Summary
Purpose
Patients with gliomas frequently present with seizures, but the factors associated with seizure development are still poorly understood. In this study, we assessed peritumoral synaptic network activity in a glioma animal model and tested the contribution of aberrant glutamate release from gliomas on glioma-associated epileptic network activity.
Methods
In vitro brain slices were made from glioma-implanted mice. Using extracellular field recordings, we analyzed peritumoral epileptiform activity induced by Mg2+-free medium in slices from tumor-bearing animals and sham-operated controls. We assessed the effect of sulfasalazine (SAS), a blocker of system xc− and glutamate release, on spontaneous and evoked activity in tumor-associated slices.
Key Findings
Tumor-associated cortical networks were hyperexcitable. The onset latency of Mg2+-free-induced epileptiform activity was significantly shorter in tumor-bearing slices, and the incidence of Mg2+-free-induced ictal-like events was higher. Block of glutamate release from system xc− decreased the response area of evoked activity and completely blocked Mg2+-free-induced ictal-like, but not interictal-like events.
Significance
Control of seizures in patients with gliomas is an essential component of clinical management; therefore, understanding the origin of seizures is vital. This work provides evidence that peritumoral synaptic network activity is disrupted by tumor masses resulting in network excitability. We show that blocking glutamate release via system xc− with SAS, a drug already approved by the US Food and Drug Administration (FDA), can inhibit Mg2+-free-induced ictal-like epileptiform events similar to other chemicals used to decrease seizure activity. We therefore suggest that further studies should consider SAS a promising agent to aid in the treatment of seizures associated with gliomas.
Keywords: Glioma, astrocytoma, sulfasalazine, epilepsy, seizure, cortex, system xc−
Introduction
Low-grade gliomas are the most epileptogenic brain tumors (Ruda et al., 2010), and antiepileptic drugs (AEDs) to control seizures are an important component of existing treatment strategies. High grade gliomas also have a poor prognosis; their infiltrative nature prevents complete excision with the majority of tumors recurring within 2 cm of their original location (Hart et al., 2010), and epileptic seizures have been also recognized as a major problem in these patients. Furthermore, epilepsy has been reported in more than 80% of low-grade gliomas (Vertosick et al. 1991) and in 30 to 60% of high-grade gliomas (Scott et al. 1980). The seizures associated with gliomas add to the potential morbidity, since patients who present with seizures at early stages are at higher risk for recurrent seizures (Glantz, 2000). Although the tumor core itself does not seem to be epileptogenic, electroencephalogram (EEG) recordings show abnormalities in the cortex adjacent to the lesion (Wolf, 1996). Several pathophysiological changes occur in the peritumoral region and investigating these alterations is vital to understanding the development of epileptogenic foci. These changes affect neuronal excitability, disrupt transmitter release (Groot et al., 2005; Ye et al., 1999), the blood-brain barrier (Furnari et al. 2007; Groothuis et al., 1983), and the balance of neurotransmitters and their receptors (Rijpkem et al., 2003; Roslin et al., 2003; Takano et al., 2001; Ye & Sontheimer, 1999). These changes result in an imbalance of network activity leading to hyperexcitability and excitotoxicity (Ye & Sontheimer, 1999) in the peritumoral region. Since epileptogenesis involves hyperexcitability of the local circuitry, AEDs are used to enhance synaptic inhibition and/or decrease excitation (Meldrum & Rogawaski, 2007) to suppress network synchronization, one of the precursors to epileptiform activity. The goal is to restore proper network function by regaining the chemical balance of the intra- and extracellular milieu. However, while new AEDs are promising both in terms of efficacy and tolerability, glioma patients remain highly resistant to AEDs (Bauer et al., 2007; Berger et al., 1993).
Imbalance in cortical networks can result in an epileptic cortex, which typically generates two patterns of neuronal discharges: interictal bursts, responsible for the spikes in EEG recordings, and ictal paroxysms, or seizures (Gibbs et al. 1936; Marsan 1961). Interictal bursts are brief synchronous neuronal discharges that occur between seizures and are rarely associated with overt behavioral disturbances (Marsan, 1961; Traub et al. 1996). On the other hand, ictal paroxysms are sustained synchronized discharges of large neuronal populations (Marsan, 1961) that are thought to occur during seizures (Traub et al. 1996). Studies of the relationship between ictal and interictal events in various epilepsy models show that generation of interictal activity requires chemical synaptic excitation, while ictal episodes can be initiated and maintained by nonsynaptic neuronal interactions (Jensen and Yaari 1988). Based on a model that utilized EEG signals from patients with mesial temporal lobe epilepsy, Wendling et al. (2005) demonstrate that the transition from interictal to ictal activity is complex and involves a time-varying ensemble interaction between pyramidal cells and local interneurons that project to either their dendritic or perisomatic regions. Importantly, AEDs prescribed for treatment of seizures block ictal-like but not interictal-like events in the hippocampus (Fueta and Avoli, 1992), hippocampus-entorhinal cortex (Brückner and Heinemann 2000), and entorhinal cortex (D’Antuono et al., 2010).
To treat glioma-associated seizures, it is important to understand how these tumors give rise to abnormal network activity. Maintaining the balance of intra- and extracelluar glutamate is critical for proper network function. Studies show that the peritumoral region has a high level of glutamate (Behrens et al., 2000; Buckingham et al., 2011), which may contribute to the impaired neuronal function and epileptiform activity associated with gliomas. A potential source of elevated extracellular glutamate is from system xc−, the cystine-glutamate antiporter, which exchanges extracellular cystine for intracellular glutamate (McBean, 2002). System xc− is a heterodimeric protein complex consisting of a catalytic L chain (xCT) and a regulatory H chain (4F2hc) (Sato et al., 1999). System xc− appears to be especially abundant in glioma cells (Kim et al., 2001), and growing tumor masses could potentially release an excitotoxic amount of glutamate into the extracellular space. Sulfasalazine (SAS), a drug already approved by the FDA, blocks system xc−.
Establishing clinically relevant animal models of gliomas that reproduce the invasive growth patterns and incidence of seizure activity in glioma patients remains a challenge. In a previous study (Buckingham et al., 2011), we established an animal model of gliomas allowing measurement of seizure activity. We demonstrated that implanting the U251 human glioma cell line or human glioma xenografts intracranially into mice induced spontaneously recurring abnormal EEGs, which were inhibited by blocking system xc− with SAS. In this study, we aimed to elucidate if gliomas modify peritumoral neuronal network activity. We hypothesized that the peritumoral neuronal network is hyperexcitable due to the release of glutamate through system xc− in glioma cells. We used SAS to block system xc− and tested its effect on tumor-associated network excitability. We present evidence that the tumor-associated brain is hyperexcitable and demonstrates a persistent change in neuronal network activity. Furthermore, we establish a partial role for glutamate release from system xc−, since SAS blocks prolonged ictal-like epileptiform activity induced by Mg2+-free solution but does not affect shorter interictal-like events. In addition, results from stimulus evoked responses show a potential alteration in inhibition.
Methods
Glioma cells - U251ffluc cells were stably transfected with pcDNA3 firefly luciferase, driven by the CMV promoter. U251GFP cells were stably transfected with pEGFP-N1 (Clontech, Mountain View, CA). The plasmid insertions were maintained with 0.25 mg/ml G418 disulfate salt (Invitrogen, Carlsbad, CA). U251 cells were incubated at 37° C, 10% C02 in 1:1 DMEM/F12 media (Invitrogen, Carlsbad, CA), and supplemented with 7% bovine growth serum (HyClone, Logan, UT) and 2 mM L-glutamine (CellGro, Herndon, VA).
In vivo Studies - Mice were bred and maintained in a pathogen-free barrier facility and all procedures were approved and performed in accordance with guidelines of the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Female C.B.17 scid mice, 8–10 wks old, were anesthetized and maintained with 2.5 % isofluorane throughout the surgery. The shaved scalp was swabbed with 70% alcohol and a 0.5 cm midline incision was made in the scalp, front to back. The animals were placed on a stereotaxic apparatus (Stoelting, Wood Dale, IL) and a small hole was bored through the skull over the left cortex, 2.5 mm posterior from Bregma and 2.5 mm lateral from the midline, using a dental drill equipped with a 1.0 mm drill bit. A needle fitted on a Hamilton syringe containing 10 μl of 5 × 105 U251GFP glioma cells in 5% methylcellulose was inserted to a depth of 1.5 mm from the skull surface, and the cell suspension was injected, 5 μl at a time separated by a 30 s interval, into the brain. Control mice were injected with 10 μl of 5% methylcellulose. The needle remained in place for 2 min after injection before removal. The incision was then closed using skin glue (Vetbond). Tumor implantation typically resulted in an organized mass as illustrated in Fig. 1C. However, in some animals, tumors spread throughout the cortex and infiltrated white matter tracts and the striatum. For consistency in this study, we used only slices that exhibited a concentrated tumor mass in the cortex; more infiltrative tumors will be studied in the future.
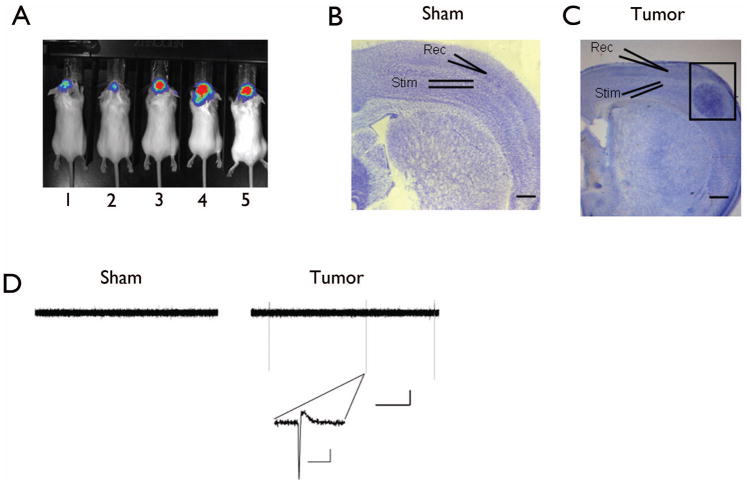
Figure 1.
Tumor-bearing slices show spontaneous epileptiform activity. Representative bioluminescence imaging of tumors in vivo. (A) Example of luciferase expressing U251ffluc glioma cells (mouse 3–5) or vehicle alone (mouse 1 and 2) implanted intracranially and visualized with an in vivo imaging using a Xenongen IVIS Bioluminescent Imaging System. Cresyl violet staining of sham-operated (B) and tumor-bearing cortex (C). Tumor-bearing slice show a pronounced tumor mass (in black box) traversing the cortical layers. Extracellular field recording electrodes positioned adjacent to tumor mass in layer II/III and stimulating electrode was placed in deeper layer IV. Electrodes were placed in a comparable region in sham-operated slices. (D) Representative samples of spontaneous extracellular field recordings from a sham (left) and tumor-bearing slice (right), scale bar: 50 sec, 0.5 mV. Inset shows individual interictal-like event in tumor-bearing slice on a faster timescale. Scale bar: 1 sec, 0.5 mV.
Slice preparation - Animals were sacrificed using a rodent guillotine. The brain was immersed in ice-cold cutting saline (CS) containing (in mM): 110 sucrose, 60 NaCl, 3 KCl, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 MgSO4, 5 glucose, and 0.6 ascorbate. Coronal slices (400 μm) were prepared with a Vibratome (The Vibratome Company, St. Louis, MO). During isolation, slices were stored in ice-cold CS. After isolation, slices were equilibrated in a mixture of 50% CS and 50% artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgSO4, and 25 glucose at room temperature for 45 min. Slices were further equilibrated in 100% ACSF for 45 min at room temperature, followed by a final incubation in 100% ACSF at 32 °C for 1 h in the recording chamber (Fine Science Tools, Foster City, CA). All solutions were saturated with 95%/5% O2/CO2.
Slice Electrophysiology - Extracellular field recordings were performed in an interface chamber (Fine Science Tools, Foster City, CA). Oxygenated ACSF (95%/5% O2/CO2) was perfused into the recording chamber at a rate of 1 ml/min. Extracellular stimuli (50 –100 ms, 0 – 300 μA) were delivered (model 2200 stimulus isolator; A-M Systems) in cortical layer IV using Teflon-coated, bipolar platinum electrodes. Slices from tumor-bearing and sham operated animals were first stimulated using a bipolar electrode placed in deeper layer IV, and field potential was recorded with an ACSF filled electrode in layer II/III, 0.5 to 2.5 mm from tumor mass over a range of increasing stimulation intensities to assess baseline synaptic transmission and slice viability (Fig. 1B, C). Spontaneous activity was recorded in layer II/III in ACSF for at least 20 min before any drug application. Mg2+-free solution was used to induce hyperexcitability. Sulfasalazine and Mg2+-free solutions were bath applied independently or co-applied depending on the specific experiment, and their effects were measured after 40 – 60 min application. In this study we defined “interictal” and “ictal” as synchronous epileptiform events with durations shorter or longer than 3 s, respectively. The duration of an epileptiform event was measured as the time interval between the first and the last population spike present in each epileptiform event.
Data collection and analysis - Electrical signals were amplified (model 1800; A-M Systems, Sequim, WA), digitized (Digidata 1320A and 1400), and stored on a PC (Clampex 9.2 and 10; Molecular Devices). Responses were filtered at 5 kHz, digitized at 10–20 kHz, and analyzed using Clampfit 10.0 software (Molecular Devices). Origin 7.5 (Microcal, Northampton, MA) was used for additional analysis and graphing. Responses were compared using Student’s t-test or analysis of variance (ANOVA) followed by Bonferroni post hoc comparisons, performed using GraphPad Instat (Graphpad Software). Significance was set at p < 0.05 . Data are reported as mean ± standard error of the mean (SEM). Concentrated stocks of D (−)-2amino-5-phosphonopentanoic acid (APV) (Tocris, Bristol) and sulfasalazine (Sigma, St. Louis, MO) were made in deionized water and stored at −20 C, and later dissolved in oxygenated ACSF at the desired concentration immediately before use.
Results
Peritumoral networks display spontaneous epileptiform activity
Using an established glioma model in which U251-GFP human glioma cells were implanted intracranially into mice (Fig. 1A), we prepared in vitro brain slices from tumor-bearing animals and sham-operated controls (vehicle alone) three weeks post-implantation to conduct extracellular field recordings. As illustrated in Fig. 1C, field recording electrodes were placed in layer II/III of the cortex 0.5 – 2.5 mm from the tumor mass, and in a comparable region in slices from sham-operated animals (Fig. 1B). Spontaneous field postsynaptic potentials (fPSPs) were assessed for 20 minutes under control conditions in standard artificial cerebrospinal fluid (ACSF), and recordings revealed the absence of spontaneous extracellular field events in slices from sham-operated control animals (Fig. 1D, left). However, only 30% of slices from tumor-bearing mice exhibited spontaneous activity (Fig. 1D, right), which is similar to the incidence in human glioblastoma patients (Oberndorfer et al., 2002). Individual events were fast and consisted of a single negative voltage change followed by a slower positive voltage change (Fig. 1D, inset on right). Tumor-bearing slices either exhibited these events continuously or not at all.
Alteration of synaptic transmission in peritumoral network
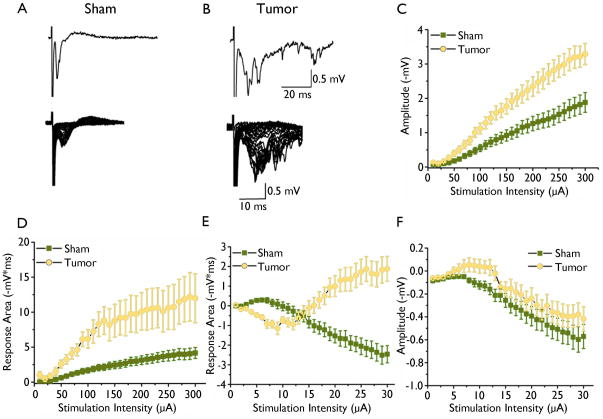
We first investigated the relative impact of tumor masses on peritumoral network activity by performing extracellular field recordings and comparing input/output curves from tumor-bearing and control slices. A stimulus electrode was used to deliver incrementally increasing stimuli to deep cortical layer IV, and fPSPs were recorded in the superficial cortical layers (II/III) within 0.5 – 2.5 mm of the tumor and in a similar region in the control slices (Fig. 1B, C). Input/output curves were constructed of the response area and amplitude as a function of stimulus intensity. Field potentials evoked in control slices were stereotypical cortical responses (Fig. 2A, lower trace). Lower stimulation intensities typically evoked responses with an “early” excitatory component; however with increasing stimulation intensities, a “later” inhibitory component emerged (Fig. 2A). Further increases in stimulus strength did not consistently increase any aspect of the response. Recordings from tumor-bearing slices varied but were always significantly larger than controls. In 16 of 25 tumor-bearing slices, only polysynaptic events were observed (Fig. 2B). Increasing stimulation resulted in greater excitatory response area and amplitude in tumor-bearing slices compared to controls (sham: n = 13 slices, 5 animals, vs tumor: n = 25 slices, 10 animals, p < 0.05, Fig. 2C, Fig. 2D, respectively). Higher stimulation intensities induced an inhibitory component of the fPSP in recordings from sham slices, but this inhibitory component was less pronounced or absent in some tumor-bearing slices. Specifically, recordings from 16 of 25 tumor-bearing slices did not show any inhibitory component of the response (Fig 2B). The remaining 9 tumor-bearing slices displayed smaller response areas (sham: n = 13 slices, 5 animals, vs tumor: n = 25 slices, 10 animals, p < 0.05, Fig 2E), but did not differ in inhibitory response amplitude (sham: n = 16 slices, 5 animals, vs tumor: n = 25 slices, 10 animals, p > 0.05, Fig 2F). Thus, there does seem to be a profound alteration in basal synaptic transmission in slices from tumor-bearing mice as indicated by extracellular recordings.
Figure 2.
Extracellular field recording in sham-operated and tumor-bearing slices. Representative traces of field recordings from sham-operated (A) and tumor-bearing (B) slices in response to increasing stimulation intensities. Upper trace in (A) shows individual trace from sham and from tumor slices in (B) in response to half maximal stimulation intensity. Graphical display of input/output curves of fPSPs recorded in superficial cortical layer II/III of the excitatory response amplitude (C) and excitatory response area (D) as a function of stimulation intensity in sham-operated (squares) compared to tumor-bearing slices (circles). Tumor-bearing slices showed significantly larger amplitude (p < 0.01) and area (p < 0.01) of the “early” excitatory component. Input/output curve of the “late” inhibitory response area (E) amplitude (F).
Tumor-associated networks are more susceptible to hyperexcitability
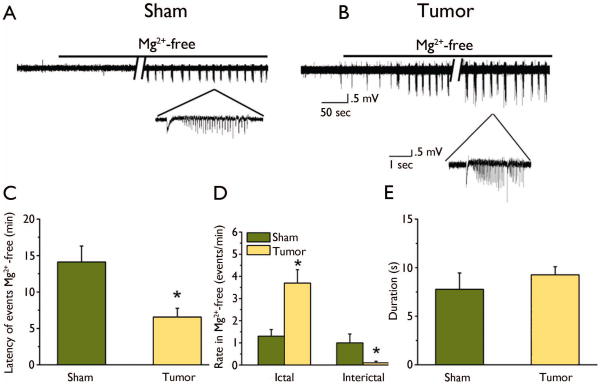
Having established that synaptic transmission is altered in peritumoral networks from the input/output curves, we further examined its vulnerability to hyperexcitability. Several studies have demonstrated that removal of Mg2+ from ACSF can induce synchronized spontaneous neuronal activity (Mody et al., 1987). This occurs because removal of Mg2+ ions, which block NMDARs, results in increased flux of depolarizing ions through NMDAR channels (Coan and Collingridge 1987; Collingridge et. al 1988). Extracellular field potential recordings were obtained for 10 minutes in standard ACSF, and then Mg2+ was omitted from the perfusate to induce epileptiform events. Epileptiform activity in both groups typically began with a negative deflection followed by a long train of individual discharges (Fig. 3A, B), which persisted while the Mg2+-free solution was used as the perfusate. While Mg2+-free ACSF generated epileptiform activity in both groups, quantitative analysis of the duration and rate of occurrence revealed pronounced differences. The onset latency of the first epileptiform discharge was significantly decreased in the tumor-bearing slices (6 ± 1 min, n = 30 slices, 12 animals) compared to controls (14 ± 2, min, n = 16 slices, 5 animals, p < 0.01, Fig. 3C). Ictal-like events were observed in slices from both groups after 60 min; however, the frequency was significantly increased in glioma-bearing slices (3.7 ± .6 events/min, n = 14 slices, 6 animals) compared to control slices (1.2 ± .3 events/min, n = 7 slices, 4 animals, p < 0.01). Additionally, there were fewer interictal-like events in glioma-bearing (0.3 ± 0.07 events/min, n =14 slices, 6 animals) compared to control slices (1 ± 0.04 events/min, p < 0.01) (Fig. 3D). The mean duration of epileptiform events was similar in glioma-bearing (6.5 ± 1 s, n = 19 slices, 8 animals) and control slices (7 ± 2 s, n = 11 slices, 5 animals, Fig. 3E).
Figure 3.
Mg2+-free solution induced epileptiform activity in control and tumor-bearing cortical slices. Representative traces of extracellular field recordings of spontaneous epileptiform events induced by Mg2+-free solution in a sham-operated (A) and tumor-bearing slice (B). Insets show traces on a magnified timescale to clearly demonstrate individual epileptiform activity in each group. (C) Mean onset latency of epileptiform event in Mg2+-free solution for each group revealed a significantly shorter latency in tumor-bearing slices compared to sham-operated. (D) Summary of the frequency of ictal-like and interictal-like events in sham-operated and tumor-bearing slices showing a significant increase in ictal-like events (p < 0.05) and a decrease in interictal-like events in tumor-bearing slices compared to controls. (E) Mean duration of epileptiform activity is similar in both groups.
Ictal-like network activity is blocked by SAS in tumor-associated cortex
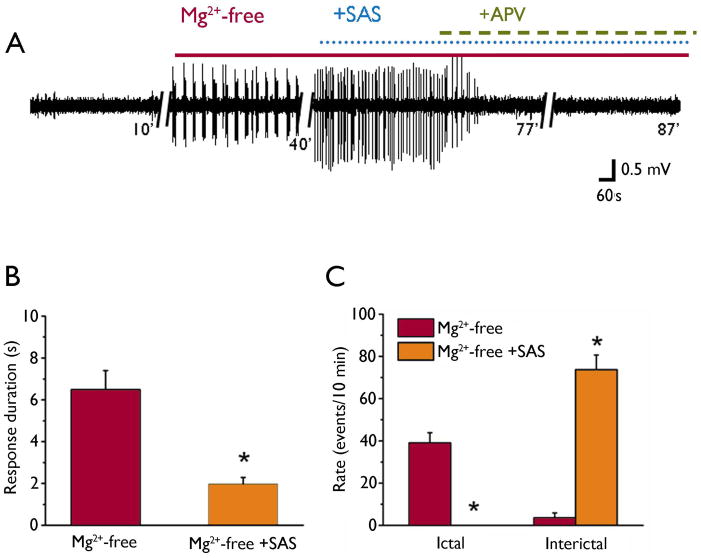
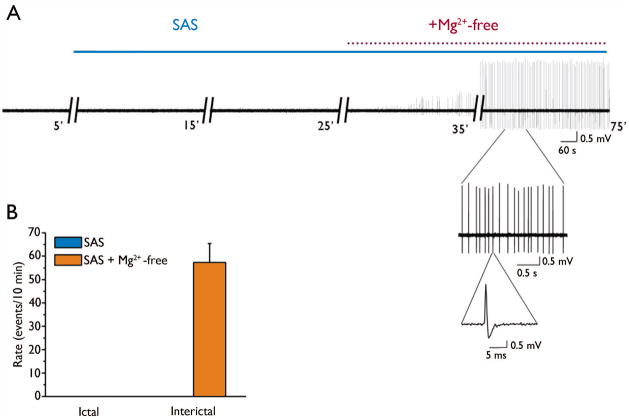
In a recent study we demonstrated that abnormal EEG events were inhibited by SAS, a blocker of the system xc− transporter, which is presumed to release glutamate from the tumor mass (Buckingham et al., 2011). Here, we wanted to determine the relative contribution of glutamate release via system xc− to peritumoral network hyperexcitability. Because previous reports demonstrate that AEDs block ictal-like events without affecting interictal events (Fueta and Avoli, 1992, Brückner and Heinemann 2000, D’Antuono et al., 2010), we tested the effect of SAS on both types of events. We first recorded fPSPs in the presence of Mg2+-free solution for 60 minutes until epileptiform activity was fully established. Next, we co-applied SAS (250 μM) to block system xc−, which produced a sustained inhibition of ictal-like events (Mg2+-free: 39 ± 5 events/10 min vs Mg2+-free + SAS: 0 events/10 min; n = 19 slices, 7 animals, Fig 4. A, C), but did not affect the emergence of interictal-like events, which significantly increased in the presence of SAS (Mg2+-free: 3.6 ± 2 events/10 min vs Mg2+-free + SAS: 73 ± 7 events/10 min, Fig 4. A, C). The duration of events decreased from 6.5 ± 1 s in Mg2+-free to 2 ± 0.3 s when SAS was co-applied for 30 min (n = 19 slices, 7 animals, Fig 4. A, B). The specificity of SAS for blocking longer ictal-like events is consistent with previous studies on AEDs currently used to treat seizures (D’Antuono et al., 2010). When APV (20 μM) was applied to block NMDARs, it completely inhibited the remaining interictal-like events (Fig. 4A), demonstrating that NMDAR activation is involved in generating these events. After demonstrating that SAS completely blocked Mg2+-free-induced ictal-like events in tumor-bearing slices, we wanted to determine whether SAS could prevent their induction. First we monitored spontaneous activity for 20 minutes in tumor-bearing slices in ACSF. Three of the 10 tumor-bearing slices showed spontaneous interictal-like events. We then treated the slices with SAS (250 μM) for 30 minutes and monitored epileptiform events. Mg2+-free solution was applied for 40 minutes in the presence of SAS (250 μM) and failed to induce ictal-like events but still induced interictal-like events (Fig. 5A, B) with a mean onset latency of 13 ± 3 minutes and mean duration of 1.6 ± 0.4 s (n = 10 slices, 5 animals). These data demonstrate that blocking system xc− with SAS preferentially inhibits Mg2+-free-induced ictal-like events, presumably by blocking the required network synchronization.
Figure 4.
Effect of SAS on synchronous activity in the peritumoral network. (A) Representative traces of field recordings in ACSF, after 30 min in Mg2+-free, followed by co-application of SAS (250 μM) and Mg2+-free and following the addition of APV (20 μM) in tumor-bearing slices (B). Summary bar graphs of the effect of SAS on the response duration of Mg2+-free-induced epileptiform activity. (C) Summary data of the experiment in (A) showing that SAS (250 μM) blocked the induction of ictal-like but not interictal-like events.
Figure 5.
SAS blocks the induction of Mg2+-free ictal-like activity. Sample traces of recordings in ACSF(A), after application of SAS (250 μM) for 30 min and following co-application of SAS (250 μM) and Mg2+-free. Insets show events on a faster timescale. (B) Summary bar graphs of the experiment in (A) showing that pretreatment with SAS (250 μM) blocked Mg2+-free-induced ictal-like but not interictal-like events.
Peritumoral evoked activity is blocked by SAS
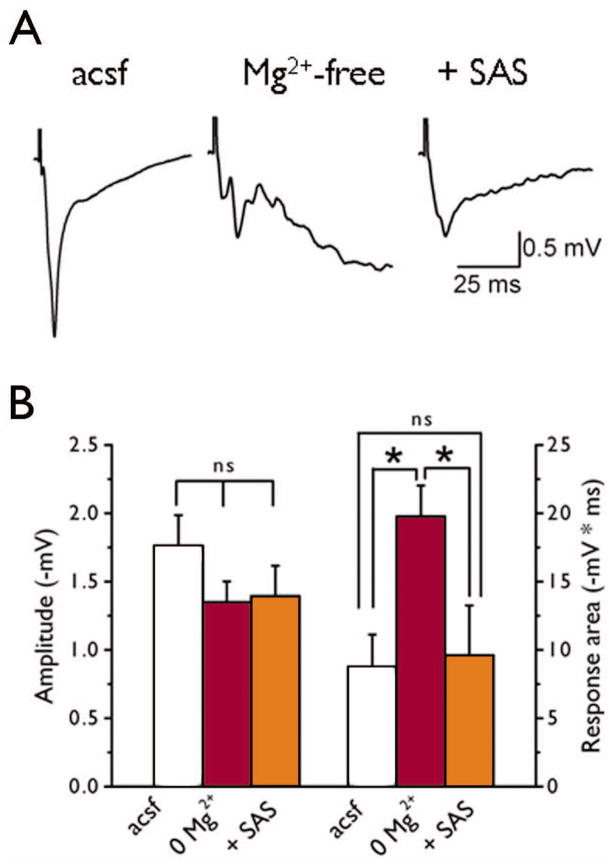
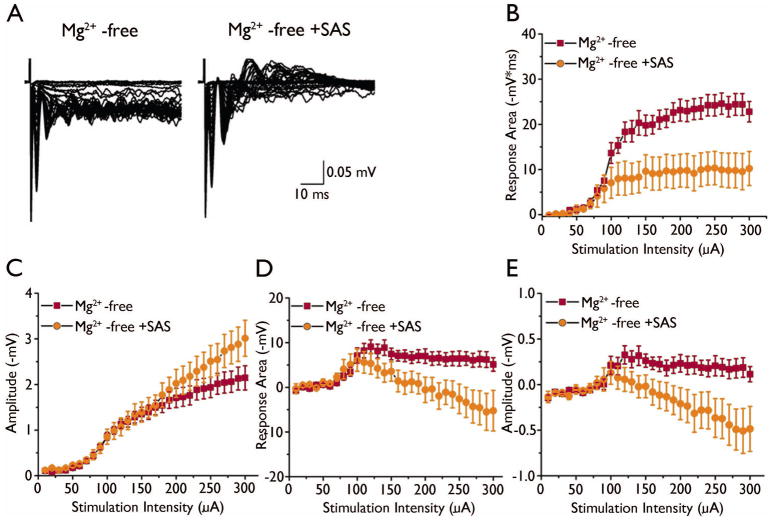
To examine the effect of SAS on evoked network activity, fPSPs were evoked in cortical layer II/III in tumor-bearing slices. Responses were elicited using a stimulation intensity that evoked half the maximal response. In ACSF, stimulation elicited large response areas, and application of Mg2+-free solution further prolonged the responses (Fig. 6A). The addition of SAS (250 μM) to the Mg2+-free solution significantly decreased the response area (Mg2+-free: −20 ± 2 ms * mV, vs Mg2+-free + SAS: −10 ± 3 ms * mV, n = 14 slices, 5 animals; F2,39 = 13, p < 0.05) but did not change the amplitude (Mg2+-free: −1.3 ± .2 mV, vs Mg2+-free + SAS: −1.4 ± .2 mV, n = 14 slices, 5 animals, F2,43 = 1.2, p > 0.05, Fig. 6B). We varied the stimulus intensity (0 – 300 μA) in order to plot input/output curves in the presence of Mg2+-free solution and during the co-application of SAS (Fig 7A). In Mg2+-free solution, stimulation at low intensities induced sustained epileptiform activity. Application of SAS (250 μM) decreased the “early” excitatory component of the response area of evoked epileptiform activity (p < 0.05, Fig 7B), but not the amplitude (p > 0.05, n = 14 slices, 5 animals, Fig 7C). Interestingly, SAS treatment evoked a “later” inhibitory component of the response which did not exist in Mg2+-free solution alone. The “later” inhibitory response area (p < 0.05, n = 14 slices, 5 animals, Fig 7D) and amplitude (p < 0.05, n =14 slices, 5 animals, Fig 7E) increased with stimulation intensity.
Figure 6.
SAS decreases evoked Mg2+-free-induced epileptiform activity. Representative traces of evoked fPSP recordings elicited by half-maximal stimulation intensity obtained from tumor-bearing slices (A) in ACSF, in the presence of Mg2+-free solution, and following co-application of SAS (250 μM). In ACSF tumor-bearing evoked responses were large with long durations. Application of Mg2+-free solution induced long-lasting polysynaptic activity and SAS (250 μM) decreased the (B) response area (p < 0.01) towards control levels but there was no change in the response amplitude (p > 0.05).
Figure 7.
SAS enhances inhibitory later “inhibitory” response in Mg2+-free solution. (A) Evoked responses to increasing stimulation intensity from tumor-bearing slices in Mg2+-free and co-application of Mg2+-free and SAS (250 μM). Summary I/O curves of the effect of SAS on the “early” excitatory response area (B) and amplitude (C) of Mg2+-free-induced activity in tumor-bearing slices. Input/output curves of the “late” inhibitory response area (D) and amplitude (E).
Discussion
This study provides a network-level analysis of the effect of glioma cell infiltration into cortical tissue on peritumoral network activity. Extracellular field recordings in acute cortical slices were used to first uncover changes in epileptic propensity at the network level in tumor-bearing and sham-operated slices and also to characterize excitability after treatment with SAS. We assessed the response of peritumoral networks to convulsant agents (Mg2+-free ACSF) due to the sporadic presence of spontaneous epileptiform activity in tumor-bearing slices. Our findings clearly demonstrate that the peritumoral tissue was hyperexcitable and more susceptible to epileptiform activity. Blockade of system xc− with SAS decreased tumor-associated epileptiform activity in an event specific manner.
The first central finding of our study is that the peritumoral network is hyperexcitable. This is in agreement with other glioma models showing epileptiform activity preferentially outside the tumor mass (Kohling et al. 2006). This excitable network could result from the culmination of several changes in peritumoral tissue, including impaired astrocytic function, a leaky blood-brain barrier (Furnari et al. 2007; Groothuis et al., 1983), abnormal transmitter release, and/or changes in receptor function and/or expression (Rijpkem et al., 2003; Roslin et al., 2003; Takano et al., 2001; Ye & Sontheimer, 1999). Impaired astrocytic function may result from astrogliosis and lead to network hyperexcitability. Many functions of astrocytes are critical in preventing neuronal excitotoxicity, especially their ability to take up extracellular glutamate and their role in spatial buffering (Anderson et al., 2003). Neuronal excitability can increase extracellular K+ to mM levels, and K+ released by neurons is thought to be sequestered primarily by glial cells (Ballanyi et al., 1987; Heinemann & Lux, 1977; Olsen et al., 2008). Several studies have shown that impaired glial K+ uptake and high [K+]o can lead to hyperexcitable conditions (Feng & Durand, 2006; Gabriel et al., 2004; Traynelis & Dingledine, 1988) and downregulation of inwardly rectifying potassium channels (Kir) channels, and a reduction in Kir currents has been reported in CNS injury and several epilepsy models (Bordey et al., 2000; Bordey et al., 2001; MacFarlane and Sontheimer, 1997; Olsen et al., 2006). We have also shown that glioma cell lines lack functional glutamate transporters, causing a reduction in glutamate uptake (Ye et al., 1999). Glutamate plays an important role in pathophysiological events that lead to neuronal dysfunction and even cell death (Choi, 1988). Significantly, Buckingham et al. (2011) measured glutamate levels in the peritumoral region using HPLC–mass spectrometry and found that the peritumoral region releases significantly higher levels of glutamate than control cortical tissue. This increase in peritumoral glutamate is consistent with that found in studies of glioma patients (Rijpkema et al., 2003; Roslin et al., 2003) and in a glioma animal model (Behrens et al., 2000). We have previously shown that glioma cells release glutamate and damage surrounding neurons (Ye et al., 1999; Ye & Sontheimer, 1999). Once in the extracellular space, elevated glutamate can activate several neurotoxic pathways. Excessive extracellular glutamate can kill neurons in the path of the tumor and overstimulate glutamatergic receptors, which leads to abnormal network functioning. Surviving neurons are left in a toxic environment conducive to seizure activity. The results of this study, in conjunction with recently published findings (Buckingham et al., 2011), suggest that glutamate release from system xc− in tumor cells is a key component driving peritumoral hyperexcitability in individual neurons and in neuronal networks.
The second important finding in this study is that blocking system xc− with SAS inhibits peritumoral ictal-like epileptiform discharges in the cortex when Mg2+ is omitted from the perfusate, while it does not block or prevent the onset of shorter interictal-like activity. This result is similar to results from D’Antuono et al. (2010), who tested the effect of three AEDs on epileptiform activity induced with the potassium channel blocker 4-aminopyridine (4AP); the drugs were able to block ictal-like events but not events shorter than 0.7 seconds. In addition, earlier studies also reported that shorter interictal-like events were resistant to AEDs (Fueta & Avoli, 1992). Although SAS completely blocked ictal-like events, there was an emergence of interictal-like activity. Other studies have reported that AEDs such as pentobarbital increased recurrent short discharges (Brückner and Heinemann, 1999), and that carbamazepine at high concentrations increased interictal discharges (D’Antuono et al. 2010) in the entorhinal cortex. The specific blockade of ictal-like events by SAS is significant because evidence from clinical studies indicates that AEDs which decrease seizure events do not affect the occurrence of interictal-like events (Gotman & Marciani, 1985; Spencer et al. 2008). The majority of AEDs are aimed at blocking voltage-dependent sodium channels and calcium channels (Rogawski & Löscher . 2004), while others either enhance the inhibitory effect of GABA or decrease the excitatory effect of glutamate (Deckers, 2000). SAS, on the other hand, is aimed directly at a specific source of glutamate to reduce hyperexcitability. In addition to blocking ictal-like events in the current study, we have previously shown that blocking system xc− with SAS can slow tumor growth and decrease the size of tumor masses (Chung et al., 2005). We also showed in Fig. 7 that SAS is effective in reducing evoked epileptiform activity. When epileptiform activity was evoked in tumor-bearing slices, responses produced either excitatory and inhibitory biphasic responses, or only monophasic excitatory responses. However, application of SAS converted excitatory monophasic responses to biphasic responses with “early” excitatory and “late” inhibitory components and increased the inhibitory component of the initial biphasic responses. This effect can be attributed to SAS’s ability to increase inhibition, decrease excitation, or both. The AED levetiracetam had a similar effects when tested on 4-AP-induced hyperexcitability in the synapsin I/II/II knockout mouse (Boido et al., 2010). This is an intriguing finding and warrants future study to uncover the role of inhibition in peritumoral excitability.
Our data together with published work suggest that the elevation in extracellular glutamate is caused by release from gliomas via system xc−, and contributes to peritumoral hyperexcitability. The blocker of system xc−, SAS, could therefore potentially represent a novel target for tumor-associated glutamate-induced hyperexcitability. Importantly, we demonstrated that SAS blocked the tumor-associated epileptiform activity in an event specific manner, similar to many AEDs (D’Antuono et al., 2010), which makes SAS an attractive and innovative candidate for treatment of glioma-associated seizures. Taken together, our results make a strong case for a role of glutamate released from gliomas via system xc− in the etiology of gliomas. Our data suggest that pharmacological blockade of system xc− suppresses hyperexcitability. The availability of an FDA approved candidate drug, SAS, is exciting, and SAS may be considered as an adjuvant treatment for tumor-associated epilepsy.
Acknowledgments
This work was supported by US National Institutes of Health grants 2RO1-NS052634, 5RO1-NS036692, and 5T32NS048039. We conducted extracellular field recordings in the Neuroscience Blueprint Core facility (Neuroscience Blueprint Core Grant NS57098). We obtained U251-MGffluc cells from M. Jensen (City of Hope National Medical Center). The authors would like to thank A. Margolies for help with histology and Eve McCutchen and Vishnu Cuddapah for editorial assistance.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Disclosure: The authors report no conflicts of interest.
References
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Grafe P, TenBruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol (London) 1987;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 2000;47:11–22. doi: 10.1023/a:1006426917654. [DOI] [PubMed] [Google Scholar]
- Boido D, Farisello P, Cesca F, Ferrea E, Valtorta F, Benfenati F, Baldelli P. Cortico-hippocampal hyperexcitability in synapsin I/II/III knockout mice: age-dependency and response to the antiepileptic drug levetiracetam. Neuroscience. 2010;171:268–83. doi: 10.1016/j.neuroscience.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Bordey A, Hablitz JJ, Sontheimer H. Reactive astrocytes show enhanced inwardly rectifying K+ currents in situ. Neuroreport. 2000;11:3151–3155. doi: 10.1097/00001756-200009280-00022. [DOI] [PubMed] [Google Scholar]
- Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J Neurophysiol. 2001;85:1719–1731. doi: 10.1152/jn.2001.85.4.1719. [DOI] [PubMed] [Google Scholar]
- Brückner C, Heinemann U. Epileptiform discharges induced by combined application of bicuculline and 4-aminopyridine are resistant to standard anticonvulsants in slices of rats. Neurosci Lett. 1999;263:163–5. doi: 10.1016/s0304-3940(99)00341-9. [DOI] [PubMed] [Google Scholar]
- Brückner C, Heinemann U. Effects of standard anticonvulsant drugs on different patterns of epileptiform discharges induced by 4-aminopyridine in combined entorhinal cortex-hippocampal slices. Brain Res. 2000;859:15–20. doi: 10.1016/s0006-8993(99)02348-3. [DOI] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17:1269–1274. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. [Review] Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Chung WJ, Sontheimer H. Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-kappaB. J Neurochem. 2009;110:182–193. doi: 10.1111/j.1471-4159.2009.06129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Herron CE, Lester RA. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Kohling R, Ricalzone S, Gotman J, Biagini G, Avoli M. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia. 2010;51:423–431. doi: 10.1111/j.1528-1167.2009.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers CL, Czuczwar SJ, Hekster YA, Keyser A, Kubova H, Meinardi H, Patsalos PN, Renier WO, Van Rijn CM. Selection of antiepileptic drug polytherapy based on mechanisms of action: the evidence reviewed. Epilepsia. 2000;41:1364–1374. doi: 10.1111/j.1528-1157.2000.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Fueta Y, Avoli M. Effects of antiepileptic drugs on 4-aminopyridine-induced epileptiform activity in young and adult rat hippocampus. Epilepsy Res. 1992;12:207–215. doi: 10.1016/0920-1211(92)90075-5. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Gibbs FA, Lennox WG, Gibbs EL. The EEG in diagnosis and in localization of epileptic seizure. Arch Neurol Psychiatry. 1936;36:1225–1235. [Google Scholar]
- Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, Grossman SA, Cairncross JG. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- Groothuis DR, Fischer JM, Pasternak JF, Blasberg RG, Vick NA, Bigner DD. Regional measurements of blood-to-tissue transport in experimental RG-2 rat gliomas. Cancer Res. 1983;43:3368–3373. [PubMed] [Google Scholar]
- Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2008;8:CD007415. doi: 10.1002/14651858.CD007415. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Lux HD. Ceiling of stimulus induced rises in extracellular potassium concentration in the cerebral cortex of cat. Brain Res. 1977;120:231–249. doi: 10.1016/0006-8993(77)90903-9. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Yaari Y. The relationship between ictal and interictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann Neurol. 1988;24:591–8. doi: 10.1002/ana.410240502. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y, Endou H. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta. 2001;1512:335–344. doi: 10.1016/s0005-2736(01)00338-8. [DOI] [PubMed] [Google Scholar]
- Kohling R, Senner V, Paulus W, Speckmann EJ. Epileptiform activity preferentially arises outside tumor invasion zone in glioma xenotransplants. Neurobiol Dis. 2006;22:64–75. doi: 10.1016/j.nbd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci. 1997;17:7316–7329. doi: 10.1523/JNEUROSCI.17-19-07316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- Marsan CA. Electrographic aspects of “epileptic” neuronal aggregates. Epilepsia. 1961;2:22–38. doi: 10.1111/j.1528-1167.1961.tb06244.x. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987;57:869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Higashimori H, Campbell SL, Hablitz JJ, Sontheimer H. Functional expression of K(ir)4.1 channels in spinal cord astrocytes. Glia. 2006;53:516–528. doi: 10.1002/glia.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M, Schuuring J, van der MY, van der GM, Bernsen H, Boerman R, van der KA, Heerschap A. Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed. 2003;16:12–18. doi: 10.1002/nbm.807. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- Roslin M, Henriksson R, Bergstrom P, Ungerstedt U, Bergenheim AT. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–160. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Scott GM, Gibberd FB. Epilepsy and other factors in prognosis of glioma. Acta Neurol Scand. 1980;61:227–230. doi: 10.1111/j.1600-0404.1980.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Kisby GE, Ross SM, Roy DN, Hugon J, Ludolph AC, Nunn PB. Guam ALS-PDC: possible causes. Science. 1993;262:825–826. doi: 10.1126/science.8235599. [DOI] [PubMed] [Google Scholar]
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- Traub RD, Borck C, Colling SB, Jeffery’s JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–91. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- Vertosick FT, Selker RG, Arena VC. Survival of patients with well differentiated astrocytoma diagnosed in the era of computed tomography. Neurosurgery. 1991;28:496–501. doi: 10.1097/00006123-199104000-00002. [DOI] [PubMed] [Google Scholar]
- Wendling F, Hernandez A, Bellanger JJ, Chauvel P, Bartolomei F. Interictal and to ictal transition in human temporal lobe epilepsy: insights from a computational model of intracerebral EEG. J Clin Neurophysiol. 2005;22:343–56. [PMC free article] [PubMed] [Google Scholar]
- Wolf HK, Roos D, Blumcke I, Pietsch T, Wiestler OD. Perilesional neurochemical changes in focal epilepsies. Acta Neuropathol. 1996;91:376–384. doi: 10.1007/s004010050439. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]