Abstract
Aims
The recent American College of Cardiology and American Heart Association Guidelines on hypertrophic cardiomyopathy (HCM) have confirmed surgical myectomy as the gold standard for non-pharmacological treatment of obstructive HCM. However, during the last 15 years, an extensive use of alcohol septal ablation has led to the virtual extinction of myectomy programmes in several European countries. Therefore, many HCM candidates for myectomy in Europe cannot be offered the option of this procedure. The purpose of our study is to report the difficulties and results in developing a myectomy programme for HCM in a centre without previous experience with this procedure.
Methods and results
The clinical course is reported of 124 consecutive patients with obstructive HCM and heart failure symptoms who underwent myectomy at a single European centre between 1996 and 2010. The median follow-up was 20.3 months (inter-quartile range: 3.9–40.6 months). No patients were lost to follow-up. A cumulative incidence of HCM-related death after myectomy was 0.8, 3.3, and 11.2% at 1, 5, and 10 years, respectively, including one operative death (procedural mortality 0.8%). The left ventricular (LV) outflow gradient decreased from 95 ± 36 mmHg before surgery to 12 ± 6 mmHg at most recent evaluation (P < 0.001), with none of the patients having a significant residual LV outflow gradient. Of the 97 patients in New York Heart Association functional class III–IV before surgery, 93 (96%) were in class I–II at most recent evaluation (P < 0.001).
Conclusion
Our results show that the development of a myectomy programme at a centre without previous experience with this procedure is feasible and can lead to highly favourable clinical results.
Keywords: Myectomy, Surgery, Hypertrophic cardiomyopathy
See page 1999 for the editorial comment on this article (doi:10.1093/eurheartj/ehs125)
Introduction
The recommendations of the American College of Cardiology and European Society of Cardiology Expert Consensus Conference on the management of hypertrophic cardiomyopathy (HCM), and the recent American College of Cardiology and American Heart Association Guidelines on HCM, have confirmed surgical septal myectomy as the gold standard for non-pharmacological treatment of obstructive HCM.1,2 However, in recent years, percutaneous alcohol septal ablation has been strongly promoted in Europe as a less invasive and safer alternative to the myectomy operation for patients with obstructive HCM.3–10 This policy has led to the virtual extinction of surgical myectomy programmes in several European countries with a long tradition for surgical treatment of patients with HCM.11 Therefore, many European patients with HCM and severe symptoms of heart failure cannot be offered the gold-standard treatment suggested by the HCM international guidelines, but only the alternative of alcohol septal ablation.
However, recent data have generated concern regarding the possibility that the transmural infarction and scar caused by septal ablation may increase the risk for life-threatening ventricular tachyarrhythmias and sudden death in HCM.12–20 Moreover, evidence has accumulated on the important role of abnormal papillary muscles and mitral valve chordae in contributing to left ventricular (LV) outflow obstruction in HCM.21–29 Such abnormalities of the mitral valve apparatus cannot be treated by alcohol septal ablation, but only with surgical intervention. In this context, we considered of interest to report our experience with developing a surgical programme for the myectomy operation.
Methods
Study population
The present investigation includes 124 consecutive symptomatic patients with obstructive HCM who underwent extended surgical myectomy at our institution between January 1996 and July 2010. Before surgery, each of the study patients had an LV outflow gradient of ≥50 mmHg at rest and disabling heart failure symptoms despite medical therapy. Operative mortality included any death within 30 days after operation. Follow-up data were obtained between January and September 2010 in each of the surviving study patients.
Diagnosis and echocardiographic evaluation
The diagnosis of HCM was based on the echocardiographic demonstration of a hypertrophied and non-dilated LV (wall thickness ≥15 mm in adults, or the equivalent relative to the body surface area in children) in the absence of another cardiac or systemic disease that could produce a comparable magnitude of LV hypertrophy.30,31 Echocardiographic measurements were obtained as described previously.30,31 Left ventricular outflow tract obstruction was defined as a peak instantaneous outflow gradient of ≥30 mmHg by continuous-wave Doppler under basal conditions.31–33
Surgical procedures
After induction of general anaesthesia, intraoperative transoesophageal echocardiography (TEE) was performed to determine the extension of the myectomy, as well as to assess the morphology of the mitral valve and the presence of associated primary mitral valve abnormalities.34 Transoesophageal echocardiography was repeated immediately after surgery, in the operating room and off cardiopulmonary bypass, in order to detect a residual LV outflow tract gradient and aortic or mitral valve regurgitation, as well as to identify potential surgical complications, such as ventricular septum perforation or coronary fistula.
The septal myectomy (in the present era commonly referred to as ‘extended myectomy’) was performed during cardiopulmonary bypass through an aortotomy. Two longitudinal incisions are started in the basal septum, 2–3 mm below the aortic valve, and extended distally to the base of the papillary muscles (equatorial zone), creating a trapezoid trough which is wider towards the apex than at the subaortic level.23,26,27,35 Great care was taken to excise the cardiac muscle specimen in one piece in order to leave a smooth surface on the remaining septum. In patients with LV outflow obstruction in whom a mid-ventricular obstruction due to markedly hypertrophied papillary muscles or muscle bundles was also present, an additional small resection was made around the base of the papillary muscle.23,27,35 After the excision of the cardiac muscle (myectomy), the operative procedure was completed by interventions on the subvalvular mitral apparatus. Fibrous or muscular structures connecting the papillary muscles to the ventricular septum or LV free wall are present in almost all patients with obstructive HCM and limit the mobility of the papillary muscles.23,27,35 Such structures, identifiable only at the time of surgery, were present and systematically excised and/or dissected free (up to the base of the papillary muscle) in each of our study patients in order to increase the papillary muscle mobility. Anomalous chordal structures or fibrous attachments of the mitral leaflets to the ventricular septum or free wall are found in a minority of patients with HCM and, whenever present in our study patients, were excised or divided.23,26,35 Anomalous attachments of the papillary muscle directly into the anterior mitral leaflet are uncommon in HCM and, in our patients, were resected when attached to the body (not the margin) of the leaflet.21,24 The extended myectomy operation was performed by the senior surgeon (P.F.) in 115 (93%) of the 124 study patients and by a surgeon in training for the myectomy operation in the remaining 9 patients (S.P.). Concomitant surgical procedures were also performed in selected patients.
During the last 3 years, myectomy was associated with surgical therapy of atrial fibrillation in patients presenting with paroxysmal or persistent atrial fibrillation. Lines of ablation were performed by using a bipolar radiofrequency energy device (Atricure, Inc., Cincinnati, OH, USA). The lesion set included an epicardial encircling of the pulmonary veins and a crux line on the external side of the left atrial appendage.
Statistical methods
The median and the inter-quartile range (IQR) of follow-up were calculated according to the reverse Kaplan–Meier method. Comparisons of continuous variables were performed with the Wilcoxon test. Analyses of change for parameters evaluated before surgery and at the most recent evaluation were performed by means of the McNemar change test and sign test, for binary and continuous variables, respectively. The crude cumulative incidence function estimates of HCM-related death were computed according to Kalbfleisch and Prentice. All reported P-values are two-sided. SPSS statistical software (SPSS, Chicago, IL, USA) and SAS System 9.2 (SAS Institute, Cary, NC, USA) were used for the calculations.
Results
Baseline characteristics
The clinical characteristics of the 124 study patients are summarized in Table 1. In the study cohort, age ranged from 2 to 81 years (mean 51 ± 17; median 54 years), with five of the patients being <18 years old and two patients <12 years old at the time of surgery. Of the 124 patients included in the study, 97 (78%) were in New York Heart Association (NYHA) functional class III–IV at the time of the operation. In 26 of the remaining 27 study patients, myectomy was performed because of the persistence of drug-refractory symptoms, including heart failure symptoms, chest pain, and/or recurrent syncopal episodes, that interfered with everyday activity and quality of life. The remaining study patient who underwent myectomy in the absence of functional class III–IV symptoms was a woman with marked LV outflow obstruction who had developed severe heart failure symptoms during her first pregnancy, but wished to have a second pregnancy. In this patient, myectomy was performed to reduce the risk of complications during pregnancy. Her second pregnancy was uncomplicated by HCM-related symptoms.
Table 1.
Baseline characteristics of the 124 study patients
| Variable | |
|---|---|
| Demographic data | |
| Age (years), mean and SD (median) | 51 ± 17 (54) |
| Male sex, n (%) | 56 (45) |
| Clinical status | |
| Shortness of breath, n (%) | 120 (97) |
| Syncope, n (%) | 24 (19) |
| NYHA functional class III or IV, n (%) | 97 (78) |
| History of prior myectomy, n (%) | 5 (4) |
| History of prior atrial fibrillation, n (%) | 27 (22) |
| Pre-operative echocardiographic data | |
| Septal LV wall thickness (mm), mean and SD | 23 ± 6 |
| LV end-diastolic cavity dimension (mm), mean and SD | 42 ± 7 |
| Resting LV outflow gradient (mmHg), mean and SD | 95 ± 36 |
| Moderate or severe mitral valve regurgitation, n (%) | 56 (45) |
| Treatment, n (%) | |
| β-Blockers | 102 (82) |
| Calcium antagonists | 12 (10) |
| Diuretics | 48 (39) |
| Amiodarone | 15 (12) |
NYHA, New York Heart Association; LV, left ventricular; SD, standard deviation.
Septal myectomy was a repeat procedure in five (4%) patients who had previously undergone a myectomy operation at other institutions, but had a significant residual LV outflow gradient under basal conditions and severe symptoms of heart failure. Septal myectomy was associated with mitral valve repair in 7 (5.6%) and mitral valve replacement in 2 (1.6%) of the 124 study patients. Of the two patients with mitral valve replacement, one was in cardiogenic shock due to rupture of the mitral valve chordae and the other had severe chronic obstructive pulmonary disease and rupture of the posterior leaflet chordae. Other operative procedures concomitant to ventricular septal myectomy, including the operative interventions on the subvalvular mitral apparatus, are summarized in Table 2. In particular, fibrous or muscular structures connecting the papillary muscles to the ventricular septum or LV free wall were present and excised in each of our study patients, in order to increase the papillary muscle mobility (Figure 1).
Table 2.
Operative procedures concomitant to septal myectomy in the 124 study patients
| Coronary artery bypass graft, n (%) | 9 (7.3) |
| Mitral valve replacement, n (%) | 2 (1.6) |
| Mitral valve repair, n (%) | 7 (5.6) |
| Aortic valve replacement, n (%) | 2 (1.6) |
| Epicardial cardioverter defibrillator, n (%) | 1 (0.8) |
| Surgical Ablation of atrial fibrillation, n (%) | 9 (7.3) |
| Unroofing of anterior descending coronary artery, n (%) | 1 (0.8) |
| Subvalvular mitral apparatus | |
| Resection of fibrous–muscular attachments between papillary muscle and ventricular septum or LV free wall, n (%) | 124 (100) |
| Excision of anomalous chordal attachments between mitral valve leaflets and ventricular septum or LV free wall, n (%)a | 11/91 (12.1) |
| Resection of anomalous attachment of the papillary muscle into the anterior mitral valve leaflet, n (%)a | 2/91 (2.2) |
aData were available in 91 of the 124 study patients.
Figure 1.
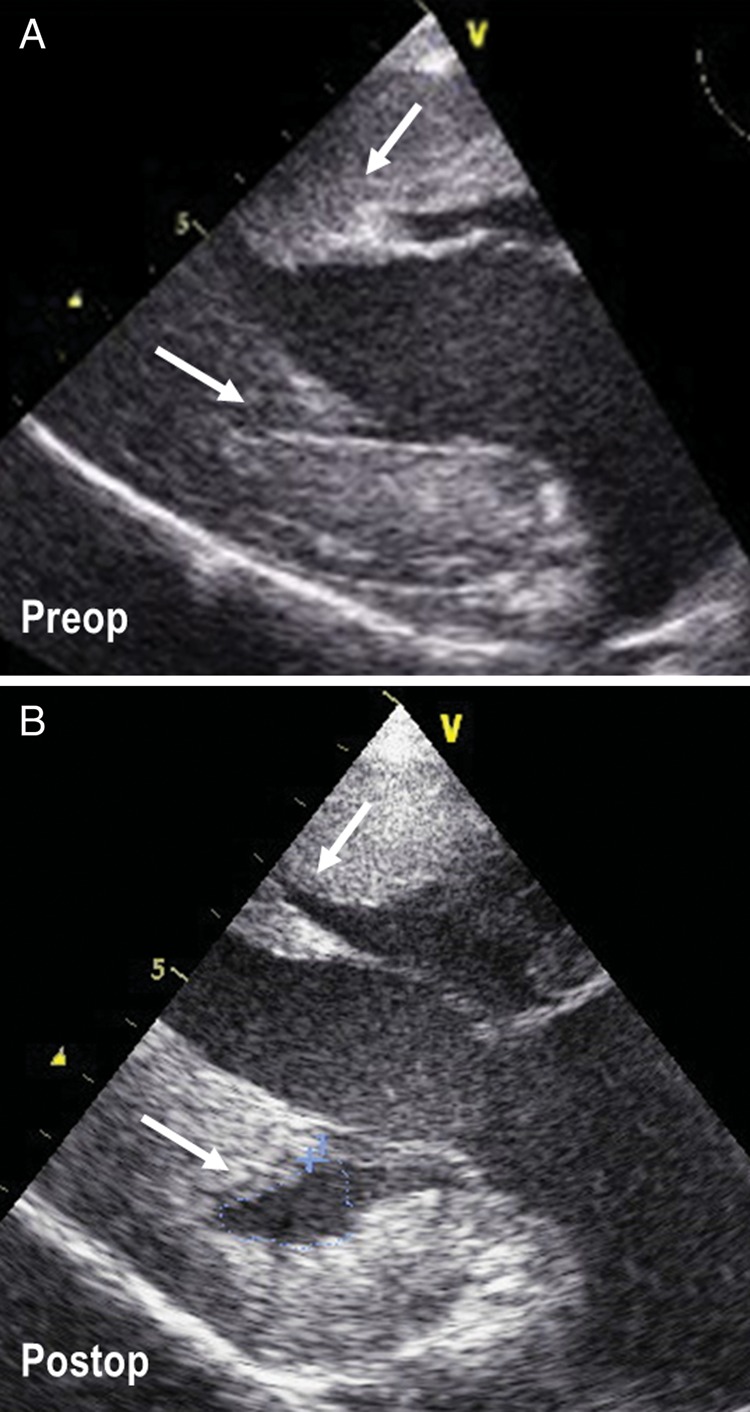
Intraoperative transoesophageal echocardiographic images before (A), and after (B), the excision of fibrous–muscular structures connecting the papillary muscles to the ventricular septum and left ventricular free wall. The area of excision of the fibrous–muscular structures is indicated by arrows.
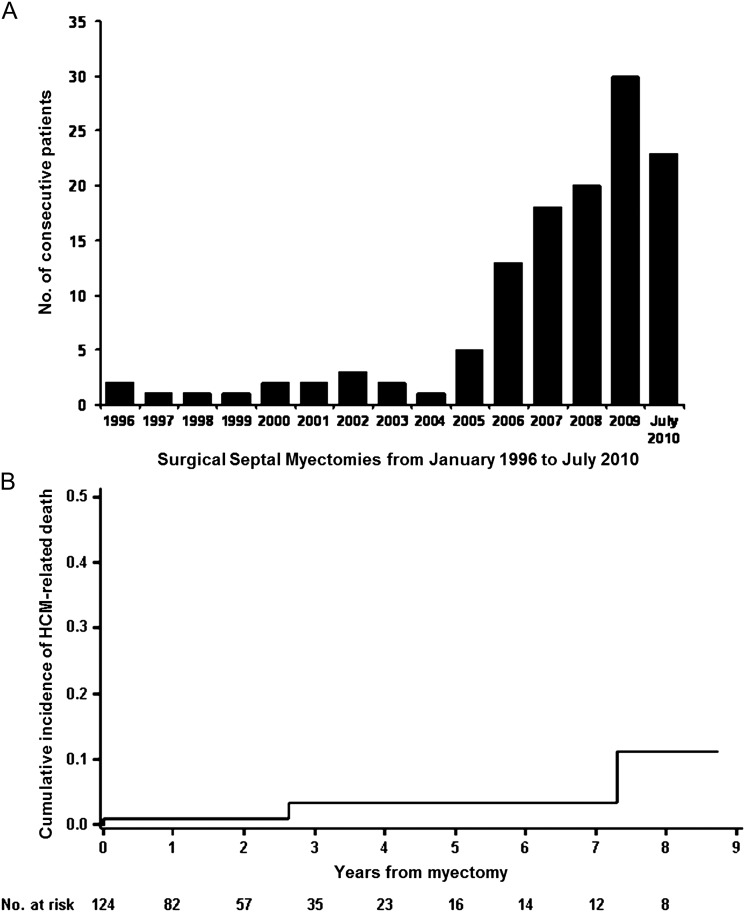
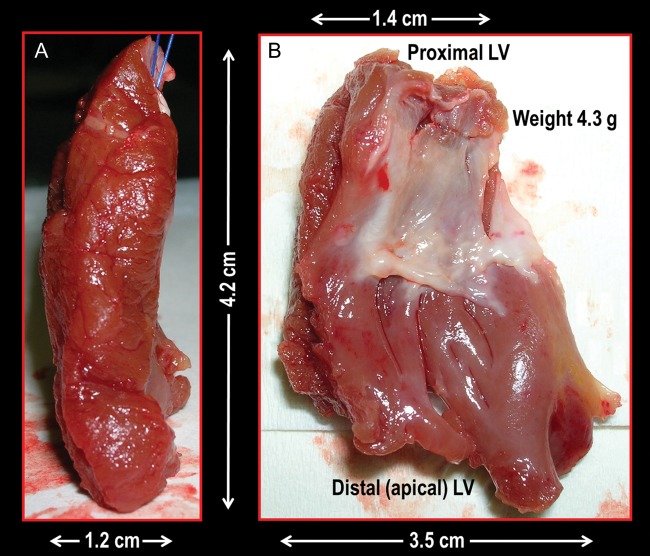
The number of septal myectomy operations was unequally distributed during the 15 years covered by the present investigation and increased exponentially in recent years, with 109 (88%) of the 124 procedures being performed during the last 5 years (Figure 2A). The mean weight of the excised myocardium at the time of surgery in the 124 study patients was 4.2 ± 1.9g. A specimen of the excised myocardium is shown in Figure 3.
Figure 2.
(A) Number of surgical septal myectomies performed between 1996 and 2010 at Dipartimento Cardiovascolare, Ospedali Riuniti, Bergamo, Italy. (B) Crude cumulative incidence of hypertrophic cardiomyopathy-related death in 124 patients who underwent surgical septal myectomy between 1996 and 2010. Both early (operative) and late deaths are included.
Figure 3.
Extended septal myectomy operation. Lateral (A) and antero-posterior (B) views of an excised cardiac muscle specimen (weight 4.3 g). The cardiac muscle is excised via two incisions in the basal septum from 2 to 3 mm below the aortic valve and extended distally to the base of the papillary muscles, creating a trapezoid trough which is wider towards the left ventricular apex than at the subaortic level.
Of the 124 study patients, 9 (7.3%) had received an implantable cardioverter defibrillator (ICD) before cardiac surgery. In one of these nine patients, the percutaneous ICD was upgraded to an epicardial cardioverter defibrillator at the time of surgery, because of massive LV hypertrophy (a maximal wall thickness of 51 mm). In one patient, an ICD was implanted during follow-up. Of the 10 (8.1%) study patients in whom a cardioverter defibrillator had been implanted either before myectomy operation or during follow-up, 1 received an appropriate intervention of the device (overdrive pacing) for ventricular tachycardia. No patients received inappropriate shocks.
Clinical course after myectomy operation
The median duration of follow-up in the 124 study patients was 20.3 months (IQR: 3.9–40.6 months). Of the 124 study patients, 1 patient (0.8%), a 78-year-old woman, died 14h after surgery as a consequence of a massive bleeding due to perforation of the free wall contiguous to the anterior septum.
A total of two HCM-related deaths occurred after hospital discharge. A 21-year-old patient with extreme LV wall thickness (a pre-operative maximal wall thickness of 36 mm) refused implantation of an ICD and died suddenly 31 months after myectomy. A 50-year-old patient died of an ischaemic cerebrovascular event 6 years after surgery. A total of two non-HCM-related deaths occurred during follow-up, one due to pneumonia in an 89-year-old patient, 7 years after surgery, and the second due to lung cancer in a 67-year-old patient, 2 years after surgery (Table 3). Including one operative death (procedural mortality 0.8%), a crude cumulative incidence of HCM-related death was 0.8, 3.3, and 11.2% at 1, 5, and 10 years, respectively (Figure 2B).
Table 3.
Major events after surgical myectomy in the 124 study patients
| Early (≤30 days after myectomy), n (%) | |
| Death | 1 (0.8) |
| Permanent pacemaker implantation | 4 (3.2) |
| Left bundle brunch block | 30 (24) |
| Late (>30 days after myectomy), n (%) | |
| Cardioverter-defibrillator implantation | 1 (0.8) |
| Permanent pacemaker implantation | 1 (0.8) |
| Mitral valve repair | 1 (0.8) |
| Cardiovascular death | 2 (1.6) |
| Sudden cardiac death | 1 (0.8) |
| Ischaemic cerebrovascular event | 1 (0.8) |
| Non-cardiovascular death | 2 (1.6) |
| Lung cancer | 1 (0.8) |
| Pneumonia | 1 (0.8) |
A clinical and echocardiographic follow-up evaluation was performed in a dedicated HCM clinic between January and September 2010. The LV outflow tract gradient before surgery and at most recent evaluation was available in 113 (91%) of the 124 study patients. The LV outflow gradient decreased from 95 ± 36 mmHg (range: 52–180 mm Hg) before surgery to 12 ± 6 mmHg at most recent evaluation (P < 0.001). None of these patients had a significant (≥30 mmHg) residual LV outflow gradient under basal conditions or at Valsalva manoeuvre at most recent evaluation. Of these 113 patients in whom resting LV outflow gradient measurements were available before surgery and at most recent evaluation, 9 had a residual gradient of ≥25 mmHg. Using colour and pulsed Doppler ultrasound, the site of the gradient was located at the mid-ventricular cavity, below the mitral valve leaflets, in each of these nine patients. At most recent evaluation, only one of these nine patients had significant heart failure symptoms (NYHA functional class III). The left atrial dimension before surgery and at most recent evaluation was available in 109 (88%) of the study patients and decreased significantly after surgery (P < 0.001). Data on NYHA functional class before surgery and at most recent evaluation were available in 116 (94%) of the study patients. Of the 97 patients in NYHA functional class III–IV before surgery, 93 (96%) were in functional class I–II at most recent evaluation (P < 0.001). Of the four patients with severe heart failure symptoms at most recent evaluation, one had shown clinical deterioration to NYHA class III–IV 18 months after surgery. In this patient, an echocardiogram identified severe mitral valve regurgitation and TEE documented perforation of the anterior mitral leaflet. At surgery, a 2 mm perforation of the anterior mitral leaflet was identified and repaired, and an anomalous attachment of the papillary muscle to a posteriorly retracted fibrous leaflet was excised. At most recent evaluation, the patient was in NYHA class II and the echocardiogram showed mild mitral valve regurgitation.
Of the 124 study patients, 27 (22%) had a pre-operative history of paroxysmal or persistent atrial fibrillation. None of the study patients were in permanent atrial fibrillation.
The patients with atrial fibrillation were older (60 years, IQR: 50–64) than patients without atrial fibrillation (51 years, IQR: 36–62) (P = 0.020) and had a significantly higher median left atrial dimension (59 mm, IQR: 56–61 vs. 46 mm, IQR: 43–50) (P < 0.001). At most recent evaluation, 25 (92%) of the 27 patients with preoperative history of paroxysmal or persistent atrial fibrillation were in sinus rhythm and 2 were in permanent atrial fibrillation. During the last 3 years, 9 of the 27 patients with a pre-operative history of atrial fibrillation had undergone surgical therapy for atrial fibrillation at the time of the myectomy operation. Each of these nine patients was in sinus rhythm at most recent evaluation.
Discussion
The ACC and ESC Expert Consensus Conference and the recent ACC and AHA guidelines on HCM have stated that surgical septal myectomy is the gold-standard and primary treatment option for patients with obstructive HCM and severe symptoms of heart failure and that alcohol septal ablation should be selected when the surgical risk is considered unacceptable because of serious co-morbidities or advanced age.1,2 However, after the introduction of percutaneous alcohol septal ablation,3 the surgical myectomy operation has almost disappeared in European countries, including some with a long and extensive experience with this operation.36–38 Therefore, many severely symptomatic European patients with obstructive HCM are denied the option of surgical septal myectomy, which is the primary treatment strategy recommended by the HCM international guidelines.1,2
Conversely, in recent years, the surgical septal myectomy operation has become the most frequently selected treatment at North American referral centres that offer both expert surgical myectomy and alcohol septal ablation as possible treatment options for patients with obstructive HCM.39–43 This preference for surgical treatment has probably been driven by the growing evidence of the favourable long-term results of myectomy, when performed by surgeons experienced with this procedure.35–37,39,40 Furthermore, morphological abnormalities of the mitral valve apparatus that limit the mobility of the papillary muscles and mitral valve leaflets are present in the majority of patients with obstructive HCM and can be treated only by surgery.23,26,27,35 These anomalies include fibrous or muscular structures connecting the papillary muscles to the ventricular septum or LV free wall, anomalous chordal structures or fibrous attachments of the mitral leaflets to the septum or free wall, or, less commonly, a direct insertion of the papillary muscle into the anterior mitral leaflet.21,23–29,35
We believe that European patients with HCM should be offered the option of surgical septal myectomy, as indicated by the HCM international guidelines. Therefore, we report here our results with developing a surgical referral programme for the myectomy operation. Our experience shows that it is possible to obtain favourable results for an elective procedure such as the myectomy operation, by relying initially on the expertise of a single surgeon and gradually establishing a dedicated surgical and cardiological HCM team through a close collaboration with cardiology centres with large experience in the clinical management of patients with HCM.
Survival was excellent in our consecutive 124 patients with HCM who underwent surgical myectomy since 1996, with a single hospital death and two HCM-related deaths during follow-up. At most recent evaluation, none of the surviving study patients had a significant residual LV outflow gradient, and the large majority had mild or no symptoms of heart failure (NYHA class I or II). In most patients, this important symptomatic improvement was already evident in the first days after myectomy. This rapid amelioration of the clinical condition after surgical myectomy is consistent with the experience of other centres with large myectomy programmes and can be explained by the underlying pathophysiology of the disease. Surgical myectomy in HCM relieves the high LV systolic pressure overload due to the LV outflow gradient, as well as the volume overload secondary to mitral valve regurgitation, in a ventricle with a high end-diastolic pressure and a small cavity but preserved ejection fraction. Therefore, in most HCM patients, LV haemodynamics and symptoms improve, often dramatically, within days after surgery.
The number of surgical septal myectomies performed at our centre has grown rapidly during the last 5 years. A similar increase in myectomy operations has also been reported in recent years by centres with a long tradition for HCM surgery in North America.35,39,40 This increasing request for surgical myectomy underlines the need for referral centres with myectomy programmes in European countries where such programmes are not available. About two to three referral centres for surgical myectomy would probably be sufficient for a country with ∼60 million people, because of the low absolute number of HCM candidates to surgery. At present, there are about five surgical referral centres for myectomy operation in the USA, a country of 300 million people.
The learning curve for surgical myectomy may be long because HCM patients with severe clinical expression of the disease are uncommon, and only a portion of such patients are surgical candidates. The specific experience required for this operation is also increased by the unusual surgical technique, which is based on an extensive cardiac muscle resection through an aortotomy, with limited visualization of the septum and the risk of causing a ventricular septal defect or damaging the aortic or mitral valve. During the last two decades, TEE guidance at the time of surgical myectomy, as well as modern cardiac preservation techniques, has contributed to make this operation easier than it was in the pioneer years of the early 1960s and have permitted to extend the septal muscle resection to the mid-ventricular level, allowing more complete reconstruction of the LV outflow tract and improving clinical results.34,44 Previous surgical experience with the treatment of congenital heart diseases associated with LV outflow tract obstruction, such as discrete subaortic membrane, subaortic fibromuscular tunnel, subaortic obstruction in complex cardiac anomalies, and post-correction of the atrio-ventricular canal, may also contribute importantly to reduce the learning curve for myectomy.
However, establishing a myectomy programme in a centre without previous experience with HCM surgery remains a complex undertaking and requires that a number of important pre-conditions are met. We believe that several components played a determinant role in our favourable results. The senior surgeon at our centre had acquired an initial experience with septal myectomy at another institution about two decades previously, and this experience was combined with an extensive expertise in reconstructive surgery for congenital heart disease and mitral valve repair. Moreover, our prolonged collaboration with cardiology centres with particular experience in the clinical management of patients with HCM allowed us to develop an integrated surgical and cardiological team for the evaluation and treatment of patients with obstructive HCM.
In conclusion, in Europe, a growing cardiological referral for septal myectomy in patients with obstructive HCM is confronted with an inadequate number of centres with experience with surgical myectomy. We believe that European patients with HCM should be granted the option of surgical septal myectomy, as indicated by the HCM international guidelines. Our results show that the initial establishment and subsequent gradual expansion of a myectomy programme at a centre without prior experience with this procedure is feasible and can lead to highly favourable clinical results, given that a number of critical preliminary conditions are met.
Funding
Funding to pay the Open Access publication charges for this article was provided by Fondazione Creberg.
Conflict of interest: none declared.
Acknowledgements
The authors are indebted to the Fondazione Creberg for covering the expenses to give free online access to this manuscript.
References
- 1.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, III, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 2.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346:211–214. doi: 10.1016/s0140-6736(95)91267-3. [DOI] [PubMed] [Google Scholar]
- 4.Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998;98:2415–2421. doi: 10.1161/01.cir.98.22.2415. [DOI] [PubMed] [Google Scholar]
- 5.Henein MY, O'Sullivan CA, Ramzy IS, Sigwart U, Gibson DG. Electromechanical left ventricular behavior after nonsurgical septal reduction in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 1999;34:1117–1122. doi: 10.1016/s0735-1097(99)00332-0. [DOI] [PubMed] [Google Scholar]
- 6.Gietzen FH, Leuner CJ, Obergassel L, Strunk-Mueller C, Kuhn H. Role of transcoronary ablation of septal hypertrophy in patients with hypertrophic cardiomyopathy, New York Heart Association functional class III or IV, and outflow obstruction only under provocable conditions. Circulation. 2002;106:454–459. doi: 10.1161/01.cir.0000022845.80802.9d. [DOI] [PubMed] [Google Scholar]
- 7.Firoozi S, Elliott PM, Sharma S, Murday A, Brecker SJ, Hamid MS, Sachdev B, Thaman R, McKenna WJ. Septal myotomy-myectomy and transcoronary septal alcohol ablation in hypertrophic obstructive cardiomyopathy. A comparison of clinical, haemodynamic and exercise outcomes. Eur Heart J. 2002;23:1617–1624. doi: 10.1053/euhj.2002.3285. [DOI] [PubMed] [Google Scholar]
- 8.van Dockum WG, Beek AM, ten Cate FJ, ten Berg JM, Bondarenko O, Götte MJ, Twisk JW, Hofman MB, Visser CA, van Rossum AC. Early onset and progression of left ventricular remodeling after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Circulation. 2005;111:2503–2508. doi: 10.1161/01.CIR.0000165084.28065.01. [DOI] [PubMed] [Google Scholar]
- 9.Roberts R, Sigwart U. Current concepts of the pathogenesis and treatment of hypertrophic cardiomyopathy. Circulation. 2005;112:293–296. doi: 10.1161/01.CIR.0000146788.30724.0A. [DOI] [PubMed] [Google Scholar]
- 10.Fifer MA, Sigwart U. Controversies in cardiovascular medicine. Hypertrophic obstructive cardiomyopathy: alcohol septal ablation. Eur Heart J. 2011;32:1059–1064. doi: 10.1093/eurheartj/ehr013. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Yacoub M, Dearani JA. Controversies in cardiovascular medicine. Benefits of surgery in obstructive hypertrophic cardiomyopathy: bring septal myectomy back for European patients. Eur Heart J. 2011;32:1055–1058. doi: 10.1093/eurheartj/ehr006. [DOI] [PubMed] [Google Scholar]
- 12.Spirito P, Maron BJ. Perspectives on the role of new treatment strategies in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 1999;33:1071–1075. doi: 10.1016/s0735-1097(98)00673-1. [DOI] [PubMed] [Google Scholar]
- 13.van Dockum WG, ten Cate FJ, ten Berg JM, Beek AM, Twisk JW, Vos J, Hofman MB, Visser CA, van Rossum AC. Myocardial infarction after percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: evaluation by contrast-enhanced magnetic resonance imaging. J Am Coll Cardiol. 2004;43:27–34. doi: 10.1016/j.jacc.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Boltwood CM, Jr, Chien W, Ports T. Ventricular tachycardia complicating alcohol septal ablation. N Engl J Med. 2004;351:1914–1915. doi: 10.1056/NEJM200410283511824. [DOI] [PubMed] [Google Scholar]
- 15.van der Lee C, ten Cate FJ, Geleijnse ML, Kofflard MJ, Pedone C, van Herwerden LA, Biagini E, Vletter WB, Serruys PW. Percutaneous versus surgical treatment for patients with hypertrophic obstructive cardiomyopathy and enlarged anterior mitral valve leaflets. Circulation. 2005;112:482–488. doi: 10.1161/CIRCULATIONAHA.104.508309. [DOI] [PubMed] [Google Scholar]
- 16.Simon RD, Crawford FA, 3rd, Spencer WH, 3rd, Gold MR. Sustained ventricular tachycardia following alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Pacing Clin Electrophysiol. 2005;28:1354–1356. doi: 10.1111/j.1540-8159.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 17.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA, 3rd, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. J Am Med Assoc. 2007;298:405–412. doi: 10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 18.Cuoco FA, Spencer WH, 3rd, Fernandes VL, Nielsen CD, Nagueh S, Sturdivant JL, Leman RB, Wharton JM, Gold MR. Implantable cardioverter-defibrillator therapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:1718–1723. doi: 10.1016/j.jacc.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 19.Noseworthy PA, Rosenberg MA, Fifer MA, Palacios IF, Lowry PA, Ruskin JN, Sanborn DM, Picard MH, Vlahakes GJ, Mela T, Das S. Ventricular arrhythmia following alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:128–132. doi: 10.1016/j.amjcard.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 20.ten Cate FJ, Soliman OI, Michels M, Theuns DA, de Jong PL, Geleijnse ML, Serruys PW. Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution. Circ Heart Fail. 2010;3:362–369. doi: 10.1161/CIRCHEARTFAILURE.109.862359. [DOI] [PubMed] [Google Scholar]
- 21.Klues HG, Roberts WC, Maron BJ. Anomalous insertion of papillary muscle directly into anterior mitral leaflet in hypertrophic cardiomyopathy. Significance in producing left ventricular outflow obstruction. Circulation. 1991;84:1188–1197. doi: 10.1161/01.cir.84.3.1188. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh CL, Maron BJ, Cannon RO, 3rd, Klues HG. Initial results of combined anterior mitral leaflet plication and ventricular septal myotomy-myectomy for relief of left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Circulation. 1992;86:II-60–II-67. [PubMed] [Google Scholar]
- 23.Schoendube FA, Klues HG, Reith S, Flachskampf FA, Hanrath P, Messmer BJ. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. Circulation. 1995;92:II122–II127. doi: 10.1161/01.cir.92.9.122. [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, Nishimura RA, Danielson GK. Pitfalls in clinical recognition and a novel operative approach for hypertrophic cardiomyopathy with severe outflow obstruction due to anomalous papillary muscle. Circulation. 1998;98:2505–2508. doi: 10.1161/01.cir.98.23.2505. [DOI] [PubMed] [Google Scholar]
- 25.van der Lee C, Kofflard MJ, van Herwerden LA, Vletter WB, ten Cate FJ. Sustained improvement after combined anterior mitral leaflet extension and myectomy in hypertrophic obstructive cardiomyopathy. Circulation. 2003;108:2088–2092. doi: 10.1161/01.CIR.0000092912.57140.14. [DOI] [PubMed] [Google Scholar]
- 26.Minakata K, Dearani JA, Nishimura RA, Maron BJ, Danielson GK. Extended septal myectomy for hypertrophic obstructive cardiomyopathy with anomalous mitral papillary muscles or chordae. J Thorac Cardiovasc Surg. 2004;127:481–489. doi: 10.1016/j.jtcvs.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: septal myectomy for obstructive hypertrophic cardiomyopathy—the Mayo Clinic experience. Nat Clin Pract Cardiovasc Med. 2007;4:503–512. doi: 10.1038/ncpcardio0965. [DOI] [PubMed] [Google Scholar]
- 28.Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124:40–47. doi: 10.1161/CIRCULATIONAHA.110.985812. [DOI] [PubMed] [Google Scholar]
- 29.Woo A, Jedrzkiewicz S. The mitral valve in hypertrophic cardiomyopathy: it's a long story. Circulation. 2011;124:9–12. doi: 10.1161/CIRCULATIONAHA.111.035568. [DOI] [PubMed] [Google Scholar]
- 30.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 31.Spirito P, Autore C, Rapezzi C, Bernabò P, Badagliacca R, Maron MS, Bongioanni S, Coccolo F, Estes NA, Barillà CS, Biagini E, Quarta G, Conte MR, Bruzzi P, Maron BJ. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 32.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 33.Autore C, Bernabò P, Barillà CS, Bruzzi P, Spirito P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J Am Coll Cardiol. 2005;45:1076–1080. doi: 10.1016/j.jacc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 34.Grigg LE, Wigle ED, Williams WG, Daniel LB, Rakowski H. Transesophageal Doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and importance in intraoperative decision making. J Am Coll Cardiol. 1992;20:42–52. doi: 10.1016/0735-1097(92)90135-a. [DOI] [PubMed] [Google Scholar]
- 35.Dearani JA, Danielson GK. Septal myectomy for hypertrophic cardiomyopathy. Oper Tech Thorac Cardiovasc Surg. 2004;9:278–292. doi: 10.1016/j.jtcvs.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 36.Schulte HD, Bircks WH, Loesse B, Godehardt EA, Schwartzkopff B. Prognosis of patients with hypertrophic obstructive cardiomyopathy after transaortic myectomy. Late results up to twenty-five years. J Thorac Cardiovasc Surg. 1993;106:709–717. [PubMed] [Google Scholar]
- 37.Schulte HD, Borisov K, Gams E, Gramsch-Zabel H, Lösse B, Schwartzkopff B. Management of symptomatic hypertrophic obstructive cardiomyopathy—long-term results after surgical therapy. Thorac Cardiovasc Surg. 1999;47:213–218. doi: 10.1055/s-2007-1013146. [DOI] [PubMed] [Google Scholar]
- 38.Franke A, Schöndube FA, Kühl HP, Klues HG, Erena C, Messmer BJ, Flachskampf FA, Hanrath P. Quantitative assessment of the operative results after extended myectomy and surgical reconstruction of the subvalvular mitral apparatus in hypertrophic obstructive cardiomyopathy using dynamic three-dimensional transesophageal echocardiography. J Am Coll Cardiol. 1998;31:1641–1649. doi: 10.1016/s0735-1097(98)00133-8. [DOI] [PubMed] [Google Scholar]
- 39.Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090. [DOI] [PubMed] [Google Scholar]
- 40.Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph-Edwards A, Rakowski H. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. doi: 10.1161/01.CIR.0000162460.36735.71. [DOI] [PubMed] [Google Scholar]
- 41.Yacoub MH. Surgical versus alcohol septal ablation for hypertrophic obstructive cardiomyopathy: the pendulum swings. Circulation. 2005;112:450–452. doi: 10.1161/CIRCULATIONAHA.105.553313. [DOI] [PubMed] [Google Scholar]
- 42.Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation. 2007;116:196–206. doi: 10.1161/CIRCULATIONAHA.107.691378. [DOI] [PubMed] [Google Scholar]
- 43.Maron BJ, Ommen SR, Nishimura RA, Dearani JA. Myths about surgical myectomy: rumors of its death have been greatly exaggerated. Am J Cardiol. 2008;101:887–889. doi: 10.1016/j.amjcard.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 44.Ommen SR, Park SH, Click RL, Freeman WK, Schaff HV, Tajik AJ. Impact of intraoperative transesophageal echocardiography in the surgical management of hypertrophic cardiomyopathy. Am J Cardiol. 2002;90:1022–1024. doi: 10.1016/s0002-9149(02)02694-2. [DOI] [PubMed] [Google Scholar]