Abstract
Focal excess sensory stimulation evokes reorganization of a sensory system. It is usually an expansion of the neural representation of that stimulus resulting from the shifts of the tuning curves (receptive fields) of neurons toward those of the stimulated neurons. The auditory cortex of the mustached bat has an area that is highly specialized for the processing of target-distance information carried by echo delays. In this area, however, reorganization is due to shifts of the delay-tuning curves of neurons away from those of the stimulated cortical neurons. Elimination of inhibition in the target-distance processing area in the auditory cortex by a drug reverses the direction of the shifts in neural tuning curves. Therefore, such unique reorganization in the time domain is due to strong lateral inhibition in the highly specialized area of the auditory cortex.
Keywords: bicuculline, centrifugal shift, centripetal shift, delay tuning, plasticity
Electric stimulation of cortical auditory neurons evokes both facilitation and inhibition of the auditory responses of cortical and subcortical neurons. The amount of facilitation and inhibition varies with the frequency of a tone burst to which the neurons respond and with the relationship in frequency tuning between the stimulated and recorded neurons. When the best frequency (BF) of a recorded neuron matches that of a stimulated cortical neuron, the response of the matched neuron to its BF is often augmented, and the responses at frequencies lower and/or higher than the BF are inhibited. When unmatched, the response of the unmatched neuron is inhibited at its BF and is facilitated at non-BFs. As a result, frequency tuning of the unmatched neuron is shifted. The shift of a BF, together with a frequency-tuning curve, is called a BF shift. Such corticofugal modulation, called egocentric selection (1), improves the input to the stimulated cortical neurons and the subcortical and cortical representations of the stimulus parameters to which the stimulated cortical neurons are tuned (2-8)
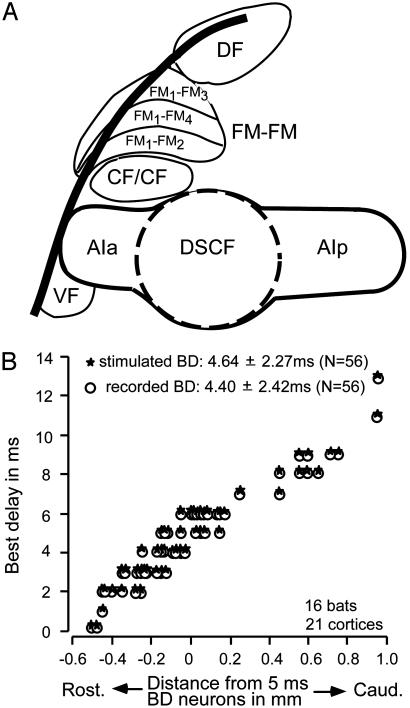
There are two types of BF shifts: centripetal and centrifugal. Centripetal BF shifts are the shifts toward the BF of electrically stimulated cortical neurons, and centrifugal BF shifts are the shifts away from the stimulated cortical BF. These two types of BF shifts occur in a specific spatial pattern along the frequency axis in the auditory cortex (AC), medial geniculate body, and inferior colliculus. The spatial pattern is basically the same in the AC and the inferior colliculus of the big brown bat (3, 5, 6, 9) and the mustached bat (1, 2, 10). In the ACs of the big brown bat (5, 6) and Mongolian gerbil (8), centripetal BF shifts occur in a large area surrounding the matched neurons, and small centrifugal BF shifts occur in the narrow zone surrounding this large centripetal area; i.e., center-surround reorganization occurs. Because centrifugal BF shifts are small, the major reorganization in these ACs is due to centripetal BF shifts. In the Doppler-shifted constant frequency (DSCF) area of the AC of the mustached bat (Fig. 1 A), however, centrifugal BF shifts occur in a large area surrounding the matched neurons (10), as in the inferior colliculus and medial geniculate body (2).
Fig. 1.
Cortical auditory areas of the mustached bat (A) and the distribution of (BD shifts) in the FM-FM area (B). A shows seven of the cortical auditory areas electrophysiologically identified. Neurons in the CF/CF area are combination-sensitive. The FM-FM area consists of three subdivisions (FM1-FM2, FM1-FM3, and FM1-FM4). AIa and AIp, anterior and posterior divisions of the primary AC; DF, dorsal fringe area; VF, ventral fringe area. Electrode penetrations for recording and electric stimulation were made in all three subdivisions of the FM-FM area. (B) BD shifts of FM-FM neurons are plotted as a function of distance from neurons tuned to a 5-ms delay. ○, BD shifts of recorded neurons. ★, BD shifts of electrically stimulated neurons. A recorded cortical neuron was also electrically stimulated after the examination of the effect of electric stimulation of other cortical neurons on its responses to paired FM sounds.
Centripetal BF shifts result in an increase in the number of neurons responding to the frequency equal to the BF of electrically stimulated cortical neurons (3, 5, 6). That is, they result in “expanded” reorganization as found in different sensory cortices of various species of animals (11-16). On the other hand, centrifugal BF shifts result in a reduced representation, which is associated with the augmentation of responses and sharpening of tuning curves of matched neurons (2). Such reorganization is named “compressed” (instead of “reduced”) reorganization (17). Compressed reorganization increases contrast in the neural representation of an auditory signal.
Expanded and compressed reorganizations in the time domain both have been found in the inferior colliculus of bats. In the big brown bat, an array of neurons tuned to certain durations of sounds has been found in the AC and the inferior colliculus (18-20). Electric stimulation of cortical duration-tuned neurons evokes centripetal best duration shifts of collicular duration-tuned neurons (21). In the mustached bat, an array of neurons tuned to certain echo delays (i.e., the time interval between two sounds) has been found in the FM-FM area of the AC (22-26) (Fig. 1 A) and the subcortical auditory nuclei (24, 27-29). Electric stimulation of cortical delay-tuned neurons evokes centrifugal best delay (BD) shifts of thalamic and collicular delay-tuned neurons (29). It is not yet known whether it also evokes centrifugal BD shifts of cortical delay-tuned neurons.
As described above, electric stimulation of cortical DSCF neurons of the mustached bat evokes the centrifugal BF shifts of collicular and cortical DSCF neurons. However, an application of bicuculline (an antagonist of inhibitory γ-aminobutyric acid type A receptors) to the stimulation site evokes centripetal BF shifts. In other words, compressed reorganization changes into expanded reorganization when cortical inhibition is removed. Electric stimulation of the posterior division of the AC of the mustached bat (Fig. 1A) evokes centripetal BF shifts of cortical neurons nearby. Bicuculline applied to the stimulation site augments these centripetal BF shifts (10). It is not yet known whether removal of cortical inhibition also changes centrifugal BD shifts into centripetal shifts.
The aims of our present article are to report our findings that electric stimulation of cortical delay-tuned neurons in the FM-FM area evokes centrifugal BD shifts of nearby cortical delay-tuned neurons and that centrifugal BD shifts in the FM-FM area are changed into centripetal shifts by blocking inhibition within this area. Our current paper provides experimental evidences supporting a principle that, in highly specialized auditory cortical areas, inhibition is strong and evokes centrifugal shifts of neural tuning regardless of the frequency or time domain.
Materials and Methods
General. Surgery, acoustic and electric stimulation, and recording of neural activity were the same as those described by Yan and Suga (1). Drug applications to a cortical area were the same as those described by Xiao and Suga (10). The Animal Studies Committee of Washington University approved the protocol for the present research.
Sixteen adult mustached bats (Pteronotus parnellii rubiginosus) from Trinidad were used. Under neuroleptanalgesia (Innovar at 4.08 mg/kg of body weight), a 1.5-cm-long metal post was glued on the dorsal surface of the bat's skull. A local anesthetic (Lidocaine HCl) and antibiotic ointment (Furacin) were applied to the surgical wound. Three to 4 d after surgery, the awake animal was placed in a polyethylene-foam body-mold, which was hung with an elastic band at the center of a 31°C soundproof room. The metal post glued on the skull was attached to a metal rod with set screws to immobilize the animal's head, and the animal's head was adjusted directly toward the loudspeakers located 74 cm away. A few holes (50-100 μm in diameter) were made in the skull covering the FM-FM area of the AC. Two pairs of tungsten-wire electrodes (≈7-μm tip diameter, 20-35 μm apart, one proximal to the other) were each inserted through two different holes ≈500-700 μm into the FM-FM area. The responses (action potentials) of FM-FM neurons to pairs of FM sounds were recorded with each electrode pair, and the BD shifts to excite the neurons were measured. Next, one electrode pair was used to electrically stimulate the neurons, and the other was used to examine the effect of electric stimulation on the neural response. After the recovery from the effect of electric stimulation, bicuculline methiodide [BMI (an antagonist of inhibitory γ-aminobutyric acid type A receptors)] was applied to the stimulation site, and its effect on the neural response at the other electrode pair was examined.
Acoustic Stimulation. The mustached bat emits orientation sounds (biosonar pulses or, simply, pulses). Each pulse consists of four harmonics, and each harmonic consists of a constant frequency (CF) and FM components. Therefore, each pulse contains eight components (CF1-4 and FM1-4). FM-FM neurons are tuned to a combination of the FM1 of the pulse stimulus and FMn (n = 2, 3, or 4) of an echo stimulus with a specific time delay from the pulse. Different types of FM-FM neurons (FM1-FM2,FM1-FM3, and FM1-FM4) are separately clustered in the FM-FM area (Fig. 1 A) (23, 26, 30, 31). Therefore, the acoustic stimuli delivered to the animal were FM1-FMn pairs. Each FM sound was 3-ms long with a 0.5-ms rise-delay time.
To generate a FM sound, the frequency of a voltage-controlled oscillator (model 134, Wavetek, San Diego, CA) was modulated with a linear voltage ramp generator to mimic the FM components in the species-specific biosonar pulse. The FM and the amplitude of each FM sound and the time interval (echo delay) between paired FM sounds were first manually varied to identify the best combination of two FM sounds (FM1-FMn) and the best echo delay to excite a given neuron. Then the FM1 sound was fixed in frequency sweep and amplitude to evoke the largest facilitation. The FMn sound was fixed in frequency sweep and in amplitude at 10 dB above the minimum threshold, i.e., the threshold of the response at the best FMn. Then FMn (echo) delay was varied with a computer and hardware (Tucker-Davis Technologies, Alachua, FL) to obtain delay-response curves. The computer-controlled delay scan consisted of 22 150-ms time-blocks: no acoustic stimulus, pulse only, echo only, and 19 pulse-echo pairs in which echo delay varied from 0 to 18 ms in 1-ms steps. An identical delay scan was delivered 50 times for the studies of neural responses.
Electric Stimulation of Cortical FM-FM Neurons. Electric stimulation was a monophasic electric pulse (0.2 ms, 100 nA) delivered at a rate of five per sec for 7 min with a constant-current stimulator (modified model A360, WPI Instruments, Waltham, MA). Such electric pulses delivered at a low rate evoke the changes in thalamic and collicular FM-FM neurons (1) and thalamic and collicular DSCF neurons (2, 10) but do not evoke any noticeable change in cochlear microphonic responses (32). The electric pulses were estimated to activate neurons within a 60-μm radius around the electrode tip (1). The distance between a recorded and an electrically stimulated neuron ranged between 100 and 1,400 μm, so it was very unlikely that recorded neurons were directly influenced by the stimulus current.
Blocking Inhibition in the AC with BMI. A glass micropipette (≈10-μm tip diameter) filled with 5.0 mM BMI in saline was placed at the site where the BD of electrically stimulated cortical FM-FM neurons was measured. Then 1.0 nl of 5.0 mM BMI was applied to that site with a Picospritzer II (General Valve, Fairfield, NJ). The Picospritzer was set at 0.67 bar (1 bar = 100 kPa) and 30 ms per BMI application. The diffusion of BMI from the application site would be very limited, because the amount of a BD shift evoked by BMI varied with the difference in BD between recorded and BMI-applied neurons, as that evoked by electric stimulation.
Data Acquisition. Action potentials of a single cortical FM-FM neuron tuned to paired FM1-FMn sounds were selected with time/amplitude/window discriminator software (Tucker-Davis Technologies). At the beginning of data acquisition, the wave-form of an action potential was stored and displayed on the monitor. This action potential (i.e., template) was compared with other action potentials obtained during data acquisition. The response of the single cortical neuron to a delay scan delivered 50 times was recorded before and after electric stimulation or BMI application and was displayed as an array of peristimulus time histograms or cumulative peristimulus time histograms. The data were stored in the computer hard drive and were used for off-line analysis. In a 1-d experiment, one or two neurons were studied for the effect of and recovery from electric stimulation or a BMI application.
Off-Line Data Processing. The magnitude of auditory responses of a neuron was expressed by a number of impulses per 50 identical stimuli and was plotted as the function of echo delays. The BD shift evoked by cortical electric stimulation or BMI was considered significant if it shifted back (i.e., recovered) to the BD in the control condition and if the auditory responses changed by the electric stimulation or BMI recovered >90%.
Results
In 16 bats, the delay-response curves of 56 cortical FM-FM neurons were studied before and after the electric stimulation of or BMI application to nearby cortical FM-FM neurons at 56 sites. The combination sensitivity of the recorded and stimulated neurons was FM1-FM2 (36 neurons), FM1-FM3 (43 neurons), or FM1-FM4 (33 neurons). All of the neurons studied were tuned to specific echo delays. Their BD shifts ranged between 0 and 13 ms (mean and SD was 4.5 ± 2.4 ms, n = 112). Neurons located rostral were tuned to short delays, whereas those located caudal were tuned to long delays. The FM-FM area has an echo-delay axis representing target-distance information along the rostro-caudal axis of the brain (Fig. 1B), as reported (25, 26).
Of the 56 neurons studied, 48 had a BD different by at least 1 ms from that of stimulated or BMI-applied neurons. They were “unmatched” neurons in BD. However, the remaining eight neurons had a BD that was the same as that of stimulated or BMI-applied neurons. They were “matched” neurons. Of the 48 unmatched neurons, 35 showed centrifugal BD shifts and 7 showed centripetal BD shifts for electric stimulation. The remaining 6 neurons had a BD that differed >6 ms from that of stimulated neurons and did not show a BD shift for electric stimulation but did show a centripetal BD shift for BMI. In the following text, the responses and delay-response curves of the 35 neurons and the changes evoked by cortical electric stimulation or BMI are first described, then those of the other neurons are described.
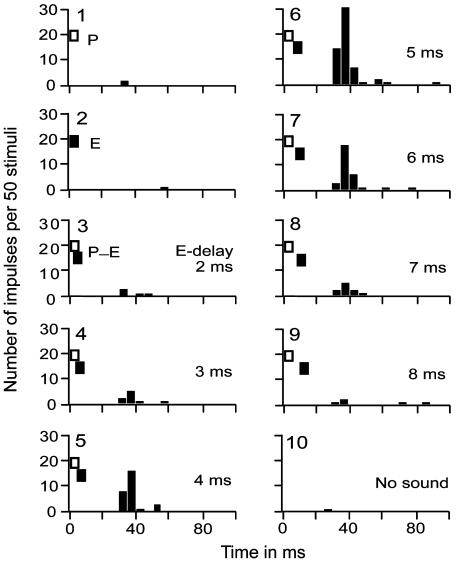
Fig. 2 shows the responses of a FM1-FM2 neuron to single or paired FM sounds. The essential components of the pulse-echo pairs for evoking the facilitatory responses of this neuron were pulse FM1 [28.5- to 22.5-kHz sweep, 40-dB sound pressure level (SPL)] and echo FM2 (58.0- to 46.0-kHz sweep, 30-dB SPL). The neuron did not respond to a FM1 pulse stimulus or FM2 echo stimulus alone, but responded to FM1-FM2 (pulse-echo) pairs with echo delays between 2 and 8 ms. The BD to evoke the largest response of the neuron was 5 ms (graph 6 in Fig. 2).
Fig. 2.
Peristimulus time histograms displaying the responses of a FM1-FM2 neuron to pulse FM1 alone (P), echo FM2 alone (E), P-E pairs with different echo delays between 2 ms and 8 ms, and no sound stimulation. The BD for the facilitatory response of the neuron was 5 ms. An identical series of stimuli was repeated 50 times, and the histograms were plotted with a computer.
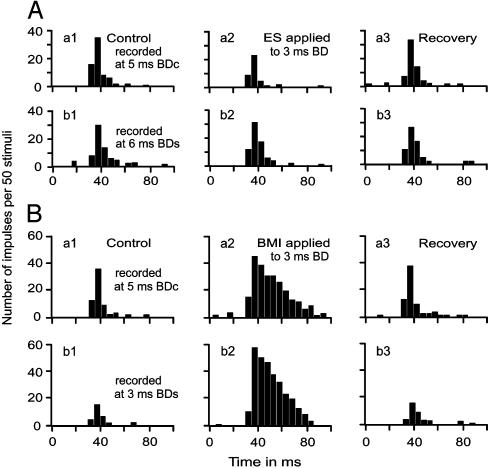
Electric stimulation or BMI applied to cortical neurons evoked delay-dependent changes in facilitatory response. Fig. 3 shows the changes in the responses of a FM1-FM2 neuron tuned to a 5-ms delay. Electric stimulation of FM1-FM3 neurons tuned to a 3-ms delay reduced the response at a 5-ms delay (Fig. 3Aa2) and increased the response at a 6-ms delay (Fig. 3Ab2). Because of such delay-dependent inhibition and facilitation, the BD of this neuron centrifugally shifted from 5 ms to 6 ms (Fig. 4A1). When BMI was applied to the neurons tuned to a 3-ms delay, the FM1-FM2 neuron tuned to a 5-ms delay showed the facilitation of the responses to all FM1-FM2 stimuli regardless of FM2 delays from FM1. However, the amount of facilitation was larger at the 3-ms delay (Fig. 3Bb2) than at the 5-ms delay (Fig. 3Ba2). Because of such delay-dependent facilitation, the BD of the neuron centripetally shifted from 5 ms to 3 ms (Fig. 4A1). The centrifugal and centripetal BD shifts of this FM1-FM2 neuron are shown by the delay-response curves in Fig. 4A1.
Fig. 3.
Effects of electric stimulation (A) and BMI (B) applied to FM1-FM3 neurons tuned to a 3-ms delay on the responses of a FM1-FM2 neuron tuned to a 5-ms delay. (a1-a3) Responses at a BD (5 ms) of the FM1-FM2 neuron in the control affected by either electric stimulation (Aa) or BMI (Ba); recovery conditions are also shown. (b1-b3) Responses at the shifted BD (6 ms in A and 3msin B) of the neuron in the control, affected, and recovery conditions. BDc, BD in the control conditions; BDs, BD in the shifted conditions.
Fig. 4.
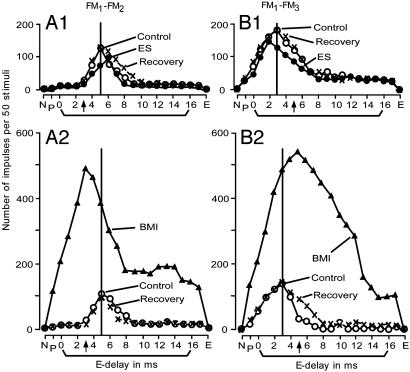
Delay-response curves of a FM1-FM2 (A) and a FM1-FM3 (B) neuron affected by electric stimulation or BMI applied to the FM1-FM3 (A)orFM1-FM2 (B) neuron. In A, electric stimulation (A1) or BMI (A2) was applied to the FM1-FM3 neurons tuned to a 3-ms delay (arrows on the abscissae). In B, electric stimulation (B1) or BMI (B2) was applied to the FM1-FM2 tuned to a 5-ms delay (arrows on the abscissae). Electric stimulation and BMI respectively evoked centrifugal and centripetal shifts of the BD of the recorded neurons.
Fig. 4B shows the delay-response curves of a FM1-FM3 neuron tuned to a 3-ms delay (open circles). When the FM1-FM2 neuron tuned to a 5-ms delay was electrically stimulated, the BD of the FM1-FM3 neuron centrifugally shifted from 3 ms to 2 ms (Fig. 4B1). For a centrifugal BD shift, the response at the shifted BD was 24% (Fig. 4A1) or 19% (Fig. 4B1) smaller than that at the control BD. In the 35 neurons, which showed centrifugal BD shifts for electric stimulation, their responses at the shifted BD shifts were 14.8 ± 11.3% smaller than those at the control BD shifts on average. When BMI was applied to cortical neurons instead of electric stimulation, the auditory responses of the recorded neuron became very large, and the centrifugal BD shift changed to a centripetal BD shift. The absolute amount of a BD shift for BMI was always larger than that for electric stimulation (Fig. 4 A2 and B2), as is further documented in Fig. 5.
Fig. 5.
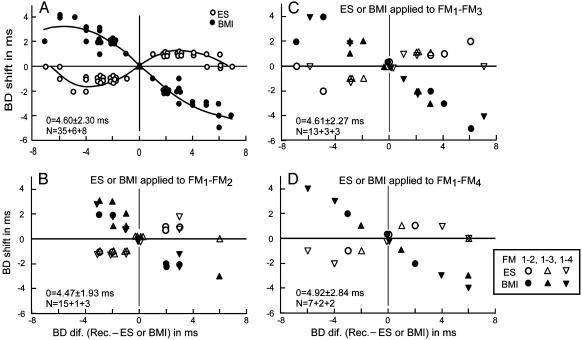
Distributions of BD shifts as a function of BD difference between recorded and electrically stimulated or BMI-applied FM-FM neurons. (A) All of the data obtained from 35 unmatched neurons showing centrifugal BD shifts for electric stimulation are plotted, together with six unmatched and eight matched neurons showing no BD shifts for electric stimulation. (B-D) The data shown in A are divided into three groups according to the type of electrically stimulated or BMI-applied FM-FM neurons: FM1-FM2 (B), FM1-FM3 (C), and FM1-FM4 (D). The mean BD and SD of stimulated or BMI-applied neurons are indicated in each figure. N, number of neurons. The open and filled symbols respectively indicate BD shifts evoked by electric stimulation or BMI.
All of the 35 centrifugal BD shifts changed to centripetal BD shifts when BMI was applied to the site where the electrically stimulated neurons were located. The amount of BD shifts varied as the function of differences in BD between recorded and stimulated or BMI applied FM-FM neurons (Fig. 5A). The maximum centrifugal BD shift was 2.0 ms for electric stimulation, and the maximum centripetal BD shift was 5.0 ms for BMI. BD shifts were largest at 2- to 5-ms BD differences for electric stimulation and at a ≈6-ms BD difference for BMI. BD shifts were larger and occurred in a wider range for BMI than for electric stimulation.
The data obtained from the six neurons that did not show a BD shift for electric stimulation but showed a centripetal shift for BMI are also plotted in Fig. 5. Their BD shifts were different from those of stimulated neurons by 5-7 ms. The absence of a BD shift for electric stimulation was probably due to these large BD differences. Because they showed as large a BD shift for BMI as the other 35 neurons that showed centrifugal BF shifts for electric stimulation, the BD shifts of the six neurons for electric stimulation were perhaps too small to be detected.
Because the FM-FM area consists of three subdivisions and each subdivision has an echo-delay axis (Fig. 1 A) (25, 26, 33), an important question is whether electric stimulation or BMI application to one of the three subdivisions evokes identical BD shifts in all three subdivisions. The data shown in Fig. 5A, which represent BD shifts evoked by electric stimulation or BMI application, were divided into three groups: FM1-FM2, FM1-FM3, or FM1-FM4 neurons. Electric stimulation or BMI applied to FM1-FM2 neurons evoked the BF shifts of all three types of FM-FM neurons. The amount of the BD shifts was similar to these three types of FM-FM neurons (Fig. 5B), as was the case for electric stimulation or BMI applied to FM1-FM3 (Fig. 5C) or FM1-FM4 neurons (Fig. 5D). The amount of BD shifts apparently depended on BD differences between recorded and stimulated or BMI-applied FM-FM neurons, not the types of recorded and stimulated or BMI-applied FM-FM neurons. These data indicate that BD shifts occurred equally along an iso-BD line across the three subdivisions of the FM-FM area.
All of the eight matched neurons showed no BD shifts for electric stimulation and BMI. Their BD shifts ranged between 3 and 7 ms (4.8 ± 1.5 ms, n = 8). The data points obtained from them are shown at the intersection of no BD difference and no BD shift lines in Fig. 5. For FM1-FM2 stimulation, the three recorded neurons were FM1-FM3 or FM1-FM4 (Fig. 5B). For FM1-FM3 stimulation, the three recorded neurons were FM1-FM2, FM1-FM3, or FM1-FM4 (Fig. 5C). For FM1-FM4 stimulation, the two recorded neurons were FM1-FM2 or FM1-FM3 (Fig. 5D). Therefore, matched neurons showed no BD shifts regardless of combinations of types of stimulated and recorded neurons.
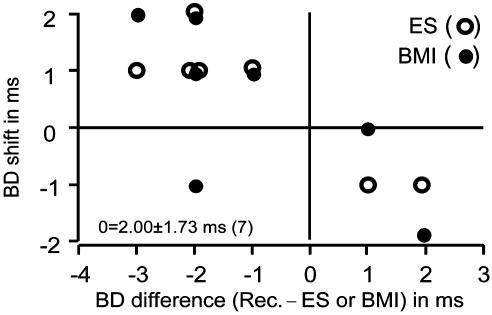
The seven FM-FM neurons that showed a centripetal BD shift for electric stimulation were located in the rostral portion of the FM-FM area. They were either FM1-FM2, FM1-FM3, or FM1-FM4. Their BD shifts were short, ranging between 0 and 4 ms (2.0 ± 1.7 ms, n = 7). The BD shifts of the electrically stimulated neurons were also short, ranging from 0 to 2 ms (1.0 ± 1.0 ms, n = 7). The absolute values of BD differences between recorded and stimulated neurons ranged between 1 and 3 ms (1.86 ± 0.69 ms, n = 7). The effect of BMI was different between them (Fig. 6): no change in BD shift (three neurons), increased BD shift (two neurons), decreased BD shift (one neuron), and centrifugal BD shift (one neuron).
Fig. 6.
The BD shifts of seven unmatched neurons that showed centripetal BD shifts for electric stimulation are plotted as a function of BD differences between recorded and stimulated (○) or BMI-applied (•) FM-FM neurons. The mean and SD of stimulated or BMI-applied neurons were 2.0 ± 1.73 ms.
Discussion
Corticofugal Modulation and Shifts in Tuning Curve or Receptive Field. Corticofugal positive feedback on subcortical matched neurons and negative feedback (lateral inhibition) on unmatched neurons have been found in the auditory (1, 2), visual (34), and somatosensory (35, 36) systems. Furthermore, it has been found that the modulation of neurons in the dorsal column nuclei and ventro-posterolateral nucleus of the somatosensory system by neurons in the motor cortex consists of the excitatory effect on neurons with a receptive field topographically corresponding (i.e., matched) to the joint controlled by neurons in the motor cortex and the inhibitory effect on these with the receptive fields topographically unmatched to that joint (37). Corticofugal positive feedback associated with lateral inhibition is apparently shared by the different sensory systems of different species of animals. However, it has not yet been demonstrated that the corticofugal feedback evokes the systematic shifts of receptive fields in the visual and somatosensory systems. This lack of data does not necessarily mean that the auditory system is different from other sensory systems in reorganization based on corticofugal modulation, because it has been demonstrated that thalamic plasticity in the somatosensory system is at least partially evoked by corticofugal feedback. Our current study demonstrates that focal cortical electric stimulation evokes cortical changes in the same way as subcortical changes.
Compressed Reorganization and Inhibition. In both the DSCF (10) and FM-FM areas (Figs. 4 and 5) of the AC of the mustached bat, the shift in frequency- or delay-tuning curve is centrifugal for focal cortical electric stimulation, but it is centripetal for BMI. The research on these two cortical areas indicates that the cortical areas highly specialized for processing specific biosonar information in the frequency or time domain are reorganized by centrifugal shifts in tuning curve for compressed reorganization, that elimination of inhibition from these specialized areas changes centrifugal shifts into centripetal shifts of the tuning curves, and that lateral inhibition is stronger in the specialized areas than nonspecialized (or less specialized) areas. We do not yet know whether these specialized areas contain more inhibitory neurons and/or more developed dendrites of inhibitory neurons than do nonspecialized cortical areas. The terms “specialized” and “nonspecialized” are somewhat arbitrary. However, the DSCF area is unique, because it contains extremely sharply frequency-tuned neurons and has the frequency vs. amplitude coordinates. It is undoubtedly specialized for processing frequency- and amplitude-modulated echoes from flying insects with spatial and temporal patterns of neural activity (38). The FM-FM area is also unique, because it consists of neurons tuned to specific echo delays, has an echo-delay axis, and is undoubtedly specialized for processing target-distance information (23, 25, 26, 39).
Compressed reorganization has been found only in the highly specialized auditory subsystems (the DSCF and FM-FM areas and the subcortical areas directly related to those cortical areas) of the mustached bat (1, 2, 10). In animals other than the mustached bat, highly specialized auditory areas have not yet been identified. Therefore, there is no known area to be examined for an additional example of compressed reorganization. In the human brain, the areas highly specialized for processing speech have been identified (40, 41). It would be an interesting project to examine how such areas are reorganized for excess stimulation with a particular speech sound. It would also be interesting to examine how the middle temporal area specialized for processing visual motion in monkeys is reorganized (42).
Suga et al. (43) hypothesized that highly focused positive feedback and strong widespread lateral inhibition evoke compressed reorganization and that facilitation of auditory responses due to positive feedback evokes centripetal shifts of tuning curves. The first hypothesis is supported by our present and previous studies (10); however, the second hypothesis remains to be tested by studies of the DSCF and FM-FM areas. If the second hypothesis is correct, one may expect that centripetal BD shifts would be evoked by electric stimulation when a BD difference between stimulated and recorded neurons is very small, say, <2 ms. Among 33 unmatched neurons showing a 1- to 3-ms BD difference, 26 showed centrifugal BF shifts for electric stimulation (Fig. 5A), and the remaining 7 showed centripetal BD shifts (Fig. 6). The data obtained thus far only partially support this hypothesis.
Reorganization of the Subdivisions of the FM-FM Area. The three subdivisions (FM1-FM2, FM1-FM3, and FM1-FM4) of the FM-FM area are distinctively different from each other in combination sensitivity but have the same delay axis. Iso-BD lines cross the three subdivisions without interruptions (25, 26, 33). As shown in our present article, electric stimulation of one of the three subdivisions evokes not only the reorganization of that stimulated subdivision but also the reorganization of the other two subdivisions. Such unified reorganization makes sense, because these three subdivisions are respectively specialized for the processing of the delays of the second, third, and fourth harmonic FM components of an echo from the first harmonic FM component of an emitted pulse (22, 23, 25, 26, 31, 44) and because the second, third, and fourth harmonics of an echo always return to the bat at the same time from a target. The reorganization of the delay map, therefore, should occur in all three subdivisions at the same time and to a same extent, as found in our present research.
In the AC of the mustached bat, there are three separate areas where delay-tuned neurons are located: FM-FM, DF, and VF areas (Fig. 1 A). The BD axis (i.e., target-range axis) goes up to ≈18 ms (310 cm) in the FM-FM area (25), ≈8 ms (140 cm) in the DF area (45), and ≈4 ms (70 cm) in the VF area (46). All of these areas consist of three types of FM-FM neurons: FM1-FM2, FM1-FM3, and FM1-FM4, and are mutually connected (47). It remains to be studied whether electric stimulation of the FM-FM area evokes the reorganization of the DF and VF areas.
Acknowledgments
We thank Drs. J. H. Casseday and J. J. Wenstrup for comments on this article. This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-00175.
Abbreviations: AC, auditory cortex; BF, best frequency; BD, best delay; BMI, bicuculline methiodide; CF, constant frequency; DSCF, Doppler-shifted CF; DF dorsal fringe area; VF, ventral fringe area.
References
- 1.Yan, J. & Suga, N. (1996) Science 273, 1100-1103. [DOI] [PubMed] [Google Scholar]
- 2.Zhang, Y., Suga, N. & Yan, J. (1997) Nature 387, 900-903. [DOI] [PubMed] [Google Scholar]
- 3.Yan, W. & Suga, N. (1998) Nat. Neurosci. 1, 54-58. [DOI] [PubMed] [Google Scholar]
- 4.Zhang, Y. & Suga, N. (2000) J. Neurophysiol. 84, 325-333. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury, S. A. & Suga, N. (2000) J. Neurophysiol. 83, 1856-1863. [DOI] [PubMed] [Google Scholar]
- 6.Ma, X. & Suga, N. (2001) J. Neurophysiol. 85, 1078-1087. [DOI] [PubMed] [Google Scholar]
- 7.Sakai, M. & Suga, N. (2001) Proc. Natl. Acad. Sci. USA 98, 3507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai, M. & Suga, N. (2002) Proc. Natl. Acad. Sci. USA 99, 7108-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, E. & Suga, N. (1998) Proc. Natl. Acad. Sci. USA 95, 12663-12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao, Z. & Suga, N. (2002) Proc. Natl. Acad. Sci. USA 99, 15743-15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godde, B., Leonhardt, R., Cords, S. M. & Dinse, H. R. (2002) Proc. Natl. Acad. Sci. USA 99, 6352-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allard, T., Clark, S. A., Jenkins, W. M. & Merzenich, M. M. (1991) J. Neurophysiol. 66, 1048-1058. [DOI] [PubMed] [Google Scholar]
- 13.Qi, H. X., Stepniewska, I. & Kaas, J. H. (2000) J. Neurophysiol. 84, 2133-2147. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong, J. D., de Belle, J. S., Wang, Z. & Kaiser, K. (1998) Learn. Mem. 5, 102-114. [PMC free article] [PubMed] [Google Scholar]
- 15.Florence, S. L., Taub, H. B. & Kaas, J. H. (1998) Science 282, 1117-1121. [DOI] [PubMed] [Google Scholar]
- 16.Irvine, D. R., Rajan, R. & McDermott, H. J. (2000) Hear. Res. 147, 188-199. [DOI] [PubMed] [Google Scholar]
- 17.Suga, N., Xiao, Z., Ma, X. & Ji, W. (2002) Neuron 36, 9-18. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich, D., Casseday, J. H. & Covey, E. (1997) J. Neurophysiol. 77, 2360-2372. [DOI] [PubMed] [Google Scholar]
- 19.Casseday, J. H., Ehrlich, D. & Covey, E. (1994) Science 264, 847-850. [DOI] [PubMed] [Google Scholar]
- 20.Wu, M. I. & Jen, P. H. (1996) J. Comp. Physiol. A 179, 385-393. [DOI] [PubMed] [Google Scholar]
- 21.Ma, X. & Suga, N. (2001) Proc. Natl. Acad. Sci. USA 98, 14060-14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suga, N., O'Neill, W. E. & Manabe, T. (1978) Science 200, 778-781. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill, W. E. & Suga, N. (1979) Science 203, 69-73. [DOI] [PubMed] [Google Scholar]
- 24.Wenstrup, J. J. (1999) J. Neurophysiol. 82, 2528-2544. [DOI] [PubMed] [Google Scholar]
- 25.Suga, N. & O'Neill, W. E. (1979) Science 206, 351-353. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill, W. E. & Suga, N. (1982) J. Neurosci. 2, 17-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen, J. F. & Suga, N. (1991) J. Neurophysiol. 65, 1275-1296. [DOI] [PubMed] [Google Scholar]
- 28.Portfors, C. V. & Wenstrup, J. J. (2001) Hear. Res. 151, 95-105. [DOI] [PubMed] [Google Scholar]
- 29.Yan, J. & Suga, N. (1996) Hear. Res. 93, 102-110. [DOI] [PubMed] [Google Scholar]
- 30.Suga, N., O'Neill, W. E. & Manabe, T. (1979) Science 203, 270-274. [DOI] [PubMed] [Google Scholar]
- 31.Suga, N., O'Neill, W. E., Kujirai, K. & Manabe, T. (1983) J. Neurophysiol. 49, 1573-1626. [DOI] [PubMed] [Google Scholar]
- 32.Xiao, Z. & Suga, N. (2002) Nat. Neurosci. 5, 57-63. [DOI] [PubMed] [Google Scholar]
- 33.Misawa, H. & Suga, N. (2001) Hear. Res. 151, 15-29. [DOI] [PubMed] [Google Scholar]
- 34.Tsumoto, T. (1978) Brain Res. 159, 85-97. [DOI] [PubMed] [Google Scholar]
- 35.Malmierca, E. & Nunez, A. (1998) Brain Res. 810, 172-180. [DOI] [PubMed] [Google Scholar]
- 36.Canedo, A. & Aguilar, J. (2000) Eur. J. Neurosci. 12, 2515-2533. [DOI] [PubMed] [Google Scholar]
- 37.Palmeri, A., Bellomo, M., Giuffrida, R. & Sapienza, S. (1999) Neuroscience 88, 135-150. [DOI] [PubMed] [Google Scholar]
- 38.Suga, N. & Manabe, T. (1982) J. Neurophysiol. 47, 225-255. [DOI] [PubMed] [Google Scholar]
- 39.Riquimaroux, H., Gaioni, S. J. & Suga, N. (1991) Science 251, 565-568. [DOI] [PubMed] [Google Scholar]
- 40.Geschwind, N. (1972) Sci. Am. 226, 76-83. [DOI] [PubMed] [Google Scholar]
- 41.Scott, S. K. & Johnsrude, I. S. (2003) Trends Neurosci. 26, 100-107. [DOI] [PubMed] [Google Scholar]
- 42.Albright, T. D. (1984) J. Neurophysiol. 52, 1106-1130. [DOI] [PubMed] [Google Scholar]
- 43.Suga, N., Gao, E., Zhang, Y., Ma, X. & Olsen, J. F. (2000) Proc. Natl. Acad. Sci. USA 97, 11807-11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawasaki, M., Margoliash, D. & Suga, N. (1988) J. Neurophysiol. 59, 623-635. [DOI] [PubMed] [Google Scholar]
- 45.Suga, N. & Horikawa, J. (1986) J. Neurophysiol. 55, 776-805. [DOI] [PubMed] [Google Scholar]
- 46.Edamatsu, H., Kawasaki, M. & Suga, N. (1989) J. Neurophysiol. 61, 202-207. [DOI] [PubMed] [Google Scholar]
- 47.Fitzpatrick, D. C., Olsen, J. F. & Suga, N. (1998) J. Comp. Neurol. 391, 366-396. [PubMed] [Google Scholar]