fukumoto K, et al. A case of Epstein-Barr virus-associated pulmonary leiomyosarcoma arising five yr after a pediatric renal transplant. Pediatr Transplant. 2010 Apr 26; doi: 10.1111/j.1399–3046. [DOI] [PubMed] [Google Scholar]
- 37.Cautero N, De Luca S, Vecchi A, et al. A. Peritoneal leiomyosarcoma in a kidney transplant patient: a case report. Transplant Proc. 2007;39(6):2038–2039. doi: 10.1016/j.transproceed.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 38.Ferri L, Fraser R, Gaboury L, Mulder D. Epstein-Barr virus-associated pulmonary leiomyosarcoma arising twenty-nine years after renal transplantation. J Thorac Cardiovasc Surg. 2003;126(3):877–879. doi: 10.1016/s0022-5223(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 39.Pritzker KP, Huang SN, Marshall KG. Malignant tumours following immunosuppressive therapy. Can Med Assoc J. 1970;103(13):1362–1365. [PMC free article] [PubMed] [Google Scholar]
- 40.Gan EC, Lau DP, Chuah KL. Epstein-Barr virus-associated smooth muscle tumour mimicking bilateral vocal process granuloma. J Laryngol Otol. 2008;122(1):100–104. doi: 10.1017/S0022215107007682. [DOI] [PubMed] [Google Scholar]
- 41.Toh HC, Teo M, Ong KW, et al. Use of sirolimus for Epstein-Barr virus-positive smooth-muscle tumour. Lancet Oncol. 2006;7(11):955–957. doi: 10.1016/S1470-2045(06)70943-3. [DOI] [PubMed] [Google Scholar]
- 42.Jeribi A, Albano L, Berguignat M, et al. Epstein-Barr virus-associated hepatic leiomyosarcoma after renal transplantation: case report. Transplant Proc. 2010;42(10):4356–4358. doi: 10.1016/j.transproceed.2010.09.122. [DOI] [PubMed] [Google Scholar]
- 43.Huang J, Loh KS, Petersson F. Epstein-Barr virus-associated smooth muscle tumor of the larynx: report of a rare case mimicking leiomyosarcoma. Head Neck Pathol. 2010;4(4):300–304. doi: 10.1007/s12105-010-0201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson PE, Wick MR, Dehner LP. Leiomyosarcoma of somatic soft tissues in childhood: an immunohistochemical analysis of six cases with ultrastructural correlation. Hum Pathol. 1991;22(6):569–577. doi: 10.1016/0046-8177(91)90234-g. [DOI] [PubMed] [Google Scholar]
- 45.Le Bail B, Morel D, Merel P, et al. Cystic smooth-muscle tumor of the liver and spleen associated with Epstein-Barr virus after renal transplantation. Am J Surg Pathol. 1996;20(11):1418–1425. doi: 10.1097/00000478-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Sharma B, Reddy M, Lee PC, Cortes G, Gumpeni R. An unusual clinical presentation of a rare renal tumour. Nephrol Dial Transplant. 2010;25(5):1713–1715. doi: 10.1093/ndt/gfp736. [DOI] [PubMed] [Google Scholar]
- 47.Yu L, Aldave AJ, Glasgow BJ. Epstein-Barr virus-associated smooth muscle tumor of the iris in a patient with transplant: a case report and review of the literature. Arch Pathol Lab Med. 2009;133(8):1238–1241. doi: 10.5858/133.8.1238. [DOI] [PubMed] [Google Scholar]
- 48.Sadahira Y, Moriya T, Shirabe T, Matsuno T, Manabe T. Epstein-Barr virus-associated post-transplant primary smooth muscle tumor of the liver: report of an autopsy case. Pathol Int. 1996;46(8):601–604. doi: 10.1111/j.1440-1827.1996.tb03660.x. [DOI] [PubMed] [Google Scholar]
- 49.Chay WY, Penafiel A, Raghuram J, et al. Dyspnea in a transplant recipient with pulmonary nodules. Chest. 2009;135(3):860–865. doi: 10.1378/chest.08-0857. [DOI] [PubMed] [Google Scholar]
- 50.Rogatsch H, Bonatti H, Menet A, et al. Epstein-Barr virus-associated multicentric leiomyosarcoma in an adult patient after heart transplantation: case report and review of the literature. Am J Surg Pathol. 2000;24:614–621. doi: 10.1097/00000478-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 51.Nur S, Rosenblum WD, Katta UD, et al. Epstein-Barr virus-associated multifocal leiomyosarcomas arising in a cardiac transplant recipient: autopsy case report and review of the literature. J Heart Lung Transplant. 2007;26(9):944–952. doi: 10.1016/j.healun.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 52.Bonatti H, Hoefer D, Rogatsch H, et al. Successful management of recurrent Epstein-Barr virus-associated multilocular leiomyosarcoma after cardiac transplantation. Transplant Proc. 2005;37(4):1839–1844. doi: 10.1016/j.transproceed.2005.03.142. [DOI] [PubMed] [Google Scholar]
- 53.Collins MH, Montone KT, Leahey AM, et al. Metachronous Epstein-Barr virus-related smooth muscle tumors in a child after heart transplantation: case report and review of the literature. J Pediatr Surg. 2001;36(9):1452–1455. doi: 10.1053/jpsu.2001.26396. [DOI] [PubMed] [Google Scholar]
- 54.Kingma DW, Shad A, Tsokos M, et al. Epstein-Barr virus (EBV)-associated smooth-muscle tumor arising in a post-transplant patient treated successfully for two PT-EBV-associated large-cell lymphomas. Case report. Am J Surg Pathol. 1996;20(12):1511–1519. doi: 10.1097/00000478-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Davidoff AM, Hebra A, Clark BJ, 3rd, Tomaszewski JE, et al. Epstein-Barr virus-associated hepatic smooth muscle neoplasm in a cardiac transplant recipient. Transplantation. 1996;61(3):515–517. doi: 10.1097/00007890-199602150-00036. [DOI] [PubMed] [Google Scholar]
- 56.Pollock AN, Newman B, Putnam PE, Dickman PS, Medina JL. Imaging of post-transplant spindle cell tumors. Pediatr Radiol. 1995;25 (Suppl 1):S118–21. [PubMed] [Google Scholar]
- 57.Somers GR, Tesoriero AA, Hartland E, et al. Multiple leiomyosarcomas of both donor and recipient origin arising in a heart-lung transplant patient. Am J Surg Pathol. 1998;22(11):1423–1428. doi: 10.1097/00000478-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Gupta S, Havens PL, Southern JF, Firat SY, Jogal SS. Epstein-Barr virus- associated intracranial leiomyosarcoma in an HIV-positive adolescent. J Pediatr Hematol Oncol. 2010;32(4):e144–147. doi: 10.1097/MPH.0b013e3181c80bf3. [DOI] [PubMed] [Google Scholar]
- 59.Berretta M, Zanet E, Taibi R, et al. Leiomyosarcoma of the parotid gland in an HIV-positive patient: therapeutic approach, clinical course and review of the literature. J Chemother. 2009;21(2):215–218. doi: 10.1179/joc.2009.21.2.215. [DOI] [PubMed] [Google Scholar]
- 60.McClain KL, Leach CT, Jenson HB, Joshi VV, et al. Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med. 1995;332(1):12–18. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- 61.Ramdial PK, Sing Y, Deonarain J, Hadley GP, Singh B. Dermal Epstein Barr Virus-Associated Leiomyosarcoma: Tocsin of Acquired Immunodeficiency Syndrome in Two Children. Am J Dermatopathol. 2011;33(4):392–396. doi: 10.1097/DAD.0b013e3181e5d16a. [DOI] [PubMed] [Google Scholar]
- 62.Sivendran S, Vidal CI, Barginear MF. Primary intracranial leiomyosarcoma in an HIV-infected patient. Int J Clin Oncol. 2011;16(1):63–66. doi: 10.1007/s10147-010-0110-5. [DOI] [PubMed] [Google Scholar]
- 63.Zevallos-Giampietri EA, Yañes HH, Orrego Puelles J, Barrionuevo C. Primary meningeal Epstein-Barr virus-related leiomyosarcoma in a man infected with human immunodeficiency virus: review of literature, emphasizing the differential diagnosis and pathogenesis. Appl Immunohistochem Mol Morphol. 2004;12(4):387–391. doi: 10.1097/00129039-200412000-00018. [DOI] [PubMed] [Google Scholar]
- 64.Barbashina V, Heller DS, Hameed M, et al. Splenic smooth-muscle tumors in children with acquired immunodeficiency syndrome: report of two cases of this unusual location with evidence of an association with Epstein-Barr virus. Virchows Arch. 2000;436(2):138–139. doi: 10.1007/pl00008213. [DOI] [PubMed] [Google Scholar]
- 65.Blumenthal DT, Raizer JJ, Rosenblum MK, et al. Primary intracranial neoplasms in patients with HIV. Neurology. 1999;52(8):1648–1651. doi: 10.1212/wnl.52.8.1648. [DOI] [PubMed] [Google Scholar]
- 66.Brown HG, Burger PC, Olivi A, et al. Intracranial leiomyosarcoma in a patient with AIDS. Neuroradiology. 1999;41(1):35–39. doi: 10.1007/s002340050701. [DOI] [PubMed] [Google Scholar]
- 67.Bejjani GK, Stopak B, Schwartz A, Santi R. Primary dural leiomyosarcoma in a patient infected with human immunodeficiency virus: case report. Neurosurgery. 1999;44(1):199–202. doi: 10.1097/00006123-199901000-00119. [DOI] [PubMed] [Google Scholar]
- 68.Norton KI, Godine LB, Lempert C. Leiomyosarcoma of the kidney in an HIV-infected child. Pediatr Radiol. 1997;27(6):557–558. doi: 10.1007/s002470050180. [DOI] [PubMed] [Google Scholar]
- 69.Morgello S, Kotsianti A, Gumprecht JP, Moore F. Epstein-Barr virus-associated dural leiomyosarcoma in a man infected with human immunodeficiency virus. Case report. J Neurosurg. 1997;86(5):883–887. doi: 10.3171/jns.1997.86.5.0883. [DOI] [PubMed] [Google Scholar]
- 70.Challapalli M. Leiomyomata and leiomyosarcomata in HIV-infected children. Diagn Cytopathol. 1993;9(3):366. doi: 10.1002/dc.2840090324. [DOI] [PubMed] [Google Scholar]
- 71.Orlow SJ, Kamino H, Lawrence RL. Multiple subcutaneous leiomyosarcomas in an adolescent with AIDS. Am J Pediatr Hematol Oncol. 1992;14(3):265–268. doi: 10.1097/00043426-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Balsam D, Segal S. Two smooth muscle tumors in the airway of an HIV- infected child. Pediatr Radiol. 1992;22(7):552–553. doi: 10.1007/BF02013014. [DOI] [PubMed] [Google Scholar]
- 73.Sabatino D, Martinez S, Young R, et al. Simultaneous pulmonary leiomyosarcoma and leiomyoma in pediatric HIV infection. Pediatr Hematol Oncol. 1991;8(4):355–359. doi: 10.3109/08880019109028809. [DOI] [PubMed] [Google Scholar]
- 74.Chadwick EG, Connor EJ, Hanson IC, et al. Tumors of smooth-muscle origin in HIV-infected children. JAMA. 1990;263(23):3182–3184. [PubMed] [Google Scholar]
- 75.Suankratay C, Shuangshoti S, Mutirangura A, et al. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis. 2005;40(10):1521–1528. doi: 10.1086/429830. [DOI] [PubMed] [Google Scholar]
- 76.Ninane J, Moulin D, Latinne D, et al. AIDS in two African children--one with fibrosarcoma of the liver. Eur J Pediatr. 1985;144(4):385–390. doi: 10.1007/BF00441784. [DOI] [PubMed] [Google Scholar]
- 77.Ritter AM, Amaker BH, Graham RS, Broaddus WC, Ward JD. Central nervous system leiomyosarcoma in patients with acquired immunodeficiency syndrome. Report of two cases. J Neurosurg. 2000;92(4):688–692. doi: 10.3171/jns.2000.92.4.0688. [DOI] [PubMed] [Google Scholar]
- 78.Kleinschmidt-DeMasters BK, Mierau GW, Sze CI, et al. Unusual dural and skull-based mesenchymal neoplasms: a report of four cases. Hum Pathol. 1998;29(3):240–245. doi: 10.1016/s0046-8177(98)90042-9. [DOI] [PubMed] [Google Scholar]
- 79.Jenson HB, Montalvo EA, McClain KL, et al. Characterization of natural Epstein-Barr virus infection and replication in smooth muscle cells from a leiomyosarcoma. J Med Virol. 1999;57(1):36–46. [PubMed] [Google Scholar]
- 80.Zetler PJ, Filipenko JD, Bilbey JH, Schmidt N. Primary adrenal leiomyosarcoma in a man with acquired immunodeficiency syndrome (AIDS). Further evidence for an increase in smooth muscle tumors related to Epstein-Barr infection in AIDS. Arch Pathol Lab Med. 1995;119(12):1164–1167. [PubMed] [Google Scholar]
- 81.Ross JS, Del Rosario A, Bui HX, Sonbati H, Solis O. Primary hepatic leiomyosarcoma in a child with the acquired immunodeficiency syndrome. Hum Pathol. 1992;23(1):69–72. doi: 10.1016/0046-8177(92)90014-t. [DOI] [PubMed] [Google Scholar]
- 82.McLoughlin LC, Nord KS, Joshi VV, DiCarlo FJ, Kane MJ. Disseminated leiomyosarcoma in a child with acquired immune deficiency syndrome. Cancer. 1991;67(10):2618–2621. doi: 10.1002/1097-0142(19910515)67:10<2618::aid-cncr2820671036>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Boman F, Gultekin H, Dickman PS. Latent Epstein-Barr virus infection demonstrated in low-grade leiomyosarcomas of adults with acquired immunodeficiency syndrome, but not in adjacent Kaposi's lesion or smooth muscle tumors in immunocompetent patients. Arch Pathol Lab Med. 1997;121(8):834–838. [PubMed] [Google Scholar]
- 84.Fizazi K, Feuillard J, Le Cesne A. Soft tissue sarcomas in HIV-infected adult patients. Eur J Cancer. 1996;32A(10):1812–1814. doi: 10.1016/0959-8049(96)00167-0. [DOI] [PubMed] [Google Scholar]
- 85.Tetzlaff MT, Nosek C, Kovarik CL. Epstein-Barr virus-associated leiomyosarcoma with cutaneous involvement in an African child with human immunodeficiency virus: a case report and review of the literature. J Cutan Pathol. 2011;38(9):731–739. doi: 10.1111/j.1600-0560.2011.01721.x. [DOI] [PubMed] [Google Scholar]
- 86.Ramdial PK, Sing Y, Deonarain J, Hadley GP, Singh B. Dermal Epstein Barr virus--associated leiomyosarcoma: tocsin of acquired immunodeficiency syndrome in two children. AM J Dermatopath. 2011;33(4):392–6. doi: 10.1097/DAD.0b013e3181e5d16a. [DOI] [PubMed] [Google Scholar]
- 87.Naresh KN, Francis N, Sarwar N, Bower M. Expression of human herpesvirus 8 (HHV-8), latent nuclear antigen 1 (LANA1) in angiosarcoma in acquired immunodeficiency syndrome (AIDS) - a report of two cases. Histopathology. 2007;51(6):861–864. doi: 10.1111/j.1365-2559.2007.02877.x. [DOI] [PubMed] [Google Scholar]
- 88.Govender PS. Atypical presentation of angiosarcoma of the scalp in the setting of human immunodeficiency virus (HIV) World J Surg Oncol. 2009;18;7:99. doi: 10.1186/1477-7819-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hyde JK, Krantz MJ, Woods JE. Intracardiac mass in a man infected with human immunodeficiency virus. Arch Pathol Lab Med. 2005;129(7):943–944. doi: 10.5858/2005-129-943-IMIAMI. [DOI] [PubMed] [Google Scholar]
- 90.Adjiman S, Zerbib M, Flam T, et al. Genitourinary tumors and HIV1 infection. Eur Urol. 1990;18(1):61–63. doi: 10.1159/000463869. [DOI] [PubMed] [Google Scholar]
- 91.Qureshi YA, Strauss DC, Thway K, Fisher C, Thomas JM. Angiosarcoma developing in a non-functioning arteriovenous fistula post-renal transplant. J Surg Oncol. 2010;101(6):520–523. doi: 10.1002/jso.21516. [DOI] [PubMed] [Google Scholar]
- 92.Farag R, Schulak JA, Abdul-Karim FW, Wasman JK. Angiosarcoma arising in an arteriovenous fistula site in a renal transplant patient: a case report and literature review. Clin Nephrol. 2005;63(5):408–412. doi: 10.5414/cnp63408. [DOI] [PubMed] [Google Scholar]
- 93.Keane MM, Carney DN. Angiosarcoma arising from a defunctionalized arteriovenous fistula. J Urol. 1993;149(2):364–365. doi: 10.1016/s0022-5347(17)36084-6. [DOI] [PubMed] [Google Scholar]
- 94.Bessis D, Sotto A, Roubert P, et al. Endothelin-secreting angiosarcoma occurring at the site of an arteriovenous fistula for haemodialysis in a renal transplant recipient. Br J Dermatol. 1998;138(2):361–363. doi: 10.1046/j.1365-2133.1998.02096.x. [DOI] [PubMed] [Google Scholar]
- 95.Byers RJ, McMahon RF, Freemont AJ, Parrott NR, Newstead CS. Angiosarcoma at the site of a ligated arteriovenous fistula in a renal transplant recipient. Nephrol Dial Transplant. 1994;9(1):112. [PubMed] [Google Scholar]
- 96.Conlon PJ, Daly T, Doyle G, Carmody M. Angiosarcoma at the site of a ligated arteriovenous fistula in a renal transplant recipient. Nephrol Dial Transplant. 1993;8(3):259–262. [PubMed] [Google Scholar]
- 97.Wehrli BM, Janzen DL, Shokeir O, et al. Epithelioid angiosarcoma arising in a surgically constructed arteriovenous fistula: a rare complication of chronic immunosuppression in the setting of renal transplantation. Am J Surg Pathol. 1998;22(9):1154–1159. doi: 10.1097/00000478-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 98.Webster P, Wujanto L, Fisher C, et al. Malignancies confined to disused arteriovenous fistulae in renal transplant patients: an important differential diagnosis. Am J Nephrol. 2011;34(1):42–48. doi: 10.1159/000328908. [DOI] [PubMed] [Google Scholar]
- 99.Murata S, Kaneko S, Kusatake K, et al. Angiosarcoma of the forearm arising in an arteriovenous fistula in a renal transplant recipient. Eur J Dermatol. 2011;21(5):792–3. doi: 10.1684/ejd.2011.1444. [DOI] [PubMed] [Google Scholar]
- 100.Ahmed I, Hamacher KL. Angiosarcoma in a chronically immunosuppressed renal transplant recipient: report of a case and review of the literature. Am J Dermatopathol. 2002;24(4):330–335. doi: 10.1097/00000372-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Kibe Y, Kishimoto S, Katoh N, et al. Angiosarcoma of the scalp associated with renal transplantation. Br J Dermatol. 1997;136(5):752–7566. [PubMed] [Google Scholar]
- 102.Dargent JL, Vermylen P, Abramowicz D, et al. Disseminated angiosarcoma presenting as a hemophagocytic syndrome in a renal allograft recipient. Transpl Int. 1997;10(1):61–64. doi: 10.1007/BF02044344. [DOI] [PubMed] [Google Scholar]
- 103.O'Connor JP, Quinn J, Wall D, et al. Cutaneous angiosarcoma following graft irradiation in a renal transplant patient. Clin Nephrol. 1986;25(1):54–55. [PubMed] [Google Scholar]
- 104.Alpers CE, Biava CG, Salvatierra O., Jr Angiosarcoma following renal transplantation. Transplant Proc. 1982;14(2):448–451. [PubMed] [Google Scholar]
- 105.Askari A, Novick A, Braun W, Steinmuller D. Late ureteral obstruction and hematuria from de novo angiosarcoma in a renal transplant patient. J Urol. 1980;124(5):717–719. doi: 10.1016/s0022-5347(17)55625-6. [DOI] [PubMed] [Google Scholar]
- 106.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22(6):683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Martelli L, Collini P, Meazza C, et al. Angiomatoid fibrous histiocytoma in an HIV-positive child. J Pediatr Hematol Oncol. 2008;30(3):242–244. doi: 10.1097/MPH.0b013e318161a9a7. [DOI] [PubMed] [Google Scholar]
- 108.Brown VL, Proby CM, Harwood CA, Cerio R. Dermatofibrosarcoma protruberans in a renal transplant recipient. Histopathology. 2003;42(2):198–200. doi: 10.1046/j.1365-2559.2003.01532_3.x. [DOI] [PubMed] [Google Scholar]
- 109.Picciotto F, Basolo B, Massara C, et al. Dermatofibrosarcoma protuberans at the site of arteriovenous fistula in a renal transplant recipient. Transplantation. 1999;68(7):1074–1075. doi: 10.1097/00007890-199910150-00034. [DOI] [PubMed] [Google Scholar]
- 110.Lai KN, Lai FM, King WW, et al. Dermatofibrosarcoma protuberans in a renal transplant patient. Aust N Z J Surg. 1995;65(12):900–902. [PubMed] [Google Scholar]
- 111.Sapadin AN, Gelfand JM, Howe KL, et al. Dermatofibrosarcoma protuberans in two patients with acquired immunodeficiency syndrome. Cutis. 2000;65(2):85–88. [PubMed] [Google Scholar]
- 112.Kanitakis J, Euvrard S, Montazeri A, et al. Atypical fibroxanthoma in a renal graft recipient. J Am Acad Dermatol. 1996:35262–264. doi: 10.1016/s0190-9622(96)90346-1. [DOI] [PubMed] [Google Scholar]
- 113.Hafner J, Kunzi W, Weinreich T. Malignant fibrous histiocytoma and atypical fibroxanthoma in renal transplant recipients. Dermatology. 1999;198(1):29–32. doi: 10.1159/000018060. [DOI] [PubMed] [Google Scholar]
- 114.So K, Macquillan GC, Adams LA, et al. Malignant fibrous histiocytoma complicating nephrogenic systemic fibrosis post liver transplantation. Intern Med J. 2009;39(9):613–617. doi: 10.1111/j.1445-5994.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- 115.Barenfanger J, Mazur JM, Mody N, Finch WT. Malignant fibrous histiocytoma of bone in a renal-transplant patient. Case report. J Bone Joint Surg Am. 1980;62(2):297–300. [PubMed] [Google Scholar]
- 116.Barroso-Vicens E, Ramirez G, Rabb H. Multiple primary malignancies in a renal transplant patient. Transplantation. 1996;61(11):1655–1656. doi: 10.1097/00007890-199606150-00020. [DOI] [PubMed] [Google Scholar]
- 117.Stein A, Hackert I, Sebastian G, Meurer M. Cutaneous malignant fibrous histiocytoma of the scalp in a renal transplant recipient. Br J Dermatol. 2006;154(1):183–185. doi: 10.1111/j.1365-2133.2005.06990.x. [DOI] [PubMed] [Google Scholar]
- 118.Shih WJ, Mitchell B, Milan P, Huang WS. 2-Deoxy-2-[F-18]fluoro-D-glucose positron emission tomography illustrates two visceral tumors in a post kidney transplant patient with multiple cutaneous malignancies. Mol Imaging Biol. 2007;9(1):1–5. doi: 10.1007/s11307-006-0070-3. [DOI] [PubMed] [Google Scholar]
- 119.Alhadab T, Alvarez F, Phillips NJ, Hauptman PJ. Malignant fibrous histiocytoma of the lung presenting as bronchial obstruction in a heart transplant recipient. J Heart Lung Transplant. 2002;21(10):1140–1143. doi: 10.1016/s1053-2498(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 120.Blatière V, De Boever CM, Jacot W, Durand L, Guillot B. Cutaneous malignant fibrous histiocytoma in an HIV-positive patient. J Eur Acad Dermatol Venereol. 2007;21(1):106–107. doi: 10.1111/j.1468-3083.2006.01796.x. [DOI] [PubMed] [Google Scholar]
- 121.Khairy-Shamel ST, Shatriah I, Adil H, et al. Orbital rhabdomyosarcoma in an HIV positive child. Orbit. 2008;27(5):388–390. doi: 10.1080/01676830802336629. [DOI] [PubMed] [Google Scholar]
- 122.Lauretti L, Montano N, Paternoster G, et al. Huge cranio-cerebral rhabdomyosarcoma in HIV-positive patient. J Neurooncol. 2010;100(1):153–155. doi: 10.1007/s11060-010-0149-1. [DOI] [PubMed] [Google Scholar]
- 123.Cheeseman Sarah H, Gang David L. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 9-1986. A 40-month-old girl with the acquired immunodeficiency syndrome and spinal-cord compression. N Engl J Med. 1986;314(10):629–640. doi: 10.1056/NEJM198603063141008. [DOI] [PubMed] [Google Scholar]
- 124.Anyiam DC, Ukah CO, Onyiaorah IV, Okafor N. Sarcoma botyroides of the cervix in a HIV positive 45-year-old woman: a case report. Niger J Clin Pract. 2010;13(3):341–343. [PubMed] [Google Scholar]
- 125.Cescon M, Grazi GL, Assietti R, et al. Embryonal rhabdomyosarcoma of the orbit in a liver transplant recipient. Transpl Int. 2003;16(6):437–440. doi: 10.1007/s00147-003-0571-9. [DOI] [PubMed] [Google Scholar]
- 126.Rheeder P, Simson IW, Mentis H, Karussett VO. Cardiac rhabdomyosarcoma in a renal transplant patient. Transplantation. 1995;60(2):204–205. [PubMed] [Google Scholar]
- 127.Lyall EG, Langdale-Brown B, Eden OB, Mok JY, Croft NM. Ewing's sarcoma in a child with human immunodeficiency virus (type 1) infection. Med Pediatr Oncol. 1993;21(2):127–131. doi: 10.1002/mpo.2950210209. [DOI] [PubMed] [Google Scholar]
- 128.Balakrishnan R, Khairullah QT, Giraldo A, Provenzano R. Extraskeletal Ewing's sarcoma in a kidney transplant patient. Am J Kidney Dis. 1999;33(6):1164–1167. doi: 10.1016/S0272-6386(99)70157-5. [DOI] [PubMed] [Google Scholar]
- 129.Gupta N, Gupta P, Sharma U, Vashishta RK, Rajwanshi A. Orbital sarcoma in HIV positive patient: a diagnostic dilemma. Diagn Cytopathol. 2010;38(1):56–58. doi: 10.1002/dc.21138. [DOI] [PubMed] [Google Scholar]
- 130.Merlo CA, Studer SM, Conte JV, et al. The course of neurofibromatosis type 1 on immunosuppression after lung transplantation: report of 2 cases. J Heart Lung Transplant. 2004;23(6):774–776. doi: 10.1016/s1053-2498(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 131.Pantanowitz L, Sen S, Crisi GM, et al. Spectrum of breast disease encountered in HIV-positive patients at a community teaching hospital. Breast. 2011;20(4):303–8. doi: 10.1016/j.breast.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 132.Grieger TA, Carl M, Liebert HP, Cotelingam JD, Wagner KF. Mediastinal liposarcoma in a patient infected with the human immunodeficiency virus. Am J Med. 1988;84(2):366. doi: 10.1016/0002-9343(88)90443-3. [DOI] [PubMed] [Google Scholar]
- 133.Manzia TM, Gravante G, Toti L, et al. Management of spermatic cord liposarcoma in renal transplant recipients: case report. Transplant Proc. 2010;42(4):1355–1357. doi: 10.1016/j.transproceed.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 134.Zavos G, Papadoukakis S, Kiriakou V, Kostakis A. Paratesticular liposarcoma in a transplanted patient. Urol Int. 2001;67(3):254–256. doi: 10.1159/000051000. [DOI] [PubMed] [Google Scholar]
- 135.Roca B, Resino E, Roca M, Vera JM. A dendritic cell tumor in an HIV-infected patient: case report. Ann Oncol. 2009;20(11):1895–1896. doi: 10.1093/annonc/mdp409. [DOI] [PubMed] [Google Scholar]
- 136.Kure K, Khader SN, Suhrland MJ. Fine needle aspiration of follicular dendritic cell sarcoma in an HIV-positive man: a case report. Acta Cytol. 2010;54(5):707–711. doi: 10.1159/000325237. [DOI] [PubMed] [Google Scholar]
- 137.Wu Q, Liu C, Lei L, et al. Interdigitating dendritic cell sarcoma involving bone marrow in a liver transplant recipient. Transplant Proc. 2010;42(5):1963–1966. doi: 10.1016/j.transproceed.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 138.Khammissa RA, Mabusela M, Wood NH, et al. Osteosarcoma of the jaw. A brief review and a case report. SADJ. 2009;64(5):220–221. [PubMed] [Google Scholar]
- 139.Danhaive O, Ninane J, Sokal E, et al. Hepatic localization of a fibrosarcoma in a child with a liver transplant. J Pediatr. 1992;120(3):434–437. doi: 10.1016/s0022-3476(05)80915-1. [DOI] [PubMed] [Google Scholar]
- 140.Flooks R, Vanacker A, Van Dorpe J, et al. Acral myxoinflammatory fibroblastic sarcoma in a renal transplant patient: a case report. Transplant Proc. 2009;41(8):3437–3497. doi: 10.1016/j.transproceed.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 141.Soper CP, Andrews PA, Bending MR, Singh L, Fisher C. Cervical myxofibrosarcoma in a renal allograft recipient treated with murine anti-CD3 monoclonal antibody therapy. Nephrol Dial Transplant. 1998;13(7):1902–1903. doi: 10.1093/ndt/13.7.1903. [DOI] [PubMed] [Google Scholar]
- 142.Buzogány I, Bagheri F, Süle N, et al. Association between carcinosarcoma and the transplanted kidney. Anticancer Res. 2006;26(1B):751–753. [PubMed] [Google Scholar]
- 143.Camargo MA, Boin I, Mainnardi JP, et al. Extragastrointestinal stromal tumor and liver transplantation: case report and review. Transplant Proc. 2008;40(10):3781–3783. doi: 10.1016/j.transproceed.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 144.Agaimy A, Wünsch PH. Gastrointestinal stromal tumours (GIST) in kidney transplant recipients--a report of two cases. Nephrol Dial Transplant. 2007;22(5):1489–90. doi: 10.1093/ndt/gfl807. [DOI] [PubMed] [Google Scholar]
- 145.Saidi RF, Sepehr A, Cosimi AB. Hertl MGastrointestinal stromal tumor in a liver transplant recipient. Transplantation. 2008;85(9):1363. doi: 10.1097/TP.0b013e31816c7e2f. [DOI] [PubMed] [Google Scholar]
- 146.Padula A, Chin NW, Azeez S, et al. Primary gastrointestinal stromal tumor of the esophagus in an HIV-positive patient. Ann Diagn Pathol. 2005;9(1):49–53. doi: 10.1053/j.anndiagpath.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 147.Kubben FJ, Kroon FP, Hogendoorn PC, et al. Absence of Epstein-Barr virus (EBV) in a gastrointestinal stromal cell tumour (GIST) in an adult human immunodeficiency virus-seropositive patient with past EBV infection. Eur J Gastroenterol Hepatol. 1997;9(7):721–724. doi: 10.1097/00042737-199707000-00014. [DOI] [PubMed] [Google Scholar]
- 148.Smith MD, Jr, Nio M, Camel JE, Sato JK, Atkinson JB. Management of splenic abscess in immunocompromised children. J Pediatr Surg. 1993;28(6):823–826. doi: 10.1016/0022-3468(93)90336-j. [DOI] [PubMed] [Google Scholar]
- 149.Garlicki M, Wierzbicki K, Przybyłowski P, et al. The incidence of malignancy in heart transplant recipients. Ann Transplant. 1998;3(4):41–47. [PubMed] [Google Scholar]
- 150.Colovic N, Jurisic V, Terzić T, Jevtovic D, Colović M. Alveolar granulocytic sarcoma of the mandible in a patient with HIV. Onkologie. 2011;34(1–2):55–58. doi: 10.1159/000317351. [DOI] [PubMed] [Google Scholar]
- 151.Rizzo M, Magro G, Castaldo P, Tucci L. Granulocytic Sarcoma(Chloroma) in a patient with HIV: a report. Forensic Sci Int. 2004;146(Suppl):S57–8. doi: 10.1016/j.forsciint.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 152.Shah UB Joshi S, Ghorpade SV, Gaikwad SN, Sundrani RM. Primary pleuro-pulmonary Synovial Carcinoma. Indian J Chest Dis Allied Sci. 2010;52(3):169–172. [PubMed] [Google Scholar]
- 153.Calderaro J, Polivka M, Gallien S, et al. Multifocal Epstein Barr virus (EBV)-associated myopericytoma in a patient with AIDS. Neuropathol Appl Neurobiol. 2008;34:115–117. doi: 10.1111/j.1365-2990.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 154.Lau PP, Wong OK, Lui PC, et al. Myopericytoma in patients with AIDS: a new class of Epstein-Barr virus-associated tumor. Am J Surg Pathol. 2009;33(11):1666–1672. doi: 10.1097/PAS.0b013e3181aec307. [DOI] [PubMed] [Google Scholar]
- 155.Metta H, Corti M, Redini L, et al. Endobronchial leiomyoma: an unusual non-defining neoplasm in a patient with AIDS. Rev Inst Med Trop Sao Paulo. 2009;51(1):53–55. doi: 10.1590/s0036-46652009000100010. [DOI] [PubMed] [Google Scholar]
- 156.Bluhm JM, Yi ES, Diaz G, Colby TV, Colt HG. Multicentric endobronchial smooth muscle tumors associated with the Epstein-Barr virus in an adult patient with the acquired immunodeficiency syndrome: a case report. Cancer. 1997;80(10):1910–1903. [PubMed] [Google Scholar]
- 157.Kaminski AM, Cameron DC, French MA. Leiomyoma of the oesophagus in an HIV-infected patient. Australas Radiol. 2001;45(1):49–51. doi: 10.1046/j.1440-1673.2001.00874.x. [DOI] [PubMed] [Google Scholar]
- 158.Kanitakis J, Carbonnel E, Chouvet B, Labeille B, Claudy A. Cutaneous leiomyomas (piloleiomyomas) in adult patients with human immunodeficiency virus infection. Br J Dermatol. 2000;143(6):1338–1340. doi: 10.1046/j.1365-2133.2000.03925.x. [DOI] [PubMed] [Google Scholar]
- 159.Karpinski NC, Yaghmai R, Barba D, Hansen LA. Case of the month: March 1999--A 26 year old HIV positive male with dura based masses. Brain Pathol. 1999;9(3):609–611. doi: 10.1111/j.1750-3639.1999.tb00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.George T, Grant J, DeLeon G, Darling CF. A 10-year-old boy, HIV positive, develops progressive weakness. Pediatr Neurosurg. 1999;30(4):211–217. doi: 10.1159/000028798. [DOI] [PubMed] [Google Scholar]
- 161.Tomà P, Loy A, Pastorino C, Derchi LE. Leiomyomas of the gallbladder and splenic calcifications in an HIV-infected child. Pediatr Radiol. 1997;27(1):92–94. doi: 10.1007/s002470050074. [DOI] [PubMed] [Google Scholar]
- 162.Yang SS, Williams RJ, Bear BJ, McCormack RR. Leiomyoma of the hand in a child who has the human immunodeficiency virus. J Bone Joint Surg Am. 1996;78(12):1904–1906. doi: 10.2106/00004623-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 163.Prévot S, Néris J, de Saint Maur PP. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch. 1994;425(3):321–325. doi: 10.1007/BF00196156. [DOI] [PubMed] [Google Scholar]
- 164.Bargiela A, Rey JL, Díaz JL, Martínez A. Meningeal leiomyoma in an adult with AIDS: CT and MRI with pathological correlation. Neuroradiology. 1999;41(9):696–698. doi: 10.1007/s002340050826. [DOI] [PubMed] [Google Scholar]
- 165.Choi S, Levy ML, Krieger MD, McComb JG. Spinal extradural leiomyoma in a pediatric patient with acquired immunodeficiency syndrome: case report. Neurosurgery. 1997;40(5):1080–1082. doi: 10.1097/00006123-199705000-00038. [DOI] [PubMed] [Google Scholar]
- 166.van Hoeven KH, Factor SM, Kress Y, Woodruff JM. Visceral myogenic tumors. A manifestation of HIV infection in children. Am J Surg Pathol. 1993;17(11):1176–1181. [PubMed] [Google Scholar]
- 167.Steel TR, Pell MF, Turner JJ, Lim GH. Spinal epidural leiomyoma occurring in an HIV-infected man. Case report. J Neurosurg. 1993;79(3):442–445. doi: 10.3171/jns.1993.79.3.0442. [DOI] [PubMed] [Google Scholar]
- 168.Jimenez-Heffernan JA, Hardisson D, Palacios J, et al. Adrenal gland leiomyoma in a child with acquired immunodeficiency syndrome. Pediatr Pathol Lab Med. 1995;15(6):923–929. doi: 10.3109/15513819509027028. [DOI] [PubMed] [Google Scholar]
- 169.Dahan H, Beges C, Weiss L, et al. Leiomyoma of the adrenal gland in a patient with AIDS. Abdom Imaging. 1994;19:259–261. doi: 10.1007/BF00203522. [DOI] [PubMed] [Google Scholar]
- 170.Radin DR, Kiyabu M. Multiple smooth muscle tumors of the colon and the adrenal gland in an adult with AIDS. AJNR. 1992;159:545–546. doi: 10.2214/ajr.159.3.1503021. [DOI] [PubMed] [Google Scholar]
- 171.Parola P, Petit N, Azzedine A, Dhiver C, Gastaut JA. Symptomatic leiomyoma of the adrenal gland in a woman with AIDS. AIDS. 1996;10:340–341. doi: 10.1097/00002030-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 172.Gibbs KE, White A, Kaleya R. Laparoscopic management of an adrenal leiomyoma in an AIDS patient. A case report and review of the literature. JSLS. 2005;9(3):345–348. [PMC free article] [PubMed] [Google Scholar]
- 173.de Chadarévian JP, Wolk JH, Inniss S, et al. A newly recognized cause of wheezing: AIDS-related bronchial leiomyomas. Pediatr Pulmonol. 1997;24(2):106–110. doi: 10.1002/(sici)1099-0496(199708)24:2<106::aid-ppul5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 174.Wachsberg RH, Cho KC, Adekosan A. Two leiomyomas of the liver in an adult with AIDS: CT and MR appearance. J Comput Assist Tomogr. 1994;18(1):156–157. doi: 10.1097/00004728-199401000-00036. [DOI] [PubMed] [Google Scholar]
- 175.Levin TL, Adam HM, van Hoeven KH, Goldman HS. Hepatic spindle cell tumors in HIV positive children. Pediatr Radiol. 1994;24(1):78–79. doi: 10.1007/BF02017675. [DOI] [PubMed] [Google Scholar]
- 176.Creager AJ, Maia DM, Funkhouser WK. Epstein-Barr virus associated renal smooth muscle neoplasm: report of a case with review of the literature. Arch Pathol Lab Med. 1998;122:277–281. [PubMed] [Google Scholar]
- 177.Wang MH, Wu CT, Hung CC, Liang JD, Chen PJ. Hepatic leiomyomatous neoplasm associated with Epstein Barr virus infection in an adult with acquired immunodeficiency syndrome. J Formos Med Assoc. 2000;99(11):873–875. [PubMed] [Google Scholar]
- 178.Chang JY, Wang S, Hung CC, Tsai TF, Hsiao CH. Multiple Epstein-Barr virus-associated subcutaneous angioleiomyomas in a patient with acquired immunodeficiency syndrome. Br J Dermatol. 2002;147(3):563–567. doi: 10.1046/j.1365-2133.2002.04818.x. [DOI] [PubMed] [Google Scholar]
- 179.Citow JS, Kranzler L. Multicentric intracranial smooth-muscle tumor in a woman with human immunodeficiency virus. Case report. J Neurosurg. 2000;93(4):701–703. doi: 10.3171/jns.2000.93.4.0701. [DOI] [PubMed] [Google Scholar]
- 180.Felix F, Gomes GA, Tomita S, et al. Painful tongue leiomyoma. Braz J Otorhinolaryngol. 2006;72(5):715. doi: 10.1016/S1808-8694(15)31032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sambol E, Patterson D, Rivera R, et al. An appendiceal leiomyoma in a child with acquired immunodeficiency syndrome. Pediatr Surg Int. 2006;22(10):865–868. doi: 10.1007/s00383-006-1773-x. [DOI] [PubMed] [Google Scholar]
- 182.Molle ZL, Moallem H, Desai N, Anderson V, Rabinowitz SS. Endoscopic features of smooth muscle tumors in children with AIDS. Gastrointest Endosc. 2000;52(1):91–94. doi: 10.1067/mge.2000.105984. [DOI] [PubMed] [Google Scholar]
- 183.Abate M, Wadhwa NK, Nord EP. Uterine leiomyoma causing urinary obstruction of the transplanted kidney. Clin Nephrol. 2010;73(4):314. doi: 10.5414/cnp73314. [DOI] [PubMed] [Google Scholar]
- 184.Zevgaridis D, Tsonidis C, Kapranos N, et al. Epstein-Barr virus associated primary intracranial leiomyoma in organ transplant recipient: case report and review of the literature. Acta Neurochir (Wien) 2009;151(12):1705–1709. doi: 10.1007/s00701-009-0307-4. [DOI] [PubMed] [Google Scholar]
- 185.Dionne JM, Carter JE, Matsell D, et al. Renal leiomyoma associated with Epstein-Barr virus in a pediatric transplant patient. Am J Kidney Dis. 2005;46(2):351–355. doi: 10.1053/j.ajkd.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 186.Sclabas GM, Maurer CA, Wente MN, Zimmermann A, Büchler MW. Case report: hepatic leiomyoma in a renal transplant recipient. Transplant Proc. 2002;34(8):3200–3202. doi: 10.1016/s0041-1345(02)03563-7. [DOI] [PubMed] [Google Scholar]
- 187.Huang J, Loh KS, Petersson F. Epstein-barr virus-associated smooth muscle tumor of the larynx: report of a rare case mimicking leiomyosarcoma. Head Neck Pathol. 2010;4(4):300–304. doi: 10.1007/s12105-010-0201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Al Hussain T, Haleem A, Alsaad KO. Synchronous hepatic, mesenteric and pulmonary Epstein-Barr virus-associated smooth muscle tumors in a renal transplant recipient. Clin Transplant. 2010;24(5):579–584. doi: 10.1111/j.1399-0012.2009.01206.x. [DOI] [PubMed] [Google Scholar]
- 189.Hara T, Tsuchida M, Takai K, Takiguchi S, Naito K. Ureteral obstruction in a transplanted kidney secondary to a subserous myoma uteri. Transplantation. 2003;75(11):1915–1916. doi: 10.1097/01.TP.0000064708.76672.E8. [DOI] [PubMed] [Google Scholar]
- 190.Liu Y, Park T, Chun KJ, Freeman LM. Uterine myoma identified on a Tc-99m MAG3 scan of a renal transplant. Clin Nucl Med. 2002;27(11):801–802. doi: 10.1097/00003072-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 191.Salamanca J, Massa DS. EBV-associated hepatic smooth muscle tumor after lung transplantation: report of a case and review of the literature. J Heart Lung Transplant. 2009;28(11):1217–1220. doi: 10.1016/j.healun.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 192.Anguita J, Rico ML, Palomo J, Muñoz P, Preciado V, Menárguez J. Myocardial Epstein-Barr virus-associated cardiac smooth-muscle neoplasm arising in a cardiac transplant recipient. Transplantation. 1998;66(3):400–401. doi: 10.1097/00007890-199808150-00020. [DOI] [PubMed] [Google Scholar]
- 193.Fernandez MP, Krejci-Manwaring J, Davis TL. Epstein-Barr virus is not associated with angioleiomyomas, or other cutaneous smooth muscle tumors in immunocompetent individuals. J Cutan Pathol. 2010;37(4):507–510. doi: 10.1111/j.1600-0560.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 194.Hatano M, Takada H, Nomura A, et al. Epstein-Barr virus- associated bronchial leiomyoma in a boy with cellular immunodeficiency. Pediatr Pulmonol. 2006;41(4):371–373. doi: 10.1002/ppul.20375. [DOI] [PubMed] [Google Scholar]
- 195.Rickinson AB, Kieff E. Epstein Barr Virus and its replication. In: Knipe DMH, Howley PM, editors. Fields Virology. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 2511–2574. [Google Scholar]
- 196.Hudnall SD, Ge Y, Wei L, et al. Distribution and phenotype of Epstein-Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol. 2005;18(4):519–527. doi: 10.1038/modpathol.3800369. [DOI] [PubMed] [Google Scholar]
- 197.McDonagh DP, Liu J, Gaffey MJ, et al. Detection of Kaposi's sarcoma-associated herpesvirus-like DNA sequence in angiosarcoma. Am J Pathol. 1996;149(4):1363–1368. [PMC free article] [PubMed] [Google Scholar]
- 198.Schmid H, Zietz C. Human herpesvirus 8 and angiosarcoma: analysis of 40 cases and review of the literature. Pathology. 2005;37(4):284–287. doi: 10.1080/00313020500169495. [DOI] [PubMed] [Google Scholar]
- 199.Remick SC, Patnaik M, Ziran NM, et al. Human herpesvirus-8-associated disseminated angiosarcoma in an HIV-seronegative woman: report of a case and limited case-control virologic study in vascular tumors. Am J Med. 2000;108(8):660–664. doi: 10.1016/s0002-9343(00)00365-x. [DOI] [PubMed] [Google Scholar]
- 200.Gessi M, Cattani P, Maggiano N, et al. Demonstration of human herpesvirus 8 in a case of primary vaginal epithelioid angiosarcoma by in situ hybridization, electron microscopy, and polymerase chain reaction. Diagn Mol Pathol. 2002;11(3):146–151. doi: 10.1097/00019606-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 201*.Chanyaputhipong J, Hock DL, Sebastian MG. Disseminated angiosarcoma of the dialysis fistula in 2 patients without kidney transplants. Am J Kidney Dis. 2011;57(6):917–920. doi: 10.1053/j.ajkd.2010.12.024. Rare case report of an angiosarcoma at the site of arteriovenous fistula prior to transplant. [DOI] [PubMed] [Google Scholar]
- 202.Jennings TA, Peterson L, Axiotis CA, et al. Angiosarcoma associated with foreign body material. A report of three cases. Cancer. 1988;62(11):2436–2444. doi: 10.1002/1097-0142(19881201)62:11<2436::aid-cncr2820621132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 203.Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562–571. doi: 10.1002/1097-0142(197208)30:2<562::aid-cncr2820300237>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 204.Cafiero, Gipponi M, Peressini A, et al. Radiation associated angiosarcoma: diagnostic and therapeutic implications—two case reports and a review of the literature. Cancer. 1996;77:2496–2502. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2496::AID-CNCR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]


