Abstract
Prenatal exposure to alcohol may exert a significant detrimental effect on the functioning of the individual’s brain, however few studies have examined this before birth. This longitudinal study examined the effect of maternal alcohol consumption on the elicited startle response of the fetus. Two groups of fetuses were examined: one whose mothers drank alcohol (approximately 10 units per week); the other whose mothers did not drink alcohol. Fetuses were examined at 29, 32 and 35 weeks gestation and their startle response observed using ultrasound in response to 2 presentations of a pink noise (70–250Hz) at 90dB(A) separated by 30 seconds. Fetuses exposed to alcohol exhibited a weaker startle response at 29 weeks gestation than did fetuses not exposed to alcohol. There was no difference in the response at 32 and 35 weeks gestation. To ensure the effects were not due to a more general effect of alcohol on fetal movement, a second experiment compared the spontaneous movements (observed on ultrasound for 45 minutes) of fetuses whose mothers drank alcohol and fetuses of mothers who didn’t drink alcohol. There were no differences in movements exhibited by the fetuses. The results suggest that exposure to alcohol delays the emergence of the elicited startle response at 29 weeks gestation but this delay has disappeared by 32 weeks gestation. The possible role of altered neural development, acute exposure to alcohol and disruptions to the fetus’s behavioural repertoire, in mediating these effects are discussed.
Keywords: Startle, Prenatal, Alcohol, CNS, Neurobehavioural, Fetus
1. INTRODUCTION
Fetal exposure to alcohol through maternal drinking may exert a long term effect on development and outcome [1], resulting in a range of effects including: growth retardation; physical and organ abnormalities; a characteristic physical appearance; and, neural, neuropsychological and neurobehavioural disorders. This range of effects is encapsulated by the term, Fetal Alcohol Spectrum Disorders [1,2]. At the most severe end of this spectrum is Fetal Alcohol Syndrome [3,4]. Perhaps the most widespread disorders that arise from prenatal exposure to alcohol are those affecting the fetus’s brain and can be evidenced through poor psychological, neural, behavioural, and social performance after birth [5]. Whilst there have been extensive studies of the effects of prenatal exposure to alcohol on the individual after birth there have been few studies examining its effects on the fetus before birth [6]; the time at which the exposure to alcohol is taking place. Such information would be beneficial in elucidating further the mediation of alcohol’s effects on the fetus, especially those affecting its brain [6]. One means of doing this is to observe the behaviour of the fetus. The behaviour of the fetus reflects the functioning and integrity of its brain [7,8] and many studies have demonstrated that the behaviour of the fetus provides an indicator of its health and well-being [9,10].
Some studies have examined the effect of maternal alcohol consumption on the behaviour of the fetus. In late pregnancy, the fetuses of mothers who drank the equivalent of 1–2 units of alcohol exhibited an immediate decrease in breathing movements upon maternal consumption and a complete cessation 30 minutes later [11,12,13]. The behavioural states of the fetus are disrupted by acute exposure to similar amounts of alcohol [14]. Studies examining the behaviour of fetuses whose mothers drink, but do not have alcohol in their system at the time of observation, reveal that fetuses exposed to alcohol exhibit more spontaneous startles than those not exposed to alcohol at 18–20 weeks gestation [15]. A longitudinal study found that as pregnancy progressed, fetuses exposed to alcohol exhibited a decrease in their level of spontaneous startles which approached the level exhibited by unexposed fetuses [16]. However, they still exhibited more spontaneous startles at 36 weeks gestation, the final age at which they were tested [16].
The elicited startle, that is a quick rapid onset movement of the whole body following the presentation of an external sound stimulus, emerges around the same time as the onset of hearing [17] at 26–27 weeks gestation. Only one previous study has examined the effect of alcohol on the elicited startle and this found that at 27 weeks gestation fetuses not exposed to alcohol were more likely to exhibit a startle in response to a vibroacoustic stimulus than fetuses exposed to alcohol [15]. However as fetuses were only examined at one age it is unknown whether there is a similar developmental catch up in the response to an external stimulus, as is observed in the spontaneous startle behaviour of fetuses exposed to alcohol [16].
Thus in this paper we examine longitudinally whether fetal exposure to alcohol via the mother’s consumption exerts an effect on the fetus’s startle response to an external auditory stimulus at 3 different gestational ages, 29, 32 and 35 weeks gestation (Experiment 1). The elicited startle obviously requires the exhibition of a movement. If a decreased startle response is observed this may be the result of alcohol influencing the fetus’s ability to exhibit a motor response [cf.11,12,13]. To examine this a further experiment was conducted to examine whether maternal consumption of alcohol affected the movements of the fetus to determine if this may account for any effects observed.
2. GENERAL MATERIALS AND METHODS
2.1 Participants
Pregnant women were recruited from the Royal Maternity Hospital, Belfast. Mothers, who did not smoke, with healthy, singleton pregnancies, with no known abnormality, were recruited at 20–22 weeks gestation by general recruitment questionnaire for research participation at the Fetal Behaviour Research Centre. Mothers were selected for this study if they indicated they either did not drink alcohol or drank, on average, 1–2 drinks per day. Based on these reports mothers were divided into two groups: mothers who drank alcohol during pregnancy and mothers who did not consume alcohol.
Details of alcohol consumption were obtained through a semi-structured interview prior to their first scan of this study and this was repeated before each scan. Mothers described their alcohol consumption over their pregnancy (“pregnancy average”) and provided detailed descriptions of their alcohol consumption over the preceding week before each scan. Their consumption for the preceding week was expressed in terms of the number of units of alcohol consumed. 1 unit of alcohol was equivalent to 1 small glass of wine, one half pint of normal strength beer, or one short (spirit) measure. All mothers reported that their alcohol consumption the week before each scan was average for them.
All fetuses were appropriately grown for gestational age. All mothers had had unremarkable ultrasound anomaly scans and mothers had no illness or condition which may affect the fetus (e.g. diabetes, epilepsy). Mothers were not taking any medications or other drugs. The following demographic details were obtained: maternal age, parity, gestational age at delivery, fetal birthweight and Apgar score at 5 minutes. None of the babies had any abnormality at birth and none had signs of Fetal Alcohol Syndrome.
Ethical approval for the study was obtained from the Medical Research Ethical Committee, Queen’s University of Belfast and each participant gave full informed consent to take part inthe study.
2.2 Apparatus
A Lion Alcometer SD-400 breath alcohol sampling device (Lion Laboratories plc, UK) was used to sample the mother’s alcohol levels immediately prior to the start of each scan.
The fetus was observed using an Ultramark 4plus (Advanced Technical Laboratories) or Dornier AI3200 ultrasound machine with a 3.5MHz curvilinear scan head. All scans were recorded on videotape for later analysis using the Observer statistical package (Noldus Technologies).
The sound stimulus (70–250Hz pink noise, 90dB(A) measured at 1 cm from the speaker) was generated from a white noise created by a waveform generator (Wavetek 100MHz model 395) passed through a dual channel filter (Stanford Research Systems SR650) and amplifier (Denon PMA-250SE) which produced the required sound intensity measured by sound level meter (Bruel & Kjaer model 2238). The sound stimuli were fed into a modified headphone speaker and surround (AKG K-160, Z-61A, Z-60A). When placed on the mother’s abdomen, this formed an effective seal between speaker and skin, but kept the speaker away from direct contact with the mother’s skin. A computer controlled the presentation of the stimulus.
3. EXPERIMENT 1
The effect of maternal alcohol consumption on the startle response of the fetus elicited by an external sound stimulus
3.1 Participants
Thirty-eight fetuses took part in this experiment. Mothers of 18 fetuses (8 male, 10 female) drank alcohol and mothers of 21 fetuses (11male and 10 female) did not. Mothers were scanned on three occasions at 29, 32 and 35 weeks gestation and their fetuses observed. Mothers had not consumed alcohol in the 24 hours preceding the scan and were breathalysed prior to the start of the scan. No mother had alcohol in her body at the time of study.
3.2 Procedure
Mothers lay in a semi-recumbent position on a couch at a 45-degree angle and a longitudinal view of the fetus was obtained, including its head, upper body, arms. This view was obtained at all gestational ages and maintained throughout the observation. The scan was recorded onto videotape for later analysis. The observed view was explained to the mother and any questions she had addressed. Following a period of 120 seconds of fetal inactivity the speaker was placed on the mother’s abdomen over the fetus’ head [18,19]. The computer then triggered the sound for 2 seconds and the fetus’s response was observed. Thirty seconds later the stimulus was presented again and the fetus’ response observed. The computer superimposed a visual indication of stimulus presentation and a centisecond timer over the ultrasound picture and this was recorded on the videotape along with the ultrasound pictures of the fetus. The identical procedure was repeated at each gestational age.
3.3 Analysis
The response of the fetus was analysed from the tape and scored according to a scale of 0 (no movement) to 4 (‘complete’ startle response):
-
0
no movement observed.
-
1
a slow, small amplitude, movement involving just 1 or 2 of the observed body parts (arms, head, or body), of long duration and may begin sometime (1–2 sec) after the stimulus is presented.
-
2
a slow, small amplitude movement of all the observed body parts (both arms, head, and body), of long duration and may begin sometime (1–2 sec) after the stimulus is presented.
-
3
a rapid, large amplitude movement often involving just 1 or 2 of the observed body parts (both arms, head, and body), of short duration and occurs almost immediately the stimulus is presented.
-
4
a rapid, large amplitude movement of all observed body parts (both arms, head, and body), of short duration and occurs almost immediately the stimulus is presented.
Analysis of the data revealed a slight negative skewing of the data and thus the raw scores obtained above were transformed using a Square Root transform [20] for the analysis. A mixed design ANOVA was undertaken to examine the fetus’s response for the between subjects factors of alcohol exposure (yes : no) and sex (male : female) and the within subject factors of gestational age at testing (29: 32 : 35 weeks gestation) and trial (first : second). To ensure there were no differences between the groups on demographic variables between subject t-tests were performed on factors of parity, gestational age at delivery, birthweight, Apgar score at 5 minutes, and maternal age.
3.4 Inter- and Intra-rater reliability
The experimenter and an experienced observer (both having completed at least 100 hours of observation of the human fetus) re-coded 20% (8 subjects, 4 from each group) of the responses of a random selection of fetuses. Both were blind to the mother’s group during this. Inter- and intra-rater reliabilities were extremely high. Intra class-coefficient ratings: Inter-rater reliability = 0.981, p<0.001 and, intra-rater reliability, 0.980, p<0.001. Although the experimenter was not ‘blinded’ to the mothers group at the time of scan the high inter- and intra-rater reliability speak to the accuracy of the data coding.
3.5 Results
There was no significant difference (at the level of 0.05) between any of the demographic variables between the alcohol exposed and non-exposed group (see Table 1). Mothers in the alcohol group consumed, on average, approximately 11 units of alcohol/week on average across pregnancy and just over 10 units/per week prior to each test (see Table 1).
Table 1.
The number of fetuses who took part in each experiment by group. NA= fetuses not exposed to alcohol, A=fetuses exposed to alcohol. The mean (and s.d.) maternal age, gestational age at delivery, birthweight and Apgar score at 5 minutes, and mean (and range) parity for participants in each Experiment by group. Alcohol consumption (pregnancy average, and each week prior to testing) is reported in mean (and s.d.) units per week.
| Experiment 1 | Experiment 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| NA | A | NA | A | |||||
| Number of fetuses | 21 m-11, 10-f |
18 m-8, f-10 |
21 m-12, f-9 |
18 m-10, f-8 |
||||
| Maternal age (yr) | 28.1 (4.35) | 27.1 (4.67) | 26.5 (4.99) | 26.1 (4.47) | ||||
| Parity | 0.71 (0–3) | 0.61 (0–2) | 0.76 (0–2) | 0.72 (0–2) | ||||
| GA at delivery | 281.2 (5.8) | 281.7 (6.5) | 282.1 (6.4) | 282.3 (7.1) | ||||
| Birthweight | 3470 (731) | 3386 (640) | 3428 (439) | 3426 (548) | ||||
| Apgar score 5min | 8.81 (0.40) | 8.78 (0.42) | 8.76 (0.44) | 8.72 (0.46) | ||||
| Alcohol consumption (pregnancy average) | 11.33 (1.53) | 11.00 (1.57) | ||||||
| Testing Age (wks GA) | 29 | 32 | 35 | 29 | 32 | 35 | 29 | 29 |
| Alcohol consumption (units/wk) | 0 | 0 | 0 | 10.0 (1.4) | 10.7 (1.9) | 10.2 (1.4) | 0 | 10.44 (1.88) |
| No. of Movements | 40.42 (13.56) | 37.5 (14.75) | ||||||
There was no significant main effect of sex (F[1,35]=0.097, p=0.757) nor any significant interactions involving sex. There was no significant effect of trial (F[1,35]=2.992, p=0.092) nor any significant interactions involving trial.
The ANOVA revealed significant main effects of: gestational age at testing (F[2,70]=67.329, p<0.001), the strength of the response increased as the fetus aged (mean (+/− s.d.) strength of response at 29 weeks = 2.667 (+/− 1.08), at 32 weeks = 3.667 (+/− 0.57), and at 35 weeks = 3.843 (+/− 0.42)); and, alcohol exposure (F[1,35]=42.718, p<0.001), fetuses not exposed to alcohol elicited a stronger startle than fetuses exposed to alcohol (mean strength of response=3.627 (+/− 0.63) and 3.120 (+/− 1.09), respectively).
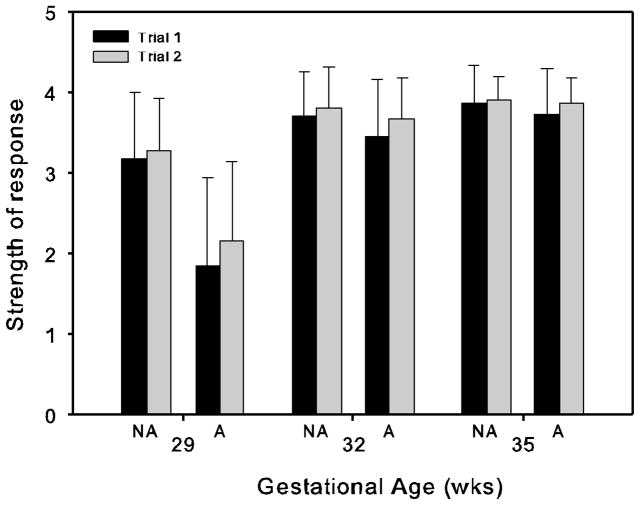
These main effects were further qualified by a significant 2 way interaction between gestational age at testing × alcohol exposure (F[2,70]=12.332, p<0.001) (see Figure 1). At 29 weeks gestational age fetuses exposed to alcohol exhibited a significantly weaker response to the stimulus on both presentations than fetuses not exposed to alcohol (p<0.001, independent t-tests, comparing responses between the two groups on trial 1, trial 2 and the mean response of trial 1 and 2). At 32 and 35 weeks gestation there was no difference between fetuses exposed to alcohol or not exposed to alcohol in the strength of their response to the stimulus. There were no other significant interactions.
Figure 1.
The mean (+/− s.d.) strength of the elicited startle response of fetuses not exposed to alcohol (NA) and exposed to alcohol (A) on trial 1 and trial 2 at each gestational age.
Across the study no response, i.e. the fetus scored 0, was only observed on 4 trials. All of these occurred at 29 weeks gestation in the alcohol exposed group. Two no responses were exhibited by females who failed to respond on the first trial but did respond on the second. Two males exhibited no response; one failed to respond on the first trial and the other failed to respond on the second trial. The low numbers precluded any formal statistical analysis.
3.6 Summary
The results indicate that exposure to alcohol reduces the strength of the fetus’ startle response at 29 weeks gestation. No differences were observed at either 32 or 35 weeks gestation suggesting that any effects are limited to the developmental emergence of the startle response to an external stimulus.
4. EXPERIMENT 2
Does exposure to alcohol through maternal consumption affect the fetus’s spontaneous movements?
Experiment 1 found that at 29 weeks gestation exposure to alcohol decreased the startle response of the fetus to an external stimulus. One possible factor that may account for this is that alcohol exposure, in some way, affected the fetus’s ability to move. The startle response comprises a motor element as its end point and if alcohol influenced the ability of the fetus to move this may result in the observation of a weaker startle. Indeed a common finding of the few studies that have examined the behaviour of the fetus in response to acute alcohol exposure is that alcohol suppresses movements [11,12,13]. To examine this more thoroughly, a second experiment, Experiment 2, compared the number of spontaneous movements exhibited by fetuses exposed, and not exposed, to alcohol at 29 weeks gestation. It might be expected that if alcohol supressed movements fewer would be observed in fetuses exposed to alcohol.
The procedure was the same as reported for Experiment 1 with the exception of the details provided below.
4.1 Participants
The fetuses of thirty nine pregnant women, who had not taken part in Experiment 1, were observed at 29 weeks gestational age. The group was divided into: 18 fetuses (10 male and 8 female) whose mothers drank alcohol; and, 21 fetuses (12 male and 9 female) whose mothers did not drink alcohol. The demographics data of this group are reported in Table 1. There were no differences in any of the demographic details between the participants of this study and that of Experiment 1, nor between the two groups (alcohol exposed : not alcohol exposed) in this study. Mothers of the alcohol group consumed a mean of 10.44 (+/− 1.88 s.d.) units of alcohol per week.
4.2 Procedure
Mothers lay on the couch and their fetus was observed using ultrasound. The head, arms and upper body were visualised and this view of the fetus recorded for a 45 minute period. The total number of movements exhibited by each fetus during this period was calculated by summing the number of individual arm, head and body movements observed. Facial movements, e.g. eye blink, or finger movements were not counted [after 21].
4.3 Analysis
To examine whether there were any differences between the number of movements exhibited by the fetus in different groups an ANOVA for the between subjects factor of alcohol exposure (yes : no) and sex (male : female) was undertaken.
4.3 Inter- and Intra-rater reliability
Inter- and intra-rater reliabilities were extremely high. The intra class-coefficient ratings were: Inter-rater reliability = 0.998, p<0.001 and, intra-rater reliability, 0.994, p<0.001.
4.5 Results
There was no significant effect of alcohol exposure (F(1,35)=0.307, p=0.583), or fetal sex F(1,35)=0.32, p=0.858) on the number of movements exhibited by the fetus. The mean (+/− s.d.) number of movements exhibited by fetuses exposed to alcohol = 37.50 (+/− 14.75) and by fetuses not exposed to alcohol = 40.43 (+/− 13.56).
4.6 Discussion
Experiment 2 was undertaken to examine the possible contribution of alcohol induced altered movement on the decreased startle response observed at 29 weeks gestation in Experiment 1. The results of Experiment 2 suggest that the difference in startle response is unlikely to be accounted for by the effects of alcohol exposure on the movements of the fetus.
There was no difference in the quantity of movements exhibited by fetuses exposed to alcohol and those not exposed to alcohol. Thus it would appear that alcohol has not supressed movements to the extent that this may account for the difference in startle response. Although the quality of movements was not formally explored, observations of the fetuses revealed no obvious differences in the quality of movements between fetuses exposed to alcohol and those not exposed. This experiment examined the frequency of specific movements related to the startle rather than just the occurrence of spontaneous startles which have been previously been shown to be affected by alcohol exposure [15,16]. It would be of interest more generally to examine whether alcohol affected spontaneous movements, examining different movements and/or using novel movement analysis systems (e.g. 22), to fully elucidate any effects of alcohol on the fetus’s behavioural repertoire.
5. GENERAL DISCUSSION
The central aim of this study was to examine whether the absence of the elicited startle response of the fetus exposed to alcohol via its mother’s consumption found at the time of its emergence was a transient or permanent effect. The findings of this study indicate that alcohol exerts an effect at the time of the emergence of the elicited startle but as the fetus matures the effect disappears. This study observed a reduced startle response in fetuses exposed to alcohol at 29 weeks gestation but there was no difference at 32 and 35 weeks gestation. This is suggestive of alcohol exerting a maturational delay on the emergence of the elicited startle which subsequently disappears. This result is similar to that observed for spontaneous startles where fetuses exposed to alcohol exhibited a developmental delay in the inhibition of the spontaneous startle response [16]. In contrast, this study found that fetuses exposed to alcohol caught up completely by 32 weeks gestation, (although see later) whereas for spontaneous startles there was still a difference present at 36 weeks gestation.
There is an apparent difference in the effect of exposure to alcohol between the exhibition of elicited startles observed in this study and spontaneous startles observed previously [15,16]. The effects of alcohol on spontaneous and elicited startle are mediated, in part, by delayed neurobehavioural development. This delayed development preserves the immature and primitive pattern of spontaneous startles resulting in more being exhibited by fetuses exposed to alcohol, however it delays the appearance of the elicited startle response (a more advanced response) resulting in fewer elicited startles being exhibited by fetuses exposed to alcohol.
The behaviour of the fetus is a reflection of the functioning of its central nervous system [7,8], and hence its investigation provides the opportunity to examine how the CNS is influenced by adverse conditions, for example, maternal ill-health [18,23], fetal abnormalities [24] or exposure to environmental agents [25]. It is well established that alcohol exposure before birth exerts an adverse impact on the functioning of the brain [26,27]. However this evidence has been gathered after birth, after the individual’s exposure to alcohol has concluded. By observing the impact of alcohol on the behaviour of the fetus a greater understanding of the effect of alcohol on the fetus’s brain may be obtained.
The underlying neural mediation of the elicited startle response in the fetus is unknown, and acknowledging the need to proceed extremely cautiously, its similarity with the auditory startle response observed after birth may suggest a similar underlying neural mediation. The auditory startle is largely a brainstem reflex involving the ponto-medullary brainstem and involves the ventral cochlear nucleus, the dorsal nucleus of the lateral lemniscus and caudal pontine reticular nucleus [28,29], with the pontine reticular nucleus being particularly important. It is most likely that alcohol is in some way acting upon these structures. One of the significant effects of prenatal alcohol exposure is the delaying of myelination of nerve fibres and growth of dendrites [30]. It may be that the chronic exposure to alcohol experienced by the fetus contributes to a maturational delay in the neural pathways responsible for the startle response.
One other possible factor that may contribute to the results is an acute effect of alcohol on the startle response. Although mothers refrained from drinking for 24 hours prior to the study, and no mother had alcohol in her system at the time of study, this does not necessarily rule out the possibility that the fetus had alcohol in its system at the time of testing. Alcohol freely crosses the placenta and fetal liver function is underdeveloped resulting in alcohol being broken down much less quickly in the fetus than the mother [31,32,33]. Alcohol may thus have suppressed responding at 29 weeks but not later as the fetus’s liver function had developed to the extent it was able to clear the alcohol. Mothers in this study were not binge drinkers but drank evenly over the week, the mean no. of days mothers drank was just over 5 (range 4–7) and, on average, mothers drank just under 2 units (range 1–3) on each occasion when drinking. Thus the mothers were consistent drinkers, drinking low levels of alcohol and it would be expected that these levels would be cleared by the fetus prior to the mother’s participation in the study. Thus although it is a possibility that acute alcohol exposure may have caused or contributed to the observed effect this seems unlikely.
It is possible that the observed effects could be mediated by a delay in the maturation of auditory function in fetuses exposed to alcohol. If fetuses were unable to hear the stimulus then this would obviously affect their response. There is little evidence to suggest alcohol exposure retards auditory development but the possibility cannot be ruled out and we are undertaking research to examine this.
The average consumption of alcohol by mothers in this study was approximately 10–11 units/week, with 2–3 units consumed on each drinking occasion. Studies of the acute effects of alcohol exposure [11,12,13,14] reveal that behaviour, breathing and behavioural states, are suppressed for over 2–3 hours following the consumption of 1–2 units of alcohol. Thus it is likely that the fetuses in this study had their behaviour disrupted when their mothers drank, and this disruption occurred on average for 5 days per week and for over two hours each time. This disruption may have contributed to the delay in startle response observed here [6]. One function of the fetus’s behavioural repertoire has been argued to be to develop neural pathways [34], a possible example of experience-dependent or experience-expectant neural development [35]. It may be that the disruption of behaviour delays the development of the neural pathways involved in the elicited startle response through lack of required ‘experiential input’.
One must be cautious with the interpretation of the catch-up exhibited here. Firstly, although the startle appeared normal following alcohol exposure at 32 and 35 weeks gestational this does not necessarily mean that the startle is back to normal. For example, rats exposed to alcohol prenatally exhibit evidence of an exaggerated startle at 35 days of age but not at 21 days of age [36]. Whether differences in startle may re-emerge at later ages is unknown. The fact that the startle returns to a similar form as exhibited by fetuses not exposed to alcohol does not necessarily mean that the underlying neural pathways are not affected more permanently. The brain is quite plastic at this age and alternative compensatory pathways may become active. Furthermore if the neural pathways are involved in other behaviours then although the elicited startle is observed as usual this does not mean that other behaviours mediated by elements of the startle neural pathways are not affected. Thus whilst the observed ‘return to normal’ of the startle response by 32 weeks gestation may be evidence of a transient developmental delay it cannot be completely ruled out that more permanent effects remain and may become evident in the individual’s behaviour later in life.
One methodological issue worthy of expansion regards the possibility that some movements recorded in the 30 second period are naturally occurring spontaneous movements as opposed to movements elicited by the stimulus. It is possible that a spontaneous movement could occur during the 30 second period after the stimulus. However the natural incidence of startles at this age is very low thus for the control group it is extremely unlikely that any startles that were recorded (i.e. scoring 3–4) were spontaneous. Previous research has suggested that startles may occur more frequently in fetuses exposed to alcohol, although their incidence is still low [16] and approaches that of non-exposed fetuses by 32 weeks gestation. Thus this may be a particular concern at 29 weeks gestation. However, if spontaneous startles did occur this would act against the hypothesis, i.e. a startle would be recorded when in fact one did not occur in response to the stimulus, thus reducing the potential difference between the groups. A slightly different concern is that a response to the stimulus may be delayed until after the second stimulus presentation. However responses scoring 1 or 2 usually occurred within 10 seconds of stimulus onset. Only 3 fetuses did not exhibit a response following the first stimulus presentation. Assuming they did exhibit a much delayed response, and giving them a score of 1 or 2 for the purposes of analysis, does not change the results nor levels of significance.
6. CONCLUSIONS
In summary, alcohol affects the developmental emergence of the elicited startle response in the human fetus at 29 weeks gestation. This effect is not mediated by peripheral processes relating to the motor components of the response but rather by the ‘central’ neural pathways involved in the startle. In terms of its behavioural presentation the startle response appears to catch up to that of fetuses unexposed to alcohol by 32 week gestation, however whether the underlying neural mediation is similarly transiently affected is unknown.
Highlights.
Maternal alcohol consumption delays the emergence the fetus’s elicited startle
Disruption in the fetus’s behaviour affect neural development
The fetal startle is highly sensitive to perturbations in its environment
Acknowledgments
This work was partially funded by a grant (RRG3.7) from the R&D Office, Dept. of Health and Social Services and Public Safety, NI, and a grant (5 U24 AA014828) from the National Institute on Alcohol and Alcohol Abuse (NIAAA) and done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Additional information about CIFASD can be found at www.cifasd.org. We thank D Wells for her contribution to inter-rater reliability. The support of the Royal Maternity Hospital, and the Wellcome Trust is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.BMA. Fetal alcohol spectrum disorders. A guide for healthcare professionals. London: BMA; 2007. [Google Scholar]
- 2.Riley EP, Infante MA, Warren KR. Fetal Alcohol Spectrum Disorders: An overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 4.Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Les enfants de parents alcooliques. Anomalies observes; A propos de 127 cas. Quest Med. 1968;21:476–82. [Google Scholar]
- 5.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioural features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepper PG. The effect of maternal consumption of alcohol on the behaviour of the human fetus: A review. Int J Dis Hum Dev. 2007;6:153–9. [Google Scholar]
- 7.Hepper PG. The behaviour of the foetus as an indicator of neural functioning. In: Lecanuet J-P, Fifer W, Krasnegor N, Smotherman W, editors. Fetal development. A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum; 1995. pp. 405–17. [Google Scholar]
- 8.Krasnegor NA, Fifer W, Maulik D, McNellis D, Romero R, Smotherman W. Fetal behavioural development: Measurement of habituation, state transitions, and movement to assess fetal well being and to predict outcome. J Mat Fetal Invest. 1998;8:51–5. [PubMed] [Google Scholar]
- 9.Lecanuet J-P, Fifer W, Krasnegor N, Smotherman W, editors. Fetal development. A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum; 1995. [Google Scholar]
- 10.Nijhuis JG, editor. Fetal behaviour: Developmental and perinatal aspects. Oxford: Oxford University Press; 1992. [Google Scholar]
- 11.Akay M, Mulder EJH. Investigating the effect of maternal alcohol intake on human fetal breathing rate using adaptive time-frequency analysis methods. Ear Hum Dev. 1996;46:153–64. doi: 10.1016/0378-3782(96)01764-1. [DOI] [PubMed] [Google Scholar]
- 12.Fox HE, Steinbrecher M, Pessel D, Inglis J, Medvid L, Angel E. Maternal ethanol ingestion and the occurrence of human fetal breathing movements. Am J Obstet Gynecol. 1978;132:354–58. doi: 10.1016/0002-9378(78)90766-4. [DOI] [PubMed] [Google Scholar]
- 13.McLeod W, Brien JF, Loomis C, Carmichael L, Probert C, Patrick J. Effects of maternal ethanol ingestion on fetal breathing movements gross body movements and heart rate at 37 to 40 weeks gestational age. Am J Obstet Gynecol. 1983;145:251–7. doi: 10.1016/0002-9378(83)90501-x. [DOI] [PubMed] [Google Scholar]
- 14.Mulder EJH, Morssink LP, van der Schee T, Visser GHA. Acute maternal alcohol consumption disrupts behavioral state organization in the near-term fetus. Ped Res. 1988;44:774–9. doi: 10.1203/00006450-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Little JF, Hepper PG, Dornan JC. Maternal alcohol consumption during pregnancy and fetal startle behavior. Physiol Behav. 2002;76:691–4. doi: 10.1016/s0031-9384(02)00804-1. [DOI] [PubMed] [Google Scholar]
- 16.Hepper PG, Dornan JC, Little JF. Maternal alcohol consumption during pregnancy may delay the development of spontaneous fetal startle behavior. Physiol Behav. 2005;83:711–4. doi: 10.1016/j.physbeh.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Hepper PG, Shahidullah S. The development of fetal hearing. Fet Mat Med Rev. 1994;6:167–179. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty NN, Hepper PG. Habituation in fetuses of diabetic mothers. Ear Hum Dev. 2000;59:85–93. doi: 10.1016/s0378-3782(00)00089-x. [DOI] [PubMed] [Google Scholar]
- 19.Joy J, McClure N, Hepper PG, Cooke I. Fetal habituation in assisted conception. Early Human Development. 2011 doi: 10.1016/j.earlhumdev.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Howell DC. Statistical methods for psychology. 6. Belmont: Thomson Wadsworth; 2007. [Google Scholar]
- 21.Hepper PG, Dornan JC, Lynch C. Sex differences in fetal habituation. Dev Science. 2012;15:373–383. doi: 10.1111/j.1467-7687.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurjak A, Miskovic B, Stanojevic M, Amiel-Tison C, Ahmed B, Azumendi G, Vasilj O, Andonotopo W, Turudic T, Salihagic-Kadic A. New scoring system or fetal neurobehavior assessed by three-and four-dimensional ultrasound. J Perinat Med. 2008;36:73–81. doi: 10.1515/JPM.2008.007. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Gonzalez NL, Medina V, Padron E, Domenech E, Gomez NMD, Armas H, Bartha JL. Fetal and neonatal habituation in infants of diabetic mothers. J Pediat. 2009;154:492–7. doi: 10.1016/j.jpeds.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 24.van Heteren CF, Boekkooi PF, Jongsma HW, Nijhuis JG. The responses to repeated vibroacoustic stimulation in a fetus with trisomy 18. Eur J Obstet Gynecol Reprod Biol. 2001;96:123–5. doi: 10.1016/s0301-2115(00)00396-1. [DOI] [PubMed] [Google Scholar]
- 25.Leader LR. The effects of cigarette smoking and maternal hypoxia on fetal habituation. In: Maeda K, editor. The fetus as a patient. Amsterdam: Elsevier; 1987. pp. 83–88. [Google Scholar]
- 26.Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcoholism. 2009;44:108–14. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behaviour. Exp Biol Med. 2005;230:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 28.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 29.Yeomans JS, Frankland PW. The acoustic startle reflex. Brain Res Rev. 1995;21:301–14. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- 30.Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Med Biol. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- 31.Brien JF, Loomis CW, Tranmer J, McGrath M. Disposition of ethanol in human maternal venous blood and amniotic fluid. Am J Obstet Gynecol. 1983;146:181–6. doi: 10.1016/0002-9378(83)91050-5. [DOI] [PubMed] [Google Scholar]
- 32.Nava-Ocampo AA, Velázquez-Armenta Y, Brien JF, Koren G. Elimination kinetics of ethanol in pregnant women. Rep Toxicol. 2004;18:613–7. doi: 10.1016/j.reprotox.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Pikkarainen PH, Raiha NC. Development of alcohol dehydrogenase activity in the human liver. Ped Res. 1967;1:165–8. doi: 10.1203/00006450-196705000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Hepper PG. Fetal psychology: an embryonic science. In: Nijhuis JG, editor. Fetal behaviour: Developmental and perinatal aspects. Oxford: Oxford University Press; 1992. pp. 129–56. [Google Scholar]
- 35.Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–59. [PubMed] [Google Scholar]
- 36.Potter BM, Berntson GG. Prenatal alcohol exposure: effects on acoustic startle and prepulse inhibition. Neurotoxicol Teratol. 1987;9:17–21. doi: 10.1016/0892-0362(87)90064-x. [DOI] [PubMed] [Google Scholar]