Abstract
Aims
Renin–angiotensin–aldosterone system (RAAS) inhibitors are well established for the reduction in cardiovascular morbidity, but their impact on all-cause mortality in hypertensive patients is uncertain. Our objective was to analyse the effects of RAAS inhibitors as a class of drugs, as well as of angiotensin-converting enzyme (ACE) inhibitors and AT1 receptor blockers (ARBs) separately, on all-cause mortality.
Methods and results
We performed a pooled analysis of 20 cardiovascular morbidity–mortality trials. In each trial at least two-thirds of the patients had to be diagnosed with hypertension, according to the trial-specific definition, and randomized to treatment with an RAAS inhibitor or control treatment. The cohort included 158 998 patients (71 401 RAAS inhibitor; 87 597 control). The incidence of all-cause death was 20.9 and 23.3 per 1000 patient-years in patients randomized to RAAS inhibition and controls, respectively. Overall, RAAS inhibition was associated with a 5% reduction in all-cause mortality (HR: 0.95, 95% CI: 0.91–1.00, P= 0.032), and a 7% reduction in cardiovascular mortality (HR: 0.93, 95% CI: 0.88–0.99, P= 0.018). The observed treatment effect resulted entirely from the class of ACE inhibitors, which were associated with a significant 10% reduction in all-cause mortality (HR: 0.90, 95% CI: 0.84–0.97, P= 0.004), whereas no mortality reduction could be demonstrated with ARB treatment (HR: 0.99, 95% CI: 0.94–1.04, P= 0.683). This difference in treatment effect between ACE inhibitors and ARBs on all-cause mortality was statistically significant (P-value for heterogeneity 0.036).
Conclusion
In patients with hypertension, treatment with an ACE inhibitor results in a significant further reduction in all-cause mortality. Because of the high prevalence of hypertension, the widespread use of ACE inhibitors may result in an important gain in lives saved.
Keywords: Hypertension, ACE inhibitor, ARB, Meta-analysis, Mortality
See page 1996 for the editorial comment on this article (doi:10.1093/eurheartj/ehs108)
Introduction
The World Health Organization describes hypertension as the number one risk factor for mortality, as worldwide annually 7.5 million deaths (13% of all deaths) are attributable to high blood pressure (BP)-related diseases, particularly cardiovascular diseases (CVD).1 For that reason, the guidelines of hypertension and cardiology societies emphasize that hypertension treatment should aim at reducing the long-term risk of (cardiovascular) morbidity and mortality.2,3 Hypertension is often referred to as the ‘silent killer’, as its presence is usually symptomless. Therefore, compliance to antihypertensive medication is a challenge for most patients, especially as adequate BP control often requires the use of multiple agents, causing additional side effects and as a result inferior adherence.2 Thus, there is a continuing need for potent medications, preferably with beneficial effects on mortality, to improve patients’ adherence to the treatment prescribed.
The benefits of antihypertensive treatment on cardiovascular morbidity are thought to be mainly due to the BP-lowering effect per se, independent of the class of drug employed, as has been demonstrated with β-blockers, diuretics, calcium channel blockers, and recently with the renin–angiotensin–aldosterone system (RAAS) inhibitors.2 Blockade of the RAAS is one of the key therapeutic targets in patients with hypertension, as an overactive RAAS is strongly associated with high BP. The RAAS controls circulating volume and electrolyte balance in the human body and is therefore an important regulator of haemodynamic stability.4 RAAS inhibitors are the most widely prescribed class of drugs for the management of hypertension. Currently, the most clinically relevant pharmacological agents that block the RAAS are angiotensin-converting enzyme (ACE) inhibitors and AT1 receptor blockers (ARBs). Both drugs block angiotensin II, but ACE inhibitors are characterized by a decrease in the degradation of bradykinin leading to a release of nitric oxide and prostaglandins resulting in additional vasodilatation. These differences in modes of action between ACE inhibitors and ARBs might have clinical implications for patients with hypertension.5
Reductions in both cardiovascular morbidity and mortality have been well demonstrated with RAAS inhibitors across specific populations that were selected and included for a criterion other than hypertension per se. For example, SOLVD (enalapril in heart failure), HOPE (ramipril in patients with high CVD risk), and EUROPA (perindopril in stable coronary disease) demonstrated significant reductions in the composite endpoint of death from cardiovascular causes, myocardial infarction or stroke with ACE inhibitors. In these trials, less than half of the patients enrolled had prevalent hypertension.6–8 The beneficial effects of RAAS inhibitors on (all-cause) mortality (a guideline-recommended goal of antihypertensive therapy)2 have not been convincingly demonstrated in the indication of hypertension. Furthermore, most (antihypertensive) trials in which the clinical effects of RAAS inhibitors were evaluated were underpowered for this endpoint.9–11 To evaluate the impact of RAAS inhibitors on all-cause and cardiovascular mortality for their main indication, hypertension, we undertook a meta-analysis of all prospective randomized clinical trials that compared RAAS inhibitors with control therapy in different populations in which the absolute majority of the patients had hypertension, and where the expected benefits would mainly come from a decrease in BP.
We hypothesized that, taken all evidence together, RAAS inhibitors would produce a significant mortality reduction compared with (contemporary) control therapy. Although the primary aim of this meta-analysis decided a priori was to evaluate RAAS inhibitors as a class of drugs, we realized that ACE inhibitors and ARBs have partly different modes of action. Therefore, we decided to also study these two classes of drugs separately.
We argued that, if a significant effect on both all-cause and cardiovascular mortality could be demonstrated, then treating physicians would have an additional argument to motivate hypertensive patients to comply with long-term treatment with these agents.
Methods
Study selection
We intended to include all publicly available morbidity–mortality prospective randomized controlled trials that compared active treatment with an ACE inhibitor or an ARB with control treatment (placebo, active control, or usual care).
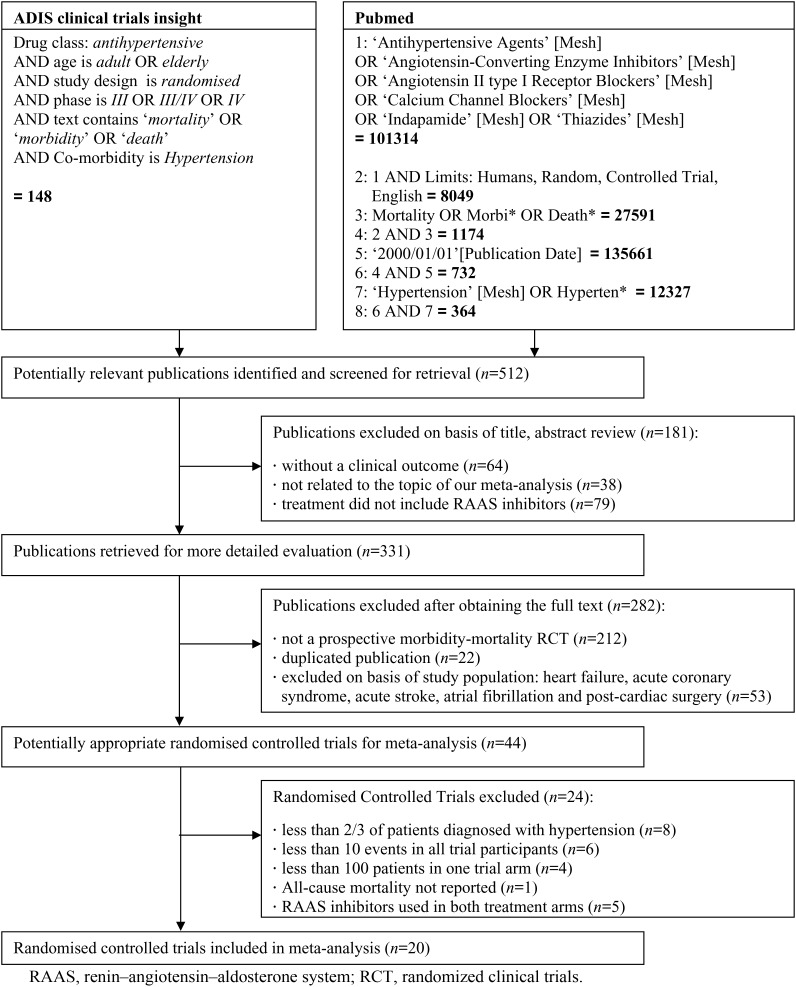
Trials were identified by a systematic search of OVID MEDLINE and (ADIS) ISI Web of Science using a broad range of key words, including ‘antihypertensive agents’, ‘angiotensin-converting enzyme inhibitors’, ‘angiotensin II Type 1 receptor blockers’, ‘hypertension’, and ‘mortality’, published in English between 1 January 2000 and 1 March 2011. We decided to start our search in the year 2000, because of our intention to evaluate the effect of RAAS inhibition on top of contemporary treatment and considered the HOPE trial to be a landmark study in this respect (published in the year 2000).7 References of identified papers and abstract listings of annual meetings of the American Heart Association, the American College of Cardiology, European Society of Cardiology, the American Society of Hypertension, the European Society of Hypertension, and the Council for High Blood Pressure Research were also examined during the same period. Each trial identified in this search was critically and independently evaluated by two investigators (L.v.V. and K.M.A.) for patient population, study treatment, protocol, and endpoints.
A total of 512 publications met the above-mentioned search criteria (Figure 1). We selected trials including different hypertensive populations for whom the benefits of RAAS inhibition would be expected to be mainly due to BP reduction. We only included the principal study publication, and excluded post hoc and subgroup analyses. Furthermore, we excluded trials in which patients were selected because of a specific disease, such as heart failure, acute coronary syndromes, acute stroke, haemodialysis, atrial fibrillation, or post-cardiac surgery patients, because of the expected benefits of RAAS inhibition beyond BP lowering in these patient populations.12,13
Figure 1.
Flow diagram of trial search and selection process. RAAS, renin–angiotensin–aldosterone system; RCT, randomized clinical trials.
Forty-four randomized controlled trials using RAAS blockade were identified that corresponded with the inclusion criteria. We additionally excluded eight trials in which less than two-thirds (66.7%) of the studied population were diagnosed with hypertension, according to the trial-specific definition. Ten trials were excluded due to either a low number of participants (n< 100) or a low incidence of all-cause death (n< 10), the primary endpoint of this study. Moreover, one trial was excluded because all-cause mortality was not reported. Finally, five trials (including INVEST, ACCOMPLISH, and ONTARGET) were excluded because RAAS inhibitors were used simultaneously in both trial arms.14–16 Thus, a total of 20 trials were included in our analysis (Figure 1), which had a follow-up duration of at least 1 year.
Data extraction
This analysis is based on data that were obtained from the papers reporting trials’ main results. Two authors (L.v.V., K.M.A.) independently extracted data from these reports, and resolved differences by consensus. For each treatment arm, we recorded the number of trial participants, the number of patients who reached the endpoint of all-cause and cardiovascular mortality, the mean age at baseline, the mean diastolic blood pressure and systolic blood pressure (SBP) at baseline, the percentage of male participants, the percentage of patients with diabetes mellitus, renal insufficiency, and hypertension, as well as the total follow-up time (until death) in years.
Endpoint definition
The endpoints of this pooled analysis were all-cause and cardiovascular mortality during long-term follow-up. Data on all-cause death were available for all trials. Data on cardiovascular death were not available for RENAAL, IDNT, MOSES, and CASE-J.
We aimed to provide estimates of the incidence of these endpoints in patients randomized to RAAS inhibitors and control therapy, as well as estimates of the absolute and relative reduction in the incidence of the endpoints by RAAS inhibitors. Since the duration of follow-up varied between the trials, we decided to base our analyses on the mortality incidence rate (IR), which was assumed to be constant over time in each of the comparison groups. The IR is defined as the number of patients who reached the endpoint in the comparison group divided by the patient-years of follow-up in the corresponding group (i.e. the sum of the follow-up times for each individual). The latter figure is equal to the number of patients multiplied by their mean follow-up duration.
To obtain the trial- and treatment-arm specific mean follow-up duration, the following five-step approach was applied. Firstly, we observed whether the mean follow-up time per treatment arm was stated in the paper. If this was not available, we then derived it from the reported death rate by dividing the total number of deaths by the annual death rate. If these data were not available, then the mean follow-up time was estimated from incidences that were derived from Kaplan–Meier curves, in combination with the number of patients that were reported to be at risk at several follow-up points. Finally, if we were not able to compute the mean follow-up duration for each treatment arm separately, we used the mean follow-up time that was reported for all trial participants together.
Statistical analysis
For each individual trial, the treatment-arm specific all-cause and cardiovascular mortality IR was determined. We evaluated the assumption that the mortality rate is constant over time by visually inspecting the Kaplan–Meier curves of the studies in this meta-analysis, comparing different time windows within each Kaplan–Meier curve. We did not find any major deviation from this assumption. Furthermore, we realized that the follow-up time within each of the trials is relatively short (the overall mean follow-up duration is 4.3 years). Thus, on average, during the course of the trial, patients became only 4 years older. In view of this fact, it seems reasonable to assume that the IRs were constant over time.
Information on follow-up times is needed to obtain estimates of absolute risks (and absolute treatment effects). However, because of the assumptions that we used, our IR estimates might be somewhat inaccurate. Therefore, we based our estimates of relative treatment effects on the hazard ratios (HRs) and confidence intervals (CIs) or standard errors that were reported for each trial. Actually, HRs were available for all trials, except for RENAAL, SCOPE, and pilot HYVET. For these trials, we calculated HRs based on the IRs in the separate treatment arms.
Because of the large variety in active (and control) treatments, we used a random-effects model to compute an overall pooled HR, even in case statistical tests for heterogeneity across trials were non-significant. Statistical heterogeneity was tested by Cochran's Q statistic,17 and a P-value <0.10 (two sided) was considered to indicate heterogeneity among trials. The degree of heterogeneity was presented as an I² value. Publication bias was assessed by visually examining funnel plot asymmetry and quantified by using an Egger regression test to calculate two-tailed P-values.18
We hypothesized that the mortality reduction by antihypertensive drugs might be influenced by age, gender, baseline SBP, BP reduction during follow-up, and follow-up time. To evaluate this hypothesis, we conducted linear regression analyses, based on trial-level data (so-called ‘meta-regression’). The trial-specific mean age, percentage of men, mean SBP, mean difference in BP reduction after 1 year of follow-up between RAAS inhibitors and control therapy, and mean follow-up time were considered as explanatory variables of the natural logarithm of the trial-specific hazard ratio (lnHR) for all-cause mortality. In this analysis, trial-level observations were weighed according to the inverse of the squared standard error of lnHR, thus taking into account the amount of ‘statistical information’ that is produced by each trial. Secondly, by including follow-up time in this analysis we were able to assess whether the mortality incidence ratio is constant over time.
Although we hypothesized that, taken all evidence together, RAAS inhibitors as a class of drugs would produce a homogenous treatment effect in terms of a mortality reduction compared with (contemporary) control therapy, we also performed stratified analyses according to the class of drug (ACE inhibitor vs. ARBs), as we realized that ACE inhibitors and ARBs have partly different modes of action. We also performed stratified analyses according to type of control (placebo vs. active treatment), and percentage of patients with diabetes mellitus or renal insufficiency at baseline (>50% vs. <50%). Pooled HRs for all-cause mortality were determined using a random effects model for each stratum, and differences between strata were studied.
All statistical tests were two-sided, and a P-value <0.05 was considered significant. We used SAS 9.2 for Windows for data analysis.
Results
Trial characteristics
A total of 20 trials fulfilled all selection criteria for this meta-analysis, and their main characteristics are presented in Table 1.9–11,19–35 In total 158 998 patients were randomized to RAAS inhibitor therapy (n= 71 401; 299 982 patient-years of follow-up) or control treatment (n= 87 597; 377 023 patient-years of follow-up). ACE inhibitors were used as the active treatment in seven trials (n= 76 615); two of these studies were placebo controlled.23,24,26,30,31,33,34 Thirteen trials, of which five were placebo-controlled, allocated participants to an ARB as the active treatment (n= 82 383).9–11,19–22,25,27–29,32,35
Table 1.
Baseline characteristics of study population in 20 trials (n= 158 998
| Trial acronym | Year | n | Active drug | Control | Mean follow-up, years | Hypertension, % | Mean SBP, mmHg | Mean age (years) | Men, % | IR in control group |
|---|---|---|---|---|---|---|---|---|---|---|
| RENAAL9 | 2001 | 1513 | Losartan | Placebo | 3.09 | 96.5 | 153 | 60.0 | 63.2 | 66.0 |
| IDNT28 | 2001 | 1715 | Irbesartan | Amlodipine or placebo | 2.86 | 100 | 159 | 58.9 | 66.5 | 54.0 |
| LIFE25 | 2002 | 9193 | Losartan with and without HCTZ | Atenolol with and without HCTZ | 4.82 | 100 | 174 | 66.9 | 46.0 | 19.5 |
| ALLHAT30 | 2002 | 33 357 | Lisinopril | Chlorthalidone or amlodipine | 5.01 | 100 | 146 | 66.9 | 53.3 | 28.5 |
| ANBP-233 | 2003 | 6083 | ACE inhibitor (enalapril) | Diuretic (HCTZ) | 4.06 | 100 | 168 | 71.9 | 49.0 | 17.1 |
| SCOPE29 | 2003 | 4937 | Candesartan | Placebo | 3.74 | 100 | 166 | 76.4 | 35.5 | 29.0 |
| pilot HYVET24 | 2003 | 1283 | Lisinopril | Diuretic or no treatment | 1.12 | 100 | 182 | 83.8 | 36.6 | 55.4 |
| JMIC-B34 | 2004 | 1650 | ACE inhibitor | Nifedipine | 2.25 | 100 | 146 | 64.5 | 68.8 | 6.2 |
| VALUE27 | 2004 | 15 245 | Valsartan | Amlodipine | 4.32 | 100 | 155 | 67.3 | 57.6 | 24.8 |
| MOSES32 | 2005 | 1352 | Eprosartan | Nitrendipine | 2.50 | 100 | 152 | 68.1 | 54.2 | 31.0 |
| ASCOT-BPLA26 | 2005 | 19 257 | Amlodipine with and without perindopril | Atenolol with and without bendroflumethiazide | 5.50 | 100 | 164 | 63.0 | 76.6 | 15.5 |
| JIKEI HEART11 | 2007 | 3081 | Valsartan | Non-ARB | 2.81 | 87.6 | 139 | 65.0 | 66.3 | 6.2 |
| ADVANCE31 | 2007 | 11 140 | Perindopril with indapamide | Placebo | 4.30 | 68.7 | 145 | 66.0 | 57.5 | 19.8 |
| HYVET23 | 2008 | 3845 | Indapamide with and without perindopril | Placebo | 2.11 | 89.9 | 173 | 83.6 | 39.5 | 59.3 |
| PRoFESS22 | 2008 | 20 332 | Telmisartan | Placebo | 2.50 | 74.0 | 144 | 66.2 | 64.0 | 29.1 |
| TRANSCEND35 | 2008 | 5926 | Telmisartan | Placebo | 4.67 | 76.4 | 141 | 66.9 | 57.0 | 25.2 |
| CASE-J20 | 2008 | 4703 | Candesartan | Amlodipine | 3.30 | 100 | 163 | 63.8 | 55.2 | 11.1 |
| HIJ-CREATE19 | 2009 | 2049 | Candesartan | Non-ARB | 4.03 | 100 | 135 | 64.8 | 80.2 | 14.3 |
| KYOTO HEART21 | 2009 | 3031 | Valsartan | Non-ARB | 2.92 | 100 | 157 | 66.0 | 57.0 | 7.2 |
| NAVIGATOR10 | 2010 | 9306 | Valsartan | Placebo | 6.10 | 77.5 | 140 | 63.8 | 49.4 | 11.5 |
HCTZ, hydrochlorothiazide; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; SBP, systolic blood pressure; IR, incidence rate per 1000 patient-years.
Patient characteristics
On average, 91% of the trial participants were hypertensive according to the definition used in each trial. The mean baseline SBP was 153 mmHg (range of the means across trials 135–182), the mean age was 67 years (range of the means across trials 59–84) and 58% of participants were man (range of this percentage across trials 36–80; Table 1).
All-cause mortality
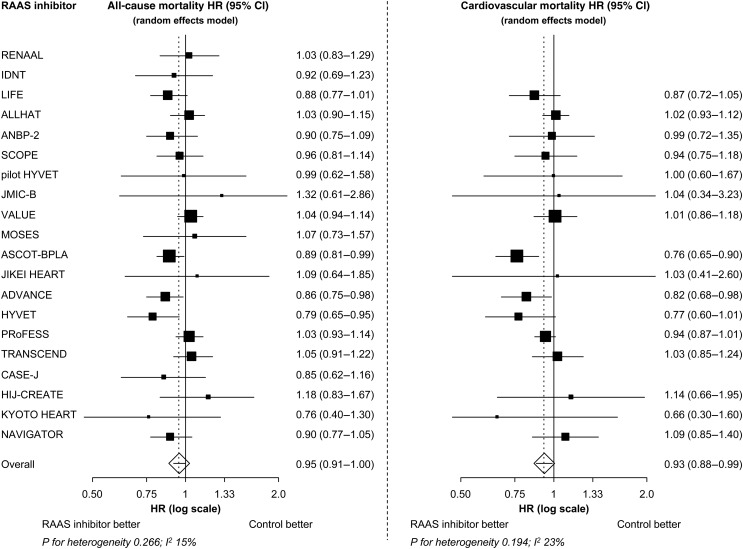
During a mean follow-up of 4.3 years, 6284 of the patients assigned to an RAAS inhibitor reached the endpoint of all-cause death. This corresponds with an IR of 20.9 deaths per 1000 patient-years. During the same period, a total of 8777 patients assigned to control therapy had all-cause death, implying an IR of 23.3 deaths per 1000 patient-years. RAAS inhibition was associated with a statistically significant reduction in all-cause mortality in three individual trials, ASCOT-BPLA, ADVANCE, and HYVET (Figure 2).23,26,31
Figure 2.
All-cause and cardiovascular mortality treatment effect of renin–angiotensin–aldosterone system blockade in all included trials. HR, hazard ratio; CI, confidence interval; RAAS, renin–angiotensin–aldosterone system. Overall P= 0.032 for all-cause mortality. Overall P= 0.018 for cardiovascular mortality.
In all 20 trials grouped together, treatment with an RAAS inhibition was associated with a statistically significant 5% reduction in all-cause mortality (HR: 0.95, 95% CI: 0.91–1.00, P= 0.032; Figure 2). The degree of heterogeneity in the treatment effect across all trials was low (I²: 15%) and non-significant (P= 0.266). No funnel-plot asymmetry was visualized, and the P-value using an Egger regression test for all-cause mortality was >0.10 (intercept −0.3, 95% CI: −1.3–0.68; P= 0.53), indicating no evidence for publication bias.
Cardiovascular mortality
Excluding the four trials that did not report on cardiovascular mortality, 2570 patients assigned to RAAS inhibition had cardiovascular death. Based on a total of 295 617 patient-years of follow-up, the IR was 8.7 per 1000 patient-years. The IR in patients assigned to control therapy was 10.1 per 1000 patient-years (3773 events; 372 105 patient-years of follow-up), resulting in a significant 7% overall reduction in cardiovascular mortality (HR: 0.93, 95% CI: 0.88–0.99, P= 0.018; Figure 2). The degree of heterogeneity in treatment effect across all trials was low (I²: 23%) and non-significant (P= 0.194). There was no evidence of publication bias.
Angiotensin-converting enzyme inhibitors vs. AT1 receptor blockers
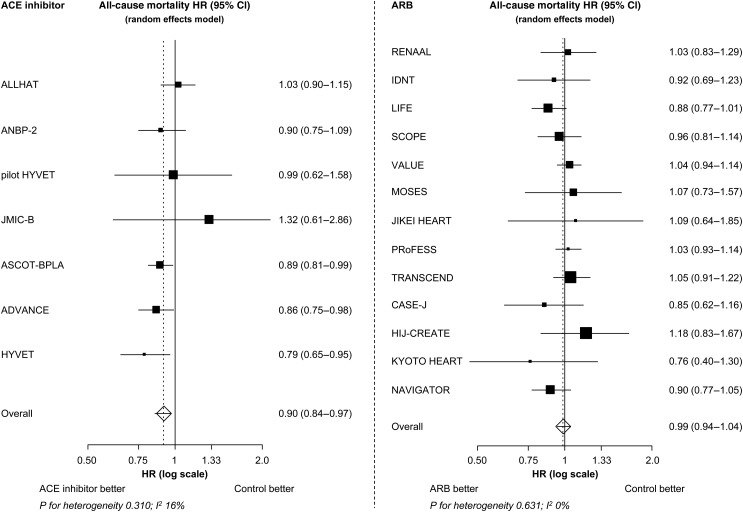
All seven trials together, ACE inhibitors were associated with a statistically significant 10% reduction in all-cause mortality (IR: 20.4 vs. 24.2 deaths per 1000 patient-years; HR: 0.90, 95% CI: 0.84–0.97, P= 0.004). No significant mortality reduction could be demonstrated with ARB treatment (13 trials; IR: 21.4 vs. 22.0 deaths per 1000 patient-years; HR: 0.99, 95% CI: 0.94–1.04, P= 0.683). This difference in the treatment effect between ACE inhibitors and ARBs was statistically significant (P-value for interaction 0.036). Apparently, the observed mortality reduction in the overall group of RAAS inhibitors was completely driven by the beneficial effect of the ACE inhibitors.
As far as the ACE inhibitor trials are concerned, the largest mortality reductions were observed in ASCOT-BPLA, ADVANCE, and HYVET, all of which studied the ACE inhibitor perindopril (pooled HR: 0.87, 95% CI: 0.81–0.93, P-value <0.001). However, there was no evidence of heterogeneity among the ACE inhibitor trials in the effect of the studied ACE inhibitor regimen on all-cause mortality (P-value for heterogeneity 0.310, I²: 16%; Figure 3). There was also no evidence of heterogeneity in the effect of ARBs (P-value for heterogeneity 0.631, I²: 0%).
Figure 3.
The all-cause mortality treatment effect of ACE inhibitor and ARB trials. HR, hazard ratio; CI, confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker. P= 0.004 for the treatment effect of ACE inhibitor on all-cause mortality. P= 0.683 for the treatment effect of ARB on all-cause mortality.
Patients randomized to an ACE inhibitor had 9.1 cardiovascular deaths per 1000 patient-years, compared with 11.2 in their controls (HR: 0.88; 95% CI: 0.77–1.00; P= 0.051). In the ARB trials, the IRs were 8.8 and 9.2 cardiovascular deaths per 1000 patient-years for patients assigned to ARB and control therapy, respectively (HR: 0.96; 95% CI: 0.90–1.01; P= 0.143). The test for heterogeneity in effects on cardiovascular mortality between ACE inhibitors and ARBs was statistically non-significant (P= 0.227).
Meta-regression
Multiple linear regression analysis showed a significant (P= 0.035) association between the trial-specific mean SBP (measured at baseline), and the relative mortality reduction by RAAS blockade. The mortality reduction was largest in trials with the highest mean baseline BP values. Secondly, there was a significant (P= 0.008) relation between the trial specific mean difference in BP between the studied RAAS inhibitor and control therapy at 1-year follow-up, and the mortality reduction produced by the RAAS inhibitor. The mortality reduction was largest in trials with the largest difference in mean SBP reduction. No significant association was found between the trial-specific mean age, man/woman ratio, mean follow-up time and the mortality reduction by RAAS blockade. Mean follow-up time was also not related to the observed mortality reduction, supporting our hypothesis that the mortality incidence ratio is constant over time (at least for the mean duration of 4.3 years).
Stratified analyses
Similar HRs for all-cause mortality were found in clinical trials that compared RAAS inhibition with placebo (HR: 0.95, 95% CI: 0.88–1.02, P= 0.177) and with active control (HR: 0.95, 95% CI: 0.91–1.01, P= 0.066; P-value for interaction 0.889). Likewise, no heterogeneity in treatment effect was observed with respect to the percentage of participants with diabetes mellitus or renal insufficiency.
Discussion
This meta-analysis, which included almost 160 000 patients, sought to evaluate the effect of RAAS inhibitors as a class of drugs on total and cardiovascular mortality in their main indication hypertension. Overall, the results show a 5% reduction in all-cause mortality during a 4-year follow-up period associated with the class of RAAS inhibitors. This mortality reduction was found when compared with placebo, as well as in comparison with other BP-lowering drugs. However, in a stratified analysis according to the class of drug, it was shown that the observed overall all-cause mortality reduction was almost completely a result of the beneficial effect of the class of ACE inhibitors (10% relative reduction in all-cause mortality), whereas the ARBs showed a neutral treatment effect. The findings are firm, as the analysis included a large number of patient-years (677 005) and endpoints (15 061 deaths). The findings are relevant to clinical practice, as they are based on data from well-designed randomized trials encompassing a broad population of patients with high BP, who were well-treated for concomitant risk factors and who represent usual hypertensive patients seen today.
Reduction in mortality is the primary goal of antihypertensive therapy.2 Paradoxically, the effect of RAAS inhibitors on mortality in hypertensive patients remained uncertain and had never been systematically evaluated. To our knowledge, no prior published meta-analysis investigated the efficacy of RAAS inhibitors on all-cause and cardiovascular mortality in their main indication of hypertension. Previous analyses in for example heart failure or coronary artery disease populations (with or without hypertension) demonstrated a reduction in cardiovascular events, stroke, and mortality.36,37 In addition, a pooled analysis of trials in patients with cardiovascular disease (including hypertension) concluded that the reduction in cardiovascular mortality and stroke with RAAS inhibitors is BP dependent.38 In our analyses, the significant reduction in cardiovascular mortality associated with RAAS inhibition supports previous literature.
As stated, the primary aim of this meta-analysis decided a priori was to test the hypothesis that RAAS inhibitors as a class of drugs would have a beneficial effect on total mortality in hypertension, when compared with contemporary control antihypertensive therapy. However, as we realized that, among the RAAS inhibitors, the ACE inhibitors and ARBs have different mechanisms of action, we also decided to study whether there was a differential effect on mortality between these two classes of drugs. Indeed, our analysis clearly showed that nearly all of the mortality reduction was observed with ACE inhibitors. Contrary, there was no clear benefit from the ARBs. This was supported by the sensitivity analysis which showed a significant stronger treatment effect in the ACE inhibitor trials compared with the ARB trials. With respect to this finding several points deserve consideration.
The reduced effect of ARBs on mortality when compared with ACE inhibitors has also previously been discussed.39,40 A recent meta-analysis of 37 ARB trials also failed to detect a reduction in all-cause or cardiovascular mortality in a broad population of patients.41 The differences in the modes of action between ACE inhibitors and ARBs, and the small-but-definite BP-independent reduction in CAD mortality with ACE inhibitors, which has not been observed with ARBs or other antihypertensive agents, might contribute to this finding.42 On the other hand, others have demonstrated that BP-dependent beneficial effects in the prevention of stroke and heart failure are similar for ACE inhibitors and ARBs. ACE inhibitors and ARBs have also been shown to be equally effective in preventing atrial fibrillation and new-onset diabetes.43,44 Furthermore, it should be emphasized that we did not design this meta-analysis to make a head-to-head comparison between ACE inhibitors and ARBs. The finding that the beneficial effect is seen in the ACE inhibitor population as opposed to the ARB population should be considered a post hoc observation. Given the nature of meta-analyses, which are per definition data-driven, the differential effect between ACE inhibitors and ARBs should be interpreted with caution to avoid overstating this subgroup finding vis-à-vis the a priori hypothesis. In this respect it should also be noted that the difference in effect on cardiovascular mortality between ACE inhibitors and ARBs was not statistically significant. Furthermore, two previous studies were designed to compare ACE inhibitors and ARBs in an hypertensive population, but both the ONTARGET (telmisartan vs. ramipril) and DETAIL (telmisartan vs. enalapril) trial did not show a differential treatment effect between ARBs and ACE inhibitors.15,45 Thus, at present, the results of this analysis do not warrant changing clinical practice treatment guidelines that recommend that an ARB may be used in ACE inhibitor-intolerant hypertensive patients.2 Hopefully, our findings will form the basis of further analysis and studies into the effects of BP treatment and total mortality which is the first line priority in the guidelines for the management of hypertension.
It might be argued that the observed 5% relative mortality reduction in the overall group of RAAS inhibitors, and the 10% relative mortality reduction in the ACE inhibitor group is small, and only found to be statistically significant in our analysis because of statistical ‘overpowering’. Indeed, in meta-analyses clinically irrelevant treatment effects might become statistically significant (i.e. the estimated effect divided by the standard error is >1.96) simply because of the large size of the aggregate (or pooled) trials. In our view, however, the observed mortality reduction in this meta-analysis is clinically relevant indeed, for several reasons. Firstly, it should be realized that the treatment effect was reached in patients who did receive a broad range of other contemporary risk-reduction therapies, including statins, antiplatelet therapy, beta-blockers, diuretics, and other BP-lowering medication (note that, as per design, we included trials that were conducted during 2000–2011). Secondly, the estimated absolute mortality reduction was 2.4 per 1000 patient-years for the RAAS inhibitors as a group and 3.8 per 1000 patient-years for the class of ACE inhibitors. This is an interesting figure, particularly since the prevalence of hypertension in Western (CAD) populations is high,46 despite the widespread use of BP-lowering medication. Thus a wider application of these agents, in particular of ACE inhibitors, may have substantial effects on the population level. Interestingly, the observed mortality reduction was largest in trials with the highest baseline SBP. The observed mortality reduction may be used as an additional argument to stimulate patients to adhere to the prescribed treatment.
Limitations
Several limitations of our analysis have to be mentioned. Firstly, there was a great deal of variation between the studied populations. For example, trials used different definitions of hypertension, different dosages of the active and control drug, different target BP levels, different follow-up times, and in several studies patients had other concomitant conditions and background therapy. Although this does not hamper the generalizability of our results, it makes it challenging to accurately estimate the effect of RAAS inhibition in a broad range of routine clinical practice situations.
Secondly, this meta-analysis is based on trial level data, rather than on individual patient data. Information on background therapy and co-morbidities were not available in several trial reports. Thus, we could not reliably analyse the relation between these factors and the observed mortality reduction. Moreover, the treatment arm-specific follow-up time was not available in all trials, we therefore derived follow-up time from either the reported death rate, Kaplan–Meier curves, or mean follow-up duration. This is an approximation of the true follow-up time, and we appreciate that our estimates of mortality incidence might be somewhat over or underestimated. However, importantly, this methodology had not influenced the estimation of the observed relative mortality reduction, which was mainly based on the HRs that were reported for the separate trials.
Finally, this meta-analysis assumed a class effect among the different ACE inhibitors and ARBs. The validity of this concept was not challenged by formal statistical tests on heterogeneity of treatment effects among the different (ACE inhibitor and ARB) trials. Still, it should be realized that differences may exist between drugs within the same class that are simply missed due to lack of statistical power. It should therefore be emphasized that our findings should be interpreted in relation to the pharmacological properties of the applied agents.
Conclusion
This meta-analysis, which involved almost 160 000 patients, demonstrated that RAAS inhibitors as a class of antihypertensive drugs were associated with a significant 5% relative reduction in all-cause mortality in populations with a high prevalence of hypertension when compared with contemporary control antihypertensive therapy. Stratified subgroup analysis according to class of drug showed a differential treatment effect between ACE inhibitors and ARBs. The overall reduction in all-cause mortality resulted almost completely from the class of ACE inhibitors, which were associated with a statistically significant 10% relative reduction in all-cause mortality, whereas no mortality reduction was observed with the ARBs. In view of the high prevalence of hypertension in the general population, widespread use of ACE inhibitors may therefore result in a considerable gain in lives saved. The results of this study provide a convincing argument to improve treatment adherence in the millions of people around the world suffering from hypertension and its sequelae.
Funding
Funding to pay the Open Access publication charges for this article was provided by the Department of Cardiology, Thoraxcenter, Erasmus MC.
Conflict of interest: M.B reports to have previously received research grants, fees, and honoraria from: Merck-Sharpe Dohme, American Medicine Company, Lilly, Servier, and Sanofi-Aventis. K.F. receives fees, and research grants from Servier Laboratories. During the previous 5 years, J.J.M. has received fees for an occasional consultancy or guest speaker meeting from various pharmaceutical companies. L.C.v.V., K.M.A., J.J.B., and E.B. have no conflict of interest to report.
References
- 1.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ Comparative Risk Assessment Collaborating G. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O'Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. The task force for the management of arterial hypertension of the European Society of H, The task force for the management of arterial hypertension of the European Society of C. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.Skeggs LT, Dorer FE, Kahn JR, Lentz KE, Levine M. The biochemistry of the renin-angiotensin system and its role in hypertension. Am J Med. 1976;60:737–748. doi: 10.1016/0002-9343(76)90888-3. [DOI] [PubMed] [Google Scholar]
- 5.Marketou M, Kintsurashvili E, Papanicolaou KN, Lucero HA, Gavras I, Gavras H. Cardioprotective effects of a selective B(2) receptor agonist of bradykinin post-acute myocardial infarct. Am J Hypertens. 2010;23:562–568. doi: 10.1038/ajh.2010.20. [DOI] [PubMed] [Google Scholar]
- 6.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 8.Fox KM Investigators EUtOrocewPiscAd. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, Investigators RS. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 10.Group NS, McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki S, Dahlof B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, Ohta M, Yamada T, Ogawa K, Kanae K, Kawai M, Seki S, Okazaki F, Taniguchi M, Yoshida S, Tajima N Jikei Heart Study g. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439. doi: 10.1016/S0140-6736(07)60669-2. [DOI] [PubMed] [Google Scholar]
- 12.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S, Investigators C. Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 14.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ, Investigators AT. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 15.Investigators O, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 16.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW, Investigators I. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasanuki H, Hagiwara N, Hosoda S, Sumiyoshi T, Honda T, Haze K, Nagashima M, Yamaguchi J, Origasa H, Urashima M, Ogawa H, Investigators H-C. Angiotensin II receptor blocker-based vs. non-angiotensin II receptor blocker-based therapy in patients with angiographically documented coronary artery disease and hypertension: the Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease (HIJ-CREATE) Eur Heart J. 2009;30:1203–1212. doi: 10.1093/eurheartj/ehp101. [DOI] [PubMed] [Google Scholar]
- 20.Ogihara T, Fujimoto A, Nakao K, Saruta T, Group C-JT. ARB candesartan and CCB amlodipine in hypertensive patients: the CASE-J trial. Expert Rev Cardiovasc Ther. 2008;6:1195–1201. doi: 10.1586/14779072.6.9.1195. [DOI] [PubMed] [Google Scholar]
- 21.Sawada T, Yamada H, Dahlof B, Matsubara H, Group KHS. Effects of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART Study. Eur Heart J. 2009;30:2461–2469. doi: 10.1093/eurheartj/ehp363. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW, Group PRS. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, Group HS. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 24.Bulpitt CJ, Beckett NS, Cooke J, Dumitrascu DL, Gil-Extremera B, Nachev C, Nunes M, Peters R, Staessen JA, Thijs L Hypertension in the Very Elderly Trial Working G. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21:2409–2417. doi: 10.1097/00004872-200312000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H Group LS. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 26.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J, Investigators A. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 27.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A group Vt. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 28.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study G. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 29.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A, Group SS. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Officers A Coordinators for the ACRGTA, Lipid-Lowering Treatment to Prevent Heart Attack T. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 31.Patel A, Group AC, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 32.Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, Zidek W, Dominiak P, Diener HC, Group MS. Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 33.Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ Second Australian National Blood Pressure Study G. A comparison of outcomes with angiotensin-converting–enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 34.Yui Y, Sumiyoshi T, Kodama K, Hirayama A, Nonogi H, Kanmatsuse K, Origasa H, Iimura O, Ishii M, Saruta T, Arakawa K, Hosoda S, Kawai C Japan Multicenter Investigation for Cardiovascular Diseases BSG. Comparison of nifedipine retard with angiotensin converting enzyme inhibitors in Japanese hypertensive patients with coronary artery disease: the Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B) randomized trial. Hypertens Res. 2004;27:181–191. doi: 10.1291/hypres.27.181. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P Telmisartan Randomised AssessmeNt Study in ACEiswcDI. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 36.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–588. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 37.Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA. 2011;305:913–922. doi: 10.1001/jama.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 40.Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 41.Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011;342:d2234. doi: 10.1136/bmj.d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trialists C, Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, Woodward M, Chalmers J, Zanchetti A, MacMahon S Blood Pressure Lowering Treatment. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951–958. doi: 10.1097/HJH.0b013e3280bad9b4. [DOI] [PubMed] [Google Scholar]
- 43.Huang G, Xu JB, Liu JX, He Y, Nie XL, Li Q, Hu YM, Zhao SQ, Wang M, Zhang WY, Liu XR, Wu T, Arkin A, Zhang TJ. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers decrease the incidence of atrial fibrillation: a meta-analysis. Eur J Clin Invest. 2011;41:719–733. doi: 10.1111/j.1365-2362.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- 44.Tocci G, Paneni F, Palano F, Sciarretta S, Ferrucci A, Kurtz T, Mancia G, Volpe M. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and diabetes: a meta-analysis of placebo-controlled clinical trials. Am J Hypertens. 2011;24:582–590. doi: 10.1038/ajh.2011.8. [DOI] [PubMed] [Google Scholar]
- 45.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J Diabetics Exposed to T, Enalapril Study G. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 46.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U, Group ES. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137. doi: 10.1097/HJR.0b013e3283294b1d. [DOI] [PubMed] [Google Scholar]