Abstract
Contaminants can be transported rapidly and over relatively long distances by atmospheric dust and aerosol relative to other media such as water, soil and biota; yet few studies have explicitly evaluated the environmental implications of this pathway, making it a fundamental but understudied transport mechanism. Although there are numerous natural and anthropogenic activities that can increase dust and aerosol emissions and contaminant levels in the environment, mining operations are notable with respect to the quantity of particulates generated, the global extent of area impacted, and the toxicity of contaminants associated with the emissions. Here we review (i) the environmental fate and transport of metals and metalloids in dust and aerosol from mining operations, (ii) current methodologies used to assess contaminant concentrations and particulate emissions, and (iii) the potential health and environmental risks associated with airborne contaminants from mining operations. The review evaluates future research priorities based on the available literature and suggest that there is a particular need to measure and understand the generation, fate and transport of airborne particulates from mining operations, specifically the finer particle fraction. More generally, our findings suggest that mining operations play an important but underappreciated role in the generation of contaminated atmospheric dust and aerosol and the transport of metal and metalloid contaminants, and highlight the need for further research in this area. The role of mining activities in the fate and transport of environmental contaminants may become increasingly important in the coming decades, as climate change and land use are projected to intensify, both of which can substantially increase the potential for dust emissions and transport.
Keywords: Dust, Aerosol, Mining, Tailings, Metals and Metalloids, Arsenic and Lead
1. Introduction
Contaminants that are persistent in the environment pose a potential risk to human health worldwide. Although natural processes can produce a number of environmental contaminants, significant amounts of contaminants are generated by anthropogenic activities such as agricultural practices, industrial manufacturing, and mining operations (Barrie et al., 1992; Lacerda, 1997; Kolpin et al., 1998; Ritter et al., 2002; Driscoll et al., 2003; Volkamer et al., 2006). Major transport pathways for contaminants in the environment are: air, water, soils, and biota. Transport of contaminants by air may occur by direct transfer of volatilized species or by particles. Atmospheric suspended particles (usually in the particle size range < 60 μm), including aerosol and dust (collectively referred here as atmospheric particulates), can play an important role in the transport of environmental contaminants, particularly those that have low volatility and low aqueous solubility and remain attached to soil particles. Transport by atmospheric particulates is an important pathway by which contaminants can be redistributed in the environment either from point sources, such as mine smelters, or by distributed sources, such as large industrial centers or urban environments. This transport mechanism will likely become more important with increased land-use activity and projected climate change (Pelletier, 2006). For example, dust storm frequency and intensity have increased in recent decades throughout many parts of the world such as Africa, Australia, and China, largely due to increased human activities and climate (Middleton et al., 1986; Tegen and Fung, 1995). Large dust events, such as the one shown in Figure 1, have the potential to transport large amounts of contaminants rapidly over long distances and large aerial extent relative to other transport pathways (water, soil and biota) and thus represent a unique risk to human health and the environment (Griffin et al., 2001; Park et al., 2004; Pope and Dockery, 2006).
Figure 1.
Schematic derived from satellite remote sensing images of 6-day transport of atmospheric dust from the Gobi Desert to the U.S. Pacific Coast during a massive dust storm in April 1998 (reprinted from Wilkening et al., 2000).
Contaminant transport by atmospheric particulates is of global concern because air masses containing large amounts of dust and aerosol frequently cross continental and international boundaries and often have adverse environmental consequences in downwind depositional areas (Barrie et al., 1992; Perry et al., 1999; Chu et al., 2003; Park et al., 2004; Gallon et al., 2011; Figure 2). Relative to the other major transport pathways (water, soil and biota), this mechanism has greatest potential to transport nonvolatile contaminants at regional and global scales because air masses generally are not confined to an appreciable extent by topographic boundaries or other barriers that might impede transport, as is the case with water, soil, and biota (Kolpin et al., 1998; Kersting et al., 1999; McGechan and Lewis, 2002; Mulligan and Yong, 2004; Braune et al., 2005). Additionally, atmospheric particulates have the greatest potential to transport contaminants rapidly (hours to days) through the environment since air masses move at much greater velocity than surface water, groundwater, and most biological vectors. Atmospheric dust and aerosol can also play an important role in the transport of contaminants over longer time periods (years to decades) and at smaller spatial scales (meters to kilometers). All of the major environmental transport pathways (i.e., water, air, soil and biota) can be important to consider when assessing the potential for contaminant transport over longer time periods (i.e., years to decades) and at local scales (i.e., meters). For example, although most transport processes in soil are inherently slow and limited in spatial extent (Weber et al., 1991; Sheppard, 2005), over sufficient time large amounts of contaminants can be transported though the soil, resulting in elevated risks to human health and the local environment. Contaminant transport by biological vectors (biota) is generally less confined by topographic barriers than transport processes in soil and thus has a greater potential to redistribute contaminants at spatial scales that extend beyond the local environment (Arthur and Markham, 1982; Hope, 1993; Braune et al., 2005). Water and fluvial sediment are also major transport pathways for the mobilization and redistribution of contaminants in the environment (Weber et al., 1991; Kolpin et al., 1998; McGechan and Lewis, 2002). Contaminant transport by water and sediments in terrestrial systems is typically confined in spatial extent by watershed boundaries, and most contaminants and associated risks are generally concentrated in riparian areas, aquatic systems, and shallow groundwater (e.g., Kolpin et al., 1998).
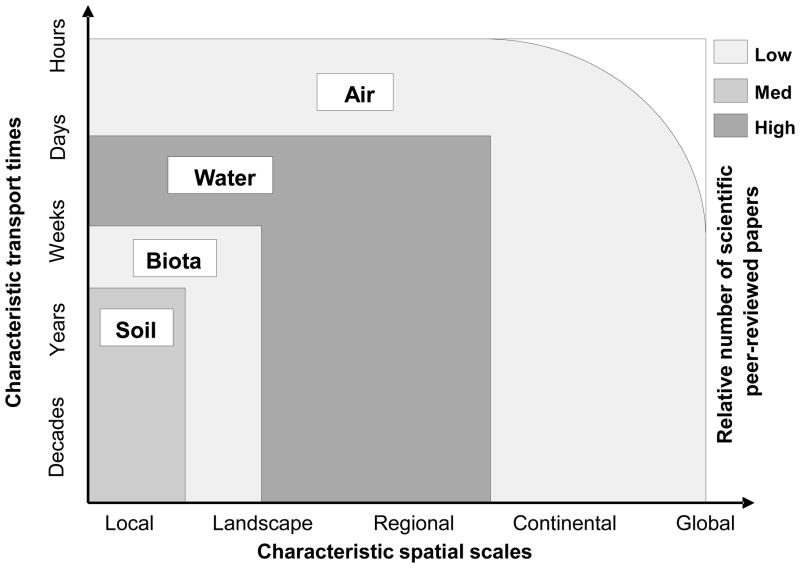
Figure 2.
Illustration of environmental contaminant transport media, their transport times and spatial extent and their relative number of scientific peer-reviewed papers (low < 1,000 studies; high > 10,000 studies).
When considering all of the major transport pathways in the context of their potential risk to human health and the environment, contaminant transport by air and atmospheric particulates is perhaps most notable due to the potential speed, distance, and aerial extent in which contaminants can be transported in the environment. Despite the potential importance of this mechanism, most studies that assess issues related to contaminant transport in the environment focus almost entirely on transport in soil and water (Figure 2). For example, in the case of contaminant transport from mining operations, there are nearly 10,000 peer-reviewed studies that explicitly focus on environmental problems associated with contaminant transport in water; about half as many studies that focus on contaminant transport in soil; and only a few hundred studies that focus on contaminant transport by atmospheric particulates. More specifically, very few studies explicitly focus on the transport of metals and metalloids in dust and aerosol from mining operations, despite the potential environmental and health risks associated with this specific transport pathway and these particular contaminants (Brotons et al., 2010; Taylor et al., 2010; Csavina et al., 2011). Data on contaminant concentrations in bulk atmospheric particulates are regularly gathered by government agencies and industrial operators in various countries at sites with suspected pollution sources. In addition, occupational samplers are used to assess worker exposure to contaminants of concern. These data are sometimes made publicly available and are used to establish possible environmental risks by comparing them with emissions and environmental standards.
The potential speed and distance through which contaminants are transported by biota are typically much less than those associated with transport by other pathways, making contaminant transport by biota more of a localized environmental problem (Arthur and Markham, 1982; Hope, 1993). Contaminant transport in soil and water is also usually more of a localized environmental problem compared to contaminant transport by air, which is more of a worldwide phenomenon that has important global implications. For example, it has been estimated that approximately 60 % of all atmospheric arsenic (As) initially originates as a point source contaminant from mining operations and then is subsequently transported and dispersed globally by atmospheric particulates (Chilvers and Peterson, 1987). This mechanism is an underappreciated and potentially understudied problem, especially with respect to other transport pathways (mainly water in the case of As). Nevertheless, atmospheric transport of metal and metalloid contaminants has important health and environmental implications from local to global scales and, therefore, should be further studied.
Atmospheric dust can be generated by natural processes associated with wind erosion, such as abrasion and deflation, as well as by anthropogenic activities and disturbances (e.g., Ravi et al., 2011). Most natural and anthropogenic sources of atmospheric dust are found in arid and semiarid regions, which account for approximately 40 % of the global land area and are inhabited by one-third of the world’s population (UNSO, 1997). These environments are particularly susceptible to wind erosion and dust emissions because vegetation cover is typically sparse, surface moisture content is generally low, and soil particle cohesion is often inherently weak due to their moisture and low organic matter content (Pye, 1987; Sivakumar, 2007). Natural sources of atmospheric mineral dust are typically associated with low levels of chemical contaminants (e.g., Reheis et al., 2009) and include dune fields and playas, desertified lands, deserts and shrublands, rangelands and grasslands, as well as forests (Tegen and Fung, 1995; Goudie and Middleton, 2006; Breshears et al., 2009). Anthropogenic sources of atmospheric mineral dust and aerosol are typically associated with higher levels of chemical contaminants (e.g., Vega et al., 2001) and include croplands and agricultural systems, feedlots and pastures, dirt roads, construction activities, landfills, mining operations, and mine tailings (Chow et al., 1992; Chow et al., 1994; Nordstrom and Hotta, 2004; Triantafyllou et al., 2006; Csavina et al., 2011).
When considering the major sources of atmospheric particulates in arid and semiarid environments, forests and grasslands typically have the lowest potential emissions followed by shrublands and deserts (Breshears et al., 2009; Okin et al., 2009; Figure 3). Because most natural sources of atmospheric particulates typically all have low concentrations of associated contaminants (Zobeck and Fryrear, 1986; van Pelt and Zobeck, 2007; Reheis et al., 2009), they typically pose a low potential risk to human health and the environment. However, disturbances that reduce or remove vegetation cover, such as fire and prolonged drought, can result in an increased potential for particulate emissions and contaminant transport (Whicker et al., 2006; Breshears et al., 2012; Ravi et al., 2011). When considering anthropogenic sources of mineral atmospheric particulates, mining operations potentially pose the greatest risk to human health and the environment because these sources are globally extensive, generate large quantities of particulates nearly continuously, and have high potential for toxic contaminants associated with the emissions (Thornton, 1996; Porcella et al., 1997; Lacerda, 1997; Chakradhar, 2004; Neuman et al., 2009; Brotons et al., 2010; Csavina et al., 2011). Agricultural practices are also globally extensive and can generate large quantities of particulates, but, unlike mining operations, agricultural dust is typically generated only in response to strong wind events and only when fields are bare or fallow (Fryrear, 1986; Stout and Zobeck, 1996). Properly managed croplands and agricultural systems are therefore not likely to generate significant quantities of atmospheric particulates throughout most the year when fields are covered by crops. In addition, croplands and agricultural systems likely have lower concentrations of toxic chemicals associated with particulate emissions than most types of mining operations and therefore may pose less potential risk to human health and the environment.
Figure 3.
Natural and anthropogenic sources of dust associated with relative amounts of emissions, contaminant concentration, and risk to human health and the environment.
Although there are numerous natural and anthropogenic sources of atmospheric particulates, mining operations pose the greatest potential risk to human health and the environment (Figure 3). In a recent comprehensive assessment of the worst environmental pollution problems, activities associated with mining operations, including artisanal gold mining; metal smelting and processing; industrial mining; and uranium mining, were identified as four of the world’s top ten pollution problems (Ericson et al., 2008). In addition, a recent assessment on the global health impacts of contaminants in the environment identified Hg, Pb, As, Cr, pesticides, and radionuclides as the six most toxic pollutants threatening human health (McCartor and Becker, 2010). Dust and aerosol emissions associated with mining operations are commonly associated with significantly elevated levels of one or more of these contaminants, including Hg, Pb, As, and Cr (Meza-Figueroa et al., 2009; Brotons et al., 2010; Csavina et al., 2011, Corriveau et al., 2011). Furthermore, contaminants commonly associated with particulates from mining operations are usually most concentrated in the finer particle size fraction (< 2 μm), which travels greater distance in the environment and poses a greater potential risks to human health than coarser particles (e.g., Ravi et al., 2011). As climate is projected to become hotter and drier and population and land-use intensity are projected to increase for many arid and semiarid regions worldwide (CCSP, 2008; IPCC, 2007; Seager et al., 2007; UNSO, 1997), the potential for atmospheric particulate emissions and associated contaminant transport from both natural and anthropogenic sources will likely increase in many parts of the world.
To address some of key emerging environmental issues related to contaminant transport by atmospheric particulates, this work provides a critical review of the literature on (i) the fate and transport of contaminants in dust and aerosols from mining operations, (ii) the current methodologies for evaluating atmospheric particulate emissions and associated contaminant concentrations, and (iii) the potential health and environmental risks associated with airborne contaminants from mining operations. More generally, this review provides an overview on the mechanisms and environmental implications of dust and aerosol generation and evaluate the potential importance of contaminant transport relative to other transport pathways (soil, water, and biota.)
2. Mechanisms and Implications of Atmospheric Particle Emissions
Wind erosion is the largest source of tropospheric aerosols (e.g., Tegen et al., 1996) and affects all major components of the biosphere (Ravi et al., 2011). Windblown dust and aerosol can be transported in the environment by three distinct mechanisms that are roughly differentiated based on the particle size: surface creep, saltation, and suspension (Bagnold, 1941). All three processes redistribute soil, nutrients, and contaminants in the environment at different spatial scales ranging from local to global (Goudie and Middleton, 2006; Li et al., 2007; Field et al., 2010). Large particles are transported by surface creep (> 2,000 μm) and saltation (60–2,000 μm) and account for the majority of mass movement at the local scale (Stout and Zobeck, 1996; Ravi et al., 2011). Smaller silt- and clay-sized particles from the soil (< 60 μm) are transported by suspension and are available for long-range transport at regional, continental, and global scales (Chadwick et al., 1999; Prospero et al., 2002). Additionally, aerosol produced by condensation of hot vapors (such as those emitted by smelters) have particle sizes that are typically < 60 μm and also are, therefore, susceptible to long-range transport. Most wind-driven particle transport from soils occurs close to the soil surface and decreases sharply with height, with surface creep and saltation accounting for the majority of total transport (Shao et al., 1993). Surface creep is characterized by particles that are too large to be lifted from the ground by aerodynamic forces and thus roll or ‘creep’ across the soil surface. Saltation is characterized by particles that are lifted from the ground but are too large to be dispersed into the atmosphere and therefore bounce along the soil surface.
Creep and saltation processes play an important role in wind erosion and the generation of dust emissions. The kinetic energy associated with the bombardment of large particles (> 60 μm) impacting the soil surface through creep and saltation may be sufficient to overcome the cohesive forces that bind fine particles together, which typically renders them unavailable for wind-driven transport (Shao et al., 1993). Creep and saltation processes can also increase dust emissions by the production and subsequent suspension of dust-sized particles through abrasion of saltating particles (Smith et al., 1991; Wright, 2001; Hagen, 2001; Mackie et al., 2006; Bullard et al., 2007). Because soil organic matter and nutrients (e.g., nitrogen, phosphorus), as well as many environmental contaminants (e.g., As, Pb), are often associated with small particles (Li et al., 2007; Csavina et al., 2011, Corriveau et al., 2011), the production and subsequent entrainment of dust-size particles in the atmosphere is an important mechanism of environmental transport that has global implications (Goudie and Middleton, 2006; Ravi et al., 2011).
Wind erosion and atmospheric particulate emissions are influenced by a variety of factors including climatic conditions, land-use, vegetation cover, and soil characteristics (Shao and Lu, 2000; Toy et al., 2002; Zobeck et al., 2003; Breshears et al., 2009; Okin et al., 2006; Ravi et al., 2011; Figure 4). Particle entrainment occurs when the wind shear velocity at the soil surface exceeds the shear strength of the soil aggregates by a certain minimum value, referred to as the threshold friction velocity or threshold shear velocity (Gillette et al., 1980; Shao, 2008). Many environmental factors, such as atmospheric humidity and surface soil moisture, can affect the minimum threshold friction velocity at which particles begin to detach from the soil surface and become available for transport by wind. Because the soil moisture in many arid and semiarid regions is typically in equilibrium with atmospheric moisture, relative humidity has a large influence on soil interparticle forces, which in turn influence the threshold friction velocity, resulting in a complex relation between relative humidity, particle size and soil erodibility (Ravi et al., 2004). Other environmental factors, such as land-use and vegetation cover, can also have a large effect on the threshold friction velocity and the potential for atmospheric particulate emissions. Vegetation cover and other non-erodible surface elements protect the soil surface by absorbing a fraction of the wind momentum flux thereby reducing the shear stress on soil particles (Stockton and Gillette, 1990). The effectiveness of vegetation in protecting the soil surface from erosion depends upon the type, cover, and arrangement of vegetation (Ravi et al., 2011). For example, in relatively undisturbed systems where land use is low, wind erosion and dust emissions are likely to be relatively low when vegetation cover is dense (> 40 %) but can increase rapidly as vegetation cover approaches intermediate levels of density (16–40 %), which results in turbulence (i.e., wake interference flow) near the soil surface, greatly increasing the potential for dust emissions (Breshears et al., 2009; Wolfe and Nickling, 1993). Soil properties, including surface roughness, clay content, particle size distribution, organic matter, and physical and biological soil crusts, also play a key role in determining the potential for wind erosion and atmospheric particle emissions under a given set of climatic conditions and vegetation cover (Gillette, 1979; Belnap and Lange, 2003; Okin et al., 2006).
Figure 4.
Factors influencing dust emissions by wind erosion and the environmental implications of dust emissions (reprinted from Ravi et al., 2011).
Atmospheric particle emissions affect all major components of the biosphere and provide important biogeochemical linkages between the atmosphere, hydrosphere, and pedosphere (Schlesinger et al., 1990; Syvitski, 2003; Munson et al., 2011). Understanding the role of aeolian processes in the biosphere is critical for managing the world’s arid and semiarid regions and for addressing emerging challenges associated with environmental transport, land-use intensification, and climate change (Field et al., 2010; Ravi et al., 2011). Land degradation and desertification by accelerated wind erosion is a major environmental problem worldwide (Schlesinger et al., 1990; Bridges and Oldeman, 1999) and will likely remain so throughout the 21st century (Lal, 2001; Valentin et al., 2005). Globally, there are approximately 430 million ha of land surface susceptible to wind erosion (Middleton and Thomas, 1997). Global estimates of soil erosion are 20–100 greater than average rates of soil renewal (Cuff and Goudie, 2009), which is particularly true in arid environments, as it takes hundreds to thousands of years to form only a few centimeters of soil (Pillans, 1997). Increased human activity is largely responsible for observed increases in wind erosion and global dust emissions that have been documented in the past (Middleton et al., 1986). Anthropogenic activities such as cultivation, livestock grazing, deforestation and vegetation shifts are estimated to account for more than half of the global atmospheric particle load and are responsible for increased inputs of aeolian sediment to mountains and marine systems worldwide (Tegen and Fung, 1995; Neff et al., 2008; Painter et al., 2010).
Wind erosion and associated particle emissions have important environmental and ecological consequences, including the loss and redistribution of soil nutrients (e.g., Zobeck and Fryrear, 1986), pollution of air masses and water bodies (e.g., Griffin et al., 2001), and direct and indirect effects on global radiation budgets and cloud condensation nuclei (e.g., Kaufman et al., 2002; Figure 4). Wind erosion has a large effect on the loss and redistribution of soil nutrients and plays a key role in exacerbating land degradation and the desertification process (Schlesinger et al., 1990; Lal, 2001; Li et al., 2007; Okin et al., 2009). This is because the erosive force of wind preferentially removes the finer silt- and clay-sized particles, which contain most of the cation-exchange capacity and water-holding capacity of the soil (Toy et al., 2002). Over time, many of these fine particles can eventually be lost from the system due to wind erosion (Gillette, 1979), resulting in local depletion of soil fertility (Zobeck and Fryrear, 1986; Li et al., 2007). Large quantities of nutrient-rich particles are deposited in terrestrial and marine systems throughout the world with important implications for global biogeochemical cycles (Okin et al., 2004; Mahowald et al., 2009). Wind erosion can also transport environmental contaminants such as metals and metalloids, pesticides, and biological pathogens, all of which are typically associated with finer atmospheric particles and tropospheric aerosols (Pope et al., 1996; Griffin et al., 2001; Csavina et al., 2011). Thus, anthropogenic contaminants such as metals and metalloids in particles that have deposited on soils in the vicinity of mining and other industrial operations can be suspended as atmospheric particulates from soils that have become susceptible to wind erosion.
Tropospheric aerosols generated by wind erosion can have both direct and indirect effects on Earth’s radiation budget (e.g., Tegen et al., 1996). Atmospheric mineral dust can absorb and reflect solar radiation and thus has direct effects on global radiation budgets by reducing the amount of radiation reaching the Earth’s surface (Andreae, 2001). Non-absorbing particulates, such as desert dust, typically result in atmospheric cooling unless iron concentration in the dust is enough to absorb visible and near-infrared wavelengths, which could result in atmospheric warming (Sokolik and Toon, 1996; Ramanathan et al., 2001). Atmospheric particulates also have direct and indirect effects on the nucleation and optical properties of clouds; an increase in local dust and aerosol emissions can result in the suppression of precipitation at landscape and regional scales (e.g., Rosenfeld et al., 2001). Atmospheric particulates act as cloud condensation nuclei and can reduce precipitation because they result in a partitioning of atmospheric moisture into a greater number of droplets within the cloud (Twomey effect) and can also result in cooling of the land surface, which causes a decrease in convection and evapotranspiration (Albrecht, 1989; Rosenfeld et al., 2001). Particles can also have an indirect effect on climate through the snow-albedo feedback (Painter et al., 2007; Steltzer et al., 2009). Atmospheric particle deposition can decrease snow albedo, causing a darker surface that absorbs solar radiation, triggering earlier and faster snowmelt, which can potentially result in less available water supplies, especially in areas where water shortages occurs. The direct and indirect effects of atmospheric particulates on radiation budgets and cloud properties can collectively lead to a weaker hydrological cycle and reduced water availability in many arid regions throughout the world (Hartmann, 1994; Ramanathan et al., 2001; Painter et al., 2007; Hui et al., 2008).
3. Mining and Smelting Operations & Environmental Assessment
3.1. Background
Modern day mining operations include excavating, crushing, grinding, separation, smelting, refining and tailings management (Figure 5). All processes produce large quantities of dust and aerosol, including the transportation of ore with haul trucks and trains (Reed and Westman, 2005). Though the vast majority of mining operations produce coarse dusts, high temperature processes produce fume and fine particulates potentially laden with metals and metalloids that are present in the ore.
Figure 5.
Coarse particulate emissions from (a) crushing and grinding and (d) wind erosion of mine tailings; fine particulate dust emissions from (b) smelting and (c) slag dumps. a. (SBM Machinery, 2010) b. (Listavia International, 2011) d. (Courtesy Blenda Machado, Mexico).
Dust and aerosol particles emitted from mining operations may mobilize dangerously high levels of metals and metalloids including the neurotoxic elements Pb and As, which can then accumulate in soils, natural waters and vegetation. It has been estimated that 40 % of the total atmospheric emissions of As arise from smelting operations (Alloway and Ayres, 1997). Accumulated contaminants in local soils and mine tailings can be further dispersed by wind erosion. As will be discussed in Section 4, high atmospheric levels of metal and metalloid-bearing atmospheric particulates can have a substantial impact on the environment and human health (Berico et al., 1997; Soukup et al., 2000; Ghio and Devlin, 2001; WHO, 2003). The magnitude of this impact is dependent both on contaminant concentration and the size of the particle.
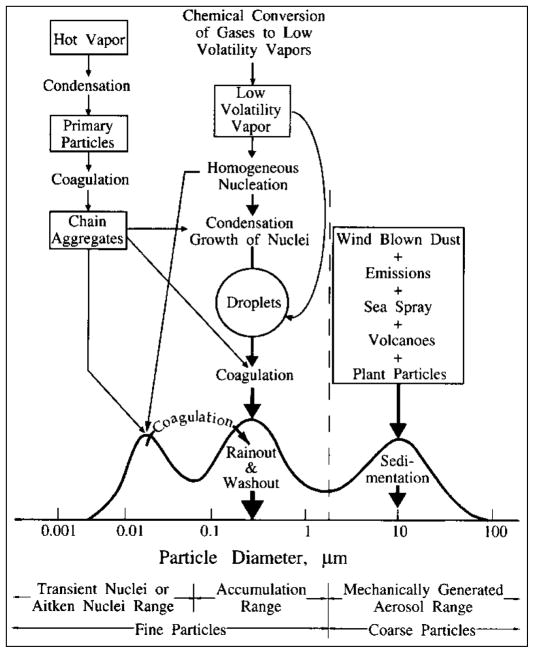
Atmospheric dust and aerosols occur in three main size ranges: the ultrafine (or Aitken), accumulative and coarse modes, as is discussed in detail by Seinfeld and Pandis (2006) (Figure 6). All three modes are of relevance to mining-related emissions: Ultra-fine particles are often generated from hot vapors, including smelting and slag dumps (Figure 5b and c), and are so small that they rapidly diffuse, coagulate and grow into the accumulation mode with characteristic residence times in the atmosphere of minutes to hours. At the other end of the spectrum, coarse particles are generated by mechanical action, including crushing and grinding of ore and wind erosion of mine tailings (Figure 5a and d), and are large enough to rapidly sediment out of the atmosphere in minutes to hours. Particles emitted from mine tailings due to wind erosion are usually in the coarse range. However, they potentially can have a wide size distribution as well as a range of metal and metalloid concentrations. In particular, efflorescent deposits, generated by evaporating water that flows to the surface of the tailings by capillarity, may contain metal and metalloid concentrations that are much higher than the surrounding tailings topsoil due to presence of crystallized soluble contaminant salts (Meza-Figueroa et al., 2009, Hayes et al., 2012). Dry, efflorescent particles are particularly susceptible to wind erosion. In the middle of the size spectrum, accumulation mode particles are too large to diffuse/coagulate at a significant rate and yet are too small to sediment by gravity so they accumulate in the atmosphere. Accumulation mode particles are highly dependent on the water cycle for the rainout/washout of the particles and therefore can have a global impact, with an average residence time of 10 days in the atmosphere (Hinds, 1999). Arid and semiarid climates can have dust storms that can transport even coarse particles tens or even hundreds of miles as discussed above. Drier climates also tend to prolong the transport of particles.
Figure 6.
Idealized description of processes that affect atmospheric aerosol formation in three size ranges or modes – ultrafine (or Aitken), accumulation, and coarse (reprinted from Seinfeld and Pandis, 2006).
Particle size also affects dust and aerosol deposition efficiency in the human respiratory system upon inhalation. Coarse particles, such as those resulting from ore crushing and grinding, deposit in the upper respiratory system and are swallowed and go through the digestive system where contaminants may be absorbed, depending on their bioavailability. In contrast, fine particles, such as those originating from smelting operations, are respired deep into the lungs where they can be transported directly to the blood stream (Krombach et al., 1997; Park and Wexler, 2008; Valiulis et al., 2008). In addition, dermal deposition and incidental ingestion are exposure mechanisms that are related to particle size. Therefore, determining the chemical composition in dust from mining operations as a function of particle size is crucial in quantifying the potential deleterious effects on human health and the environment.
3.2. Dust and Aerosol Monitoring
Various types of aerosol and dust samplers have been used in studies to characterize atmospheric particulates originating in mining operations. Example studies presented in this section and Sections 3.3 and 3.4 are not necessarily related to mining operations due to the relative lack of research in this area, but provide an example application that can be used for such investigations.
Bulk particle collection (without size fractionation) allows for the quantification of the total atmospheric particulates and contaminants in the ambient air. Some studies simply collect fallout or settleable particulate matter (>10–20 m) by open collection containers (e.g., Munksgaard and Parry, 1998; Deb et al., 2002). Other studies investigate atmospheric particulates by the deposition onto top soil, which requires a consistent method of soil collection (e.g., Taylor et al., 2010). Additionally, for the understanding of saltation from soils or wind erosion, passive samplers are often used, including a Modified Wilson and Cook (MWAC) and Big Spring Number Eight catcher (BSNE) (Fryrear, 1985; Goossens et al., 2000; Goossens and Offer, 2000). High volume samplers such as total suspended particle (TSP) samplers have been used to collect particles with size up to 250 μm (Benin et al., 1999; Shaheen et al., 2005; Garcia et al., 2008). These measurements can be used to understand the magnitude of the contaminant environmental hazards and for comparative measurements with other analyses.
Concentrations of particulate matter (PM) with aerodynamic diameters less than 10 μm (PM10) and 2.5 μm (PM2.5) are measured using dichotomous samplers with size-selective inlets. These concentrations are widely used for regulatory work due to the health concerns associated with these particles (Lv et al., 2006; Moreno et al., 2007; Kon et al., 2007; Tursic et al., 2008). Recent epidemiological studies indicate that smaller size fractions may be most intimately involved in causing hazards to human health; therefore, studies with PM2.5 measurements may be relevant (Shaheen et al., 2005; Moreno et al., 2006; Querol et al., 2007).
Particle counters and mobility particle sizers are also used to collect atmospheric particulate samples (Neuman et al., 2009). The particle number concentration as a function of diameter can be measured using a scanning mobility particle sizer (SMPS) system (Wang and Flagan, 1990; Csavina et al., 2011). Optical particle counters measure mass concentration of aerosols and can be set to respond to different particle diameters with a selective inlet (< PM1, < PM2.5, and < PM10) (e.g., Chan et al., 2005). Locating these at various horizontal and vertical positions can provide measurements of horizontal and vertical dust and aerosol flux transport (e.g., Gillies et al., 2005).
For size-fractionated dust and aerosol collection, multi-stage cascade impactors have been used (Querol et al., 2000; Tursic et al., 2008). Size-fractioned samples can be individually analyzed both physically (e.g., microscopically, gravimetrically) and chemically. The mass distributions of individual chemical species can be determined by analyzing the chemical composition of particles collected in each size range, as described in the next section. Spear et al. (1998) utilized an Andersen cascade impactor to study Pb concentrations around a Pb smelter. Corriveau et al. (2011) used a Proton Induced X-ray Emission Spectroscopy Cascade Impactor (PCI) sampler to assess As contamination from a mine-tailing site. Micro-orifice uniform deposit impactors (MOUDI) have been used in several studies (Querol et al., 2000; Allen et al., 2001; Tursic et al., 2008; Csavina et al., 2011). In one model (MSP model #110), the nozzle plates rotate relative to the impaction plates to achieve near uniform particle deposition on the collecting substrates over the impaction area. Marple et al. (1991) described in detail the calibration and use of MOUDI samplers. The sharp cut-points (particle diameters where 50 % collection efficiency is achieved) for the size fractions are: 0.056, 0.10, 0.16, 0.32, 0.56, 1.0, 1.8, 3.2, 5.6, 10 and 18 μm, respectively. An after-filter is placed after the 10th (smallest particle diameter) stage to collect all particles smaller than 0.056 μm in aerodynamic diameter, whereas a size-selective inlet collects particles larger than 18 μm by impaction. At the typical operating flow rate (30 L/min), the internal wall loss is very low.
Previous field investigations have shown that metals and metalloids, including As, Pb and Cr, have been detected worldwide in clouds, fog and snow (e.g., Tatsumoto and Patterson, 1963; Schemenauer and Cereceda, 1992; Barbaris and Betterton, 1996; Cherif et al., 1998; Rattigan et al., 2002; Mancinelli et al., 2005; Hutchings et al., 2009), with mining activities as possible sources. The effect of mining and smelting emissions in the 19th and 20th centuries from the Australian sites of Broken Hill and Port Pirie on Pb emissions on remote southern hemisphere pristine environments has been detailed in several studies on Antarctic ice cores (e.g., Vallelonga et al., 2003; Burn-Nunes et al., 2011.)
Other sampling techniques exist beyond those reviewed here; however, these are widely used methods in other areas and have best application for the field. Most advantageous is having multiple samplers to provide a comparison measurement to ensure no bias in sampling methods. Additionally, a holistic approach with multiple forms of analyses discussed in the next section provides for the best understanding of ambient aerosol source and transport vectors.
3.3. Contaminant Analysis
Digestion is typically used to extract contaminants of concern from collected dust and aerosols into the aqueous phase. The contaminant and analytical method chosen will determine the type of digestion to use. For example, for ion chromatography work, deionized water is typically used to preserve the composition of aerosols. Acid digestion, with HF and HNO3, HNO3 and HClO4, or HNO3 and HCl, is used to extract metal and metalloid species from dust and aerosol samples (Harper et al., 1983; Shaheen et al., 2005; Moreno et al., 2007; Garcia et al., 2008). Extraction is sometimes enhanced by heating and/or agitation. The aqueous extracts can then be subjected to a suite of chemical analysis, including inductively coupled plasma – mass spectrometry (ICP-MS) (Querol et al., 2000; Allen et al., 2001; Herner et al., 2006), inductively coupled plasma – atomic emission spectrometry (ICP-AES) (Lv et al., 2006; Li et al., 2007), and atomic absorption spectrophotometry (AA) (Benin et al., 1999; Shaheen et al., 2005; Garcia et al., 2008). Depending on the sampler and analysis to be done, various sampler substrates can be used, such as cellulose membrane filters (Querol et al., 2000; Pekney et al., 2006), quartz fiber filters (Lv et al., 2006; Querol et al., 2007), glass fiber filters (Shaheen et al., 2005), aluminum foil substrates (Csavina et al., 2011) and polycarbonate filters (Moreno et al., 2006; Csavina et al., 2011).
For water soluble ions, ion chromatography (IC) and capillary electrophoresis (CE) have been used to measure chloride, sulfate and nitrate (Querol et al., 2000; Lv et al., 2006; Tursic et al., 2008). For soluble cation measurements, colorimetry-flow injection analysis (FIA) and ICP-AES have been used (Querol et al., 2000; Lv et al., 2006).
Gravimetric analysis often requires an ultra-microbalance with a filter attachment for mass quantities that are small (< 100 μg). This is often the case for size-fractionated samples. For larger quantities of mass such as those found with TSP and PM10 samplers, a balance with the sensitivity of 1 mg is often sufficient.
X-ray diffraction has been used to examine the mineralogy of non-soluble materials in dust (Spear et al., 1998; Querol et al., 2000; Moreno et al., 2007). Moreno et al. (2007) identified silica, kaolinite, alunite, jarosite, illite and iron oxides in PM10 particles in the vicinity of an ore processing plant near a gold mine in Southeastern Spain. In comparison, after a spill of mining heavy metals in Southwestern Spain, Querol et al. (2000) identified pyrite as the main component, along with clay, quartz, calcite, gypsum, and plagioclase. X-ray absorption spectroscopy has also been used to characterize As-laden mine tailings (Paktunc et al., 2003; Slowey et al., 2007).
Morphological and chemical characterization of particles by scanning electron microcopy (SEM) and field emission SEM (FESEM), have been used to examine the microscopic structures of aerosol particles (Csavina et al., 2011). Using polycarbonate filters, Querol et al., (2007) identified silica, Al-Si clay, sulfates (with K and Al), as well as K, Fe and Ti. Three metalloids, As, Sb, and Te, were also identified, possibly derived from mine tailings.
Isotope concentrations can provide an understanding of the geological nature of elements in ores (Day et al., 2010). They are often used for source apportionment when isotope concentrations in dust and aerosols are compared to the mined ore body. Pb is often associated with mined ores, and therefore Pb isotope ratios are commonly used for source apportionment (e.g., Munksgaard and Parry, 1998; Hsu et al., 2006; Taylor et al., 2010). Pb isotopes can be analyzed by thermal ionization mass spectrometry or ICP-MS (Cheng and Hu, 2010). For example, the Burn-Nunes et al. (2011) study of East Antarctica Law Dome ice cores confirmed that the isotopic Pb signature of samples dated to the late 19th century was identical to the Pb emitted from Broken Hill and Port Pirie, Australia, at that time.
In addition to isotope analysis, source apportionment can also be done utilizing chemical concentrations. Some examples of this include measurements of enhanced contaminant concentration according to wind patterns consistent with the source plume (Fernandez-Camacho et al., 2010), ratios of metal concentrations of the source compared to background (Beavington et al., 2004; Bi et al., 2006), or enrichment factors (Meza-Figueroa et al., 2009). Modeling discussed in the next section can also be used for source apportionment by trajectory of dust emissions.
3.4. Modeling
Dust and aerosol suspension and dispersion can be best investigated by an integration of field measurements, physical approaches and computer modeling. The pioneer work developed by Bagnold (1941) and investigations by Chepil and others (Chepil, 1962; Woodruff and Siddoway, 1965) served as starting point to numerous models (Goudie and Middleton, 2006). Existing models use mathematical equations that are continuously adapted to physical and/or empirical approaches to describe the factors and processes that are involved in wind erosion. A comprehensive numerical model of steady-state saltation (COMSALT) was one of the first physically-based models that could reproduce a wide range of experimental observations using a minimum of empirical equations (Kok and Renno, 2009). The model takes into account gravity, drag, particle spin, fluid shear, turbulence and a parameterization of the splashing of surface particles by impacting saltating particles; the latter is considered a stochastic process. The key issue is to identify the concepts behind the parameterization of soil (i.e. understand the transport mechanisms that contribute to dust emissions), vegetation, and land management effects on the susceptibility of landscapes to wind erosion (Webb and McGowan, 2009). A disadvantage of empirical models is that most rely upon field-measured inputs, which are not available at broad spatial scales (Woodruff and Siddoway, 1965; Fryrear et al., 1998; Webb and McGowan, 2009). On the other hand, physically based models typically do not account for temporal variations in soil erodibility (Webb and McGowan, 2009). Currently, there are a limited number of integrated dust models that can provide forecasts in both time and space, such as the Dust Regional Atmospheric Model (DREAM).
The DREAM model is a deterministic numerical model that simulates the emission, transport and deposition of dust and aerosol with predicted atmospheric conditions, and is coupled with the Weather Research and Forecasting (WRF) model (Nickovic et al., 2001). WRF is a state-of-the art weather forecasting model that can be run at horizontal resolutions of 1–10 km (Skamarock et al., 2005). The DREAM model is validated with in-situ PM2.5 and PM10 data usually from USEPA monitoring stations, satellite images of the particle plumes, and ground-based optical sensing of vertical profiles and visibility or other light extinction monitoring instruments. In addition to this, the DREAM model performance has been tested for various dust storm events in various places using gridded analysis or forecasting fields from various sources for initial and boundary conditions (Nickovic et al., 2001).
A current strategy to model wind and particle flows from areas susceptible to wind erosion involves the use of computational fluid dynamic models (CFD), which can be run at relatively high resolutions (< 1 m) (Holmes, 2006; Diego et al., 2009). Unlike most currently used regulatory air quality models, CFD simulations are able to treat topographical details such as terrain variations and building structures in urban areas, as well as local aerodynamics and turbulence. Although CFD can provide high resolutions, it can become computationally expensive. Feng and Ning (2010) used CFD and the Owen (1964) model to determine saltation fluxes. FLUENT CFD was used to reproduce wind patterns over a complex with microtopography (60 m × 60 m) in the Chihuahuan desert that were previously obtained by the Quick Urban & Industrial Complex (QUIC) field model developed by Bowker et al. (2006). Wind profiles using FLUENT CFD agreed well with measured wind speeds. The authors also simulated sand fluxes at several positions of a dune brick in Minquin, China under two different wind directions. The Owen (1964) model was linked to FLUENT’s CFD codes to simulate sand fluxes. More recently, FLUENT CFD software has become an approved air pollution tool by The EPA National Exposure Research Laboratory. In a different study, Torno et al. (2011) calculated dust emissions using CFD and realistic topography obtained from Light Detection and Ranging (LIDAR) techniques, which provides high resolution topographic data, and wind events from a fixed direction correlated well with wind speed measurements. Badr and Harion (2005, 2007) used CFD to model fugitive dust emissions from stockpiles. Their calculations showed that effects of terrain geometry on wind flow patterns play an important role in erosion and particle transport assessment.
To date, there have been few attempts to model atmospheric particulate emission from mine tailings piles. Kon et al. (2007) developed a wind erosion model designed to predict dust emission rates of flat dry tailings prone to wind erosion, taking into account fluctuations in wind velocity. The performance of the wind erosion model was assessed using wind tunnel and field data reported in the literature. It was found that analytical saltation models derived for agricultural lands or desert dunes can be applied to industrial minerals that are crushed and have a density different from that of quartz. Field experiments undertaken by Kon et al. (2007) on the tailings dumps of Mantos Blancos copper mine, which is located in Atacama Desert in Chile, demonstrated that the model predicts satisfactorily the occurrence and the magnitude of wind erosion events on mine tailings.
3.5. Case Studies
In many cases, towns are built around mines in remote areas and so the source of contaminated atmospheric particulates is easy to discern. However, in those cases where mining occurs along with other industries, source apportionment can become more difficult. For example, Barcan (2002) performed a study in the Severonickel Smelter Complex in Monchegorsk, Russia, in which samples were obtained directly from the smelter stack. An important finding from their study was that the fine particle (< 2.5 μm) fractions were enriched in Pb, Zn, and As oxides. Santacatalina et al. (2010) surveyed Southeast Spain for fugitives emissions of particulate matter and used PM10 data in a Positive Matrix Factorization (PMF) receptor model to identify the main sources of mineral, road traffic, secondary, sulfate, petroleum coke, sea spray and industry, with x-ray diffraction (XRD) and scanning electron microscopy-energy dispersive x-ray spectroscopy (SEM-EDX) techniques. Similarly, Allen et al. (2001) performed principal component analysis (PCA) on metal concentrations from MOUDI size-fractionated samples collected in the United Kingdom. Their study identified main components of sources to be road traffic, utility and industrial combustion, resuspension and industrial activities.
Munksgaard and Parry (1998) were able to show differences in pre- and post-mining Pb isotope ratios and ore-derived Pb versus local soil-derived Pb in a mining town. Bi et al. (2006) examined Pb isotope ratios to distinguish the difference between flue gas particles and waste from a zinc smelter in western Guizhou, China. Depending on the types of sources that can be impacting the samples collection and the accessibility to equipment may make one analysis better than others.
Meza-Fiegueroa et al. (2009) analyzed soils in Sonora, Mexico around the Pilares mine Nacozari tailings site for metal and metalloid content, which showed that contaminant dispersion took place primarily by surface run off, but enrichment factors for Hg, Cu, As, Zn and Pb in residential soils arose from wind erosion. Benin et al. (1999) reported that the impacts of smelting and refining were particularly acute adjacent to the Torreón site in Mexico where a large active nonferrous smelter is located. As shown in Figure 7, concentrations of As, Pb, and Cd in roadside dust decreased with distance from the stack. Differences in the decay shape of curve might be a consequence of differences in the particle size distribution for the three species. In addition, metal and metalloid concentrations were significantly higher to the south and west of the site, but lower to the east. This was attributed to either the effect of prevailing wind or other factors such as transportation, unloading and distribution of ore within the complex.
Figure 7.
Plots of contaminant concentrations in roadside dust versus distance from the smelter stack (reference point), approximately the emissions of the industrial complex in Torreon, Mexico. (A) Arsenic; (B) cadmium; and (C) lead (reprinted from Benin et al., 1999).
Earlier studies have shown that distance from the source and meteorological factors play important roles in exposure to potentially toxic metals and metalloids. For example, a study by Landrigan et al. (1975) showed that Pb, Cd, Zn and As in airborne particulates near a large ore smelter in El Paso fell rapidly with distance and reached background values 4 – 5 km from the smelter.
A recent study of soil and outdoor and indoor dust around the Torreón smelter by Soto-Jiménez and Flegal (2011) showed that contaminant concentrations were greatest near the smelter, with the highest levels corresponding to the prevailing wind direction. Soil and dust Pb concentrations were orders of magnitude above background concentrations of 7.3–33.3 μg g−1. Atmospheric Pb deposition rates in the city was also significantly elevated (130 to 1350 μg m−2 d−1), with values greatest at distances less than 1 km from the smelter. Emission sources were not only evident in environmental samples but in Pb isotope measures of neighboring children blood, which were indistinguishable from the smelted Pb-ores and the environmental samples.
The effect of prevailing winds in redistributing contaminants in the vicinity of mining and smelting operations is not a new observation, with other studies demonstrating that distance from the source and meteorological factors play significant roles in the soil accumulation of metals and metalloids (e.g., Cartwright et al., 1977; van Alphen, 1999; Sterckeman et al., 2000; Stafilov et al., 2010; Fernandez-Camacho et al., 2010; Taylor et al., 2010; Sánchez de la Campa et al., 2011; Ojelede et al., 2012). Moreover, dispersion plumes have been shown to correlate with smelter stack height and smelter capacity (e.g., Knight and Henderson, 2006).
Utilizing an atmospheric dispersion model and spatial modeling of urban surface soil concentrations, Taylor et al. (2010) found that elevated soil metals were a consequence of mining emissions from the Xstrata Mount Isa Mines lease in Queensland, Australia, where elevated concentrations of Cd, Cu, Pb, and Zn were observed. At a goldmine in Ghana, Amasa (1975) analyzed soils samples for As to account for As concentrations in human hair samples, which were as high as 1,940 and 268 ppm for mine workers and citizens, respectively. Soil samples were found to be 147, 67.2, and 96.5 ppm at 300 yards, 1.5 mi, and 9 mi away from the smelting stack, respectively. In Humberside, UK, Rawlins et al. (2006) measured Pb and Sn in an area of land around a former smelter and showed that significant amounts of metals were deposited up to 24 km from the smelter by the prevailing wind with an estimated 2500 and 830 ton of excess Pb and Sn, respectively, deposited in the area. Soil sampling allows for a quick method to discovering the contaminants present due to mining operations.
TSP results from Beavington et al. (2004) in Port Kembla, New South Wales, Australia, showed more than 74 % reduction in atmospheric metal concentration around a smelter from 1978 to 2001–2002 due to improved environmental emissions control. However, even with improvements, the air quality still showed intermediate to high enrichment in metals and metalloids (Cd, Se, Cu, Pb, An, Br, As and Ni).
Fall-out was assessed by Deb et al. (2002) in atmospheric particulates from an urban area in central India. They found the total annual flux of As to be as high as 1.12 kg km−2 yr−1 with the largest emission of atmospheric As from pyrometallurgical processes employed in the production of non-ferrous metals such as Pb, Cu and Zn. Munksgard and Parry (1998) were able to show that Pb fall-out from settling atmospheric particulates increased significantly at a rate consistent with proximity to the McArthur River Mine, Northern Territory, Australia. A specific collector was designed by Brotons et al. (2010) to measure mining waste in southeast Spain transported by wind into dust traps placed at three heights and from eight wind directions. The results showed that nearby towns, farming areas and beaches would be affected by high levels of wind-eroded dust carrying high concentrations of metals, especially Zn and Pb. Still, without size-fractionated measurements, it is difficult to make any inferences to how these particles will transport in the human respiratory system and environment.
Fernandez-Camacho et al. (2010) used PM10 and PM2.5 to assess emissions from a copper smelter in the city of Huelva, Southwest Spain. Though the smelter accounted for only 8 % of the bulk mass of PM10, the majority of the mass was attributed to the metals and metalloids of environmental interest (As, Se, Bi, Cu, Zn). Additionally, they found that 85 % of the total PM10 As was in the PM2.5 size fraction. In southwestern Spain, Querol et al. (2000) studied suspended particles derived from heavy metal mining wastes. For Cr, Cu, Mn, Ni and Zn, the mass in the PM2.5 fraction comprised 17 to 36 % of that in the TSP, whereas this fraction increased to 59 to 70 % for the mass in the PM10 fraction. In comparison, for As, Cd, Co, Pb, Sb, Se and Tl, the mass fraction in the TSP was 20–48 % for PM2.5 and 80–93 % for PM10. These generally higher percentages suggest that the second group of contaminants exist preferentially in the finer fractions of particles due to their physicochemical properties. Further, the two studies differed in that Fernandez-Camacho et al.’s was near a smelter while Querol et al.’s was only around mine waste. The high temperatures of the smelting process produce fine metal and metalloids particulates that result in relatively high concentrations of metal and metalloids in the fine size fraction.
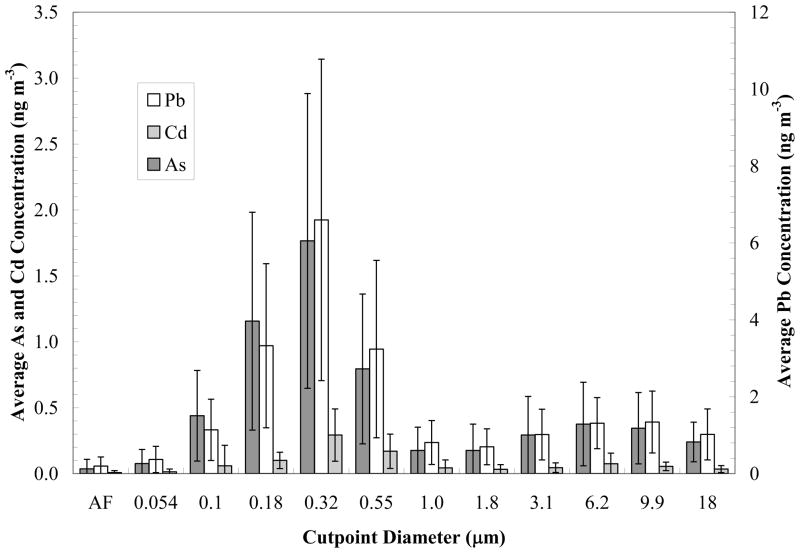
Few size-resolved studies have been published for mine-related dust and aerosol. Both Csavina et al. (2011) and Spear et al. (1998) showed that the majority of atmospheric metals and metalloids around smelting operations are in the fine to ultrafine size fraction. Spear et al. (1998) used sequential chemical extractions to show that the solubility of combined bulk smelter particulate Pb compounds was < 7 %. However, analysis of coarse airborne dust from the blast and furnace processes showed that 65 % of the total Pb was exchangeable. Figure 8 illustrates the size distribution for As, Cd, and Pb concentrations in atmospheric particulate around a Cu smelter in Arizona, US, studied by Csavina et al. (2011). These results show that approximately 75 % of metals and metalloids exist in the PM range below 1 m. Spear et al. (1998) used an Andersen impactor to investigate the chemical speciation of Pb-laden dust associated with a Pb smelter in Montana, US. This study similarly found the largest percentage of Pb in the fine size fraction, which also had higher percentages of bio-available Pb. Corriveau et al. (2011) used a PCI sampler to analyze atmospheric particulate near an As-rich abandoned mine tailings site in Nova Scotia, Canada. They found that As speciation in atmospheric particulates corresponded to soil collected from the tailings. Even though As concentrations were the highest in coarse size fractions (> 8 μm), they found significant As presence in finer particulates (< 2 μm).
Figure 8.
Annual averaged Pb, As and Cd concentrations from MOUDI observations at the Hayden site for the period December 2008 through November 2009. Data represent average concentrations over thirty six 96-hour sampling periods; AF denotes after filter sample; error bars represent standard deviation of measurements (reprinted from Csavina et al., 2011).
Transport of metals and metalloids from mining operations also can occur over large spatial scales. Andrade et al. (2006) studied Cu concentrations in a marine environment off the coast of Northern Chile in Chañaral Bay contaminated by mining activities, particularly by windblown mine tailings which remain untreated. Seawater samples showed dissolved Cu concentrations up to 500 nM.
4. Health and Environmental Impacts
Contaminated dust and aerosol from mining operations can contribute to transport and accumulation of contaminants in soil, water and biota. Interactions between transported particles and repository media may affect speciation, mineralogy and bioavailability of contaminants. For example, Beaulieu and Savage (2005) determined that arsenic oxide particles released from a smelter were further oxidized, dissolved and the As then adsorbed onto soil minerals and colloids upon deposition.
Ingestion of contaminated soils from mining operations emissions or inhalation of emitted particulates may result in contaminant doses that could have deleterious consequences. Some metals and metalloids are known carcinogens (e.g. As, Cd, Cr) and can reduce mental and nervous system function, cause lower energy levels, damage vital organs (ATSDR, 2011), and cause DNA damage (Yáñez et al., 2003). Long-term exposure to these contaminants may mimic degenerative diseases such as Alzheimer’s disease, Parkinson’s disease, muscular dystrophy, and multiple sclerosis (IOSHIC, 1999). The neurotoxic effects of Pb have been well established in the scientific literature: high levels are known to cause a range of effects ranging from comas, seizures and death (Woolf et al., 2007). Due to their increased uptake of contaminants when compared to adults, children are particularly at risk of neurotoxic effects such as learning or behavioral problems including speech, intelligence, attention, behavior, and mental processing problems (Woolf et al., 2007).
Given that most As and Pb exposures are potentially avoidable and owing to their serious childhood environmental health risk factors, special attention is often given to the problem of these elements in human environments. Lubin et al. (2008) found a direct correlation between lung cancer and inhaled As in copper-smelter workers. Multiple studies of Pb exposure and, to a lesser extent, As, have shown these two contaminants are debilitating neurotoxins that cause adverse effects on children’s cognitive function and behavior (Calderón et al., 2001; Wright et al., 2006; Rosado et al., 2007; Wasserman et al., 2007; Munksgaard et al., 2010). In particular, childhood Pb poisoning at blood Pb levels (PbB) > 0.1 g/cm3 is a common occurrence in smelting and mining towns (Wichmann et al., 1997; Tong et al., 2000; Needleman, 2004; Munksgaard et al., 2010; Taylor et al., 2010; Soto-Jimenez and Flegal, 2011), whose communities face a disproportionate exposure risk, with marked losses in intelligence quotient (Baghurst et al., 1992) and also consequentially later childhood emotional and behavior problems (Burns et al., 1999). Laidlaw and Filippelli (2008) presented evidence that atmospheric resuspension of Pb-enriched soil in urban environments represents a persistent source of Pb poisoning in children.
The pervasive effect of environmental Pb emissions from all industrial sources on human exposure was demonstrated by Flegal and Smith (1992) who showed that the natural PbB level in the prehistoric human was as low as 0.16 ng/cm3. This value is some 625 times lower than the current National Health and Medical Research Council (NHMRC, 2009), Centers for Disease Control (CDC, 1991) and World Health Organization (WHO, 1995) blood Pb level of concern (0.10 μg/cm3) in humans. Recent research has indicated that levels well below the currently accepted PbB intervention may result in significant impairment in terms of neurocognitive functioning (Lanphear et al., 2000; Dietrich et al., 2004; Lanphear et al., 2005a; Woolf et al., 2007; Jusko et al., 2008). Specifically, exposure to low levels of Pb has been linked with decreased Intelligence Quotient (IQ) and academic achievement, as well as a range of socio-behavioural problems such as attention deficit hyperactivity disorder (ADHD), learning difficulties, oppositional/conduct disorders, and delinquency (Lanphear et al., 2000; Tong et al., 2000; Braun et al., 2006; Gilbert and Weiss, 2006; Bellinger, 2008; Wright et al., 2008). These are serious mental health issues that are associated with significant life interference and distress. Furthermore, there is evidence to suggest that childhood disorders do not tend to remit with age but continue into adulthood (Rutter, 1989; Keller et al., 1992; Schwartz, 1994; Wright et al., 2008).
There is significant evidence that there is no safe threshold for the adverse consequences of Pb exposure (Schwartz, 1994), supporting NHMRC’s (2009) recent statement that there are no benefits of human exposure to Pb and that all demonstrated effects of such exposure are adverse. Indeed, Lanphear et al.’s (2005a) log-linear modeling of pooled data from multiple studies revealed that not only did there appear to be no lower effects threshold but that the slope of effect of Pb exposure is greater in the range below 0.10 μg/cm3. These patterns of exposure are not unique to environmental Pb. Numerous other environmental contaminants have shown toxicity at very low levels (e.g. As, dioxins, methyl mercury, polychlorinated biphenyls,). Further, many environmental contaminants have a dose-response curve that shows greater impacts per increment of exposure at lower levels than at higher levels (Stayner et al., 2003; Lanphear et al., 2005b). Consequently, there is increasing pressure to lower or totally eradicate the use of known environmental toxicants because no safe level of exposure can be established and in many cases exposures are preventable (Lanphear et al., 2005b). The effect of Pb exposure and its inherent risk was summarized by the US EPA after their 2008 decision to lower the Pb-in-air guideline for 1.5 μg/m3 to 0.15 μg/m3 (USEPA, 2008).
Particulate matter itself, irrespective of chemical compositions, is regulated under the US National Ambient Air Quality Standards (NAAQS) PM10 and PM2.5 limited to 150 and 35 g/m3, respectively, for a 24-hour period (USEPA, 2011). Further toxicity, potential human exposure and US regulations and guidelines have been summarized by ATSDR (2011).
Dust and soil ingestion through hand-to-mouth activity is an exposure pathway for humans, especially in the case of children who can exhibit pica behavior (Filippelli and Laidlaw, 2010). Calabrese et al. (1997) showed that the levels of metals and metalloids were elevated in blood and/or urinary samples of children living in contaminated areas, when only an estimated 200 mg soil/day were ingested. Pica can result in 5–50 g soil per episode ingested, with one episode being sufficient to leave children at or above the acute human lethal dose (Calabrese et al., 1997). Of particular importance is the fact that enhanced presence of As and Pb in smaller particle size fractions (< 63 m), which preferentially adhere to skin over large particles, might lead to contaminant uptakes that surpass expected levels from overall soil concentration (Bergstrom et al., 2011). Carrizales et al. (2006) reported elevated concentrations of Pb and As in the blood of children living near a copper smelter in San Luis Potosí, Mexico. These concentrations correlated with the relatively high contaminant content in the soil.
The physical and chemical properties and size distribution of the inhaled aerosols are necessary to assess more completely risks associated with contaminant exposure (Spear et al., 1998). Absorption of the contaminant from the respiratory and/or gastrointestinal tract is influenced by particle size, the site of deposition, and bioavailability, which is often related to solubility. In particular, the size of particles can be used as a predictor for the efficiency and region of deposition in the respiratory tract. Using a multi-breath inhalation simulation, Figure 9 shows the predicted deposited particle fraction as a function of particle size (Park and Wexler, 2008). Here, the respiratory system is divided into an extrathoracic (the nasal and oral passages, pharynx, and larynx), a conducting (bronchial generations 0–16), and a pulmonary region (generations 17–23). For the ultrafine fraction particles (< 0.1 m), diffusive mobility increases and so does the fraction deposited. Accumulation mode particles (0.1–1 m) have the lowest deposited fraction in all the regions due to a combination of relatively low diffusivity and high inertia. They are transported to the lungs since they are too large to undergo significant diffusion and too small to be substantially affected by sedimentation (Hinds, 1999; Schulz et al., 1996) but are relatively easily exhaled too. Coarse particles are already removed in the upper respiratory airways due to inertial deposition in the extrathoratic region (Park and Wexler, 2008).
Figure 9.
Total multi-breath deposited particle fraction (solid lines) and regional deposition fraction in the extrathoratic, conducting, and pulmonary airways (dotted lines) (adapted from Park and Wexler, 2008).
Particle size also affects the bioavailability of the contaminant. From a study on the bioavailability of Pb according to size fraction from ambient aerosols around a Pb smelter, Spear et al. (1998) found that not only was the highest percentage of Pb concentration in the fine size fraction, but also it contained the largest percentage of bio-available Pb. Therefore, finer size fraction particles that deposit in the lungs and are transported to the blood stream via macrophages have a higher bioavailability (Krombach et al., 1997) and have been shown to contain the largest concentrations of metals and metalloids from smelter emissions (Spear et al., 1998; Csavina et al., 2011).
Similar to the relationship between airborne contaminant level and distance, numerous studies have found an inverse relationship between contaminant (e,g., Pb, As) levels in blood/urine and the distance of home or school environment (for biological sampling) from metal smelters and other mining operations. Based on prior studies, Benin et al. (1999) predicted that the blood Pb levels in children at all three sites studied were likely to be well above the 10 g/dL threshold of concern for children set by the Center for Disease Control and Prevention. As a result, they suggested that metal contamination in these areas is sufficient to pose a health hazard to the human population. Hwang et al. (1997) measured As in children’s urine samples, soil samples and indoor floor dust near a former copper smelter in Montana. Arsenic concentration in soil, as well as in urine, was found to be significantly related to the proximity to the smelter site and wind direction, as seen in Figure 10. Goix et al. (2011) reported a correlation between schoolchildren’s hair metal and metalloid content (Ag, As, Cu, Pb and Sb) and smelting activities in a region in the Bolivian Altiplano.
Figure 10.
Arsenic levels in soil of bare areas in yards, in interior floor dust and speciated urinary arsenic by proximity index of residence to smelter site (GM±GSE) (reprinted from Hwang et al., 1997).
Many studies have found negative effects from metals and metalloids on plant matter (e.g., Chardonnens et al., 1999; Ernst and Nelissen, 2000; Alonso et al., 2002; Kim et al., 2003; Yousefi et al., 2011). The delayed and reduced reproduction of plants may have substantial impacts at the population, community and ecosystem level (Ryser and Sauder, 2006). Vegetation has also been shown to accumulate metals and metalloids (Müller and Anke, 1994; Hooda et al., 1997; Cobb et al., 2000; Mattina et al., 2003; Hough et al., 2004; Zhou et al., 2005). Studies have shown an important remobilization of legacy metals pollution from forest fires that combust the soils and vegetation that contain industrial-sourced contaminants (Biswas et al., 2007; Obrist et al., 2008; Odigie and Flegal, 2011). While living organisms generally need trace amounts of a variety of elements for good health, large amounts of those same elements may cause chronic or acute toxicity (e.g., Trepka et al., 1997; Benin et al., 1999; Küpper et al., 2002). Athar and Ahmad (2002) found that metals in soils brought about significant reduction in plant growth and grain yield of wheat, with Cd being the most toxic metal followed by Cu, Ni, Zn, Pb and Cr. Similarly, Ryser and Sauder (2006) found that lower leaf production and plant mass were correlated with high contaminant levels. Bi et al. (2009) observed elevated concentrations of Pb and Cd in maize plants impacted by smelting emissions.
Metals and metalloids taken up by plants may enter the food chain in significant amounts (Wolnik et al., 1983; Nasreddine and Parent-Massin, 2002; Intawongse and Dean, 2006). In examining the impacts of the Australian Port Pirie mine on the neighboring environment, Merry (1981) showed that wheat plants and grain concentrations of Cd, Pb and Zn exceeded background values mostly within 30 km of the smelter, depending on wind direction. The greatest impact occurred toward the south east, in the direction of the prevailing winds. Indeed, the levels of Pb and Cd contamination were so high that the wheat required blending with uncontaminated wheat before it was suitable for export quality standards. In a study on cattle from a low level environmental contamination of metals and metalloids, Alonso et al. (2002) found correlations of Cd, Pb, and As and trace elements in the tissue and blood of the cattle most likely as the result of contaminated soil and plant ingestion.
5. Research Priorities and Insights
Table 1 summarizes case studies discussed in this review. Dust and aerosol emissions from mining operations have received much recent attention in a wide variety of locations. Table 1 also highlights the focus that has been given to four particular locations of US, Australia, Spain, and UK, despite the global scale of the issue. Studies for bulk collection show the scale of the problem of contamination from mining operations but do not fully unravel the environmental health hazards associated with the contamination. For this, size-resolved chemical analysis of the emissions from mining operations is needed, yet from Table 1 it is evident that only a handful of studies have looked into size-fractioned aerosols. Additionally, very few studies take a holistic approach at understanding dust from the mining operations, including source apportionment. This knowledge, when fully understood and correctly parameterized, can be incorporated into existing models (e.g., Yin et al., 2005; Yin et al., 2007) to forecast dust generation, contaminant transport by windblown dust and accurately assess human health risk associated with contaminants on airborne particulate.
Table 1.
Case studies according to sample collection methods, location and year published.
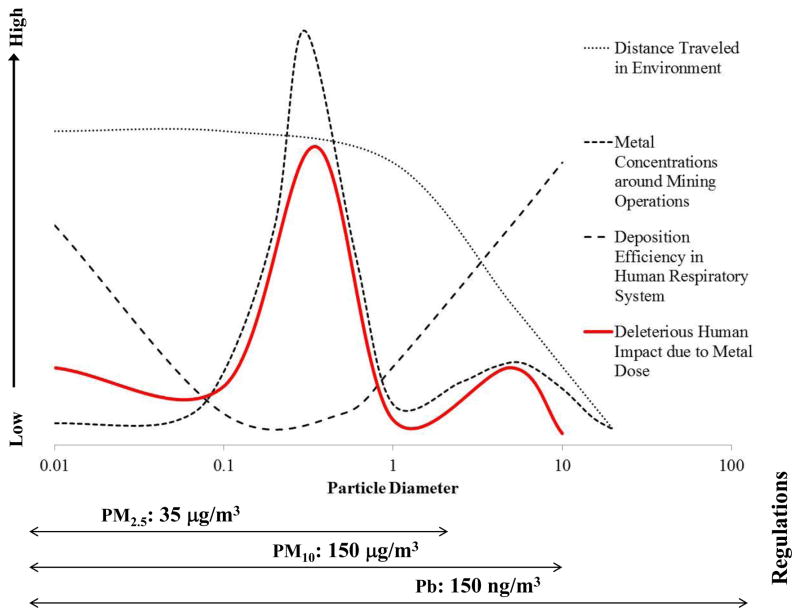
Figure 12 shows impact of contaminants from mining operations in atmospheric particulate, taking into account relative metal and metalloid concentrations, deposition efficiency and location of deposition in the human respiratory system, as well as the distance traveled in the environment. The fine particle fraction (< 1 μm) can travel further and may have higher contaminant concentrations around smelting operations. As discussed in section 4, the deposition efficiency is lower in the 0.1–1 μm size range. However, the inhaled fine particles deposit in the lungs as opposed to the mouth and nose, which are then transported to the blood stream and therefore have a high dose associated with them. The coarse particles have higher deposition efficiency in the upper respiratory system, which might imply uptake in the gastric system, and therefore could be associated with lower doses. Further, fine particulates have a higher relative surface area which results in higher dissolution rates and thus greater bioavailability. Despite the human health hazards associated with fine particulate, regulations are focused on coarse particles.
Future research should prioritize understanding the fine particle size fraction in atmospheric particulates, as well as take a holistic approach to understanding contaminant sources across the particle size spectrum. Smelter emissions, slag piles, ore transport, mine tailings and other ore-handling operations should be considered as potential sources of contaminated dust and aerosol near mining and refining sites. In particular, generation of contaminated fine size particles from gaseous emissions and wind erosion of contaminated soils, including efflorescence regions in mine tailings, should be viewed as a priority in establishing potential effects to huam and ecosystem health. Although most studies do tend to focus on a specific aspect of the problem, such as soil contamination, few focus on the small particle size range. Research should utilize methods that are suitable for assessing the fine particle size fraction, such as impactors with metal and metalloid analysis similar to the work developed by Allen et al. (2001), Spear et al. (1998) and Csavina et al. (2011). Studies should also better integrate field studies with modeling to predict environmental health risks associated with mining operations.
Figure 11.
A schematic representation of the deleterious impact of contaminants from emissions of mining operations on humans due to metal dose, taking into account relative metal concentrations around mining operations, deposition efficiency and location of deposition in the human respiratory system, and the distance traveled in the environment for the scope of human impact with US air quality regulations.
Highlights.
Metal and metalloid contaminants in atmospheric particulate generated by mining operations may pose health and environmental risks
Fate, transport, measurement and occurrence of metal and metalloid contaminants (arsenic, lead, etc.) in dust and aerosol from mining operations are reviewed
Recent research on health and environmental implications of contaminated dust and aerosol are reviewed.
Acknowledgments
This work was supported by grant number P42 ES04940 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). The views of authors do not necessarily represent those of the NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht BA. Aerosols, cloud microphysics, and fractional cloudiness. Science. 1989;245:1227–30. doi: 10.1126/science.245.4923.1227. [DOI] [PubMed] [Google Scholar]
- Allen AG, Nemitz E, Shi JP, Harrison RM, Greenwood JC. Size distributions of trace metals in atmospheric aerosols in the United Kingdom. Atmos Environ. 2001;35:4581–91. [Google Scholar]
- Alloway BJ, Ayres DC. Chemical principles of environmental pollution. New York: Blackie Academic & Professional; 1997. [Google Scholar]
- Alonso ML, Benedito JL, Miranda M, Castillo C, Hernandez J, Shore RF. Interactions between toxic and essential trace metals in cattle from a region with low levels of pollution. Arch Environ Con Tox. 2002;42:165–72. doi: 10.1007/s00244-001-0012-7. [DOI] [PubMed] [Google Scholar]
- Amasa SK. Arsenic pollution at Obuasi goldmine, town, and surrounding countryside. Environ Health Persp. 1975;12:131–5. doi: 10.1289/ehp.7512131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade S, Moffett J, Correa JA. Distribution of dissolved species and suspended particulate copper in an intertidal ecosystem affected by copper mine tailings in Northern Chile. Mar Chem. 2006;101:203–12. [Google Scholar]
- Andreae MO. The dark side of aerosols. Nature. 2001;409:671–2. doi: 10.1038/35055640. [DOI] [PubMed] [Google Scholar]
- Arthur WJ, Markham OD. Radionuclide export and elimination by coyotes at two radioactive waste disposal areas in southeastern Idaho. Health Phys. 1982;43:493–00. doi: 10.1097/00004032-198210000-00003. [DOI] [PubMed] [Google Scholar]
- Athar R, Ahmad M. Heavy metal toxicity: Effect on plant growth and metal uptake by wheat, and on free living Azotobacter. Water Air Soil Poll. 2002;138:165–80. [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry. Atlanta: 2011. [Google Scholar]
- Badr D, Harion JL. Numerical modeling of flow over stockpiles: Implications on dust emissions. Atmos Environ. 2005;39:5576–84. [Google Scholar]
- Badr D, Harion JL. Effect of aggregate storage piles configuration on dust emissions. Atmos Environ. 2007;41:360–8. [Google Scholar]
- Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, et al. Environmental exposure to lead and children’s intelligence at the age of seven years. The Port Pirie cohort study. New Engl J Med. 1992;327:1279–84. doi: 10.1056/NEJM199210293271805. [DOI] [PubMed] [Google Scholar]
- Bagnold RA. The physics of blown sand and desert dunes. London: Chapman and Hall; 1941. [Google Scholar]
- Barbaris B, Betterton EA. Initial snow chemistry survey of the Mogollon Rim in Arizona. Atmos Environ. 1996;30:3093–103. [Google Scholar]
- Barcan V. Nature and origin of multicomponent aerial emissions of the copper-nickel smelter complex. Environ Int. 2002;28:451–6. doi: 10.1016/s0160-4120(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Barrie LA, Gregor D, Hargrave B, Lake R, Muir D, Shearer R, et al. Artic contaminants - sources, occurrence and pathways. Sci Total Environ. 1992;122:1–74. doi: 10.1016/0048-9697(92)90245-n. [DOI] [PubMed] [Google Scholar]
- Beaulieu BT, Savage KS. Arsenate adsorption structures on aluminum oxide and phyllosilicate mineral surfaces in smelter-impacted soils. Environ Sci Technol. 2005;39:3571–9. doi: 10.1021/es048836f. [DOI] [PubMed] [Google Scholar]
- Beavington F, Cawse PA, Wakenshaw A. Comparative studies of atmospheric trace elements: improvements in air quality near a copper smelter. Sci Total Environ. 2004;332:39–49. doi: 10.1016/j.scitotenv.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotox. 2008;29:828–32. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnap J, Lange OL. Structure and functioning of biological soil crusts: A synthesis. In: Belnap J, Lange OL, editors. Biological soil crusts: Structure, function and management. Berlin: Springer-Verlag; 2003. pp. 471–479. [Google Scholar]
- Benin AL, Sargent JD, Dalton M, Roda S. High concentrations of heavy metals in neighborhoods near ore smelters in northern Mexico. Environ Health Persp. 1999;107:279–84. doi: 10.1289/ehp.99107279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom C, Shirai J, Kissel J. Particle size distributions, size concentration relationships, and adherence to hands of selected geologic media derived from mining, smelting, and quarrying activities. Sci Total Environ. 2011;409:4247–56. doi: 10.1016/j.scitotenv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Berico M, Luciani A, Formignani M. Atmospheric aerosol in an urban area - measurements of TSP and PM10 standards and pulmonary deposition assessments. Atmos Environ. 1997;31:3659–65. [Google Scholar]
- Bi XY, Feng XB, Yang YG, Qiu GL, Li GH, Li FL, et al. Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environ Int. 2006;32:883–90. doi: 10.1016/j.envint.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Bi XY, Feng XB, Yang YG, Li X, Shin GPY, Li F, et al. Allocation and source attribution of lead and Cd in maize (Zea mays L) impacted by smelting emissions. Environ Pollut. 2009;157:834–9. doi: 10.1016/j.envpol.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Biswas A, Blum JD, Klaue B, Keeler GJ. Release of mercury from Rocky Mountain forest fires. Global Biogeochem Cy. 2007;21:GB1002. [Google Scholar]
- Bowker GE, Gillette DA, Bergametti G, Marticorena B. Modeling flow patterns in a small vegetated area in the northern Chihuahuan Desert using QUIC (Quick Urban & Industrial Complex) Environ Fluid Mech. 2006;6:359–84. [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in US children. Environ Health Persp. 2006;114:1904–9. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braune B, Outridge P, Fisk A, Muir D, Helm P, Hobbs K, et al. Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: An overview of spatial and temporal trends. Sci Total Environ. 2005;351–352:4–56. doi: 10.1016/j.scitotenv.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Breshears DD, Kirchner TB, Whicker JJ, Field JP, Allen CD. Modeling aeolian transport in response to succession, disturbance and future climate: Dynamic long-term risk assessment for contaminant redistribution. Aeol Res. 2012;3:445–57. [Google Scholar]
- Breshears DD, Whicker JJ, Zou CB, Field JP, Allen CD. A conceptual framework for dryland aeolian sediment transport along the grassland-forest continuum: Effects of woody plant canopy cover and disturbance. Geomorphol. 2009;105:28–38. [Google Scholar]
- Bridges EM, Oldeman LR. Global assessment of human-induced soil degradation. Arid Soil Res Rehab. 1999;13:319–25. [Google Scholar]
- Brotons JM, Diaz AR, Sarria FA, Serrato FB. Wind erosion on mining waste in southeast Spain. Land Degrad Dev. 2010;21:196–209. [Google Scholar]
- Bullard JE, McTainsh GH, Pudmenzky C. Factors affecting the nature and rate of dust production from natural dune sands. Sedimentology. 2007;54:169–82. [Google Scholar]
- Burn-Nunes LJ, Loss RD, Burton GR, Edwards R, Rosman KJR, Vallelonga P, et al. Seasonal variability in the input of lead, barium and indium to Law Dome, Antarctica. Geochim Cosmochim Acta. 2011;75:1–20. [Google Scholar]
- Burns JM, Baghurst PA, Sawyer MG, McMichael AJ, Tong SL. Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11–13 years. The Port Pirie Cohort Study. Am J Epidemiol. 1999;149:740–9. doi: 10.1093/oxfordjournals.aje.a009883. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Stanek EJ, James RC, Roberts SM. Soil ingestion: A concern for acute toxicity in children. Environ Health Persp. 1997;105:1354–8. doi: 10.1289/ehp.971051354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón J, Navarro M, Jimenez-Capdeville M, Santos-Diaz M, Golden A, Rodriguez-Leyva I, et al. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res. 2001;85:69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Carrizales L, Razo I, Téllez-Hernández JI, Torres-Nerio R, Torres A, Batres LF, et al. Exposure to arsenic and lead of children living near a copper-smelter in San Luis Potosi, Mexico: Importance of soil contamination for exposure of children. Environ Res. 2006;101:1–10. doi: 10.1016/j.envres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Cartwright B, Merry RH, Tiller KG. Heavy metal contamination of soils around a lead smelter at Port Pirie, South Australia. Aust J Soil Res. 1977;15:69–81. [Google Scholar]
- CCSP (Climate Change Science Program) The effects of climate change on agriculture, land resources, water resources, and biodiversity in the United States. Washington, DC: 2008. [Google Scholar]
- CDC (Centers for Disease Control) Strategic plan for the elimination of childhood lead poisoning. Atlanta: 1991. [Google Scholar]
- Chadwick OA, Derry LA, Vitousek PM, Huebert BJ, Hedin LO. Changing sources of nutrients during four million years of ecosystem development. Nature. 1999;397:491–7. [Google Scholar]
- Chakradhar B. Fugitive dust emissions from mining areas. J Environ Sys. 2004;31:279–88. [Google Scholar]
- Chan CY, Xu XD, Li YS, Wong KH, Ding GA, Chan LY, et al. Characteristics of vertical profiles and sources of PM2. 5, PM10 and carbonaceous species in Beijing. Atmos Environ. 2005;39:5113–24. [Google Scholar]
- Chardonnens AN, Bookum WM, Vellinga S, Schat H, Verkleij JAC, Ernst WHO. Allocation patterns of zinc and cadmium in heavy metal tolerant and sensitive Silene vulgaris. J Plant Physiol. 1999;155:778–87. [Google Scholar]
- Cheng H, Hu Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ Pollut. 2010;158:1134–46. doi: 10.1016/j.envpol.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Chepil WS, Siddoway FH, Armbrust DV. Climatic factor for estimating wind erodibility of fram fields. J Soil Water Conserv. 1962;17:162–5. [Google Scholar]
- Cherif S, Millet M, Sanusi A, Herckes P, Wortham H. Protocol for analysis of trace metals and other ions in filtered and unfiltered fogwater. Environ Pollut. 1998;103:301–8. [Google Scholar]
- Chilvers DC, Peterson PJ. Global cycling of arsenic. In: Hutchinson TC, Meema KM, editors. Lead, mercury, cadmium, and arsenic in the environment. Chichester: Wiley; 1987. pp. 279–302. [Google Scholar]
- Chow JC, Watson JG, Lowenthal DH, Solomon PA. PM10 source apportionment in California’s San Joaquin Valley. Atmos Eviron A. 1992;26:3335–54. [Google Scholar]
- Chow JC, Watson JG, Fujita EM, Lu ZQ, Lawson DR, Ashbaugh LL. Temporal and spatial variations of PM(2. 5) and PM(10) aerosol in the southern California air-quality study. Atmos Environ. 1994;28:2061–80. doi: 10.1007/BF00546199. [DOI] [PubMed] [Google Scholar]
- Chu DA, Kaufman YJ, Zibordi G, Chern JD, Mao J, Li C, et al. Global monitoring of air pollution over land from the Earth Observing System-Terra Moderate Resolution Imaging Spectroradiometer (MODIS) J Geophys Res. 2003;108:4. ACH. [Google Scholar]
- Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E. Accumulation of heavy metals by vegetables grown in mine wastes. Environ Toxicol Chem. 2000;19:600–7. [Google Scholar]
- Corriveau MC, Jamieson HE, Parsons MB, Campbell JL, Lanzirotti A. Direct characterization of airborne particles associated with arsenic-rich mine tailings: Particle size, mineralogy and texture. Appl Geochem. 2011;26:1639–48. [Google Scholar]
- Csavina J, Landázuri A, Wonaschütz A, Rine K, Rheinheimer P, Barbaris B, et al. Metal and Metalloid Contaminants in Atmospheric Aerosols from Mining Operations. Water Air Soil Pollut. 2011;221:145–57. doi: 10.1007/s11270-011-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff DJ, Goudie AS. The Oxford companion to global change. New York: Oxford University Press; 2009. [Google Scholar]
- Day JMD, Pearson DG, Macpherson CG, Lowry D, Carracedo JC. Evidence for distinct proportions of subducted oceanic crust and lithosphere in HIMU-type mantle beneath El Hierro and La Palma, Canary Islands. Geochim Cosmochim Acta. 2010;74:6565–89. [Google Scholar]
- Deb MK, Thakur M, Mishra RK, Bodhankar N. Assessment of atmospheric arsenic level in airborne dust particulates of an urban city of Central India. Water Air Soil Pollu. 2002;140:57–71. [Google Scholar]
- Diego I, Pelegry A, Torno S, Toraño J, Menendez M. Simultaneous CFD evaluation of wind flow and dust emission in open storage piles. Appl Math Mod. 2009;33:3197–207. [Google Scholar]
- Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, Rhoads GG, et al. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics. 2004;114:19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Whitall D, Aber J, Boyer E, Castro M, Cronan C, et al. Nitrogen pollution in the Northeastern United States: Sources, effects, and management options. Bioscience. 2003;53:357–74. [Google Scholar]
- Ericson B, Hanrahan D, Kong V. The World’s worst pollution problems: Top ten of the toxic twenty. New York: Blacksmith Institute; 2008. [Google Scholar]
- Ernst WHO, Nelissen HJM. Life-cycle phases of a zinc- and cadmium-resistant ecotype of Silene vulgaris in risk assessment of polymetallic mine soils. Environ Pollut. 2000;107:329–38. doi: 10.1016/s0269-7491(99)00174-8. [DOI] [PubMed] [Google Scholar]
- Feng S, Ning H. Computational simulations of blown sand fluxes over the surfaces of complex microtopography. Environ Model Software. 2010;25:362–7. [Google Scholar]
- Fernandez-Camacho R, de la Rosa J, de la Campa AMS, Gonzalez-Castanedo Y, Alastuey A, Querol X, et al. Geochemical characterization of Cu-smelter emission plumes with impact in an urban area of SW Spain. Atmos Res. 2010;96:590–601. [Google Scholar]
- Field JP, Belnap J, Breshears DD, Neff JC, Okin GS, Whicker JJ, et al. The ecology of dust. Front Ecol Environ. 2010;8:423–30. [Google Scholar]
- Filippelli GM, Laidlaw MAS. The elephant in the playground. Confronting lead-contaminated soils as an important source of lead burdens to urban populations. Perspect Biol Med. 2010;53:31–45. doi: 10.1353/pbm.0.0136. [DOI] [PubMed] [Google Scholar]
- Flegal AR, Smith DR. Lead levels in preindustrial humans. New Engl J Med. 1992;326:1293–4. [PubMed] [Google Scholar]
- Fryrear DW. Soil cover and wind erosion. T ASAE. 1985;28:781–4. [Google Scholar]
- Fryrear DW. Wind erosion and its effects on plant-growth. Ann Arid Zone. 1986;25:245–54. [Google Scholar]
- Fryrear DW, Saleh A, Bilbro JD. A single event wind erosion model. T ASAE. 1998;41:1369–74. [Google Scholar]
- Gallon C, Ranville MA, Conaway CH, Landing WM, Buck CS, Morton PL, et al. Asian industrial lead inputs to the North Pacific evidenced by lead concentrations and isotopic compositions in surface waters and aerosols. Environ Sci Technol. 2011;45:9874–82. doi: 10.1021/es2020428. [DOI] [PubMed] [Google Scholar]
- Garcia R, Torres MC, Baez A. Determination of trace elements in total suspended particles at the Southwest of Mexico City from 2003 to 2004. Chem Ecol. 2008;24:157–67. [Google Scholar]
- Ghio AJ, Devlin RB. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Resp Crit Care Med. 2001;164:704–8. doi: 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 mu g/dL. Neurotoxicol. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette DA. Environmental factors affecting dust emission by wind erosion. In: Morales C, editor. Saharan Dust. New York: Wiley; 1979. pp. 71–94. [Google Scholar]
- Gillette DA, Adams J, Endo A, Smith D, Kihl R. Threshold velocities for input of soil particles into the air by dessert soils. J Geophy Res Oceans Atmos. 1980;85:5621–30. [Google Scholar]
- Gillies JA, Etyemezian V, Kuhns H, Nikolic D, Gillette DA. Effect of vehicle characteristics on unpaved road dust emissions. Atmos Environ. 2005;39:2341–7. [Google Scholar]
- Goix S, Point D, Oliva P, Polve M, Duprey JL, Mazurek H, et al. Influence of source distribution and geochemical composition of aerosols on children exposure in the large polymetallic mining region of the Bolivian Altiplano. Sci Total Environ. 2011;412–413:170–84. doi: 10.1016/j.scitotenv.2011.09.065. [DOI] [PubMed] [Google Scholar]
- Goossens D, Offer Z, London G. Wind tunnel and field calibration of five aeolian sand traps. Geomorphol. 2000;35:233–52. [Google Scholar]
- Goossens D, Offer ZY. Wind tunnel and field calibration of six aeolian dust samplers. Atmos Environ. 2000;34:1043–57. [Google Scholar]
- Goudie A, Middleton N. Desert dust in the global system. Berlin: Springer; 2006. [Google Scholar]
- Griffin D, Kellogg C, Shinn E. Dust in the wind: Long range transport of dust in the atmosphere and its implications for global public and ecosystem Health. Global Change Human Health. 2001;2:20–33. [Google Scholar]
- Hagen LJ. Processes of soil erosion by wind. Ann Arid Zone. 2001;40:233–50. [Google Scholar]
- Harper SL, Walling JF, Holland DM, Pranger LJ. Simplex optimization of multielement ultrasonic extraction of atmospheric particulates. Anal Chem. 1983;55:1553–7. [Google Scholar]
- Hartmann DL. Global Physical Climatology. San Diego: Academic Press; 1994. [Google Scholar]
- Hayes S, Webb SM, Bargar J, O’Day PA, Maier RM, Chorover J. Geochemical weathering increases lead bioaccessibility in semi-arid mine tailings. Environ Sci Technol. 2012 doi: 10.1021/es300603s. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herner JD, Green PG, Kleeman MJ. Measuring the trace elemental composition of size-resolved airborne particles. Environ Sci Technol. 2006;40:1925–33. doi: 10.1021/es052315q. [DOI] [PubMed] [Google Scholar]
- Hinds WC. Aerosol Science and Technology. New York: John Wiley & Sons; 1999. [Google Scholar]
- Holmes L. A review of dispersion modelling and its application to the dispersion of particles: An overview of different dispersion models available. Atmos Environ. 2006;40:5902–28. [Google Scholar]
- Hooda PS, McNulty D, Alloway BJ, Aitken MN. Plant availability of heavy metals in soils previously amended with heavy applications of sewage sludge. J Sci Food Agric. 1997;73:446–54. [Google Scholar]
- Hope B. Estimating contaminant transport by biological vectors. Chemosphere. 1993;27:817–24. [Google Scholar]
- Hough RL, Breward N, Young SD, Crout NMJ, Tye AM, Moir AM, et al. Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environ Health Persp. 2004;112:215–21. doi: 10.1289/ehp.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Liu SC, Jeng WL, Chou CCK, Hsu RT, Huang YT, et al. Lead isotope ratios in ambient aerosols from Taipei, Taiwan: Identifying long-range transport of airborne Pb from the Yangtze Delta. Atmos Environ. 2006;40:5393–404. [Google Scholar]
- Hui WJ, Cook BI, Ravi S, Fuentes JD, D’Odorico P. Dust-rainfall feedbacks in the West African sahel. Water Resour Res. 2008;44:W05202. [Google Scholar]
- Hutchings L, Roberts MR, Verheye HM. Marine environmental monitoring programmes in South Africa: a review. South African J Sci. 2009;105:94–102. [Google Scholar]
- Hwang YH, Bornschein RL, Grote J, Menrath W, Roda S. Environmental arsenic exposure of children around a former copper smelter site. Environ Res. 1997;72:72–81. doi: 10.1006/enrs.1996.3691. [DOI] [PubMed] [Google Scholar]
- Intawongse M, Dean JR. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit Contam. 2006;23:36–48. doi: 10.1080/02652030500387554. [DOI] [PubMed] [Google Scholar]
- IOSHIC (International Occupational Safety and Health Information Centre) Metals. Geneva: 1999. [Google Scholar]
- IPCC (International Pannel for Climate Change) Climate change 2007: Climate change impacts, adaptation and vulnerability. New York: Cambridge University Press; 2007. Working group II contribution to the intergovernmental panel on climate change fourth assessment report. [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 mu g/dL and child intelligence at 6 years of age. Environ Health Persp. 2008;116:243–8. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman YJ, Tanre D, Boucher O. A satellite view of aerosols in the climate system. Nature. 2002;419:215–23. doi: 10.1038/nature01091. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Wunder J, Beardslee WR, Schwartz CE, Roth J. Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Psy. 1992;31:595–9. doi: 10.1097/00004583-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Kersting AB, Efurd DW, Finnegan DL, Rokop DJ, Smith DK, Thompson JL. Migration of plutonium in ground water at the Nevada Test Site. Nature. 1999;397:56. [Google Scholar]
- Kim CG, Bell JNB, Power SA. Effects of soil cadmium on Pinus sylvestris L seedlings. Plant Soil. 2003;257:443–9. [Google Scholar]
- Knight RD, Henderson PJ. Smelter dust in humus around Rouyn-Noranda, Quebec. Geochem Explor Environ Anal. 2006;6:203–14. [Google Scholar]
- Kok JF, Renno NO. A comprehensive numerical model of steady state saltation (COMSALT) J Geophy Res Atmos. 2009;114:D17204. [Google Scholar]
- Kolpin DW, Barbash JE, Gilliom RJ. Occurrence of pesticides in shallow groundwater of the United States: Initial results from the National water-quality assessment program. Environ Sci Technol. 1998;32:558–66. [Google Scholar]
- Kon LC, Durucan S, Korre A. The development and application of a wind erosion model for the assessment of fugitive dust emissions from mine tailings dumps. Int J Mining Reclam Environ. 2007;21:198–218. [Google Scholar]
- Krombach F, Munzing S, Allmeling AM, Gerlach JT, Behr J, Dorger M. Cell size of alveolar macrophages: An interspecies comparison. Environ Health Persp. 1997;105:1261–3. doi: 10.1289/ehp.97105s51261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Šetlík I, Spiller M, Küpper FC, Prášil O. Heavy metal-induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation. J Phycol. 2002;38:429–41. [Google Scholar]
- Lacerda LD. Global mercury emissions from gold and silver mining. Water Air Soil Pollut. 1997;97:209–21. [Google Scholar]
- Laidlaw MAS, Filippelli GM. Resuspension of urban soils as a persistent source of lead poisoning in children: A review and new directions. Appl Geochem. 2008;23:2021–39. [Google Scholar]
- Lal R. Soil degradation by erosion. Land Degrad Devel. 2001;12:519–39. [Google Scholar]
- Landrigan PJ, Gehlbach SH, Rosenblum BF, Shoults JM, Candelaria RM, Barthel WF, et al. Epidemic lead absorption near an ore smelter - role of particulate lead. New Engl J Med. 1975;292:123–9. doi: 10.1056/NEJM197501162920302. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations < 10 mu g/dL in US children and adolescents. Pub Health Rep. 2000;115:521–9. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurstl P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Persp. 2005a;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Vorhees CV, Bellinger DC. Protecting children from environmental toxins - Toxicity testing of pesticides and industrial chemicals is a crucial step. Plos Med. 2005b;2:203–8. doi: 10.1371/journal.pmed.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Okin GS, Alvarez L, Epstein H. Quantitative effects of vegetation cover on wind erosion and soil nutrient loss in a desert grassland of southern New Mexico, USA. Biogeochem. 2007;85:317–32. [Google Scholar]
- Listavia International. Smelting Industry. G3M Solutions Ltd; 2011. [Google Scholar]
- Lubin JH, Moore LE, Fraumeni JF, Cantor KP. Respiratory cancer and inhaled inorganic arsenic in copper smelters workers: A linear relationship with cumulative exposure that increases with concentration. Environ Health Persp. 2008;116:1661–5. doi: 10.1289/ehp.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W, Wang YX, Querol X, Zhuang XG, Alastuey A, Lopez A, et al. Geochemical and statistical analysis of trace metals in atmospheric particulates in Wuhan, central China. Environ Geol. 2006;51:121–32. [Google Scholar]
- Mackie DS, Peat JM, McTainsh GH, Boyd PW, Hunter KA. Soil abrasion and eolian dust production: Implications for iron partitioning and solubility. Geochem Geophy Geosys. 2006;7:Q12Q03. [Google Scholar]
- Mahowald NM, Engelstaedter S, Luo C, Sealy A, Artaxo P, Benitez-Nelson C, et al. Atmospheric iron deposition: Global distribution, variability, and human perturbations. Annu Rev Marine Sci. 2009;1:245–78. doi: 10.1146/annurev.marine.010908.163727. [DOI] [PubMed] [Google Scholar]
- Mancinelli V, Decesari S, Facchini MC, Fuzzi S, Mangani F. Partitioning of metals between the aqueous phase and suspended insoluble material in fog droplets. Ann Chim. 2005;95:275–90. doi: 10.1002/adic.200590033. [DOI] [PubMed] [Google Scholar]
- Marple VA, Rubow KL, Behm SM. A microorifice uniform deposit impactor (MOUDI) - description, calibration, and use. Aerosol Sci Tech. 1991;14:434–46. [Google Scholar]
- Mattina MI, Lannucci-Berger W, Musante C, White JC. Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ Pollut. 2003;124:375–8. doi: 10.1016/s0269-7491(03)00060-5. [DOI] [PubMed] [Google Scholar]
- McCartor A, Becker D. World’s worst pollution problems report 2010. New York: Blacksmith Institute; 2010. Top six toxic threats. [Google Scholar]
- McGechan M, Lewis D. Transport of particulate and colloid-sorbed contaminants through soil, Part 1: General principles. Biosyst Eng. 2002;83:255–73. [Google Scholar]
- Merry R. Contamination of wheat crops around a lead-zinc smelter. Environ Pollut B. 1981;2:37–48. [Google Scholar]
- Meza-Figueroa D, Maier RM, de la O-Villanueva M, Gomez-Alvarez A, Moreno-Zazueta A, Rivera J, et al. The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere. 2009;77:140–7. doi: 10.1016/j.chemosphere.2009.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton N, Goudie A, Wells G. Aeolian geomorphology. Boston: Allen and Unwin; 1986. [Google Scholar]
- Middleton N, Thomas D. World atlas of desertification. London: Arnold Publishing; 1997. [Google Scholar]
- Moreno T, Oldroyd A, McDonald I, Gibbons W. Preferential fractionation of trace metals-metalloids into PM10 resuspended from contaminated gold mine tailings at Rodalquilar, Spain. Water Air Soil Pollut. 2007;179:93–105. [Google Scholar]
- Moreno T, Querol X, Alastuey A, Viana M, Salvador P, de la Campa AS, et al. Variations in atmospheric PM trace metal content in Spanish towns: Illustrating the chemical complexity of the inorganic urban aerosol cocktail. Atmos Environ. 2006;40:6791–803. [Google Scholar]
- Müller M, Anke M. Distribution of cadmium in the food chain (soil-plant-human) of a cadmium exposed area and the health risks of the general population. Sci Total Environ. 1994;156:151–8. doi: 10.1016/0048-9697(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Mulligan CN, Yong RN. Natural attenuation of contaminated soils. Environ Int. 2004;30:587–601. doi: 10.1016/j.envint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Munksgaard NC, Parry DL. Lead isotope ratios determined by ICP-MS: Monitoring of mining-derived metal particulates in atmospheric fallout, Northern Territory, Australia. Sci Total Environ. 1998;217:113–25. [Google Scholar]
- Munksgaard NC, Taylor MP, Mackay A. Recognising and responding to the obvious: the source of lead pollution at Mount Isa and the likely health impacts. Med J Aust. 2010;193:131–2. doi: 10.5694/j.1326-5377.2010.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Munson SM, Belnap J, Okin GS. Responses of wind erosion to climate-induced vegetation changes on the Colorado Plateau. PNAS. 2011;108:3854–9. doi: 10.1073/pnas.1014947108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine L, Parent-Massin D. Food contamination by metals and pesticides in the European Union. Should we worry? Toxicol Lett. 2002;127:1–3. doi: 10.1016/s0378-4274(01)00480-5. [DOI] [PubMed] [Google Scholar]
- Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–22. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- Neff JC, Ballantyne AP, Farmer GL, Mahowald NM, Conroy JL, Landry CC, et al. Increasing eolian dust deposition in the western United States linked to human activity. Nature Geosci. 2008;1:189–195. [Google Scholar]
- Neuman CM, Boulton JW, Sanderson S. Wind tunnel simulation of environmental controls on fugitive dust emissions from mine tailings. Atmos Environ. 2009;43:520–9. [Google Scholar]
- NHMRC (National Health and Medical Research Council) Blood lead levels for Australians. Canberra: 2009. [Google Scholar]
- Nickovic S, Kallos G, Papadopoulos A, Kakaliagou O. A model for prediction of desert dust cycle in the atmosphere. J Geophys Res. 2001;106:18113–29. [Google Scholar]
- Nordstrom KF, Hotta S. Wind erosion from cropland in the USA: a review of problems, solutions and prospects. Geoderma. 2004;121:157. [Google Scholar]
- Obrist D, Moosmuller H, Schurmann R, Chen LWA, Kreidenweis SM. Particulate-phase and gaseous elemental mercury emissions during biomass combustion: Controlling factors and correlation with particulate matter emissions. Environ Sci Technol. 2008;42:721–7. doi: 10.1021/es071279n. [DOI] [PubMed] [Google Scholar]
- Odigie KO, Flegal AR. Pyrogenic remobilization of historic industrial lead depositions. Environ Sci Technol. 2011;45:6290–5. doi: 10.1021/es200944w. [DOI] [PubMed] [Google Scholar]
- Ojelede ME, Annegarn HJ, Kneen MA. Evaluation of aeolian emissions from gold mine tailings on the Witwatersrand. Aeolian Res. 2012;3:477–86. [Google Scholar]
- Okin GS, Gillette DA, Herrick JE. Multi-scale controls on and consequences of aeolian processes in landscape change in arid and semi-arid environments. J Arid Environ. 2006;65:253–75. [Google Scholar]
- Okin GS, Mahowald N, Chadwick OA, Artaxo P. Impact of desert dust on the biogeochemistry of phosphorus in terrestrial ecosystems. Global Biogeochem Cy. 2004;18:GB2005. [Google Scholar]
- Okin GS, Parsons AJ, Wainwright J, Herrick JE, Bestelmeyer BT, Peters DC, et al. Do changes in connectivity explain desertification? Bioscience. 2009;59:237–44. [Google Scholar]
- Owen PR. Saltation of uniform grains in air. J Fluid Mech. 1964;20:225–42. [Google Scholar]
- Painter TH, Barrett AP, Landry CC, Neff JC, Cassidy MP, Lawrence CR, et al. Impact of disturbed desert soils on duration of mountain snow cover. Geophys Res Lett. 2007;34:L12502. [Google Scholar]
- Painter TH, Deems JS, Belnap J, Hamlet AF, Landry CC, Udall B. Response of Colorado River runoff to dust radiative forcing in snow. PNAS. 2010;107:17125–30. doi: 10.1073/pnas.0913139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paktunc D, Foster A, Laflamme G. Speciation and characterization of arsenic in Ketza River mine tailings using x-ray absorption spectroscopy. Environ Sci Technol. 2003;37:2067–74. doi: 10.1021/es026185m. [DOI] [PubMed] [Google Scholar]
- Park RJ, Jacob DJ, Field BD, Yantosca RM, Chin M. Natural and transboundary pollution influences on sulfate-nitrate-ammonium aerosols in the United States: Implications for policy. J Geophys Res. 2004;109:D15204. [Google Scholar]
- Park SS, Wexler AS. Size-dependent deposition of particles in the human lung at steady-state breathing. J Aerosol Sci. 2008;39:266–76. [Google Scholar]
- Pekney NJ, Davidson CI, Bein KJ, Wexler AS, Johnston MV. Identification of sources of atmospheric PM at the Pittsburgh Supersite, Part I: Single particle analysis and filter-based positive matrix factorization. Atmos Environ. 2006;40:S411–3. [Google Scholar]
- Pelletier JD. Sensitivity of playa windblown-dust emissions to climatic and anthropogenic change. J Arid Environ. 2006;66:62–75. [Google Scholar]
- Perry KD, Cahill TA, Schnell RC, Harris JM. Long-range transport of anthropogenic aerosols to the National Oceanic and Atmospheric Administration baseline station at Mauna Loa Observatory, Hawaii. J Geophys Res. 1999;104:18521–33. [Google Scholar]
- Pillans B. Soil development at snail’s pace: evidence from a 6 Ma soil chronosequence on basalt in north Queensland, Australia. Geoderma. 1997;80:117–28. [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–42. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Pope P, VanEeckhout E, Rofer C. Waste site characterization through digital analysis of historical aerial photographs. Photogram Eng Remote Sens. 1996;62:1387–94. [Google Scholar]
- Porcella DB, Ramel C, Jernelov A. Global Mercury Pollution and the Role of Gold Mining: An Overview. Water Air Soil Pollut. 1997;97:205–7. [Google Scholar]
- Prospero JM, Ginoux P, Torres O, Nicholson SE, Gill TE. Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev Geophys. 2002;40:1002. [Google Scholar]
- Pye K. Aeolian Dust and Dust Deposits. Boca Raton: Academic Press; 1987. [Google Scholar]
- Querol X, Alastuey A, Lopez-Soler A, Plana F. Levels and chemistry of atmospheric particulates induced by a spill of heavy metal mining wastes in the Donana area, Southwest Spain. Atmos Environ. 2000;34:239–53. [Google Scholar]
- Querol X, Viana M, Alastuey A, Amato F, Moreno T, Castillo S, et al. Source origin of trace elements in PM from regional background, urban and industrial sites of Spain. Atmos Environ. 2007;41:7219–31. [Google Scholar]
- Ramanathan V, Crutzen PJ, Kiehl JT, Rosenfeld D. Atmosphere - aerosols, climate, and the hydrological cycle. Science. 2001;294:2119–24. doi: 10.1126/science.1064034. [DOI] [PubMed] [Google Scholar]
- Rattigan OV, Mirza MI, Ghauri BM, Khan AR, Swami K, Yang K, et al. Aerosol sulfate and trace elements in urban fog. Energy Fuels. 2002;16:640–6. [Google Scholar]
- Ravi S, D’Odorico P, Breshears DD, Field JP, Goudie A, Huxman TE, et al. Aeolian processes and the biosphere: Interactions and feedback loops. Rev Geophys. 2011;49:RG3001. [Google Scholar]
- Ravi S, D’Odorico P, Over TM, Zobeck TM. On the effect of air humidity on soil susceptibility to wind erosion: The case of air-dry soils. Geophysical Research Letters. 2004;31:1–4. [Google Scholar]
- Rawlins BG, Lark RM, Webster R, O’Donnell KE. The use of soil survey data to determine the magnitude and extent of historic metal deposition related to atmospheric smelter emissions across Humberside, UK. Environ Pollut. 2006;143:416–26. doi: 10.1016/j.envpol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Reed WR, Westman EC. A model for predicting the dispersion of dust from a haul truck. Int J Surf Mining Reclam Environ. 2005;19:66–74. [Google Scholar]
- Reheis MC, Budahn JR, Lamothe PJ, Reynolds RL. Compositions of modern dust and surface sediments in the Desert Southwest, United States. J Geophys Res Earth Surf. 2009;114:F01028. [Google Scholar]
- Ritter L, Solomon K, Sibley P, Hall K, Keen P, Mattu G, et al. Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the Walkerton Inquiry. J Toxicol Environ Health A. 2002;65:1–142. doi: 10.1080/152873902753338572. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, et al. Arsenic Exposure and Cognitive Performance in Mexican Schoolchildren. Environ Health Persp. 2007;115:1371–5. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld D, Rudich Y, Lahav R. Desert dust suppressing precipitation: A possible desertification feedback loop. PNAS. 2001;98:5975–80. doi: 10.1073/pnas.101122798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Pathways from childhood to adult life. J Child Psycol Psyc. 1989;30:23–51. doi: 10.1111/j.1469-7610.1989.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Ryser P, Sauder WR. Effects of heavy-metal-contaminated soil on growth, phenology and biomass turnover of Hieracium piloselloides. Environ Pollut. 2006;140:52–61. doi: 10.1016/j.envpol.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Sánchez de la Campa AM, de la Rosa J, Fernández-Caliani JC, González-Castanedo Y. Impact of abandoned mine waste on atmospheric respirable particulate matter in the historic mining district of Rio Tinto (Iberian Pyrite Belt) Environ Res. 2011;111:1018–23. doi: 10.1016/j.envres.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Santacatalina M, Reche C, Minguillon MC, Escrig A, Sanfelix V, Carratala A, et al. Impact of fugitive emissions in ambient PM levels and composition: A case study in Southeast Spain. Sci Total Environ. 2010;408:4999–5009. doi: 10.1016/j.scitotenv.2010.07.040. [DOI] [PubMed] [Google Scholar]
- SBM Machinery. SBM Processing Equipment. 2010. Ore Grinder. [Google Scholar]
- Schemenauer RS, Cereceda P. Monsoon cloudwater chemistry on the Arabian Peninsula. Atmos Environ A. 1992;26:1583–7. [Google Scholar]
- Schlesinger WH, Reynolds JF, Cunningham GL, Huenneke LF, Jarrell WM, Virginia RA, et al. Biological feedbacks in global desertification. Science. 1990;247:1043–8. doi: 10.1126/science.247.4946.1043. [DOI] [PubMed] [Google Scholar]
- Schulz H, Schulz A, Heyder J. Influence of intrinsic particle properties on the assessment of convective gas transport by aerosol bolus technique. Exp Lung Res. 1996;22:393–407. doi: 10.3109/01902149609046031. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air-pollution and daily mortality - A review and meta analysis. Environ Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Seager R, Ting MF, Held I, Kushnir Y, Lu J, Vecchi G, et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science. 2007;316:1181–4. doi: 10.1126/science.1139601. [DOI] [PubMed] [Google Scholar]
- Seinfeld JH, Pandis SN. Atmospheric chemistry and physics: From air pollution to climate change. New York: Wiley; 2006. [Google Scholar]
- Shaheen N, Shah MH, Jaffar M. A study of airborne selected metals and particle size distribution in relation to climatic variables and their source identification. Water Air Soil Pollut. 2005;164:275–94. [Google Scholar]
- Shao Y. Physics and Modeling of Wind Erosion. Dordrecht: Springer; 2008. [Google Scholar]
- Shao Y, Raupach MR, Findlater PA. Effect of saltation bombardment on the entrainment of dust by wind. J Geophys Res Atmos. 1993;98:12719–26. [Google Scholar]
- Shao YP, Lu H. A simple expression for wind erosion threshold friction velocity. J Geophy Res Atmos. 2000;105:22437–43. [Google Scholar]
- Sheppard SC. Assessment of long-term fate of metals in soils: Inferences from analogues. Can J Soil Sci. 2005;85:1–18. [Google Scholar]
- Sivakumar MVK. Interactions between climate and desertification. Agric Forest Meteorol. 2007;142:143–55. [Google Scholar]
- Skamarock WC, Klemp JB, Dudhia J, Gill DO, Barker DM, Wang W, et al. A description of the advanced research WRF Version 2. Ft. Belvoir: Defense Technical Information Center; 2005. [Google Scholar]
- Slowey AJ, Johnson SB, Newville M, Brown GE. Speciation and colloid transport of arsenic from mine tailings. Appl Geochem. 2007;22:1884–98. [Google Scholar]
- Smith BJ, Wright JS, Whalley WB. Simulated aeolean abrasion of Pannonian sands and its implications for the origins of Hungarian Loess. Earth Surf Proc Land. 1991;16:745–52. [Google Scholar]
- Sokolik IN, Toon OB. Direct radiative forcing by anthropogenic airborne mineral aerosols. Nature. 1996;381:681–3. [Google Scholar]
- Soto-Jimenez MF, Flegal AR. Childhood lead poisoning from the smelter in Torreon, Mexico. Environ Res. 2011;111:590–6. doi: 10.1016/j.envres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Soukup JM, Ghio AJ, Becker S. Soluble components of Utah Valley particulate pollution alter alveolar macrophage function in vivo and in vitro. Inhal Toxicol. 2000;12:401–14. doi: 10.1080/089583700196112. [DOI] [PubMed] [Google Scholar]
- Spear TM, Svee W, Vincent JH, Stanisich N. Chemical speciation of lead dust associated with primary lead smelting. Environ Health Persp. 1998;106:565–71. doi: 10.1289/ehp.98106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafilov T, Sajn R, Pancevski Z, Boev B, Frontasyeva MV, Strelkova LP. Heavy metal contamination of topsoils around a lead and zinc smelter in the Republic of Macedonia. J Haz Mat. 2010;175:896–914. doi: 10.1016/j.jhazmat.2009.10.094. [DOI] [PubMed] [Google Scholar]
- Stayner L, Steenland K, Dosemeci M, Hertz-Picciotto I. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health. 2003;29:317–24. doi: 10.5271/sjweh.737. [DOI] [PubMed] [Google Scholar]
- Steltzer H, Landry C, Painter TH, Anderson J, Ayres E. Biological consequences of earlier snowmelt from desert dust deposition in alpine landscapes. PNAS. 2009;106:11629–34. doi: 10.1073/pnas.0900758106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterckeman T, Douay F, Proix N, Fourrier H. Vertical distribution of Cd, Pb and Zn in soils near smelters in the North of France. Environ Pollut. 2000;107:377–89. doi: 10.1016/s0269-7491(99)00165-7. [DOI] [PubMed] [Google Scholar]
- Stockton PH, Gillette DA. Field measurement of the sheltering effect of vegetation on erodible land surfaces. Land Degrad Devel. 1990;2:77–85. [Google Scholar]
- Stout JE, Zobeck TM. The Wolfforth field experiment: A wind erosion study. Soil Sci. 1996;161:616. [Google Scholar]
- Syvitski JPM. Supply and flux of sediment along hydrological pathways: research for the 21st century. Global Planet Change. 2003;39:1–11. [Google Scholar]
- Tatsumoto M, Patterson CC. Concentrations of common lead in some Atlantic and Mediteraranean waters and in snow. Nature. 1963;199:350–2. [Google Scholar]
- Taylor MP, Mackay AK, Hudson-Edwards KA, Holz E. Soil Cd, Cu, Pb and Zn contaminants around Mount Isa city, Queensland, Australia: Potential sources and risks to human health. Appl Geochem. 2010;25:841–55. [Google Scholar]
- Tegen I, Fung I. Contribution to the atmospheric mineral aerosol load from land-surface modification. J Geophys Res Atmos. 1995;100:18707–26. [Google Scholar]
- Tegen I, Lacis AA, Fung I. The influence on climate forcing of mineral aerosols from disturbed soils. Nature. 1996;380:419–22. [Google Scholar]
- Thornton I. Impacts of mining on the environment; some local, regional and global issues. J Int Assoc Geochem Cosmochem. 1996;11:355–61. [Google Scholar]
- Tong S, von Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull WHO. 2000;78:1068–77. [PMC free article] [PubMed] [Google Scholar]
- Torno S, Torano J, Menendez M, Gent M, Allende C. Prediction of particulate air pollution from a landfill site using CFD and LIDAR techniques. Environ Fluid Mech. 2011;11:99–112. [Google Scholar]
- Toy TJ, Foster GR, Renard KG. Soil erosion: Processes, prediction, measurement, and control. New York: John Wiley & Sons; 2002. [Google Scholar]
- Trepka MJ, Heinrich J, Krause C, Schulz C, Lippold U, Meyer E, et al. The internal burden of lead among children in a smelter town - A small area analysis. Environ Res. 1997;72:118–30. doi: 10.1006/enrs.1996.3720. [DOI] [PubMed] [Google Scholar]
- Triantafyllou A, Zoras S, Evagelopoulos V. Particulate matter over a seven year period in urban and rural areas within, proximal and far from mining and power station operations in Greece. Environ Monitor Assess. 2006;122:1–3. doi: 10.1007/s10661-005-9162-9. [DOI] [PubMed] [Google Scholar]
- Tursic J, Grgic I, Berner A, Skantar J, Cuhalev I. Measurements of size-segregated emission particles by a sampling system based on the cascade impactor. Environ Sci Technol. 2008;42:878–83. doi: 10.1021/es071094g. [DOI] [PubMed] [Google Scholar]
- UNSO (United Nations Sudano-Sahelian Office) Aridity zones and dryland populations : an assessment of population levels in the World’s drylands. New York: Office to Combat Desertification and Drought; 1997. [Google Scholar]
- US Environmental Protection Agency. National Ambient Air Quality Standards (NAAQS) 2011. Washington, D.C: 2011. [Google Scholar]
- US Environmental Protection Agency. Environmental Protection Agency 40 CFR Parts 50, 51, 53 and 58. Federal Register; 2008. National Ambient Air Quality Standards for Lead; p. 73. [Google Scholar]
- Valentin C, Poesen J, Li Y. Gully erosion: Impacts, factors and control. Catena. 2005;63:132–53. [Google Scholar]
- Valiulis D, Sakalys J, Plauskaite K. Heavy metal penetration into the human respiratory tract in vilnius. Lithuanian J Phys. 2008;48:349–55. [Google Scholar]
- Vallelonga P, Candelone JP, Van de Velde K, Curran MAJ, Morgan VI, Rosman KJR. Lead, Ba and Bi in Antarctic Law Dome ice corresponding to the 1815 AD Tambora eruption: an assessment of emission sources using Pb isotopes. Earth Planet Sci Lett. 2003;211:329. [Google Scholar]
- van Alphen M. Atmospheric heavy metal deposition plumes adjacent to a primary leadzinc smelter. Sci Total Environ. 1999;236:119–34. doi: 10.1016/s0048-9697(99)00272-7. [DOI] [PubMed] [Google Scholar]
- van Pelt R, Zobeck T. Chemical constituents of fugitive dust. Environ Monitor Assess. 2007;130:1–3. doi: 10.1007/s10661-006-9446-8. [DOI] [PubMed] [Google Scholar]
- Vega E, Mugica V, Reyes E, Sanchez G, Chow JC, Watson JG. Chemical composition of fugitive dust emitters in Mexico City. Atmos Environ. 2001;35:4033–9. [Google Scholar]
- Volkamer R, Jimenez JL, San Martini F, Dzepina K, Zhang Q, Salcedo D, et al. Secondary organic aerosol formation from anthropogenic air pollution: Rapid and higher than expected. Geophys Res Lett. 2006;33:L17811. [Google Scholar]
- Wang SC, Flagan RC. Scanning electrical mobility spectrometer. Aerosol Sci Technol. 1990;13:230–40. [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Persp. 2007;115:285–9. doi: 10.1289/ehp.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb N, McGowan H. Approaches to modelling land erodibility by wind. Prog Phys Geo. 2009;33:587–613. [Google Scholar]
- Weber WJ, McGinley PM, Katz LE. Sorption phenomena in subsurface systems - Concepts, models, and effects on contaminant fate and transport. Water Res. 1991;25:499–528. [Google Scholar]
- Whicker JJ, Pinder JE, Breshears DD, Eberhart CF. From dust to dose: Effects of forest disturbance on increased inhalation exposure. Sci Total Environ. 2006;368:2–3. doi: 10.1016/j.scitotenv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Wichmann HE, Trepka MJ, Heinrich J, Ihme W, Mekel O, Lin JH. From epidemiologic exposure and risk assessment to probabilistic models: Experience with the investigation of health effects of soil contamination in Germany. Int J Toxicol. 1997;16:391–418. [Google Scholar]
- Wilkening KE, Barrie LA, Engle M. Atmospheric science: trans-Pacific air pollution. Science. 2000;290:65. doi: 10.1126/science.290.5489.65. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Nickling WG. The protective role of sparse vegetation in wind erosion. Progr Phys Geo. 1993;17:50–68. [Google Scholar]
- Wolnik KA, Fricke FL, Capar SG, Braude GL, Meyer MW, Satzger RD, et al. Elements in major raw agricultural crops in the United States. 2. Other elements in lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J Agric Food Chem. 1983;31:1244. doi: 10.1021/jf00120a025. [DOI] [PubMed] [Google Scholar]
- Woodruff NP, Siddoway FH. A wind erosion equation. Soil Sci Soc Am Proc. 1965;29:602–8. [Google Scholar]
- Woolf AD, Goldman R, Bellinger DC. Update on the clinical management of childhood lead poisoning. Pediatr Clin North Am. 2007;54:271–94. doi: 10.1016/j.pcl.2007.01.008. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Updating and revision of the air quality guideline for Europe. EUR/ICP/EHAZ 94 05/PB01, Meeting of the working group “classical” air pollutants; 1995. [Google Scholar]
- WHO (World Health Organization) Report on a WHO working group. 2003. Health aspects of air pollution with particulate matter, ozone and nitrogen dioxide. [Google Scholar]
- Wright J. Making loess-sized quartz silt: data from laboratory simulations and implications for sediment transport pathways and the formation of ‘desert’ loess deposits associated with the Sahara. Quater Int. 2001;76–77:7–19. [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. Plos Med. 2008;5:732–40. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicol. 2006;27:210–6. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Yáñez L, García-Nieto E, Rojas E, Carrizales L, Mejía J, Calderón J, et al. DNA damage in blood cells from children exposed to arsenic and lead in a mining area. Environ Res. 2003;93:231–40. doi: 10.1016/j.envres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Yin D, Nickovic S, Barbaris B, Chandy B, Sprigg WA. Modeling wind-blown desert dust in the southwestern United States for public health warning: A case study. Atmos Environ. 2005;39:6243–54. [Google Scholar]
- Yin D, Nickovic S, Sprigg WA. The impact of using different land cover data on wind-blown desert dust modeling results in the southwestern United States. Atmos Environ. 2007;41:2214–24. [Google Scholar]
- Yousefi N, Chehregani A, Malayeri B, Lorestani B, Cheraghi M. Investigating the effect of heavy metals on developmental stages of anther and pollen in Chenopodium botrys L (Chenopodiaceae) Bio Trace Elem Res. 2011;140:368–76. doi: 10.1007/s12011-010-8701-6. [DOI] [PubMed] [Google Scholar]
- Zhou DM, Hao XZ, Wang YJ, Dong YH, Cang L. Copper and Zn uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere. 2005;59:167–75. doi: 10.1016/j.chemosphere.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Zobeck TM, Fryrear DW. Chemical and physical characteristics of windblown sediment, II: chemical characteristics and total soil and nutrient discharge. T ASAE. 1986;29:1037–41. [Google Scholar]
- Zobeck TM, Sterk G, Funk R, Rajot JL, Stout JE, Van Pelt RS. Measurement and Data Analysis Methods for Field-Scale Wind Erosion Studies and Model Validation. J Brit Geomorphol Res Group. 2003;28:1163–88. [Google Scholar]