Abstract
Replacing the tissue lost after a stroke potentially provides a new neural substrate to promote recovery. However, significant neurobiological and biotechnological challenges need to be overcome to make this possibility into a reality. Human neural stem cells (hNSCs) can differentiate into mature brain cells, but require a structural support that retains them within the cavity and affords the formation of a de novo tissue. Nevertheless, in our previous work, even after a week, this primitive tissue is void of a vasculature that could sustain its long-term viability. Therefore, tissue engineering strategies are required to develop a vasculature. Vascular endothelial growth factor (VEGF) is known to promote the proliferation and migration of endothelial cells during angio- and arteriogenesis. VEGF by itself here did not affect viability or differentiation of hNSCs, whereas growing cells on poly(D,L-lactic acid-co-glycolic acid) (PLGA) microparticles, with or without VEGF, doubled astrocytic and neuronal differentiation. Secretion of a burst and a sustained delivery of VEGF from the microparticles in vivo attracted endothelial cells from the host into this primate tissue and in parts established a neovasculature, whereas in other parts endothelial cells were merely interspersed with hNSCs. There was also evidence of a hypervascularization indicating that further work will be required to establish an adequate level of vascularization. It is therefore possible to develop a putative neovasculature within de novo tissue that is forming inside a tissue cavity caused by a stroke.
Keywords: Stroke, Neural Stem Cells, Cell Transplantation, PLGA, Microparticle, Tissue Engineering, Neo-Vascularization, VEGF, Angiogenesis, Arteriogenesis
1. Introduction
Replacing brain tissue lost due to a stroke remains a major challenge. Behavioral impairments after a stroke are caused by the death of neurons. Replacing these lost neurons therefore could potentially reverse behavioral impairments [1]. Neural stem cells can differentiate into neurons upon implantation into the brain, but their intracerebral injection does not replace the lost tissue. Cells integrate into the remaining host brain and promote some behavioral recovery by integrating into existing neuronal networks and/or by stimulating angiogenesis that in turn improves neuronal functioning [2]. In contrast, implanted tissue pieces from fetuses into the stroke cavity form a tissue with some connections between the developing fetal tissue in the stroke cavity and the adult host tissue [3, 4]. However, for clinical translation fetal tissue is logistically and ethically challenging. To replace the lost brain tissue, it is therefore important to recreate the essential components required to form brain tissue.
One key factor for this is the provision of a structural support. Scaffolding poly(D,L-lactic acid-co-glycolic acid) (PLGA) microparticles can provide this support and allow implanted cells to fill the lesion cavity [5, 6]. In fetal, as well as in adult tissue, this structural support is provided by the extracellular matrix that constitutes approximately 20% of brain volume. Integration of human neural stem cells (hNSCs) with de-cellularized extra-cellular matrix (ECM) bioscaffolds can also achieve an efficient distribution of neural stem cells within the lesion cavity [7]. Although these strategies provide the required structural support to retain implanted cells within the infarct cavity, there is no vascular ingrowth from the host that can sustain the long-term development of this de novo tissue formation. It is thus essential that an additional strategy is incorporated that promotes the recruitment of a vascular supply.
A variety of chemical factors are known to be involved and control angiogenesis in tumors [8], but also in developing tissues [9]. One of the most potent angiogenic molecules is vascular endothelial growth factor (VEGF) [10]. Endothelial cells expressing the VEGF receptors proliferate and migrate in response to a gradient of VEGF-A in the brain [11]. VEGF-secreting cell grafts have been shown to promote angiogenesis after a stroke [12] and its release from PLGA microparticles is known to promote neovascularization after hindlimb ischemia [13, 14]. Pre-stroke implantation of a VEGF-releasing hydrogel also produced an almost complete neurological and anatomical protection [15], whereas post-stroke injection of VEGF-releasing biomaterials increased the colonization of the scaffold by astrocytes and endothelial cells in the lesion cavity [16].
Implantation of VEGF-releasing PLGA microparticles therefore could stimulate angiogenesis (i.e. the formation of new blood vessels), potentially providing a neovascularization of the de novo tissue forming inside the lesion cavity and thus increase the survival and integration of transplanted cells. To investigate this hypothesis, the effect of neuroscaffolds with and without human recombinant VEGF incorporation on hNSCs proliferation and differentiation was established in vitro prior to implantation into a rat model of stroke. These studies provide the fundamental basis to potentially guide and control in situ tissue engineering in the brain.
2. Material and Methods
2.1. VEGF-PLGA microparticle formulation
Poly(D,L lactic acid-co-glycolic acid) (PLGA) microparticles were fabricated using a double emulsion technique. The optimized method for the production of 50–100 μm-sized particles consisted of 1 g PLGA polymer (85:15 PLGA, Lakeshore Biomaterials, USA) being dissolved in 5 ml of dichloromethane (DCM) at room temperature overnight in a glass scintillation vial. For a 0.1% loading, 1 mg of VEGF (Pepro Tech, UK) was dissolved in 80 μl of 1% (w/v) bovine serum albumin (BSA) before being added to the polymer solution and emulsified using a vortex mixer (VM20 vortex mixer, scale 5) for 2 minutes to form the primary water in oil (O/W) emulsion. 5 ml of a pre-filtered 0.3% (w/v) polyvinyl alcohol (PVA) solution (Whatman No.1 paper) was then slowly added to the vial before the second emulsification process using a VM20 vortex mixer at scale 5 for 2 minutes. This secondary (W/O/W) emulsion was then immediately poured into a beaker containing 800 ml of the pre-filtered 0.3% (w/v) PVA solution and stirred constantly (IKA magnetic stirrer, speed 3) for 8 hours in a fume hood to allow the solvent to evaporate. Following the complete evaporation of the solvent, the solidified microparticles were washed three times with distilled water and harvested via vacuum filtration using Whatman No. 1 filter paper. Freeze dried microparticles were sieved and separated by a Retsch AS200 sieve shaker (amplitude 1.40, 40 s interval time and 10 minutes total running time). Long-term storage of microparticles was achieved using vacuum-sealed packaging and storage within a silica-filled desiccator. The size distribution of the pre-fractionated microparticles was determined by laser diffraction. Using a Coulter LS230 particle size analyzer (Beckman, UK), the particle size distribution was then determined as a function of the particle diffraction using the Coulter software (version 2.11a) and plotted as a function of volume percentage. Details regarding surface treatment via plasma polymerization can be found in Bible et al.[17]
2.2. Entrapment efficiency for VEGF
The measurement of the entrapment efficiency for VEGF within the MPs was modified from a protocol previously described by Deluca et al[18, 19]. In brief, 10 mg of PLGA microparticles were digested in 1 ml of 1 mM sodium hydroxide (NaOH) for 2 hours and then neutralized with 1ml of 1mM hydrochloric acid (HCl). The solution was then centrifuged (16000 × g, Sigma 1–16K) for 15 minutes at room temperature. VEGF quantification was achieved using an ELISA Quantikine Kit (R&D Systems, UK). For this, 50 μl of the assay diluent (RD1W) and 200 μl of the sample solution (or the assay standards) were added to a 96 well plate. Wells were pre-coated with a mouse monoclonal antibody against VEGF for 2 hours at room temperature. After three washes with buffer, 200 μl/well of a horseradish peroxidase polyclonal antibody against VEGF was added for 2 hours at room temperature. After another washing step, 200 μl/well of a substrate solution composed of 1:1 hydrogen peroxide and TMB chromogen was added for 20 minutes. Finally, the reaction was stopped using 50 μl of 2N sulphuric acid. The optical density was measured at 450 nm using a Tecan infinite M 200. VEGF concentrations in the samples were determined using a standard curve of known concentrations of VEGF supplied with the kit.
2.3. Human neural stem cells (hNSCs)
The cmyc-ERTAM conditionally immortalized human neural stem cell line STROC05 (ReNeuron Ltd.) [20–22] was isolated from the whole ganglionic eminence of a 12 week old fetal human brain. Transfection was performed using a retroviral vector pLNCX-2 (Clontech, Palo Alto) encoding the cmyc-ERTAM gene. Transfected cell colonies were isolated following neomycin selection before being expanded into a cell line. To ensure proliferation through the conditional immortalization gene, 4-hydroxy-tamoxifen ($-OHT); 100nM/ml, Sigma-Aldrich) was added to all subsequent culture media.
Expansion and maintenance of STROC05 was achieved in chemically defined media, consisting of Dulbecco’s Modified Eagle’s Medium/Ham’s with F12 medium (DMEM:F12, Gibco) supplemented with a range of components [20]. To maintain proliferation through the conditional immortalization gene, 4-hydroxy-tamoxifen (4-OHT, Sigma-Aldrich) and growth factors (basic fibroblast growth factor-2, bFGF, 10ng/ml, and epidermal growth factor, EGF, 20 ng/ml, Peprotech) were added to media in laminin-coated tissue culture flasks (mouse, 1:100, 10 mg/ml, Trevigen). For cell differentiation, growth factors and 4-OHT were withdrawn.
2.4. Attachment of cells to microparticles
As previously described in detail [5, 17], charged PLGA microparticles (20mg/ml) were coated in a single well of a sterile flat-bottom tissue culture-treated polystyrene 24 well plate (Costar, Corning) on a shaker (Grant-Bio PMR-30) with laminin (1:100, Cultrex, USA) for 1 hour prior to cell seeding (1×106 cells/ml). For cell colonization of coated microparticles, these were transfered to a new well. No laminin-coating was applied to this well to promote cell attachment to the particles rather than the tissue culture plate. To achieve a homogenous cell attachment, shaking (30 rpm, 5 min) and static (25 min) periods were alternated for 4 hours and cells/particles were then left in motion (35 rpm) overnight in a 24 well plate in the incubator.
2.5. Viability
To establish if the release of VEGF from PLGA microparticles could influence cell viability, STROC05 cells were grown on microparticles with (VEGF+) or without VEGF (VEGF−). Monocultures (30,000 cells in 400 μl media) with or without human recombinant VEGF165 (10 ng/ml, 100–20, Peprotech) were also used to control for potential effects of microparticle biodegradation on cell viability. A comparison of cell growth on microparticles +/− VEGF was also essential to account for difference in cell seeding, growth and counting on microparticles compared to monocultures. For viability measurements at 1 and 4 days, cells were incubated for 1 hr at room temperature using the Live/Dead® kit (Invitrogen) in 4μM ethidium/2μM calcein-AM. Samples were mounted in the incubation media and 5 random images from each of 3 coverslips were captured live using a x10 objective on an Axioplan (Zeiss, USA) microscope.
2.6. Proliferation and maintenance of neural stem cell characteristics
STROC05 cells growing on microparticles or in monoculture were fixed at 1 and 4 days using 4% paraformaldehyde (Pioneer) for 15 minutes. Cells were stained by incubation with a rabbit anti-Ki67 antibody (1:500, Abcam) indicating proliferating cells and a goat anti-Sox-2 (1:100, Abcam), a marker of stemness, for 2 hrs at RT. Visualization of the primary antibody was achieved by incubation with the relevant secondary (1:500, donkey anti-rabbit 488; 1:1000, donkey anti-goat 555, Chemicon) for 2 hrs at RT. Omission of the primary antibody provided a negative control to account for unspecific binding of the secondary antibody. Images were captured using a 20x objective. For microparticles, 10 random images were taken, whereas for monocultures 5 images per technical replicate were sufficient. An increase in the number of images for analysis for microparticles was due to the lower number of cells remaining attached to these after the 3 × 5 minutes of PBS rinsing steps, as well as the smaller number of cells within each focal plane. The number of Ki67+ or Sox2+ cells was expressed as a percentage of the total number of DAPI+ nuclei.
2.7. STROC05 neuronal differentiation
After withdrawal of the growth factors (bFGF & EGF) and tamoxifan (5-OHT), STROC05 cells were differentiated either on microparticles or in monoculture for 7 days prior to fixation with 4% paraformaldehyde for 15 minutes. To assess their neuronal differentiation, cells were incubated for 2 hrs at RT with the neuronal marker β-III-tubulin (Tuj, 1:500, Abcam), the astrocytic marker GFAP (1:1000, DAKO) or nestin (1:1000, Abcam), a marker for neuronal progenitor cells. Appropriate secondary antibodies (1:500, donkey anti-rabbit 488; 1:1000, donkey anti-goat 555, Chemicon) were incubated for 2 hrs at RT to visualize the primary antibodies. The number of β-III-tubulin+, GFAP+ or nestin+ cells was expressed as a percentage of the total number of DAPI+ nuclei.
2.8. Middle Cerebral Artery Occlusion (MCAO)
All animal procedures complied with the UK Animals (Scientific) Procedures Act (1986) and the Ethical Review Process of the Institute of Psychiatry. Sprague-Dawley rats (n=56, Harlan, UK) were maintained on a 12 h light/dark schedule with food available ad libitum. One week after arrival, 36 rats (weight 250–280g at surgery) had their right MCA transiently occluded for 60 minutes by insertion of a propylene filament (Doccol Corporation, USA), as previously described [23]. Isoflurane (4% for induction, 2% for maintenance in 30% oxygen) was used for anesthesia during insertion and removal of the filament, with temperature held at 37±1° C by rectal probe and heating pad. During the recovery period of two weeks, animals were monitored for neurological deficits [24].
2.9. Magnetic Resonance Imaging (MRI)
Animals that underwent MCAo surgery were scanned 10 days post-infarction using a spin echo T2-weighted MRI scan (TR = 4000 ms, TE = 60 ms, averages = 10, FOV = 40 × 40 mm, matrix = 128×128, 45 slices at 0.6 mm slice thickness). Scans were converted from the flexible data file (fdf) format to the Analyze 7.5 format for analysis using Jim5.0 (Xynapse Systems). Based on exclusion criteria [2] consisting of a hyperintense area <10 mm3 or the presence of a hemorrhage (hypointensity on T2), 12 animals were excluded.
2.10. Implantation procedure
Two weeks after MCAO, animals were allocated to their experimental groups (Table 1). Anesthesia was induced by intraperitoneal (i.p.) injection of 0.25ml/100g of body weight of a mixture of 0.1ml medetomidine hydrochloride (Dormitor, Pfizer, UK), 0.23 ml ketamine hydrochloride (Ketaset, Wyeth, UK), in 1ml sterile H2O. Animals were placed in a stereotactic frame and based on MRI coordinates a burr hole was drilled at appropriate co-ordinates for each animal.
Table 1. Number of rats in experimental groups.
Normal or rats with middle cerebral artery occlusion (MCAo) were transplanted either under control conditions with vehicle (no cells) or cells (STROC05). Vehicle injections into normal animals were not done (n.d.). Microparticles with (+) or without (−) vascular endothelial growth factor (VEGF) were implanted either with or without cells. A total of 44 rats were used for this study.
| Experimental Conditions | Normal | MCAO | |
|---|---|---|---|
| Suspension | No cells | n.d. | 4 |
| Cells | 4 | 4 | |
| Microparticles | VEGF− | 4 | 4 |
| VEGF+ | 4 | 4 | |
| Microparticles+ cells | VEG− & cells | 4 | 4 |
| VEGF+ & cells | 4 | 4 | |
| Total | 20 | 24 | |
To avoid tissue damage due to a large injection volume, normal rats received a single deposit of a 10 μl suspension (2μl/min) at the following bregma coordinates: AP −1.3mm; L −3.5mm; V −6.5. Injections either consisted of cell suspensions (50,000 cells/μl), microparticles (20 μg/μl) or microparticles with attached cells (20 μg/μl with 10,000 cells/μl). For MCAo animals, injection co-ordinates were determined from MR images with reference to the rat brain atlas [25] to correspond to the center of the lesion cavity [17]. A 30 μl volume (= 30 mm3) of HBSS (Hanks balanced salt solution, Gibco, UK)/NAC (N-acetyl cysteine, Sigma, UK) was injected directly into a 45 mm3 lesion at a speed of 2 μl/min.
After grafting, animals were injected with 0.1 ml / 100 g of atipamezole hydrochloride (Anti-Sedan, Pfizer, UK) to counteract anesthesia. After suturing, a local analgesic (Emla cream, UK) was applied to minimize discomfort. Post-operative care included analgesia by i.p. injection of 0.1ml/100g buprenorphine, fluid replacement (4 ml 0.18% glucosaline solution i.p.) and a supplementary mashed diet. Animals were immunosuppressed by daily injections of cyclosporine A (CsA, Sanimmun, Novartis 10mg/kg, diluted in saline) from the day prior to transplantation until perfusion.
2.11. Histology
Animals were euthanized 7 days after transplantation by a sodium pentobarbital (60 mg/kg i.p.) overdose. Transcardial perfusion with 0.9% saline flushed out blood followed by 4% ice-cold paraformaldehyde in 0.2M phosphate buffered saline to fix tissues. Brains were dissected out, post-fixed overnight (4% paraformaldehyde), and placed in a cryoprotective solution (30% sucrose in PBS) for at least 24 hr. Sectioning of the brains was achieved by rapidly freezing these on dry ice followed by cutting sections (40 μm thickness) on a cryostat (Leica) directly onto glass slides.
For immunohistochemistry, parallel series of sections from each animal were incubated with the astrocytic marker glial fibrillary astrocytic protein (polyclonal rabbit anti-GFAP, 1:1000, DAKO), the rat endothelial cell antigen (RECA-1) marker (mouse anti-RECA1, 1:100, Abcam), the basal membrane marker collagen-IV (polyclonal goat anti-mouse collagen-IV, 1:50, Chemicon) and the microglia marker CD11b (rat anti-CD11b, 1:100, Abcam). To identify transplanted human cells, sections were stained with human nuclear antigen (mouse anti-HNA, 1:400, Millipore) or human-specific heat shock protein 27 (rabbit anti-HSP27, 1:200, Cambridge Bioscience). Sections were incubated in 10% of the appropriate blocking sera and 0.3% PBS-Triton for 40 min prior to overnight incubation with primary antibodies (in 10% of the appropriate blocking sera and 0.3% PBS-Triton) at 4° C. Sections were washed 3×5 min in PBS followed by incubation of the appropriate secondary antibody (1:500, Alexa488/555/647, Invitrogen) for 2 hours at RT. Omission of the primary antibody provided a negative control to account for unspecific binding of the secondary antibody. Sections were rinsed 3×5 min in PBS before application of Vectashield with DAPI (Vector Labs) and coverslips. Images were captured on a Zeiss Axioplan and images captured using Axiovision software.
2.12. Statistical Analysis
Statistical analyses of cell counts and measurements were performed on mean values by using two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc analysis and expressed as means ± standard error of means (SEM) with Prism 4 (GraphPad). Three technical replicates for each of three biological replicates were averaged for mean values. A p value of <.05 was considered significant.
3. Results
3.1. In vitro assessment of microparticle-release of VEGF on human neural stem cell fate
The local delivery of growth factors, such as vascular endothelial growth factor (VEGF), released from PLGA particles can significantly influence cellular functions, such as viability, proliferation and phenotypic differentiation (Figure 1). As microparticles provide a 3 dimensional growth environment, this could also affect cell morphology and phenotype. It is therefore important to also compare the effects of VEGF on cell growth in a monoculture environment in vitro. VEGF by itself here did not affect the viability of human neural stem cells (hNSCs) in monoculture, but viability was decreased (2.5%, P<.01) when cells were grown on particles (Figure 2A). VEGF released from particles also slightly decreased viability, but was statistically equivalent to microparticles not releasing VEGF. Therefore growing cells on PLGA microparticles can affect measures of viability and VEGF release can slightly increase this effect, although VEGF by itself does not affect viability of hNSCs.
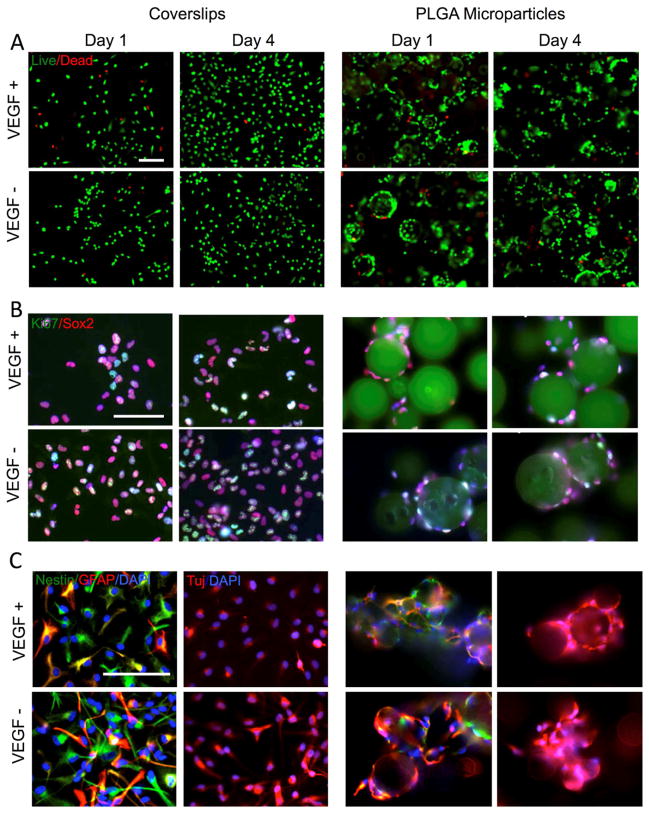
Figure 1. In vitro phenotypic characterization of cell fate after microparticle attachment.
A. Live/Dead stain of hNSCs grown on coverslips and PLGA microparticles in the absence (−) or presence (+) of vascular endothelial growth factor (VEGF). The overwhelming majority of cells are viable (green), with only a small fraction of cells being dying (red). B. Proliferation of cells (Ki67+ cells in green) was more pronounced when grown on coverslips compared to PLGA particles. VEGF did not affect proliferation on coverslips but further reduced proliferation when cells were grown on PLGA particles. Concomitantly with a reduction in proliferation, also fewer cells remained in their neural stem cell state (SOX2+ cells in red) when grown on particles. C. While hNSCs differentiate VEGF maintained a larger population of cells within their stem cell state as indicated by the number of Nestin+ cells (in green) on coverslips, but exerted the opposite effect and decreased the number of undifferentiated neural stem cells. This can also been seen in the increase of hNSCs differentiating into GFAP+ (red) and Tuj+ cells (red) indicating that microparticle enhance the differentiation of hNSCs.
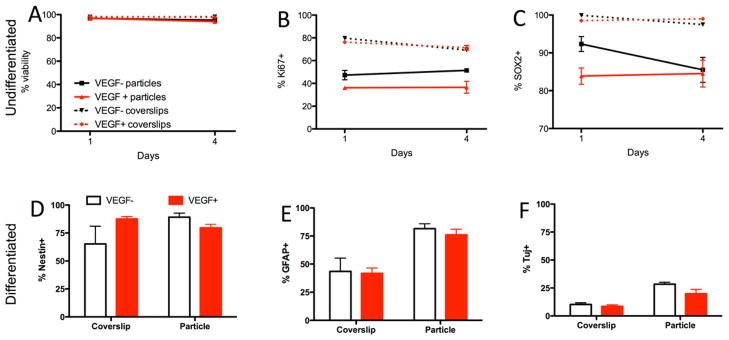
Figure 2. In vitro release of VEGF affects human neural stem cell fate.
A. Attachment of human neural stem cells (hNSCs) to PLGA microparticles did not result in any effect of viability after attachment for 24 hours. However, a minor 2% decrease in viability was evident after 4 days. No significant effect of vascular endothelial growth factor (VEGF) on cell viability was evident on coverslips or on microparticles. B. The number of proliferating cells (Ki67+ cells) was lower on microparticles compared to cells grown on coverslips. VEGF release from the microparticles further decreased the proportion of proliferating cells on particles, but not on coverslips. C. Attachment of cells to PLGA microparticles reduces the proportion of cells expressing SOX2 as a marker of undifferentiated neural stem cells. VEGF release from the particles further decrease this proportion, although there was no effect on the cells when VEGF was present on coverslips. D. When cells were grown for 4 days under differentiating conditions, VEGF increased the proportion of cells remaining in their stem cell state when grown as a monolayer on coverslips, but decreased the proportion of undifferentiated cells when cells are attached to particles. E. Cells attached to microparticles exhibited an increased differentiation into astrocytes compared with cells grown on coverslips, but there was no effect of VEGF under either condition. F. Neuronal differentiation was also increased when cells were grown on PLGA microparticles, with a slight reduction of Tuj+ cells in the presence of VEGF.
Growing hNSCs on microparticles dramatically reduced the number of proliferating cells (Ki67+ cells) by approximately half (Figure 2B). VEGF-release from microparticles also significantly reduced the number of proliferating cells compared with cells growing on non-releasing microparticles. However, VEGF itself did not affect proliferating cells in a monoculture environment. These effects were also evident in the number of cells expressing Sox2, a transcription factor essential to maintain self-renewal of undifferentiated stem cells. VEGF did not affect the stem cell phenotype when present in a monoculture, but growing cells on microparticles reduced the number of Sox2+ cells, hence reducing the proportion of undifferentiated dividing cells (Ki67+, Figure 2C). VEGF-release from microparticles further decreased this proportion of cells, but by 4 days in culture was equivalent to the proportion of neural stem cells present on non-releasing microparticles. These results indicate that growing hNSCs on PLGA microparticles reduced the number of SOX2+ neural stem cells coinciding with a reduction of proliferating cells. VEGF-release from microparticles potentiates this effect, but VEGF by itself neither affected proliferation, nor the stemness of neural stem cells.
hNSCs grown under differentiating conditions on PLGA microparticles exhibited a significant increase in nestin, a protein expressed during the early stages of development in the central nervous system that is replaced with phenotype-specific proteins upon differentiation (Figure 2D). In monoculture, this protein was upregulated in the presence of VEGF, suggesting that it promoted a neural progenitor state, whereas upon release from PLGA microparticles, nestin was slightly downregulated compared with non-releasing microparticles. Differentiation on microparticles doubled the proportion of astrocytes (GFAP+, Figure 1E) and neurons (Tuj+, Figure 2F). VEGF did not significantly affect astrocytic or neuronal differentiation in monoculture or on microparticles. Differentiating hNSCs on PLGA microparticles therefore improved their neuronal differentiation.
3.2. Angiogenesis after hNSC implantation
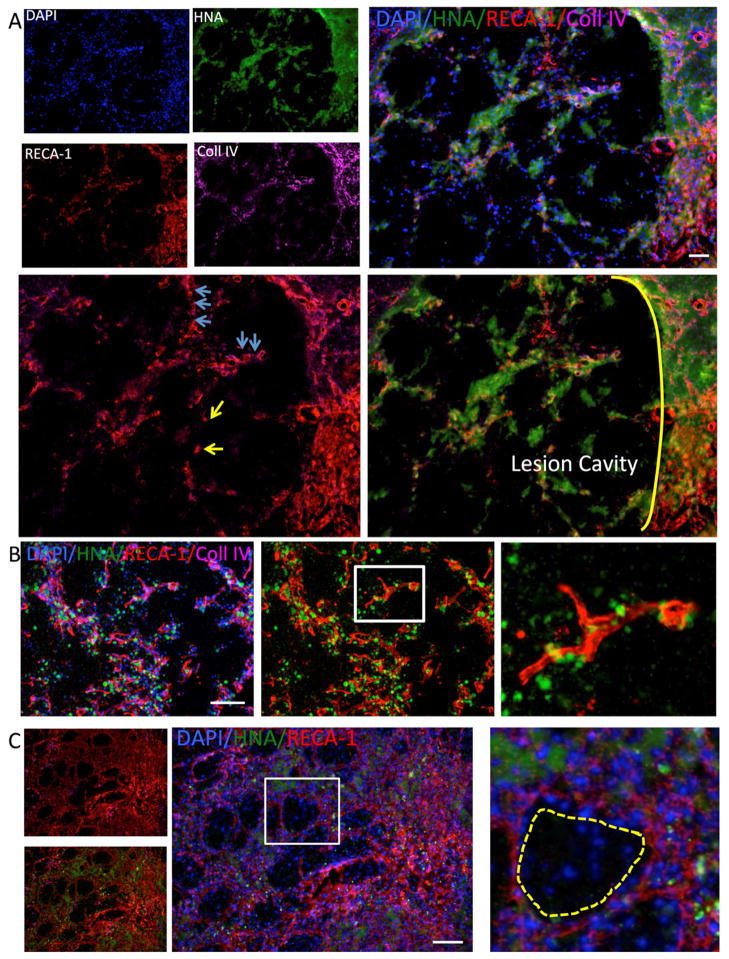
Implantation of hNSCs in suspension into the striatum of normal rats produces a local increase in angiogenesis, but newly generated vessels lack basal lamina that express collagen IV by 7 days (Figure 3A). Middle cerebral artery occlusion (MCAO) also produced a local angiogenesis in the peri-infarct area (data not shown). Implantation of hNSCs in the peri-infarct striatum coincides with areas of angiogenesis (Figure 3B). However, there is no growth of blood vessels into the lesion cavity. Immediately adjacent to the lesion, proliferating endothelial cells (ECs) can be found without being organized into a vascular structure (Figure 3C). Areas undergoing angiogenesis are preferentially colonized with hNSCs indicating the intricate interaction between neovascularization and neural stem cells in newly developing neurovascular units. However, not all areas undergoing angiogenesis contain hNSCs. There are large peri-infarct areas that do no exhibit any angiogenesis. To ensure a homogenous neovascularization of the lesion cavity through neovascularization, it is therefore essential to attract and support vessels, as well as to stimulate angiogenesis in some peri-infarct areas. The release of VEGF from PLGA particles could therefore provide a structural support for hNSCs, as well as promote a neo-vascularization of the cavity.
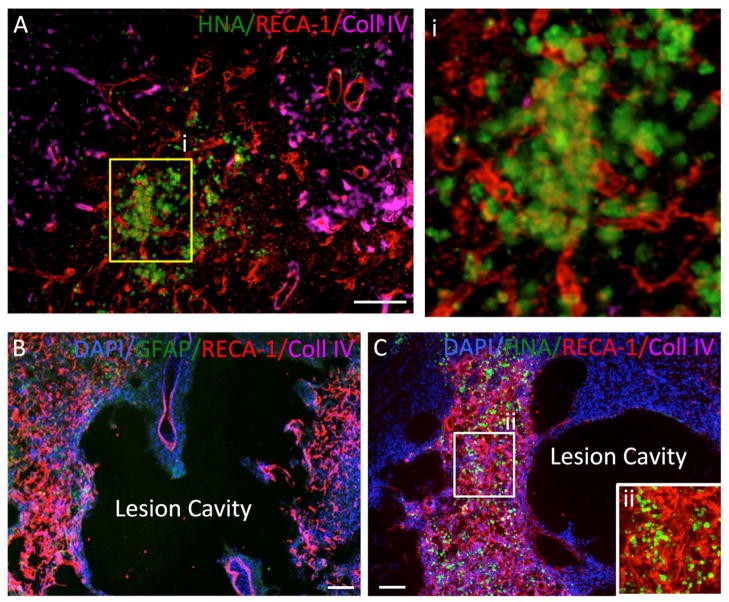
Figure 3. Implantation of hNSC into normal and stroke-damaged brain.
A. Human neural stem cells (HNA in green) injected into the normal rat striatum induce an angiogenic response (Reca-1 in red) at their site of injection with blood vessels penetrating the graft, but also individual endothelial cells being present (boxed area enlarged in i). Importantly these newly formed vessels do not yet contain a basement membrane as indicated by collagen IV (in pink). B. Stroke induces a dramatic angiogenic response in the peri-infarct area and this is associated with astrocytes (GFAP in green). C. A peri-lesion invasion of injected human hNSCs (HNA in green) indicates a co-localization with an area undergoing neovascularization. This neovascularization of the peri-infarct area is a gradual process and it is evident that other areas have not yet developed a dense vascular network. No ingrowth into the lesion cavity can be observed after injection of hNSCs. A higher magnification of the area undergoing angiogenesis indicates that implanted hNSCs are integrated within the mesh of newly forming blood vessels (ii).
3.2. Angiogenesis after microparticle-released VEGF
An injection of PLGA particles (VEGF −) with hNSCs attached into the normal rat brain attracts ECs into the neuroscaffold (Figure 4A), but only a small number of blood vessels are formed. This level of angiogenesis is insufficient to attract blood vessels into the lesion cavity. By contrast, the release of VEGF from PLGA particles recruits a substantial number of ECs into the neuroscaffold within the lesion cavity (Figure 4B).
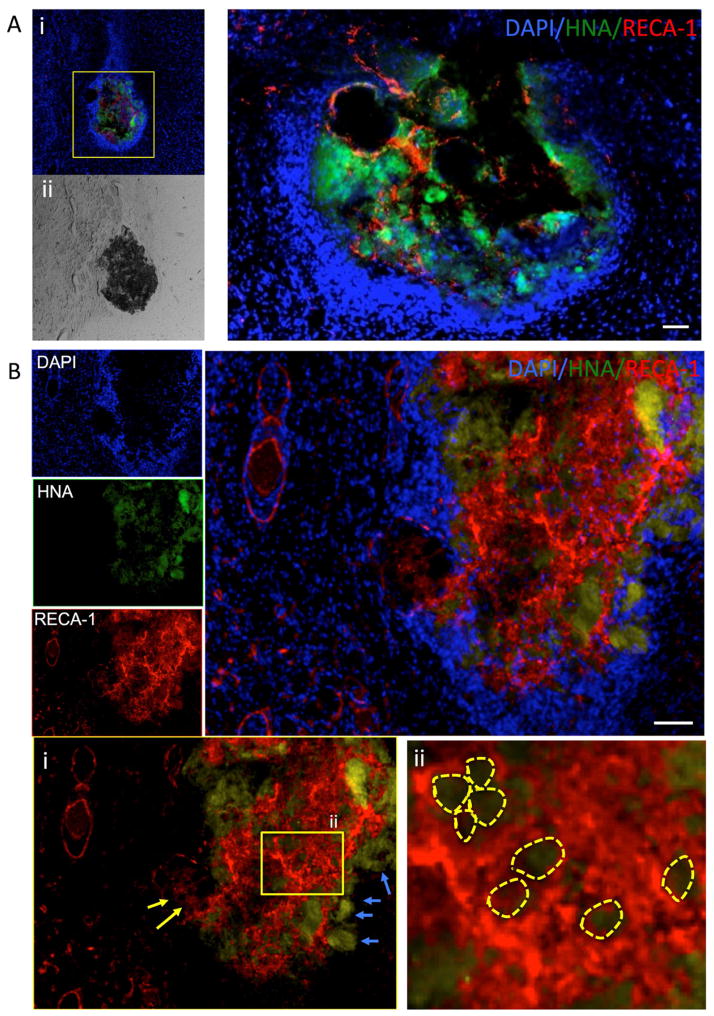
Figure 4. Implantation of VEGF-releasing microparticles in normal rat brain.
A. Injection of PLGA particles with human neural stem cells (HNA in green) results in the formation/attraction of only a few endothelial cells (RECA-1 in red) (i). The brightfield image shows the dense deposit of PLGA particles within the normal brain (ii). A higher magnification of the deposit indicates that endothelial cells invade and intermix with human neural stem cells around PLGA particles. A dense population of cells (DAPI+) around the injection site can be observed reflecting glial scarring. B. Incorporation and release of VEGF from VEGF particles attracts a significant population of endothelial cells into the injection site. The source of these endothelial cells is the peri-injection environment (yellow arrows, i) and results in the accumulation of endothelial cells around VEGF-releasing PLGA microparticles (yellow circles, ii). However, there is not just migration into the area of injection, as human neural stem cells can also been seen to move out of the site of injection (blue arrows, i). However, it is important to note that there is no significant migration of hNSCs from the site of injection.
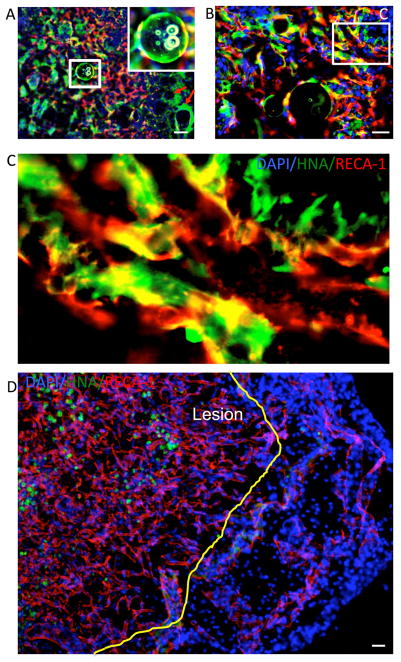
3.3. Neo-vascularization of the lesion cavity
An injection of VEGF releasing microparticles into the lesion cavity promotes the recruitment of ECs into the lesion cavity (Figure 5A). In many instances, ECs are loosely interspersed with hNSCs, but a high proportion of ECs also express collagen IV. Some loosely interspersed ECs organize into tube-like structures that could represent the early parts of blood vessels forming within the lesion cavity (Figure 5B). In other instances, where there is a denser population of hNSCs, a more organized endothelial network is apparent (Figure 5C). However, there is a high regional variability in endothelial infiltration and the formation of neurovascular units. PLGA microparticles are still present within these densely populated areas and create islands that are void of cells. It is, however, unclear at this stage of tissue development if these microparticles are still required to provide a structural support.
Figure 5. Injection of VEGF-releasing PLGA particles into the stroke-damaged brain.
Injection of human neural stem cells attached to PLGA microparticles releasing vascular endothelial growth factor (VEGF) into the lesion cavity provided a structural support for cells to remain within the tissue void, as well as attracting host-derived endothelial cells. Endothelial cells interspersed with hNSC (yellow arrows) and upon sufficient accumulation of endothelial cells aligned to form endothelial rods of interconnected cells (blue arrows). All endothelial cells were host derived with hNSCs (in green) being tightly linked to the vascular cells. B. In between particles a network of hNSC and EC emerged. In some cases these only formed small interconnected elements (boxed area), whereas in others these gradually integrated. C. At the poles of the lesion cavity a more structured environment emerged where a clear web of interconnects endothelial cells can be seen with hNSCs populating the areas in between. PLGA particles (yellow line) maintained a structural support (boxed area) and provide the basis for endothelial cells to invade the lesion cavity.
The neovascularization of the lesion cavity is a gradual process where there is a dynamic interplay between hNSC attached to PLGA microparticles and the invading ECs (Figure 6A). Although the VEGF releasing microparticles attract ECs (Figure 6B), they are not the focus of vascular network development. Detachment of hNSC from the PLGA microparticles leads to an interaction with ECs to form primitive neurovascular units (Figure 6C). These neurovascular units do not necessarily form functional blood vessels and most commonly do not link up within other similar units. However, in other cases, these units interlink and potentially are the building blocks of a functional vascular network that anastomoses with the host vasculature. The number of ECs and neurovascular units found within the lesion cavity is higher than in surrounding intact host tissue (Figure 6D). In some regions, this leads to a hypervascularization, a process akin to the peri-infarct area that contains glial scarring.
Figure 6. Hyper-vascularization.
A. Vascularization of the lesion cavity is gradual process with cells migrating in and gradually covering the lesion space. Therefore some areas contain human neural stem cells (in green) attached to particles (red arrows), but no endothelial cells (Reca-1 in red). Some particles are still clearly visible as whole particle (boxed insert) with cells attached. B. In some instance, it is evident that a primitive neuro-vascular environment is emerging where hNSC support the formation of tubules formed by endothelial cells. However, these are non-functional elements as these remained incomplete. C. However, the density of endothelial cells and newly forming blood vessels is much denser than found in normal tissue. It is unclear here if this is part of an attempt to establish a vascular network that for instance, will eventually prune some unnecessary branches or if this could remain as a hyper-vascularized tissue. One potential side effect of this hypervascularization is that hNSCs might preferentially differentiate into astrocytes.
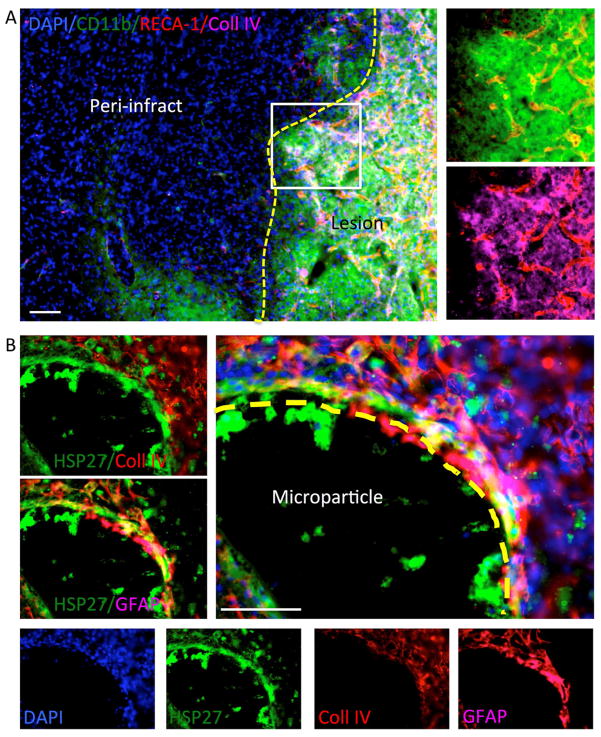
There is also a significant overlap of the area undergoing a neovascularization and the presence of microglia (CD11b+ cells, Figure 7A). Areas with a strong microglia presence exhibited a more consistent pattern of vascular formation, rather than loosely seeded ECs. This was further evidenced by the presence of collagen IV in most vascular formations in areas of microglia reactivity. However, some collagen IV was not associated with ECs. hNSCs predominantly expressed GFAP as an indication of astrocytic differentiation, but these were generally collagen IV negative (Figure 7B). These results indicate that a host-derived neo-vascularization of de novo tissue in the lesion cavity is achievable using VEGF-releasing PLGA microparticles seeded with hNSCs.
Figure 7. Inflammation and astrocytic differentiation.
A. It is important to note that it is not only endothelial cells that infiltrate the grafted area, but also inflammatory cells. Microglial/macrophages (CD11b+) infiltrate the implant site extensively. It is known that collagen IV activates microglia. Microglia are also known to be an essential component of a regenerative tissue rich in collagen. Collagen IV provides a basement membrane to newly forming blood vessels, but is also associated with glial scarring and a foreign body response when not associated with blood vessels. B. Almost all implanted hNSCs (HSP27+ cells) express GFAP as an indicator that these cells differentiated into astrocytes, potentially forming glial scarring within the lesion cavity. Collagen IV, however, is not expressed in the implanted cells, but is present in host cells.
4. Discussion
Replacing lost tissue after a stroke will require in situ tissue engineering. Implanted neural stem cells and biomaterials can form a primitive de novo tissue, but fail to generate a vasculature that could guarantee the long-term survival of this tissue. The injection of PLGA microparticles releasing VEGF is efficient to recruit endothelial cells (ECs) from the host brain and to facilitate a neovascularization in the lesion cavity. This approach demonstrates that release of growth factors from microparticles is a promising direction for in situ tissue engineering in the brain.
4.1. Promoting neo-vascularization
A functional vasculature is an essential component of a viable tissue. As in the case of stroke, without the supply of nutrients and oxygen, the tissue will die. Replacing this lost tissue therefore crucially depends on re-establishing a vascular supply to the area lost due to ischemia. Implantation of human neural stem cells (hNSCs) on biomaterials, such as PLGA microparticles or interspersed with an ECM bioscaffold, is sufficient to generate de novo tissue formation, but is insufficient to attract a vascular supply [5, 7]. Therefore additional tissue engineering is required to ensure a vascular supply of nutrients and oxygen.
As demonstrated here, VEGF-release from PLGA particles can attract endothelial cells into this newly forming tissue. Endothelial cells are derived from the host and gradually invade the new tissue. Importantly, it is not individual blood vessels that invade this area, but individual ECs and these are found to interact with hNSCs. The continued release of VEGF continually attracts ECs and promotes their migration, as well as their proliferation [26]. VEGF is known to induce angiogenesis, but also to create a permeable blood-brain barrier (BBB) [11]. VEGF therefore is a useful growth factor to rapidly attract ECs to the neuroscaffold, but its continued presence and release might lead to an overexpansion of individual unorganized ECs, whilst also permitting the invasion of inflammatory cells through a compromised BBB. Nevertheless, the presence of microglial cells, as also reported here, has been suggested to play an active role in the neovascularization of allografts [27]. A secretion of additional factors, such as hepatocyte growth factor and angiotensin-1, known to organize and stabilize blood vessels, from PLGA microparticles [13] might therefore be more appropriate to establish a well organized neovasculature with a functional BBB.
However, the presence of a large number of unorganized ECs is potentially a preliminary step prior to their organization into neurovascular units. Within the neuroscaffold, some ECs organized in tube-like structures in the presence of hNSCs. It is possible that this organization is dependent on the amount of ECs that infiltrated this area, as the ratio of EC versus NSCs is known to reciprocally influence their differentiation [28]. Conversely, it is also conceivable that the range of gradients and diffusion of VEGF from PLGA microparticles affected the behavior of ECs [29, 30]. In sparsely colonized areas, this appeared to result in small elements of prototypical neurovascular units. In contrast, in extensively populated areas, an extensive neovascularization was evident. Commonly these areas, however, were hypervascularized compared to normal tissue.
Hypervascularization has been associated with excessive amounts of VEGF [31], but it remains unclear if a hypervascularization is potentially beneficial or detrimental. The advantage of a hypervascularized de novo tissue is that its nutrient demands are met and that potentially gradually the vasculature will evolve to include larger vessels, as well as microvessels. There are indications that merely the formation of microvessels is insufficient to supply a tissue with nutrients and oxygen, although in certain circumstances some microvessels can mature into larger vessels [32]. Given the establishment of some vasculature here, longer-term studies can now be envisaged to fine-tune the attraction of ECs and their maturation into a functional vascular network. It is, however, already evident here that regardless of establishing a functional vasculature, the neuronal differentiation of hNSCs will pose additional challenges, as almost all of the hNSCs differentiated into astrocytes [33]. For in situ tissue engineering, further developments are therefore required to instruct implanted cells to form an appropriate tissue.
4.2. In situ tissue engineering
The generation of functional tissue within the lesion cavity will be a complex endeavor that requires the coordination of various processes. For instance, the predominant astrocytic differentiation of hNSCs here indicates that further engineering will be required to promote a neuronal differentiation. Although fetal striatal tissue appropriately differentiates when implanted into an adult lesioned brain [34, 35], partially differentiated cells, as well as the extracellular matrix present within these tissues, might provide sufficient cues to direct cellular differentiation. The lack of positional specification and signals in our implantation paradigm with NSCs requires guidance of cells towards a striatal phenotype. This can, for example, be achieved by the prolonged activation of the hedgehog pathway using a stable small molecule, such as purmorphamine, that has been shown to promote striatal output neurons in these striatal hNSC [22]. Apart from the sustained release of factors that attract and stabilize a vasculature, it will therefore also be essential to provide clear instructions to implanted hNSCs to differentiate into striatal neurons rather than astrocytes. PLGA microparticles can be engineered to secrete multiple factors over different time courses [13]. They are hence an appropriate delivery 1devise for factors required to drive in situ tissue engineering.
Astrocytes are also the likely source of further challenges, once an appropriate neuronal differentiation has been achieved. Specifically, the dense astrocytic scar that forms around the cavity is a tightly packed barrier that is unlikely to allow an easy penetration of cells or projections into the host brain, although there has been evidence in fetal tissue transplants that this is possible [36]. Although chondroitinase can attenuate this glial scarring by removing chondroitin sulfate proteoglycans (CSPGs) [37] and allow a penetration of axons, this would, nevertheless, interfere with the development of an extracellular matrix required for tissue formation in the lesion cavity. Therefore alternative strategies that would target the proliferation of astrocytes, such as a cyclin-dependent kinase inhibitor, are more likely to produce a compromised glial scar [38] through which connections between the host and graft could be established, as well as reducing astrocytic differentiation of transplanted cells.
The local delivery of factors through biomaterials will be crucial to advance in situ tissue engineering. Although PLGA microparticles exhibit a good biocompatibility [6, 39] and are excellent candidates to encapsulate growth factors and release these upon biodegradation [39, 40], the decease in viability of cells grown on PLGA microparticles over time here requires some consideration. It is conceivable that degradation products of the microparticles here could have affected the viability of hNSCs and this is not yet evident 1 day post-attachment. It might therefore be cautious to reduce the amount of PLGA implanted into the brain to avoid delayed effects. Nevertheless, in the brain there was no clear evidence of any deleterious effects, although further consideration to this should be given in future studies. Importantly, differentiation of neural stem cells was doubled in vitro on PLGA microparticles. In vitro differentiation of cells on PLGA microparticles for delivery into the brain is therefore conceivable. Further investigations are also required to determine if cellular attachment is indeed required for tissue engineering or if similar results can be achieved simply by mixing PLGA micro- or nanoparticles with neural stem cells prior to injection, akin to the process involved in combining a ECM bioscafffold with hNSCs [7].
5. Conclusion
To sustain a de novo tissue in the lesion cavity, nutrient and oxygen supply need to be guaranteed through a vascular supply. The implantation of hNSCs on VEGF-releasing PLGA microparticles here attracted host ECs to invade this neuroscaffolding and develop a vascular network. However, three main future challenges will need further consideration to create a vascular supply akin to normal tissue: 1. a reduction of the hypervascularization created by the sustained delivery of VEGF; 2. the establishment of a functional blood-brain barrier; 3. the generation of larger vessels that can provide an efficient vascular delivery system for a volume the size of an adult striatum. Additional challenges in guiding the differentiation of hNSCs are also evident and will need to be part of a more general in situ tissue engineering strategy for the brain. Although one might never achieve the integration and functionality of a developing brain using these approaches, in situ engineering of striatal tissue might nevertheless provide a suitable environment for implanted cells that could influence some aspects of behavioral recovery that cannot be provided by intraparenchymal transplants.
Acknowledgments
This work was supported by a BBSRC project grant (BB/D014808/1) and a NIBIB Quantum Grant (1 P20 EB007076-01).
Footnotes
Conflict of Interest Statement
The authors declare competing financial interests. Kevin Shakesheff is CSO of Regentec. Mike Modo received grant and personnel support from ReNeuron Ltd.
Author’s Contribution
Ellen Bible: design of study, conducted all in vitro and in vivo experiments, MRI, conducted histological analyses, contributed to the draft of the manuscript; OQ: produced and characterized the microparticles, contributed to the draft of the manuscript; David Chau: produced and characterized the microparticles, contributed to the draft of the manuscript, Morgan Alexander: Plasma Polymerization, contributed to the draft of the manuscript; Kevin Shakesheff: design of study, financial support, contributed to the draft of the manuscript; Mike Modo: design of study, acquired histological images, financial support, drafted the manuscript, final approval of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dihne M, Hartung HP, Seitz RJ. Restoring neuronal function after stroke by cell replacement: anatomic and functional considerations. Stroke. 2011;42:2342–50. doi: 10.1161/STROKEAHA.111.613422. [DOI] [PubMed] [Google Scholar]
- 2.Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, et al. Implantation Site and Lesion Topology Determine Efficacy of a Human Neural Stem Cell Line in a Rat Model of Chronic Stroke. Stem Cells. 2011 doi: 10.1002/stem.1024. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen JC, Grabowski M, Zimmer J, Johansson BB. Fetal neocortical tissue blocks implanted in brain infarcts of adult rats interconnect with the host brain. Exp Neurol. 1996;138:227–35. doi: 10.1006/exnr.1996.0061. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski M, Sorensen JC, Mattsson B, Zimmer J, Johansson BB. Influence of an enriched environment and cortical grafting on functional outcome in brain infarcts of adult rats. Exp Neurol. 1995;133:96–102. doi: 10.1006/exnr.1995.1011. [DOI] [PubMed] [Google Scholar]
- 5.Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30:2985–94. [Google Scholar]
- 6.Newman KD, McBurney MW. Poly(D,L lactic-co-glycolic acid) microspheres as biodegradable microcarriers for pluripotent stem cells. Biomaterials. 2004;25:5763–71. doi: 10.1016/j.biomaterials.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Bible E, Dell’acqua F, Solanky B, Balducci A, Crapo PM, Badylak SF, et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials. 2012;33:2858–71. doi: 10.1016/j.biomaterials.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 9.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–64. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–29. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 12.Yano A, Shingo T, Takeuchi A, Yasuhara T, Kobayashi K, Takahashi K, et al. Encapsulated vascular endothelial growth factor-secreting cell grafts have neuroprotective and angiogenic effects on focal cerebral ischemia. J Neurosurg. 2005;103:104–14. doi: 10.3171/jns.2005.103.1.0104. [DOI] [PubMed] [Google Scholar]
- 13.Saif J, Schwarz TM, Chau DY, Henstock J, Sami P, Leicht SF, et al. Combination of injectable multiple growth factor-releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscler Thromb Vasc Biol. 2010;30:1897–904. doi: 10.1161/ATVBAHA.110.207928. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wei YT, Zu ZH, Ju RK, Guo MY, Wang XM, et al. Combination of hyaluronic acid hydrogel scaffold and PLGA microspheres for supporting survival of neural stem cells. Pharm Res. 2011;28:1406–14. doi: 10.1007/s11095-011-0452-3. [DOI] [PubMed] [Google Scholar]
- 15.Emerich DF, Silva E, Ali O, Mooney D, Bell W, Yu SJ, et al. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010;19:1063–71. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Hayashi T, Tsuru K, Deguchi K, Nagahara M, Hayakawa S, et al. Vascular endothelial growth factor promotes brain tissue regeneration with a novel biomaterial polydimethylsiloxane-tetraethoxysilane. Brain Res. 2007;1132:29–35. doi: 10.1016/j.brainres.2006.09.117. [DOI] [PubMed] [Google Scholar]
- 17.Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. Attachment of stem cells to scaffold particles for intra-cerebral transplantation. Nat Protoc. 2009;4:1440–53. doi: 10.1038/nprot.2009.156. [DOI] [PubMed] [Google Scholar]
- 18.Deluca P, Jiang G, Woo B. Office USP. (Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres. United States: University of Kentucky Research Foundation; 2007. A61K 9/50 ed. [Google Scholar]
- 19.Jiang G, Woo BH, Kang F, Singh J, DeLuca PP. Assessment of protein release kinetics, stability and protein polymer interaction of lysozyme encapsulated poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2002;79:137–45. doi: 10.1016/s0168-3659(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 20.Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–54. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- 21.Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–55. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 22.El-Akabawy G, Medina LM, Jeffries A, Price J, Modo M. Purmorphamine Increases DARPP-32 Differentiation in Human Striatal Neural Stem Cells Through the Hedgehog Pathway. Stem Cells Dev. 2011;20:1873–87. doi: 10.1089/scd.2010.0282. [DOI] [PubMed] [Google Scholar]
- 23.Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. Journal of neuroscience methods. 2000;104:99–109. doi: 10.1016/s0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- 24.Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. J Neurosci Methods. 2000;104:99–109. doi: 10.1016/s0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam, NL: Academic Press; 2007. [Google Scholar]
- 26.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–43. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennell NA, Streit WJ. Colonization of neural allografts by host microglial cells: relationship to graft neovascularization. Cell Transplant. 1997;6:221–30. doi: 10.1177/096368979700600305. [DOI] [PubMed] [Google Scholar]
- 28.Lippmann ES, Weidenfeller C, Svendsen CN, Shusta EV. Blood-brain barrier modeling with co-cultured neural progenitor cell-derived astrocytes and neurons. J Neurochem. 2011;119:507–20. doi: 10.1111/j.1471-4159.2011.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivron NC, Vrij EJ, Rouwkema J, Le Gac S, van den Berg A, Truckenmuller RK, et al. Tissue deformation spatially modulates VEGF signaling and angiogenesis. Proc Natl Acad Sci U S A. 2012;109:6886–91. doi: 10.1073/pnas.1201626109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibuya M. Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. Febs J. 2009;276:4636–43. doi: 10.1111/j.1742-4658.2009.07175.x. [DOI] [PubMed] [Google Scholar]
- 31.Flamme I, von Reutern M, Drexler HC, Syed-Ali S, Risau W. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995;171:399–414. doi: 10.1006/dbio.1995.1291. [DOI] [PubMed] [Google Scholar]
- 32.Scholz D, Cai WJ, Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4:247–57. doi: 10.1023/a:1016094004084. [DOI] [PubMed] [Google Scholar]
- 33.Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–47. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naimi S, Jeny R, Hantraye P, Peschanski M, Riche D. Ontogeny of human striatal DARPP-32 neurons in fetuses and following xenografting to the adult rat brain. Exp Neurol. 1996;137:15–25. doi: 10.1006/exnr.1996.0002. [DOI] [PubMed] [Google Scholar]
- 35.DiFiglia M, Schiff L, Deckel AW. Neuronal organization of fetal striatal grafts in kainate- and sham-lesioned rat caudate nucleus: light- and electron-microscopic observations. J Neurosci. 1988;8:1112–30. doi: 10.1523/JNEUROSCI.08-04-01112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deacon TW, Pakzaban P, Burns LH, Dinsmore J, Isacson O. Cytoarchitectonic development, axon-glia relationships, and long distance axon growth of porcine striatal xenografts in rats. Exp Neurol. 1994;130:151–67. doi: 10.1006/exnr.1994.1194. [DOI] [PubMed] [Google Scholar]
- 37.Hyatt AJ, Wang D, Kwok JC, Fawcett JW, Martin KR. Controlled release of chondroitinase ABC from fibrin gel reduces the level of inhibitory glycosaminoglycan chains in lesioned spinal cord. J Control Release. 2010;147:24–9. doi: 10.1016/j.jconrel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Redecker C, Yu ZY, Xie MJ, Tian DS, Zhang L, et al. Rat focal cerebral ischemia induced astrocyte proliferation and delayed neuronal death are attenuated by cyclin-dependent kinase inhibition. J Clin Neurosci. 2008;15:278–85. doi: 10.1016/j.jocn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y, Ang M, Foo S, Lee WS, Ma Z, Venkatraman SS, et al. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS One. 2011;6:e22507. doi: 10.1371/journal.pone.0022507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhang SH, Lim JS, Choi CY, Kwon YK, Kim BS. The behavior of neural stem cells on biodegradable synthetic polymers. J Biomater Sci Polym Ed. 2007;18:223–39. doi: 10.1163/156856207779116711. [DOI] [PubMed] [Google Scholar]