Abstract
Rationale
Churg-Strauss syndrome (CSS) and hypereosinophilic syndrome (HES) overlap considerably in clinical presentation. A reliable means of distinguishing between these groups of patients is needed, especially in the setting of glucocorticoid therapy.
Methods
A retrospective chart review of 276 adult subjects referred for evaluation of eosinophilia >1500/μl was performed, and subjects with a documented secondary cause of eosinophilia or a PDGFR-positive myeloproliferative neoplasm were excluded. The remaining subjects were assessed for the presence of American College of Rheumatology (ACR) criteria. Laboratory and clinical parameters were compared between subjects with biopsy-proven vasculitis (CSS; n=8), ≥4 ACR criteria (probable CSS; n=21), HES with asthma and/or sinusitis without other CSS-defining criteria (HESwAS; n=20), HES without asthma or sinusitis (HES; n=18), and normal controls (n=8). Serum biomarkers reported to be associated with CSS were measured using standard techniques.
Results
There were no differences between the subjects with definite or probable CSS or HES with respect to age, gender, or maintenance steroid dose. Serum CCL17, IL-8 and eotaxin levels were significantly increased in eosinophilic subjects as compared to normal controls, but were similar between the eosinophilic groups. Serum CCL17 correlated with eosinophil count (p<0.0001, r=0.73), but not with prednisone dose.
Conclusions
In patients with a history of asthma and sinusitis, distinguishing between ANCA-negative CSS and PDGFR-negative HES is difficult due to significant overlap in clinical presentation and biomarker profiles.
Keywords: Churg-Strauss syndrome, Hypereosinophilic syndrome, Eosinophilia
Introduction
Churg-Strauss syndrome (CSS) and hypereosinophilic syndrome (HES) are rare eosinophilic disorders that overlap considerably in clinical presentation. Corticosteroids are considered first-line therapy for both disorders; however, corticosteroid dosing, recommended steroid-sparing agents, disease course and prognosis can be quite different. Accurate diagnosis is therefore important.
CSS is characterized by the development of clinical manifestations of eosinophilic vasculitis in an eosinophilic patient with long-standing asthma and sinusitis. However, definitive diagnosis of CSS relies on the demonstration of vasculitis in tissue. In practice, this is often difficult since many patients present on chronic corticosteroid therapy for asthma control, and affected tissues may not be easily accessible for biopsy. HES is defined by unexplained blood eosinophilia >1500/μL on two separate occasions at least one month apart and evidence of end-organ involvement attributed to eosinophilia(1). Since asthma and sinusitis are common in the general population, distinction between CSS and HES with asthma and/or sinusitis can be extraordinarily difficult in the absence of clear pathologic evidence of vasculitis. Although perinuclear anti-neutrophil antibodies (p-ANCA) are associated preferentially with CSS, these are absent in up to 40–60% of patients with biopsy-proven CSS(2, 3)
The American College of Rheumatology (ACR) criteria for CSS were developed to distinguish CSS from other forms of vasculitis for inclusion of subjects in research studies and are based on the following clinicopathologic findings: 1) asthma with a history of wheezing or diffuse high-pitched expiratory rhonchi, 2) eosinophilia >10% on differential white blood cell count, 3) mono- or polyneuropathy, 4) migratory or transitory pulmonary infiltrates, 5) paranasal sinus abnormality with a history of acute or chronic paranasal sinus pain, or radiographic opacification of the paranasal sinuses, and 6) extravascular eosinophils noted on a biopsy that includes an artery, arteriole or venule(4). In the setting of documented vasculitis, the presence of 4 or more ACR criteria had a sensitivity of 95% and specificity of 99.2% for CSS(4).
The ACR criteria are often used to support a clinical diagnosis of CSS in individual patients; however, the utility of this approach has not been validated. Clearly, alternative means of distinguishing between patients with HES and CSS are needed, especially in the setting of glucocorticoid therapy. Various studies have assessed the utility of biomarkers to distinguish between quiescent and active CSS(5–8), however, few have investigated biomarkers to distinguish between CSS and HES. In the present study, clinical manifestations, routine laboratory parameters and serum biomarkers previously associated with CSS were examined in subjects with biopsy-proven CSS, probable CSS, HES with sinusitis or asthma and HES without sinusitis or asthma in order to assess their potential utility in the diagnosis of CSS.
Methods
Study Subjects
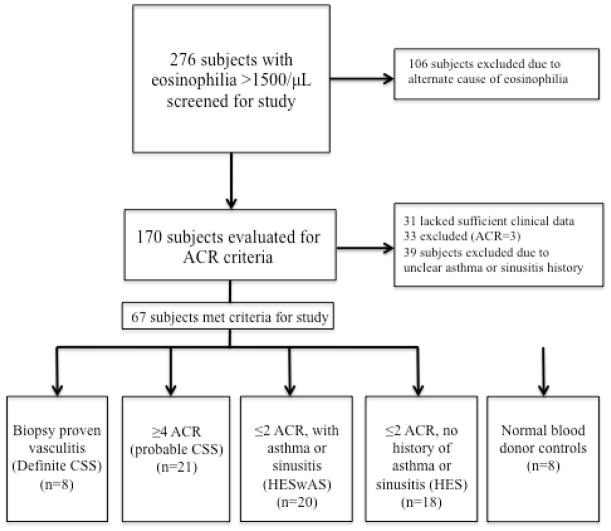
Medical records were reviewed retrospectively from all subjects evaluated at the NIH between June 9th, 1999 and June 14th, 2010 on an NIAID IRB-approved protocol to study eosinophilia (NCT00001406). Informed consent was obtained from all subjects. Of the 276 adult subjects with 10% eosinophilia and absolute eosinophil count (AEC) >1500/μL, 106 had a documented cause of eosinophilia (i.e. drug hypersensitivity, helminth infection, myeloproliferative neoplasm) and were excluded from further study (Figure 1). ACR criteria were assessed in the remaining 170 subjects. Subjects for whom clinical data was lacking (n=31), with uncertain asthma and/or sinus disease status (n=39) or who had asthma and/or sinusitis with migratory infiltrates, neuropathy or perivascular eosinophils on biopsy, but who did not meet ≥4 ACR criteria (n=33) were excluded. The remaining subjects were classified into 4 study groups: biopsy-proven vasculitis defined by the presence of two of the following criteria: 1) eosinophils surrounding or infiltrating a blood vessel, 2) granulomas in or around a vessel, and 3) disruption of the elastica as assessed by immunohistochemistry (definite CSS; n=8), ≥4 ACR criteria without biopsy-proven vasculitis (probable CSS; n=21), eosinophilia with asthma and/or sinusitis but no other CSS-defining features (HESwAS; n=20), and eosinophilia without asthma or sinusitis (HES; n=18). Normal blood bank donors were used as controls for laboratory studies (n=8).
Figure 1.
Subject screening and evaluation for study. Abbreviations: CSS- Churg Strauss Syndrome, HESwAS-Hypereosinophilic syndrome with asthma and/or sinusitis, HES-Hypereosinophilic syndrome.
Clinical and Serologic Assessments
A standardized set of clinical data was collected for all subjects from the medical record (Supplement A). Clinical data presented reflects information obtained throughout the course of disease until the time of record review. When possible, missing records, such as CT scans, were obtained by calling subjects. However, information obtained via means of telephone was only used if objective evidence was obtained (e.g. CT scans showing polyps). Equivalent prednisone dosage was calculated for subjects being treated with other corticosteroid preparations.
Serum samples were collected prospectively from all subjects at each protocol visit and stored at −80°C. The earliest available serum sample was used for analysis. Serum levels of eotaxin 1, GM-CSF, IFN-γ, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, soluble IL-2 receptor (sIL-2R), soluble intracellular adhesion molecule-1 (s-ICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble E (sE)-selectin and myeloperoxidase (MPO) were measured by suspension array technology in multiplex (Millipore Corp, St. Charles, MO) according to the manufacturer’s instructions. Serum TARC/CCL17 levels were evaluated by ELISA (R&D, Minneapolis, MN). Atopic status was assessed by self-report and by serum specific IgE levels (Phadiotop, now Thermo Fisher Scientific, Waltham, MA) since sensitization status by skin prick testing was not available for all subjects. Results are expressed as Phadiatop Arbitrary units (PAU/I) with greater values indicating increased sensitization.
Statistical Methods
Fisher’s exact test and Mann-Whitney U test were used to compare prevalence and group means, respectively. Cytokine values are expressed as geometric means (GM) with 95% CI, and associations between groups assessed using a 1 way ANOVA (Kruskall Wallis test) with Dunn’s correction for multiple comparisons. Correlations of biomarkers with eosinophil counts were performed using Spearman Rank sum.
Results
Diagnostic classification of subjects
Among the 29 subjects who met ACR criteria for CSS, only 8 had biopsy-proven vasculitis (definite CSS). Of the remaining 21 subjects, 6 had no tissue biopsies performed. Fourteen subjects underwent biopsy of at least one organ other than skin (lung (n=9), nerve (n=3) cardiac (n=2), gastrointestinal (n=2), salivary gland (n=1) and gallbladder (n=1)). Although no vasculitis was demonstrated in any of the tissues, lung biopsies in 2 subjects showed perivascular eosinophils. Ten subjects were known to be taking corticosteroids at the time of the biopsy.
Clinical and demographic characteristics
Age and gender were similar between the subjects with definite CSS, probable CSS or HES. Forty subjects (60%) were taking corticosteroids at the time of initial evaluation: 6/8 (75%) subjects with CSS, 13/21 (60%) subjects with probable CSS, 11/20 (55%) subjects with HESwAS and 10/18 (56%) subjects with HES. None of the normal volunteers were on steroid therapy. Median maintenance corticosteroid doses were low (≤10 mg daily) in all 4 groups, but were increased in the probable and definite CSS groups (10mg daily in both groups) compared to the two HES groups (2.5mg and 2.8mg daily; p=0.04, Mann-Whitney U test) (Table 1). Asthma was present in all of the subjects with definite or probable CSS and in 13 (65%) of the subjects with HESwAS. Sinusitis (diagnosed clinically or by CT scans) was present in all but one subject with definite CSS (n=7; 87.5%), as well as in most subjects with probable CSS (n=16; 76.2%), and half of the subjects with HESwAS (n=10; 50%). All subjects with biopsy-proven CSS met the ACR criteria, and none of the subjects in any of the groups were ANCA-positive at any time during their clinical course.
Table 1.
Demographic and laboratory characteristics of subjects
| Definite CSS (n=8 ) | Probable CSS (n=21) | HES <2 ACR with asthma or sinusitis (n=20) | HES <2 ACR No asthma or sinusitis (n=18) | Normals (n=8) | |
|---|---|---|---|---|---|
| Median age§ (range) | 45 (22–55) | 55 (22–75) | 49 (19–82) | 59 (30–76) | 43 (28–54) |
| Gender (M/F) | 5/3 | 7/14 | 9/11 | 10/8 | 2/6 |
| Median serum IgE§ (range) | 128 (9–856) | 213 (18–2600) | 266 (4–23000) | 99.1 (14–5022) | ND |
| Median equivalent prednisone dose§ (mg) | 10 (0–40) | 10 (0–60) | 3.6 (0–60) | 2.8 (0–20) | 0 |
| Median CRP§ (range) | 1.03 (0.51–31) | 2.9 (0.91–29.7) | 1.42 (0.43–18.5) | 0.7 (0.28–5.73) | ND |
| Median ESR§ (range) | 11 (2–72) | 14 (3–77) | 7.5 (2–90) | 7 (2–90) | ND |
| Median BVAS§ (range) | 5.5 (2–13) | NA | NA | NA | NA |
| Median B12§ (range) | 834 (339–1312) | 742.5 (285–1699) | 681 (201–1383) | 637 (316–1603) | ND |
| Median AEC§ (range) | 515 (115–5810) | 570 (0–3162) | 547 (40–6878) | 1145 (30–3680) | 140 (37–340) |
| Median peak eosinophil count¶ (range) | 9452 (1300–17000) | 4900 (716–48080) | 3400 (1000–40000) | 5366 (1800–21080) | NA |
| Median duration of eosinophilia¶ (years) | 5 | 4 | 3 | 6 | NA |
| Median ALC§ (range) | 1603 (824–4111) | 1812.5 (950–5090) | 1600 (340–3940) | 1490 (618–3940) | ND |
| Median tryptase§ (range) | 5.4 (1.78–10.5) | 4.24 (1.31–28.1) | 6.51 (1.47–33.1) | 5.65 (1.67–6067) | ND |
Abbreviations: ACR, American College of Rheumatology, AEC, absolute eosinophil count, ALC- absolute lymphocyte count, BVAS- Birmingham vasculitis activity score CRP- C reactive protein, CSS, Churg Strauss Syndrome, ESR-Erythrocyte sedimentation rate, HES- Hypereosinophilic syndrome, HESwAS-Hypereosinophilic syndrome with asthma and/or sinusitis. ND-No data, NA-Not applicable.
Data collected at the time of the serum collection
Data collected over the course of the disease process and follow-up.
Although there were no significant differences between subjects with definite or probable CSS with respect to migratory infiltrates or neuropathy, these CSS-defining manifestations were significantly less common in the subjects with HES. Subjects with HESwAS were not included in this analysis because, by definition, they could not have additional manifestations of CSS. Migratory infiltrates were noted in 3 subjects (37%) with definite CSS and 14 subjects (66%) with probable CSS, but no subjects (0%) with HES (p<0.001 compared to definite and probable CSS, Fisher’s exact test). Similarly, neuropathy was diagnosed in 62.5% (5/8) of subjects with CSS and 57.1% (12/21) subjects with probable CSS, but only 22% (4/18) of subjects with HES (p=0.012, Fisher’s exact test). Neuropathy was confirmed by NCV/EMG in 3 cases. Nerve biopsy was performed on two subjects, both taking corticosteroids, and showed no evidence of vasculitis.
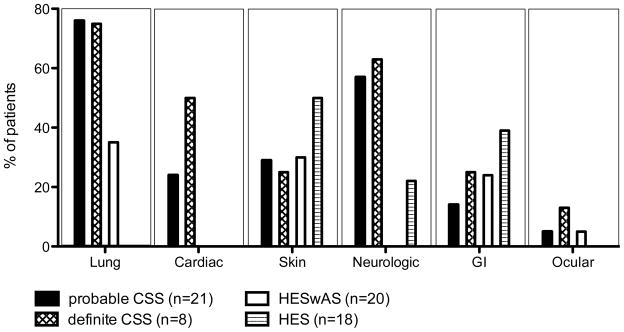
In general, the pattern of organ involvement was similar between subjects with definite CSS, probable CSS and HESwAS with the exception of cardiac involvement, which was seen exclusively in the CSS groups (Figure 2). Clinical manifestations in subjects with HES were predominantly dermatologic and gastrointestinal, including eosinophilic hepatitis.
Figure 2.
End organ manifestations demonstrated prior to evaluation at our institution and over the duration of follow-up. The bars represent the percentage of subjects in each group with involvement of the indicated organ system. Abbreviations: CSS-Churg Strauss Syndrome, GI-Gastrointestinal, HES-Hypereosinophilic syndrome, HESwAS- Hypereosinophilic syndrome with asthma and/or sinusitis.
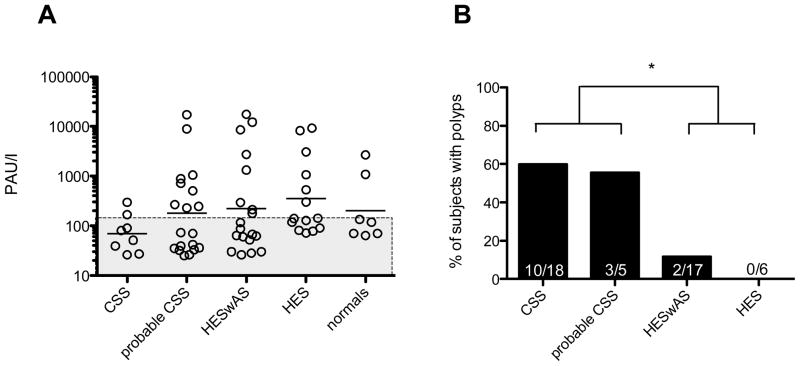
There was no significant difference in atopic status between any of the groups studied (Figure 3), including the normal group. However, polyps were present in significantly higher numbers in the definite and the probable CSS groups (n=13) as compared to the subjects with HES (n=2; p=0.0004, Fisher’s Exact Test).
Figure 3.
Atopy and Polyp Status within groups. (A) Phadiotop results expressed as Phadiotop Arbitrary Units/liter (PAU/l) were comparable between groups. Open circles represent individual subjects within groups with geometric mean. Subjects in shaded region are not considered atopic by Phadiotop. (B) Proportion of polyps is higher in both probable CSS and definite CSS (n=13) as compared to the subjects with HES (n=2; * p=0.0004, Fisher’s Exact Test). Abbreviations: CSS- Churg Strauss Syndrome, HESwAS-Hypereosinophilic syndrome with asthma and/or sinusitis, HES- Hypereosinophilic syndrome.
Blood and Serum Results
Routine laboratory parameters, including inflammatory markers (CRP, ESR), duration of eosinophilia and peak eosinophil count, were similar between the various groups (Table 1). Serum samples were available for 64/67 eosinophilic subjects and all normal controls. Two subjects with probable CSS and one subject with HES did not have serum samples available for analysis and were included in the clinical, but not the serological assessment.
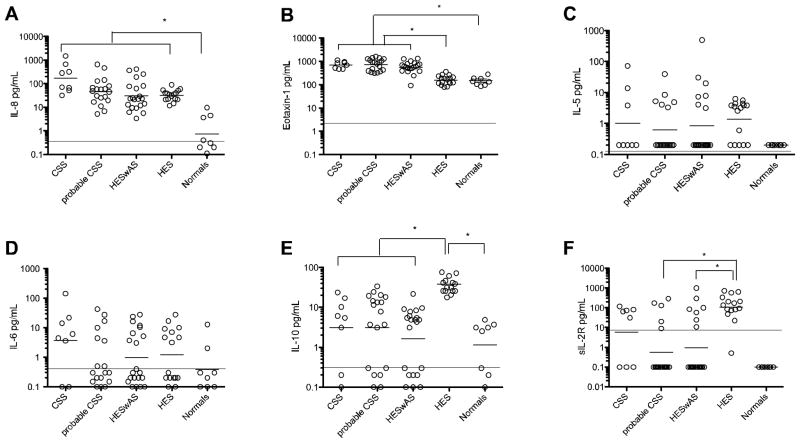
Serum IL-8 and eotaxin-1 levels were significantly increased in all eosinophilic subjects as compared to normal controls (Figure 4a,b). Serum IL-5 was elevated in some, but not all, subjects with eosinophilia and in none of the normal subjects (Figure 4c). Serum levels of inflammatory cytokines, including IL-6, were not increased in subjects with probable or definite CSS compared with normal subjects, consistent with the lack of a difference in CRP and ESR levels between the groups (Figure 4d, Table 1). Serum levels of sIL-2R, a previously described marker of CSS, were elevated in eosinophilic subjects of all groups compared to normals and were correlated with AEC (R=0.48; p<0001). Serum sIL-2R levels were significantly elevated in subjects with HES (GM 110.5pg/mL [43.9–278]) compared to subjects with probable CSS (GM 0.54 pg/mL [0.12–2.3]), and HESwAS (GM 0.95 pg/mL [0.21–4.4], p<0.05 by 1 way ANOVA), but not to subjects with CSS (GM 5.89 pg/mL [0.34–99]) (Figure 4f). This was true even when levels were adjusted for AEC (Supplement B). GM IL-10 levels were elevated in eosinophilic subjects without asthma or sinusitis (38.48pg/mL [29.6–49.4]; p<0.05) compared to eosinophilic patients with asthma or sinusitis, including probable CSS (3.2 pg/mL [1.1–8.9]), definite CSS (2.8pg/mL [0.5–14.3]), and HESwAS (1.6pg/mL [0.6–3.7]). (Figure 4e)
Figure 4.
Serum cytokine values. Serum evaluation of (A) IL-8 (B) Eotaxin-1, (C) IL-5, (D) IL-6, (E) IL-10 and (F) sIL-2 levels in groups with probable CSS, definite biopsy proven CSS, HES with asthma and/or sinusitis, HES without asthma or sinusitis and normal subjects. * p<0.05 by 1 way ANOVA. Horizontal line denotes mean minimal detectable levels. Mean minimal detectable values are as follows: IL-8 (0.3pg/mL), IL-6 (0.4pg/mL), Eotaxin-1 (2.1pg/mL), IL-10 (0.3pg/mL), IL-5 (0.1pg/mL), and sIL-2R (7.5pg/mL). Abbreviations: CSS-Churg Strauss Syndrome, HESwAS-Hypereosinophilic syndrome with asthma and/or sinusitis, HES-Hypereosinophilic syndrome.
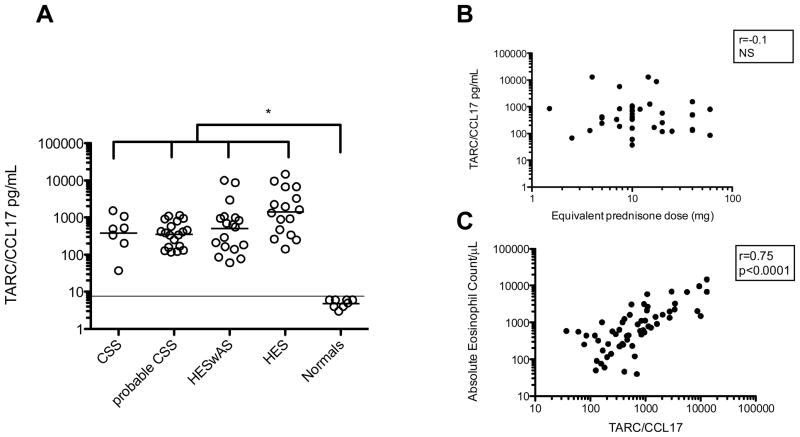
Serum CCL17 levels were elevated in all eosinophilic groups with the highest levels (1408pg/mL [663.6–2987]) in the HESwAS group. Serum CCL17 correlated with eosinophil count (p<0.0001, r=0.735), but not with prednisone dose (p=0.8; NS) (Figure 5a,b). Adjusting for AEC did not change the overall results (Supplement B).
Figure 5.
CCL17/TARC levels. (A) TARC/CCL17 levels by group, and (B) Lack of correlation of TARC/CCL17 levels with equivalent prednisone dose for treated subjects, and (C) positive correlation of TARC/CCL17 with absolute eosinophil count. *p<0.05 by 1 way ANOVA. Horizontal line denotes minimal detectable level of TARC/CCL17 (7.8pg/mL). Abbreviations: CSS- Churg Strauss Syndrome, HESwAS-Hypereosinophilic syndrome with asthma and/or sinusitis, HES- Hypereosinophilic syndrome.
Detectable levels of GM-CSF, IL-12, IL-13 and IL-17 were similar between the groups (data not shown). There were no differences between the CSS, probable CSS, HESwAS, and HES groups for any of the circulating endothelial markers or adhesion molecules evaluated (Supplement C).
Discussion
Differentiating between CSS and HES is remarkably difficult at the individual patient level due to the overlap in clinical manifestations between the two disorders and the fact that many patients present on glucocorticoid therapy, which can mask underlying vasculitis. The subjects in our series were all ANCA-negative and none had renal manifestations, likely reflecting the fact that subjects are referred to our center for eosinophilic disorders, including eosinophilic vasculitis, rather than for vasculitis itself. It is these ANCA-negative patients with eosinophilia that are most difficult to classify.
The primary aim of our study was to identify biomarkers to distinguish patients with eosinophilia and vasculitis (CSS) from those with eosinophilia >1500/μL, features of CSS (asthma and/or sinusitis), but no evidence of vasculitis (HESwAS). To this end, we selected groups across the clinical spectrum, ranging from biopsy-proven CSS (definite CSS) to PDGFR-negative HES without features of CSS (HES). No clinical differences were identified between subjects with definite or probable CSS. In contrast, migratory infiltrates, cardiac and neurologic complications were seen exclusively in subjects in these two groups as compared to those with HES without asthma or sinusitis. Although subjects with HESwAS were excluded from this analysis because they could not, by definition, have migratory infiltrates or neurologic complications (ACR criteria), subjects with HES who met 3 ACR criteria (n=33) showed an intermediate clinical phenotype with migratory infiltrates, cardiac and neurologic manifestations in 15%, 12% and 18% of subjects, respectively (data not shown). This is consistent with reports in the literature, which describe cardiac and neurologic manifestations in 10–12% and 8–21%, respectively, of patients with PDGFR-negative HES (9, 10) Nasal polyps were also more common in the definite and probable CSS groups, but were present in some subjects with HESwAS. Thus, although our data suggest a higher prevalence of migratory infiltrates, cardiac and neurologic manifestations and nasal polyps in subjects with CSS as compared to those with HES, the pattern of organ involvement is not very useful for diagnosis at the individual patient level.
Multiple studies have confirmed the specificity and sensitivity of the Phadiatop assay in distinguishing between atopic and non-atopic individuals (11, 12). Despite the increased prevalence of sinusitis, polyps and asthma in the subjects with CSS, Phadiotop positivity was comparable between the subjects with probable or definite CSS and those with HES.
A number of serum biomarkers, including serum levels of cytokines, cytokine receptors and chemokines, have been proposed as markers of active CSS(5, 7, 13–15). Elevated levels of some of these, including IL-10, and sIL2R, have also been reported in patients with CSS in remission(16, 17). Although many of these same biomarkers have also been reported to be elevated in idiopathic HES(18, 19), their utility in distinguishing between CSS and HES has not been systematically explored. Among the serum biomarkers examined in the present study, none were useful in differentiating between subjects with CSS (definite or probable) and HESwAS. Serum levels of IL-8 and IL-5, cytokines that can be produced by a variety of cell types, including eosinophils themselves, were increased in eosinophilic subjects compared to normal controls, but did not distinguish between the eosinophilic subgroups. Similarly, serum levels of TARC/CCL17, a chemokine released by mononuclear cells and dendritic cells(20) and reported to be elevated in patients with active CSS disease(5) and lymphocytic variant HES(21), were increased in all eosinophilic groups as compared to the normal subjects and correlated positively with absolute eosinophil count. Of note, corticosteroids did not appear to impact the ability to detect elevated TARC/CCL17 in serum.
Several serum biomarkers appeared to distinguish eosinophilic subjects with asthma and/or sinusitis (definite CSS, probable CSS and HESwAS) from those without evidence of asthma and/or sinusitis (HES). Eotaxin-1 levels were increased in subjects with asthma and/or sinusitis compared with HESwAS and normal controls. There is little data on serum eotaxin-1 levels in sinusitis; however, lower serum levels in subjects with asthma have been associated with better asthma control(22, 23). In contrast, levels of IL-10 and sIL-2R were decreased in subjects with evidence of asthma and/or sinusitis. Although prior studies have demonstrated elevated serum IL-10 levels in patients with CSS (either active or in remission)(17) as well as increased CD4+CD25+ IL-10 producing T cells in patients with CSS in remission(24) compared to normal controls, elevated serum IL-10 was not observed in subjects with definite or probable CSS in our study. More importantly, serum IL-10 levels in the subjects with definite or probable CSS were similar to levels in subjects with HESwAS, precluding the use of serum IL-10 levels as a biomarker of CSS. The significantly increased serum IL-10 levels in the HES group without asthma or sinusitis is puzzling and suggests a negative association between serum IL-10 and asthma and/or sinusitis in subjects with eosinophilia. A similar pattern was seen with sIL-2R, a marker of endothelial damage and T cell activation, with elevated levels in all of the eosinophilic groups and significantly increased levels in subjects with HES without asthma or sinusitis.
In summary, there is significant clinical overlap between ANCA-negative CSS and PDGFR-negative HES. Although serum levels of eotaxin-1, IL-10 and sIL-2R may be useful in distinguishing between eosinophilic patients with and without asthma and/or sinusitis, none of the biomarkers studied were preferentially detected in subjects with definite or probable CSS as compared to those with HESwAS. Whether HES with asthma and sinusitis and ANCA-negative CSS share a common pathogenesis accounting for the similarity in biomarker profiles remains to be elucidated.
Acknowledgments
Funding:
Division of Intramural Research, NIAID, National Institutes of Health; Clinical Trials: NCT00001406. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN2610080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the National Institute of Allergy and Infectious Diseases.
Footnotes
No conflicts of Interest
Author contributions:
PK and ADK conceived and designed the study; PK, PZ, ED, CSS, CTW, and NCH were involved in acquisition and/or interpretation of data. PK drafted the article, ADK was instrumental in revisions, and all authors were involved in critical review of content. All authors approved the final version to be published.
References
- 1.Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(1):45–49. doi: 10.1016/j.jaci.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA) Autoimmunity. 2005;38(1):93–103. doi: 10.1080/08916930400022673. [DOI] [PubMed] [Google Scholar]
- 3.Pagnoux C, Guillevin L. Churg-Strauss syndrome: evidence for disease subtypes? Curr Opin Rheumatol. 2010;22(1):21–28. doi: 10.1097/BOR.0b013e328333390b. [DOI] [PubMed] [Google Scholar]
- 4.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33(8):1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 5.Dallos T, Heiland GR, Strehl J, Karonitsch T, Gross WL, Moosig F, et al. CCL17/thymus and activation-related chemokine in Churg-Strauss syndrome. Arthritis Rheum. 2010;62(11):3496–3503. doi: 10.1002/art.27678. [DOI] [PubMed] [Google Scholar]
- 6.Guilpain P, Auclair JF, Tamby MC, Servettaz A, Mahr A, Weill B, et al. Serum eosinophil cationic protein: a marker of disease activity in Churg-Strauss syndrome. Ann N Y Acad Sci. 2007;1107:392–399. doi: 10.1196/annals.1381.041. [DOI] [PubMed] [Google Scholar]
- 7.Polzer K, Karonitsch T, Neumann T, Eger G, Haberler C, Soleiman A, et al. Eotaxin-3 is involved in Churg-Strauss syndrome--a serum marker closely correlating with disease activity. Rheumatology (Oxford) 2008;47(6):804–808. doi: 10.1093/rheumatology/ken033. [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Tsurikisawa N, Tsuburai T, Oshikata C, Akiyama K. Cytokine production profile of CD4+ T cells from patients with active Churg-Strauss syndrome tends toward Th17. Int Arch Allergy Immunol. 2009;149 (Suppl 1):61–65. doi: 10.1159/000210656. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 10.Pardanani A, Brockman SR, Paternoster SF, Flynn HC, Ketterling RP, Lasho TL, et al. FIP1L1-PDGFRA fusion:prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood. 2004;104(10):3038–3045. doi: 10.1182/blood-2004-03-0787. [DOI] [PubMed] [Google Scholar]
- 11.Paganelli R, Ansotegui IJ, Sastre J, Lange CE, Roovers MH, de Groot H, et al. Specific IgE antibodies in the diagnosis of atopic disease. Clinical evaluation of a new in vitro test system, UniCAP, in six European allergy clinics. Allergy. 1998;53(8):763–768. [PubMed] [Google Scholar]
- 12.Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol. 2001;86(2):196–202. doi: 10.1016/S1081-1206(10)62691-9. [DOI] [PubMed] [Google Scholar]
- 13.Taira T, Matsuyama W, Mitsuyama H, Kawahara KI, Higashimoto I, Maruyama I, et al. Increased serum high mobility group box-1 level in Churg-Strauss syndrome. Clin Exp Immunol. 2007;148(2):241–247. doi: 10.1111/j.1365-2249.2007.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Mitsuyama H, Matsuyama W, Iwakawa J, Higashimoto I, Watanabe M, Osame M, et al. Increased serum vascular endothelial growth factor level in Churg-Strauss syndrome. Chest. 2006;129(2):407–411. doi: 10.1378/chest.129.2.407. [DOI] [PubMed] [Google Scholar]
- 15.Zwerina J, Bach C, Martorana D, Jatzwauk M, Hegasy G, Moosig F, et al. Eotaxin-3 in Churg-Strauss syndrome: a clinical and immunogenetic study. Rheumatology (Oxford) 2011;50(10):1823–1827. doi: 10.1093/rheumatology/keq445. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt WH, Csernok E, Kobayashi S, Klinkenborg A, Reinhold-Keller E, Gross WL. Churg-Strauss syndrome: serum markersof lymphocyte activation and endothelial damage. Arthritis Rheum. 1998;41(3):445–452. doi: 10.1002/1529-0131(199803)41:3<445::AID-ART10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Schonermarck U, Csernok E, Trabandt A, Hansen H, Gross WL. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA-associated vasculitides. Clin Exp Rheumatol. 2000;18(4):457–463. [PubMed] [Google Scholar]
- 18.Prin L, Plumas J, Gruart V, Loiseau S, Aldebert D, Ameisen JC, et al. Elevated serum levels of soluble interleukin-2 receptor: a marker of disease activity in the hypereosinophilic syndrome. Blood. 1991;78(10):2626–2632. [PubMed] [Google Scholar]
- 19.Kanbe N, Kurosawa M, Igarashi N, Tamura A, Yamashita T, Kurimoto F, et al. Idiopathic hypereosinophilic syndrome associated with elevated plasma levels of interleukin-10 and soluble interleukin-2 receptor. Br J Dermatol. 1998;139(5):916–918. doi: 10.1046/j.1365-2133.1998.02525.x. [DOI] [PubMed] [Google Scholar]
- 20.Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J Biol Chem. 1996;271(35):21514–21521. doi: 10.1074/jbc.271.35.21514. [DOI] [PubMed] [Google Scholar]
- 21.de Lavareille A, Roufosse F, Schandene L, Stordeur P, Cogan E, Goldman M. Clonal Th2 cells associated with chronic hypereosinophilia: TARC-induced CCR4 down-regulation in vivo. Eur J Immunol. 2001;31(4):1037–1046. doi: 10.1002/1521-4141(200104)31:4<1037::aid-immu1037>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Chu YT, Chiang W, Wang TN, Hung CH, Jong YJ, Wu JR. Changes in serum eotaxin and eosinophil cationic protein levels, and eosinophil count during treatment of childhood asthma. J Microbiol Immunol Infect. 2007;40(2):162–167. [PubMed] [Google Scholar]
- 23.Hoffmann HJ, Nielsen LP, Harving H, Heinig JH, Dahl R. Asthmatics able to step down from inhaled corticosteroid treatment without loss of asthma control have low serum eotaxin/CCL11. Clin Respir J. 2008;2(3):149–157. doi: 10.1111/j.1752-699X.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsurikisawa N, Saito H, Tsuburai T, Oshikata C, Ono E, Mitomi H, et al. Differences in regulatory T cells between Churg-Strauss syndrome and chronic eosinophilic pneumonia with asthma. J Allergy Clin Immunol. 2008;122(3):610–616. doi: 10.1016/j.jaci.2008.05.040. [DOI] [PubMed] [Google Scholar]