Abstract
Lone atrial fibrillation (AF) is associated with various ion channel gene sequence variants, notably the common S38G loss-of-function polymorphism in the KCNE1 K+ channel ancillary subunit gene. New-onset postoperative AF (POAF) generally occurs 48–72 hours after major surgery, particularly following procedures within the chest, but its molecular bases remain poorly understood. To begin to address this gap in knowledge, we analyzed molecular changes in the left atrium (LA) in relation to simultaneous changes in hemodynamics, LA effective refractory period (ERP), and the capacity to sustain electrically-induced AF following left upper lung lobectomy in swine. Relative to control pigs (no previous surgery), 3 days after lobectomy higher values for mean pulmonary artery pressure (16 ± 1 vs 22 ± 2 mm Hg; P = 0.045) and pulmonary vascular resistance (273 ± 47 vs 481 ± 63 dyn·s/cm5; P = 0.025) were evident, whereas other hemodynamic variables were unchanged. LA ERP trended toward reduction in lobectomy animals (187 ± 16 vs 170 ± 20 ms, P > 0.05). None of the lobectomy pigs developed spontaneous POAF as assessed by telemetric ECG. However, all lobectomy pigs, but none of the controls, were able to sustain AF induced by a 10 s burst of rapid pacing for ≥30 s (P=0.0079), independent of LA ERP; AF was sustained ≥60 s in 3/5 postoperative pigs versus 0/5 controls and correlated with a shorter ERP overall (P=0.023). Transcriptomic analysis of LA tissue revealed 23 up-regulated and 10 down-regulated transcripts (≥1.5-fold, P<0.05) in lobectomy pigs. Strikingly, of the latter, KCNE1 down-regulation showed the statistically strongest link to surgery (2.0-fold, P=0.009), recapitulated at the protein level with western blotting (P=0.039), suggesting KCNE1 down-regulation as a possible common mechanistic factor in POAF and lone AF. Of the up-regulated transcripts, while Teneurin-2 was the strongest linked (1.5-fold change, P=0.001), DSCR5 showed the highest induction (2.7-fold, P=0.02); this and other hits will be targeted in future functional studies.
Keywords: arrhythmia, IKs, KCNQ1, MinK, postoperative atrial fibrillation
1. Introduction
Atrial fibrillation (AF) is a cardiac arrhythmia associated with rapid, irregular atrial activation. AF represents an enormous burden in terms of healthcare costs, with an estimated 2.3 million sufferers in the United States alone. While most cases of AF are linked to underlying structural defects in the heart, lone or idiopathic AF, i.e., that in the absence of structural heart disease, has been associated with variants in genes encoding ion channel subunits. For example, a rare variant in the gene encoding the KCNE1 ancillary (β) subunit, which together with the KCNQ1 pore-forming (α) subunit forms the cardiac IKs potassium channel complex, was recently found to associate with AF [1], while individuals with two G alleles at the KCNE1 S38G polymorphic site (38GG) were found in one study to have a >10-fold increased risk of lone AF compared to other genotypes [2].
Another common form of AF, new-onset postoperative AF (POAF), increases postoperative stays and in-hospital patient mortality [3]. Not surprisingly, the highest incidence, up to 65%, has been reported following cardiac surgery where the pericardium was opened and the atria directly manipulated. However, even following non-cardiac surgery in the chest such as lung resection, a POAF incidence of >30% has been reported in some cohorts, with most episodes typically occurring within the first 2–3 postoperative days [4]. As inflammation and activation of the sympathetic nervous system are considered particularly important factors in the etiology of POAF, it is thought to be mechanistically somewhat distinct from other forms of AF, but the molecular basis for POAF is still incompletely understood. To begin to address this, we developed a large animal lung resection model of susceptibility to POAF. Here we report findings from a global whole-transcript analysis of postoperative gene remodeling in left atrial (LA) tissue obtained 3 days after removal of the left upper lung lobe in swine. Of the 25,388 genes probed, 10 genes were down-regulated ≥1.5-fold in the postoperative pigs compared to controls. Strikingly, KCNE1 exhibited the strongest statistical link to postoperative remodeling.
2. Methods
Young adult Sinclair swine were divided into ‘control’ (n = 4) and ‘postop’ (n = 5) groups, the latter undergoing thoracotomy, removal of the left upper lung lobe, and implant of a transmitter for telemetric ECG recording prior to study. As the first procedure in controls and on the third postoperative day for the postop group, animals were anesthetized and instrumented for measurement of baseline systemic and pulmonary hemodynamic variables. After ganglionic blockade to isolate the myocardium from autonomic nervous input and its potential effects on ERP and AF induction, as we previously described [5], left atrial (LA) effective refractory period (ERP) was measured from a 300 ms cycle length. Atrial fibrillation was the then induced by rapid burst pacing (10s) and the duration of sustained AF after cessation of pacing determined. Animals were then euthanized, tissue harvested, and total RNA and total protein isolated from tissue homogenates for whole-transcript genomics and western blotting studies, respectively. Global whole-transcript analysis was performed using a GeneAtlas microarray system (Affymetrix) and Porcine Gene 1.1 ST Array Strips (Affymetrix), each array comprising 572,667 probes to probe 25,388 genes (median probe set per transcript of 25, hence ‘whole-transcript’ analysis). Gene expression changes between control and surgery groups were analyzed in an unbiased fashion, using a ≥1.5-fold change, P<0.05 significance (students t-test), and mean transcript signal intensity in either group of ≥5.0 as cutoffs. Detailed Methods are available in the online Supplementary Methods section.
3. Results
In postop pigs, mean pulmonary artery pressure was increased relative to control (16 ± 1 vs 22 ± 2 mm Hg;, P = 0.045) as was pulmonary vascular resistance index (273 ± 47 vs 481 ± 63 dyn·s/cm5; P = 0.025). There were no significant differences in other measured hemodynamic variables (Supplementary Table 1). LA ERP trended toward reduction in postoperative animals (from 187 ± 16 to 170 ± 20 ms, P>0.05).
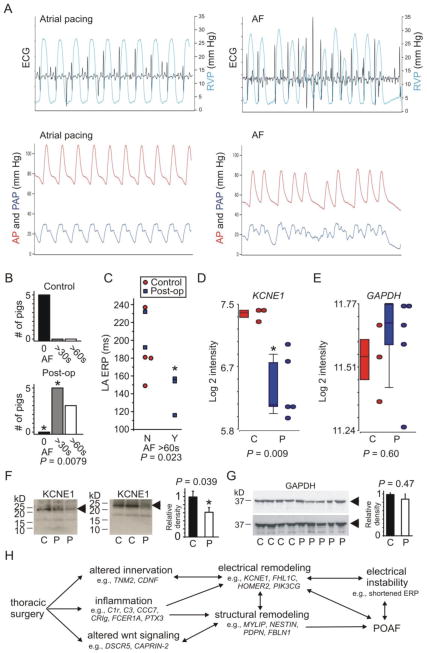
Rapid burst pacing was sufficient to induce AF with characteristic hemodynamic effects (Figure 1A). All the postop pigs, but none of the controls, sustained AF for ≥30 s following rapid burst pacing (P = 0.0079), independent of LA ERP (Figure 1B). AF ≥60 s occurred in 3/5 postoperative pigs versus 0/5 controls and correlated with a shorter LA ERP when values from control and postoperative pigs were pooled (P = 0.023) (Figure 1C).
Figure 1. Atrial KCNE1 downregulation in porcine postoperative atrial fibrillation.
A. Representative tracings of ECG, right ventricular pressure (RVP), systemic arterial pressure (AP), and pulmonary arterial pressure (PAP) during atrial pacing at a rate of 80/min and following induction of AF
B. Incidence of sustained AF in Control and 3-days postoperative pigs. *P=0.0079 versus Controls
C. Left atrial effective refractory period duration versus incidence of AF sustained >60 s after 10 s rapid pacing (Y, yes versus N, no) plotted for control and 3-days postoperative pigs as indicated
D. Mean KCNE1 transcript intensity in LA tissue of Control (C) and postoperative (P) pigs; n = 3–5
E. Mean GAPDH transcript intensity in LA tissue of Control (C) and postoperative (P) pigs; n = 3–5
F. Left, exemplar western blots using anti-KCNE1 antibody to probe lysates from LA tissue of Control (C) and postoperative (P) pigs; right, band densitometry for blots as in left; n = 4–5, each performed in triplicate
G. Left, exemplar western blots using anti-GAPDH antibody to probe lysates from LA tissue of Control (C) and postoperative (P) pigs; right, band densitometry for blots as in left; n = 4–5, each performed in triplicate
H. Hypothetical framework of POAF etiology drawing from the findings herein and previous functional analyses of the genes listed.
Whole-transcript microarray analysis of LA tissue revealed 33 genes with expression changes ≥1.5-fold in postoperative pigs (Table 1). Of the 10 transcripts down-regulated ≥1.5-fold, KCNE1 showed the statistically strongest link to postoperative changes (2.01-fold reduced, P = 0.009) (Figure 1D, E), followed by NPPB (Natriuretic peptide B precursor) and PTX3 (Pentraxin 3), which showed more variability but the strongest down-regulation (4.08-fold, P = 0.03). KCNE1 transcript down-regulation was reflected at the protein level, with a 40% reduction in KCNE1 protein band density in LA tissue from 3-day postoperative pigs compared to controls (n = 4–5, P = 0.039) (Figure 1F), while GAPDH protein expression was unchanged (n = 4–5, P = 0.47) (Figure 1G), recapitulating the microarray data (P = 0.88) (Figure 1E). Left atrial transcripts for 23 genes were upregulated ≥1.5-fold in postoperative pigs, the strongest-correlated being TNM2 (Teneurin 2) with a signal-to-noise ratio (F) of 35.3; and the most up-regulated (2.65-fold) being DSCR5 (Down syndrome critical region 5) (Table 1).
Table 1. Transcripts with ≥1.5-fold altered expression in left atrial tissue of 3-day postoperative pigs versus controls.
Transcripts are separated into down-regulated (upper) and upregulated (lower), and sorted by F (signal-to-noise ratio). Pre-filtered to remove low-intensity signals (<5.0 in both Control and Postop groups). KHDRBS3, KH domain containing, RNA binding, signal transduction associated 3; CAPRIN 2, Cytoplasmic activation/proliferation-associated protein 2; SH3BGRL, SH3 domain binding glutamic acid-rich protein like.
| DOWNREGULATED POST-OP
| ||||
|---|---|---|---|---|
| Gene name | Fold-change | P value | F | Protein name |
| KCNE1 | −2.01 | 0.009 | 14.4 | KCNE1 (MinK) |
| NPPB | −2.15 | 0.01 | 13.8 | Natriuretic peptide B precursor |
| PTX3 | −4.08 | 0.03 | 8.05 | Pentraxin 3 |
| NPR3 | −1.51 | 0.03 | 7.93 | Natriuretic peptide receptor C |
| AOPEP | −1.69 | 0.03 | 7.78 | Aminopeptidase O isoform 1 |
| ADCY5 | −1.55 | 0.041 | 6.68 | Adenylyl cyclase type V |
| FHL1C | −1.51 | 0.045 | 6.34 | FHL1 isoform C |
| NESTIN | −1.55 | 0.045 | 6.34 | Nestin |
| PDPN | −1.89 | 0.046 | 6.32 | Podoplanin |
| HOMER2 | −1.59 | 0.049 | 6.07 | Homer 2 |
|
| ||||
| UPREGULATED POST-OP
| ||||
| Gene name | Fold-change | P value | F | Protein name |
|
| ||||
| TNM2 | 1.52 | 0.001 | 35.3 | Teneurin-2 |
| PDGFRL | 1.61 | 0.005 | 19.1 | Platelet-derived growth factor receptor-like |
| CCC7 | 2.02 | 0.006 | 16.7 | Complement Component C7 |
| MYLIP | 1.66 | 0.01 | 13.9 | Myosin regulatory light chain interacting protein |
| CD34 | 1.71 | 0.02 | 11.1 | Hematopoietic progenitor cell antigen CD34 |
| FBLN1 | 1.76 | 0.02 | 11.0 | Fibulin 1 |
| FCER1A | 1.56 | 0.02 | 10.7 | IgE Fc receptor subunit gamma |
| DSCR5 | 2.65 | 0.02 | 10.3 | Down Syndrome Critical Region 5 |
| ALOX5 | 1.54 | 0.02 | 9.8 | Arachidonate 5-lipoxygenase |
| CRIg | 2.03 | 0.02 | 9.8 | Complement receptor of the Ig superfamily |
| TBC1D5 | 1.56 | 0.02 | 9.7 | TBC1 domain family, member 5 |
| ATRNL1 | 1.68 | 0.02 | 9.1 | Attractin-like protein 1 |
| ASAH1 | 1.57 | 0.03 | 8.8 | Acid ceramidase |
| PIK3CG | 1.59 | 0.03 | 8.7 | PIP3-kinase catalytic subunit g |
| KHDRBS3 | 1.51 | 0.03 | 7.6 | KHDRBS3 |
| C1R | 1.91 | 0.03 | 7. 4 | Complement C1r |
| MS4A6D | 2.05 | 0.04 | 7. 2 | 4-span TM protein 3 |
| C3 | 1.58 | 0.04 | 6.9 | Complement C3a anaphylatoxin |
| CAPRIN-2 | 1.52 | 0.04 | 6.6 | CAPRIN 2 |
| CDNF | 1.94 | 0.04 | 6.6 | Cerebral dopamine neurotrophic factor |
| TNNI1 | 1.84 | 0.046 | 6.3 | Troponin I |
| SH3BGRL | 1.58 | 0.047 | 6.2 | SH3BGRL |
| EMCN | 1.68 | 0.049 | 6.0 | Endomucin |
4. Discussion
To our knowledge this is the first study to examine global atrial tissue transcript remodeling in the acute postoperative period following open-chest surgery (cardiac or non-cardiac). The strong correlation of atrial KCNE1 down-regulation to the postoperative state revealed here, measurable just 3 days after surgery, is of great interest given multiple prior studies indicated a correlation between lone AF and the KCNE1 38G polymorphism (e.g., [2]), which reduces KCNQ1-KCNE1 (IKs) current density in vitro [6]. Whether KCNE1 downregulation in POAF is a compensatory response or a factor contributing to the substrate for POAF remains to be established, but our findings provide the first evidence of this common molecular factor in lone AF and POAF, and suggest the possibility that KCNE1 polymorphisms might influence incidence of POAF in human subjects who have undergone cardiothoracic surgery.
Lone AF-associated point mutations in KCNE1 are generally thought to increase IKs density and thereby shorten atrial myocyte action potentials [1]. In the present study of POAF, shortened LA ERP associated with the highest propensity for AF (Figure 1C), yet atrial KCNE1 expression was reduced in the postoperative group. The loss-of-function effects of the KCNE1 38G allele were suggested, based on in silico modeling, to have the potential to predispose to early after-depolarizations [6], and this is potentially occurring in the current POAF model. However, one of two further alternatives is considered more likely: either the reduced KCNE1 expression alters repolarization in such a way as to provide a substrate for reentry by increasing the dispersion of ERP, or the KCNE1 remodeling we observe is not contributing to POAF but is instead a compensatory response to shortening of the LA ERP by other mechanisms. Lastly, while it is important to recognize that there are profound differences in the molecular correlates of the K+ currents in the atria of large animals versus mice, it is theoretically possible that reduced KCNE1 expression actually increases porcine atrial myocyte K+ current by mechanisms independent of KCNQ1, as observed in the Kcne1 null mouse, which exhibits AF in the absence of structural heart disease, and increased atrial IK [7].
In addition to KCNE1, the results suggests a number of other possible contributors to POAF susceptibility, and additional work will now be directed toward understanding cause and effect relationships versus compensatory remodeling scenarios for these other transcripts (Table 1). Briefly, it is fascinating that PTX3 and NPPB (and, likewise, NPR3) were down-regulated in the postoperative group, given the strong positive correlation of the protein products of these genes with myocardial damage and their widespread use as biomarkers; it is suggested that the decrease in PTX3 reflects increased utilization (and therefore release) of inflammation competent cells. Among the other proteins whose transcripts were down-regulated, FHL1C (Four and a half LIM domain protein isoform C) reduction could increase atrial Kv1.5 activity [8], potentially shortening shorten the LA ERP. Aminopeptidase O isoform 1 is a metalloproteinase putatively involved in the renin angiotensin pathway [9]; Nestin is implicated in myocardial regeneration [10]; podoplanin is implicated in sinoatrial node development [11]; Homer 2 inhibits RyR2 channel function, and therefore Homer 2 downregulation could result in increased RyR2 activity, as was recently observed in chronic AF patients [12].
For the proteins encoded by the postoperatively up-regulated transcripts, as might be expected many are involved in inflammation such as those in the complement cascade, consistent with an inflammatory component to POAF [13]. Also of note, PIK3CG synthesizes phosphatidylinositol(3,4,5)-trisphosphate (PIP3), known to regulate atrial myocyte K+ currents [14]; Troponin I is involved in Ca2+-sensitive regulation of cardiac muscle contraction. Perhaps most intriguingly, Teneurin 2 (the transcript expression level of which was the most strongly correlated of all to the postoperative state) is a transmembrane signaling protein almost exclusively studied for its roles in the brain, but recently found to facilitate neuromuscular junction formation in Drosophila [15], suggesting a hypothetical novel role in cardiac innervation and a possible (but at this point speculative) role for Teneurin in mediating sympathetic nervous system changes potentially contributing to POAF. A hypothetical framework for POAF in the light of our transcriptomics findings, intended to stimulate debate and functional verification analyses, is presented (Figure 1H).
Supplementary Material
Acknowledgments
Sources of funding This work was supported by the US National Institutes of Health, National Heart, Lung and Blood Institute HL079275 and HL101190 (G.W.A. and D.J.C.) and University of California, Irvine setup funds (G.W.A.). In addition, support was provided by the Memorial Sloan-Kettering Steps for Breath Fund (PMH).
Footnotes
Disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olesen MS, Bentzen BH, Nielsen JB, Steffensen AB, David JP, Jabbari J, et al. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC medical genetics. 2012;13:24. doi: 10.1186/1471-2350-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prystupa A, Dzida G, Myslinski W, Malaj G, Lorenc T. MinK gene polymorphism in the pathogenesis of lone atrial fibrillation. Kardiologia polska. 2006;64:1205–11. discussion 12–3. [PubMed] [Google Scholar]
- 3.Roselli EE, Murthy SC, Rice TW, Houghtaling PL, Pierce CD, Karchmer DP, et al. Atrial fibrillation complicating lung cancer resection. The Journal of thoracic and cardiovascular surgery. 2005;130:438–44. doi: 10.1016/j.jtcvs.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Raman T, Roistacher N, Liu J, Zhang H, Shi W, Thaler HT, et al. Preoperative left atrial dysfunction and risk of postoperative atrial fibrillation complicating thoracic surgery. The Journal of thoracic and cardiovascular surgery. 2012;143:482–7. doi: 10.1016/j.jtcvs.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Heerdt PM, Gandhi CD, Dickstein ML. Disparity of isoflurane effects on left and right ventricular afterload and hydraulic power generation in swine. Anesthesia and analgesia. 1998;87:511–21. doi: 10.1097/00000539-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich JR, Zicha S, Coutu P, Hebert TE, Nattel S. Atrial fibrillation-associated minK38G/S polymorphism modulates delayed rectifier current and membrane localization. Cardiovascular research. 2005;67:520–8. doi: 10.1016/j.cardiores.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Temple J, Frias P, Rottman J, Yang T, Wu Y, Verheijck EE, et al. Atrial fibrillation in KCNE1-null mice. Circulation research. 2005;97:62–9. doi: 10.1161/01.RES.0000173047.42236.88. [DOI] [PubMed] [Google Scholar]
- 8.Poparic I, Schreibmayer W, Schoser B, Desoye G, Gorischek A, Miedl H, et al. Four and a half LIM protein 1C (FHL1C): a binding partner for voltage-gated potassium channel K(v1.5) PloS one. 2011;6:e26524. doi: 10.1371/journal.pone.0026524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axton R, Wallis JA, Taylor H, Hanks M, Forrester LM. Aminopeptidase O contains a functional nucleolar localization signal and is implicated in vascular biology. Journal of cellular biochemistry. 2008;103:1171–82. doi: 10.1002/jcb.21497. [DOI] [PubMed] [Google Scholar]
- 10.Drapeau J, El-Helou V, Clement R, Bel-Hadj S, Gosselin H, Trudeau LE, et al. Nestin-expressing neural stem cells identified in the scar following myocardial infarction. Journal of cellular physiology. 2005;204:51–62. doi: 10.1002/jcp.20264. [DOI] [PubMed] [Google Scholar]
- 11.Mahtab EA, Vicente-Steijn R, Hahurij ND, Jongbloed MR, Wisse LJ, DeRuiter MC, et al. Podoplanin deficient mice show a RhoA-related hypoplasia of the sinus venosus myocardium including the sinoatrial node. Developmental dynamics: an official publication of the American Association of Anatomists. 2009;238:183–93. doi: 10.1002/dvdy.21819. [DOI] [PubMed] [Google Scholar]
- 12.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–70. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imazio M, Brucato A, Ferrazzi P, Rovere ME, Gandino A, Cemin R, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124:2290–5. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 14.Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie GS, et al. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110gamma. Molecular cell. 2011;42:84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker RP, Martin D, Kos R, Chiquet-Ehrismann R. The expression of teneurin-4 in the avian embryo. Mechanisms of development. 2000;98:187–91. doi: 10.1016/s0925-4773(00)00444-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.