Abstract
About a decade ago, cell membranes from the electric organ of Torpedo and from the rat brain were transplanted to frog oocytes, which thus acquired functional Torpedo and rat neurotransmitter receptors. Nevertheless, the great potential that this method has for studying human diseases has remained virtually untapped. Here, we show that cell membranes from the postmortem brains of humans that suffered Alzheimer's disease can be microtransplanted to the plasma membrane of Xenopus oocytes. We show also that these postmortem membranes carry neurotransmitter receptors and voltage-operated channels that are still functional, even after they have been kept frozen for many years. This method provides a new and powerful approach to study directly the functional characteristics and structure of receptors, channels, and other membrane proteins of the Alzheimer's brain. This knowledge may help in understanding the basis of Alzheimer's disease and also help in developing new treatments.
Keywords: γ-aminobutyric acid receptors, sodium channels, calcium channels, postmortem brain
Ever since the original description of the dementia of Auguste D. by Alois Alzheimer in 1906, many different hypotheses have been proposed to explain Alzheimer's disease (AD) (1–6). However, despite extensive investigation using many different approaches, the cause of this disease still remains unclear. A central feature of AD in its late stages is a somewhat specific loss of neurons that seems to depend on at least two factors: amyloid β-peptide deposition and synaptic dysfunction (ref. 6 and references therein). Neurotransmitter receptors are key players in the process of synaptic transmission, and, in view of the neuronal loss, it is not surprising that the numbers of several types of receptors are reduced in the AD brain (7–14). However, most previous studies of receptors in AD have used biochemical and immunocytochemical methods, and because of many technical difficulties, almost nothing is known about the functional characteristics of the neurotransmitter receptors of the AD brain.
Over a score of years, we have developed two comparatively simple methods that allow us to study neurotransmitter receptors and ion channels from the human brain in great structural and functional detail. One well established method consists of isolating mRNA from the brain and expressing the encoded foreign receptors in Xenopus oocytes, using the oocyte's own protein-synthesizing machinery (15–18). The other method, less known but perhaps more important for the study of AD and other diseases, involves the microtransplantation of cell membranes from the brain to the membrane of frog oocytes (19, 20). In this procedure, instead of the mRNA, cell membranes are injected into the oocytes. Within an hour, the foreign membranes, carrying their receptors and other proteins, fuse with the oocyte's own plasma membrane, where the incorporated receptors can be functionally characterized. It should be noted that, besides its simplicity and directness of approach, one important advantage of this method is that it allows us to study the original receptors and associated molecules while they are still embedded in their natural lipid environment.
Materials and Methods
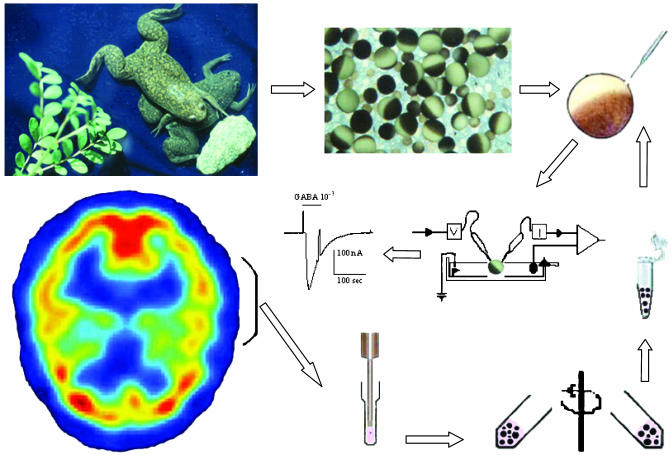
Transplanting Brain Membranes. The microtransplantation method is illustrated diagrammatically in Fig. 1. For the present experiments, we used small portions of the temporal cerebral cortices of four severe AD and five non-AD age-matched subjects (61–65 years old). In all cases, the brains had been obtained within 2.5–5.2 h after death and had been kept deep-frozen for 1–9 years. Using a glass/glass tissue grinder, cell membranes were prepared from the cerebral cortex samples (≈1 g each) in an ice-cold solution containing 200 mM glycine-NaOH, pH 9, 150 mM NaCl, 50 mM EGTA, 50 mM EDTA, and 300 mM sucrose with protease inhibitors (Sigma P2714). The homogenate was centrifuged (9,500 × g, 15 min), and the supernatant was centrifuged (100,000 × g, 2 h). The final pellets were suspended in glycine (5 mM) at 2–4 mg of protein per ml and kept frozen at -80°C until injected into the oocytes as described (19, 20). Protein concentrations were determined by the Coomassie protein assay (Pierce).
Fig. 1.
Method for microtransplanting receptors from the human brain to frog oocytes. Part of the human temporal cortex is homogenized and centrifuged to prepare membrane fragments that are injected into an oocyte isolated from a Xenopus frog. Subsequent voltage-clamp recording and exposure to GABA show that the oocyte incorporated human GABA receptors, which elicit desensitizing GABA currents.
Electrophysiology. From 1 h to >2 weeks after injection, membrane currents were recorded from oocytes voltage-clamped at -60 or, more commonly, -80 mV by using two microelectrodes filled with 3 M KCl (21). Neurotransmitters and other substances were superfused over the oocytes at a rate of 5–10 ml/min (chamber volume ≈0.1 ml; see refs. 19–21 for more details).
Results and Discussion
Microtransplantation of Human γ-Aminobutyric Acid (GABA) Receptors. When membrane-injected oocytes were exposed to GABA, the main inhibitory neurotransmitter in the human brain, the frog oocytes generated membrane currents that were clearly caused by activation of incorporated human GABA receptors because noninjected oocytes did not respond to GABA. The amplitude of the GABA currents increased with time after injection, reached a peak within 3 days, and then became progressively smaller. Nevertheless, it is important to note that some receptors remained functional for >2 weeks after membrane injection.
In earlier studies, when we began injecting membranes from different cell sources into frog oocytes, we noticed that some membrane preparations were ootoxic and many oocytes died 1 or 2 days after injection. This was more common when the injected membranes were relatively concentrated and had a protein content of >3–5 mg/ml. The same applied now to the human brain membranes. Therefore, we have adopted the practice of injecting all new membrane preparations at various dilutions to select a concentration suitable for subsequent electrophysiological recordings. In future experiments, it will be important to determine whether the AD and control human brain membranes have different ootoxicities.
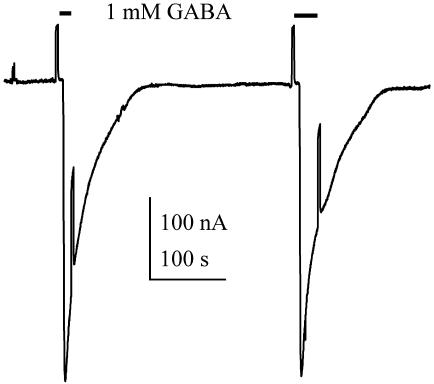
Properties of GABA Receptors from the AD Brain. During relatively prolonged applications of high concentrations of GABA (e.g., 1 mM), the GABA current desensitized rapidly (Fig. 2), but after superfusing the oocyte with normal Ringer's solution for a few minutes, the current recovered its original amplitude or became even larger. Moreover, during repetitive applications of GABA, at 3- to 5-min intervals, the GABA currents increased progressively, sometimes more than doubling their initial amplitude after 10 applications. This potentiation was observed with both AD and non-AD receptors, but so far, the number of samples examined is too small to tell whether there are any significant differences between them. Nevertheless, this potentiation contrasts sharply with the marked run-down of GABA currents seen in oocytes expressing receptors from the epileptic brain (18).
Fig. 2.
Two consecutive responses to GABA recorded from an oocyte injected with control (C1) brain membranes. For this and subsequent figures, the membrane potential was clamped at -80 mV, unless otherwise specified.
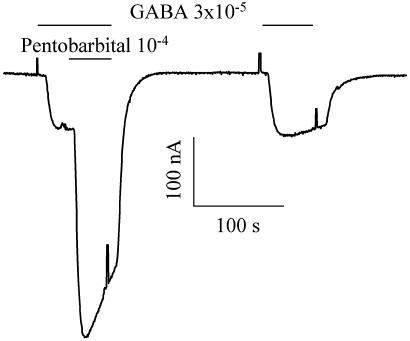
A brief characterization of the AD and control GABA receptors was made. When GABA currents were recorded at different membrane potentials, the currents were found to reverse sign at ≈-30 mV, indicating that both types of receptors open membrane channels that are mainly permeable to chloride ions (compare with refs. 15, 16, 22, and 23). Furthermore, in both cases the GABA currents were reversibly blocked by the specific GABAA receptor antagonist bicuculline and were potentiated by pentobarbital (Fig. 3) and benzodiazepines. All of these features indicate clearly that the GABA receptors generating the currents are ionotropic and of the GABAA type.
Fig. 3.
Pentobarbital potentiation of the GABA current elicited in an oocyte injected with control (C2) brain membranes. Note that pentobarbital increased the amplitude and rate of desensitization of the GABA current.
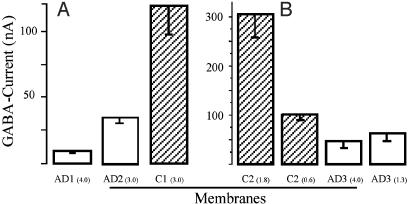
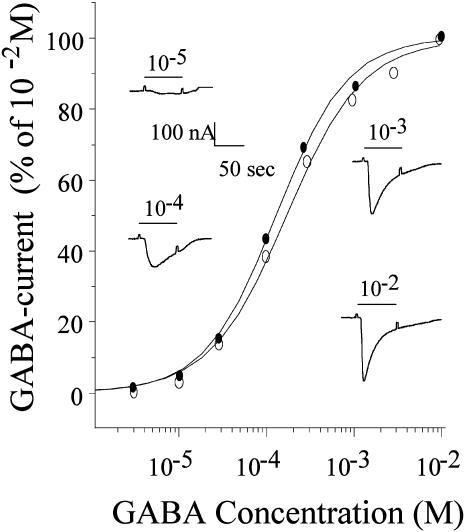
Interestingly, the amplitude of the currents elicited by GABA were consistently smaller in the oocytes injected with membrane vesicles derived from the AD brains than in oocytes injected with non-AD brain membranes, even when the latter were injected at lower protein concentrations. This is illustrated in Fig. 4, which shows results obtained with membranes from three AD and two control brains injected into oocytes from two frogs; similar results were obtained with other membranes prepared from the same brain samples, always injecting AD and control membranes into the same batches of oocytes. Despite the smaller GABA currents, the GABA dose–response relationships of AD and non-AD oocytes were very similar (Fig. 5), suggesting that the affinity of the receptors for GABA is not greatly altered by the disease. Several factors may account for the lower amplitude of GABA currents in the oocytes injected with AD-brain membranes (e.g., a lower content of receptors in the membranes or a lower efficiency of membrane incorporation). Alternatively, the reduced action of GABA on AD oocytes may reflect impaired receptor–channel function rather than decreased numbers because previous immunocytochemical, biochemical, and spectroscopic studies indicate that GABAA receptors are not generally reduced in number in the AD brain (24–26). However, GABAA receptors (8) and the GABA α1 and β3 subunits are decreased in the hippocampus (27, 28). Further experiments are needed to shed some light on these apparent discrepancies.
Fig. 4.
Amplitude of currents elicited by GABA (10-3 M) in oocytes from two frogs injected with membranes from three AD (1–3) and two control (1, 2) brains. The numbers in brackets show the protein concentrations of the injected membranes; the columns show the mean and SEM of 3, 5, and 14 (A), and 16, 20, 13, and 15 (B) oocytes. Noninjected oocytes from this and other frogs did not elicit GABA currents >≈1 nA.
Fig. 5.
GABA dose–current response relations of two oocytes, one injected with membranes from an AD brain (•, AD2) and the other from a control brain (○, C1). The respective EC50 values were 130 and 172 μM, and the Hill coefficients were 1.3 and 1.1. (Insets) Sample currents from the oocyte injected with the control brain membranes.
Microtransplanting Voltage-Gated Ion Channels. In addition to incorporating GABA receptors, the oocytes injected with human brain membranes acquired receptors to glutamate, as well as voltage-gated Ca2+ and Na+ channels. In the experiments carried out so far, the currents elicited by glutamate and kainate were comparatively small, but the currents caused by the influx of Ca2+ ions through voltage-gated channels were substantial and were amenable to more detailed study.
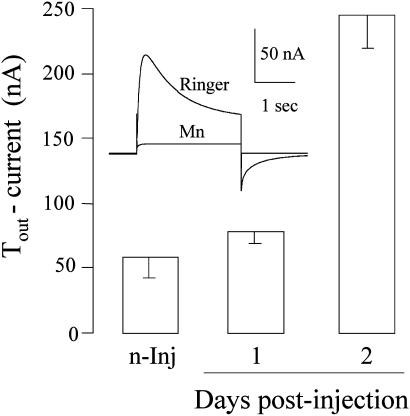
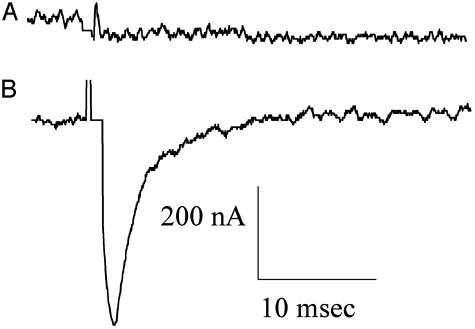
It is known that Xenopus oocytes have native Cl- channels that are gated when the intracellular concentration of Ca2+ is increased (21). Moreover, the oocytes also possess native voltage-gated Ca2+ channels that open when the membrane is depolarized. The consequent influx of Ca2+ opens the Cl- channels, generating a transient outward Cl- current (Tout) that disappears when the Ca2+ channels are blocked, e.g., by manganese (ref. 21 and Fig. 6 Inset). Therefore, the Tout current is a simple and convenient monitor of the presence of Ca2+ channels in the oocyte membrane. As shown in Fig. 6, the Tout current generated by oocytes injected with human brain membranes increased with time after injection and was greater than the native Tout current of noninjected oocytes from the same frog. This indicates that functional Ca2+ channels from the human brain had been incorporated into the oocyte plasma membrane. The oocytes also incorporated functional human Na+ channels that could be blocked by tetrodotoxin (Fig. 7). The Tout and Na+ currents generated in oocytes injected with AD-brain membranes were generally smaller and more variable in time course than those of oocytes injected with the control brain membranes, but here again a larger sample of brains is needed to assess the significance of these results.
Fig. 6.
Tout currents in oocytes from one frog. The columns show the mean amplitudes and SEM (n = 7, 3, and 4) of the currents blocked by manganese in noninjected oocytes and 1 and 2 days after injection of membranes from the AD (2) brain. (Inset) Sample currents for depolarizing pulses from -100 to +20 mV, in normal Ringer's solution and after adding Mn2+ (5 mM).
Fig. 7.
Na+ currents elicited by depolarizing pulses from -100 to -10 mV. The two records show the inward currents blocked by tetrodotoxin (300 nM for 1min) in a noninjected oocyte (A) and in an oocyte injected with membranes from the C1 control brain (B).
It is important to mention here that electrophysiological and ultrastructural studies have shown that amyloid β proteins form Ca2+ channels in various types of membranes (29, 30), channels that are the basis of the channel hypothesis of AD (5, 31). If these channels were present, and open, in the AD-brain membranes that we injected, one might expect the injected oocytes to have a lower resting membrane potential and input resistance than the oocytes injected with non-AD membranes. So far, we have not observed a clear difference.
There Is Great Wealth in the AD Brain Banks. In an earlier work, we showed that, even 21 h after death, the rat brain still yields mRNAs capable of expressing various types of functional receptors and channels in Xenopus oocytes (32). However, it was not known then whether the receptors themselves remained functional in the postmortem brain. The present work demonstrates the striking fact that some human brain neurotransmitter receptors, as well as Na+ and Ca2+ channels, are indeed able to survive for -5 h after death and that they resume function after they have been kept frozen for many years.
Clearly, more control and AD samples need to be examined, and their receptors and channels need to be further characterized. Nonetheless, the present results already show that it is possible to study directly the function and structure of neurotransmitter receptors, voltage-gated channels, and other membrane proteins from normal and AD brains. This opens the way to the wealth of tissues stored in many brain banks; using the methods described here, we can derive much-needed functional information that will help in understanding the etiology of AD. We are only at the beginning of a new and exciting road that may lead to a better understanding of the bases of many human diseases, and may thus help toward their treatment.
Acknowledgments
We are grateful to Drs. Eleonora Palma, John Heuser, and Juan Carlos Lopez for critically reading the manuscript. The brain tissues used in this project were provided by the University of California, Irvine, Institute for Brain Aging and Dementia Tissue Repository. This work was supported by the National Science Foundation, Neural and Glial Mechanism Grant 998285 (to R.M.), Programa de Apoyo a Proyectos de Investigacion e Innovacion Tecnológica-Universidad Nacional Autónoma de México Grant 212702 (to A.M.-T. and R.M.), Consejo Nacional de Ciencia y Tecnologia Grant 41309Q and the Ministero Istruzione Università Ricerca and Ministero della Salute (F.E.). Zulma Dueñas is a PEW Latin American Fellow.
Abbreviations: AD, Alzheimer's disease; GABA, γ-aminobutyric acid.
References
- 1.Maccioni, R. B., Muñoz, J. P. & Barbeito, L. (2001) Arch. Med. Res. 32, 367-381. [DOI] [PubMed] [Google Scholar]
- 2.Arendt, T. (2001) Neuroscience 102, 723-765. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe, D. J. (2001) Physiol. Rev. 81, 741-766. [DOI] [PubMed] [Google Scholar]
- 4.Small, D. H., Mok, S. S. & Bornstein, J. C. (2001) Nat. Rev. Neurosci. 2, 595-598. [DOI] [PubMed] [Google Scholar]
- 5.Kagan, B. L., Hirakura, Y., Azimov, R., Azimova, R. & Lin, M. C. (2002) Peptides 7, 1311-1315. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe, D. J. (2002) Science 298, 789-791. [DOI] [PubMed] [Google Scholar]
- 7.Davies, P. & Maloney, A. J. (1976) Lancet 2, 1403 (lett.). [DOI] [PubMed] [Google Scholar]
- 8.Chu, D. C., Penney, J. B. & Young, A. B. (1987) Neurosci. Lett. 82, 246-252. [DOI] [PubMed] [Google Scholar]
- 9.Zilles, K., Qu, M., Schleicher, A., Schroeter, M., Kraemer, M. & Witte, O. W. (1995) Arzneim. Forsch. 45, 361-366. [PubMed] [Google Scholar]
- 10.Ikonomovic, M. D., Mizukami, K., Warde, D., Sheffield, R., Hamilton, R., Wenthold, R. J. & Armstrong, D. M. (1999) Exp. Neurol. 160, 194-204. [DOI] [PubMed] [Google Scholar]
- 11.Ikonomovic, M. D., Nocera, R., Mizukami, K. & Armstrong, D. M. (2000) Exp. Neurol. 166, 363-375. [DOI] [PubMed] [Google Scholar]
- 12.Hynd, M. R., Scott, H. L. & Dodd, P. R. (2001) J. Neurochem. 78, 175-182. [DOI] [PubMed] [Google Scholar]
- 13.Sze, C.-I., Bi, H., Kleinschmidt-DeMasters, B. K., Filley, C. M. & Martin, L. J. (2001) J. Neurol. Sci. 182, 151-159. [DOI] [PubMed] [Google Scholar]
- 14.Mulugeta, E., Karlsson, E., Islam, A., Kalaria, R., Mangat, H., Winblad, B. & Adem, A. (2003) Brain Res. 960, 259-262. [DOI] [PubMed] [Google Scholar]
- 15.Miledi, R., Parker, I. & Sumikawa, K. (1989) Fidia Research Foundation Neuroscience Award Lecture (Raven, New York), Vol. 3, pp. 57-90. [Google Scholar]
- 16.Gundersen, C. B., Miledi, R. & Parker, I. (1984) Nature 308, 421-424. [DOI] [PubMed] [Google Scholar]
- 17.Umbach, J. A. & Gundersen, C. B. (1989) Mol. Pharmacol. 36, 582-588. [PubMed] [Google Scholar]
- 18.Palma, E., Esposito, V., Mileo, A. M., Di Gennaro, G., Quarato, P., Giangaspero, F., Scoppetta, C., Onorati, P., Trettel, F., Miledi, R. & Eusebi, F. (2002) Proc. Natl. Acad. Sci. USA 99, 15078-15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsal, J., Tigyi, G. & Miledi, R. (1995) Proc. Natl. Acad. Sci. USA 92, 5224-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miledi, R., Eusebi, F., Martinez-Torres, A., Palma, E. & Trettel, F. (2002) Proc. Natl. Acad. Sci. USA. 99, 13238-13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miledi, R. (1982) Proc. R. Soc. London Ser. B 215, 491-497. [DOI] [PubMed] [Google Scholar]
- 22.Kusano, K., Miledi, R. & Stinnakre, J. (1982) J. Physiol. 328, 143-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer, M., Koeppe, R. A., Frey, K. A., Foster, N. L. & Kuhl, D. E. (1995) Arch. Neurol. 52, 314-317. [DOI] [PubMed] [Google Scholar]
- 24.Mizukami, K., Ikonomovic, M. D., Grayson, D. R., Rubin, R. T., Warde, D., Sheffield, R., Hamilton, R. L., Davies, P. & Armstrong, D. M. (1997) Exp. Neurol. 147, 333-345. [DOI] [PubMed] [Google Scholar]
- 25.Ohyama, M., Senda, M., Ishiwata, K., Kitamura, S., Mishina, M., Ishii, K., Toyama, H., Oda, K. & Katayama, Y. (1999) Ann. Nucl. Med. 13, 309-315. [DOI] [PubMed] [Google Scholar]
- 26.Howell, O., Atack, J. R., Dewar, D., McKernan, R. M. & Sur, C. O. (2000) Neuroscience 98, 669-675. [DOI] [PubMed] [Google Scholar]
- 27.Mizukami, K., Ikonomovic, M. D., Grayson, D. R., Sheffield, R. & Armstrong, D. M. (1998) Mol. Brain Res. 799, 148-155. [DOI] [PubMed] [Google Scholar]
- 28.Mizukami, K., Grayson, D. R., Ikonomovic, M. D., Sheffield, R. & Armstrong, D. M. (1998) Mol. Brain Res. 56, 268-272. [DOI] [PubMed] [Google Scholar]
- 29.Arispe, N., Pollard, H. B. & Rojas, E. (1993) Proc. Natl. Acad. Sci. USA 90, 10573-10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, H., Bhatia, R. & Lal, R. (2001) FASEB J. 15, 2433-2444. [DOI] [PubMed] [Google Scholar]
- 31.Arispe, N., Pollard, H. B. & Rojas, E. (1994) Mol. Cell. Biochem. 140, 119-125. [DOI] [PubMed] [Google Scholar]
- 32.Ragsdale, D. & Miledi, R. (1991) Proc. Natl. Acad. Sci. USA 88, 1854-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]