SUMMARY
The hippocampus is an integral brain region for affective disorders. TRIP8b knockout mice lacking functional HCN channels as well as both HCN1 and HCN2 knockout mice have been shown to display antidepressant-like behaviors. The mechanisms or brain regions involved in these alterations in behavior, however, are not clear. We developed a lentiviral shRNA system to examine whether knockdown of HCN1 protein in the dorsal hippocampal CA1 region is sufficient to produce antidepressant-like effects. We found that knockdown of HCN1 channels increased cellular excitability and resulted in physiological changes consistent with a reduction of Ih. Rats infused with lentiviral shRNA-HCN1 in the dorsal hippocampal CA1 region displayed antidepressant- and anxiolytic-like behaviors associated with widespread enhancement of hippocampal activity and upregulation of BDNF-mTOR signaling pathways. Our results suggest that HCN1 protein could be a potential target for treatment of anxiety and depression disorders.
INTRODUCTION
The limbic-cortical system in the brain is considered to be the neural substrate for the action of antidepressants (Mayberg, 1997; Mayberg et al., 2000). Chronic administration of antidepressant drugs in depressed patients changed regional glucose metabolism measured by positron emission tomography in the hippocampus (Kennedy et al., 2001; Mayberg et al., 2000). There are several lines of evidence suggesting that dorsal hippocampus is an integral brain region for the action of antidepressants (Lu et al., 2006; McLaughlin et al., 2007; Padovan and Guimaraes, 2004). Functional neuroimaging of depressed patients has shown that the volume of posterior hippocampus, which corresponds to the dorsal hippocampus in rodents (Colombo et al., 1998), was significantly reduced (Campbell et al., 2004), resulting in impaired spatial learning and memory (Gould et al., 2007). It is often observed that patients with depression also have anxiety-like symptoms (Jacobi et al., 2004; Lamers et al., 2011). This comorbidity of depression and anxiety disorders in some patients was effectively treated with chronic administration of fluoxetine (Sonawalla et al., 2002). In addition, mice chronically injected with fluoxetine displayed antidepressant- and anxiolytic-like behaviors (Dulawa et al., 2004), suggesting depression and anxiety might share common neural substrates.
It has been reported that brain-derived neurotrophic factor (BDNF) protein expression in the hippocampus of postmortem depressed patients was significantly reduced (Dwivedi et al., 2003) and this can be reversed by antidepressant treatments (Chen et al., 2001), suggesting an important role of BDNF in major depression. Studies in animals also have shown that BDNF and mammalian target of rapamycin (mTOR) signaling pathways are important for antidepressant effects of ketamine (Autry et al., 2011; Li et al., 2010). A single sub-anesthesia dose of ketamine (i.p. 10 ~ 15 mg/kg) produced early onset and long lasting therapeutic antidepressant-like effects, which required upregulation of BDNF-mTOR signaling pathways and suggesting a cellular mechanism underlying the antidepressant-like effects of ketamine (Autry et al., 2011; Duman and Monteggia, 2006; Li et al., 2010; Liu et al., 2011).
Voltage-gated ion channels are non-uniformly distributed in the CA1 pyramidal neurons (Magee, 1999b). They regulate the processing of input information and the induction of synaptic plasticity (Frick and Johnston, 2005). Membrane currents generated by hyperpolarization-activated, cyclic nucleotide gated nonselective cation channels (h-channels) are characterized by 1) cyclic nucleotide-mediated modulation, 2) Na+ and K+ permeability, and 3) activation by membrane hyperpolarization (Pape, 1996). Although there are four isoforms of HCN channels (HCN1-HCN4), HCN1 is the predominant isoform expressed in hippocampus, neocortex, and cerebellar cortex (Brewster et al., 2007; Monteggia et al., 2000). In the hippocampal CA1 region, the expression of HCN1 shows a gradient of increasing channel density from the soma to the distal apical dendrites (Lorincz et al., 2002). This is consistent with an increase in Ih density by cell-attached recordings across the somatodendritic compartments (Magee, 1998). A portion of h-channels is active at rest leading to the generation of a depolarizing non-inactivating inward current that contributes to the resting membrane potential, input resistance (Rin), and membrane time constant (τm) of CA1 pyramidal neurons. Ih also contributes to the intrinsic resonance properties, which influence how CA1 neurons respond to oscillating inputs (Hu et al., 2002; Narayanan and Johnston, 2007). Blockade of Ih by Cs+ or ZD7288 enhances synaptic summation, indicating a key role in the integration of subthreshold synaptic inputs (Magee, 1999a). Loss of functional Ih by deletion of the HCN1 gene causes a change in behavioral phenotypes (Nolan et al., 2004; Nolan et al., 2003). Global HCN1 knockout mice showed impaired motor learning and memory (Nolan et al., 2003), whereas forebrain-specific HCN1 knockout mice displayed improved short- and long-term spatial learning and memory (Nolan et al., 2004).
A recent report demonstrated that reduction of Ih in three different lines of knockout mice (TRIP8b, HCN1, and HCN2) showed antidepressant-like behaviors, suggesting that reduction of h-channel function might result in antidepressant effects (Lewis et al., 2011). However, the mechanisms or brain regions underlying these effects are unknown. Given the lack of HCN1-specific blockers or genetic animal models that offer region-specific manipulation of HCN1 channels, we developed a lentiviral shRNA system that provides sequence-specific manipulation of HCN1 with spatial and temporal control (Elbashir et al., 2001). We found that shRNA-HCN1-infected dorsal CA1 pyramidal neurons had altered intrinsic membrane properties and increased cellular excitability, consistent with the reduction of HCN1 protein expression in the shRNA-HCN1-infected CA1 region. Remarkably, rats infused with lentiviral shRNA-HCN1 in the dorsal CA1 regions displayed anxiolytic- and antidepressant-like behaviors associated with upregulation of BDNF and mTOR signaling. We further found that knockdown of HCN1 in the dorsal CA1 region resulted in widespread enhancement of hippocampal activity using voltage sensitive dye (VSD) imaging, consistent with an increase in synaptic transmission. Taken together, knockdown of HCN1-by lentiviral shRNA-HCN1 in the dorsal hippocampal CA1 region enhanced cellular excitability, upregulated BDNF-mTOR signaling, increased hippocampal activity and produced anxiolytic- and antidepressant-like behaviors. Our findings suggest that targeting HCN1 channels might provide an alternative therapy for treating depression and anxiety disorders.
RESULTS
Knockdown of HCN1 channels in the dorsal hippocampal CA1 region
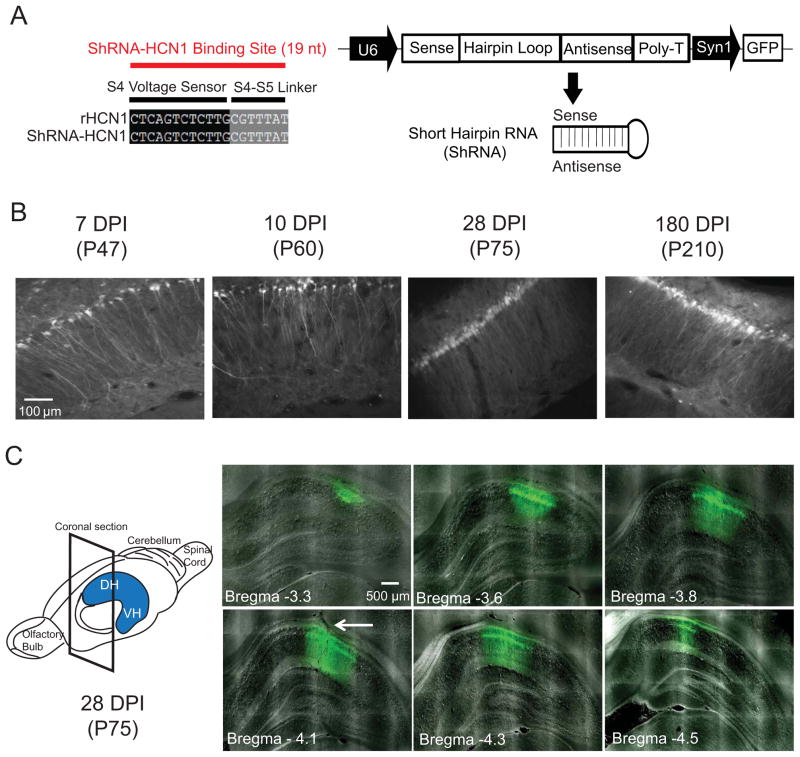
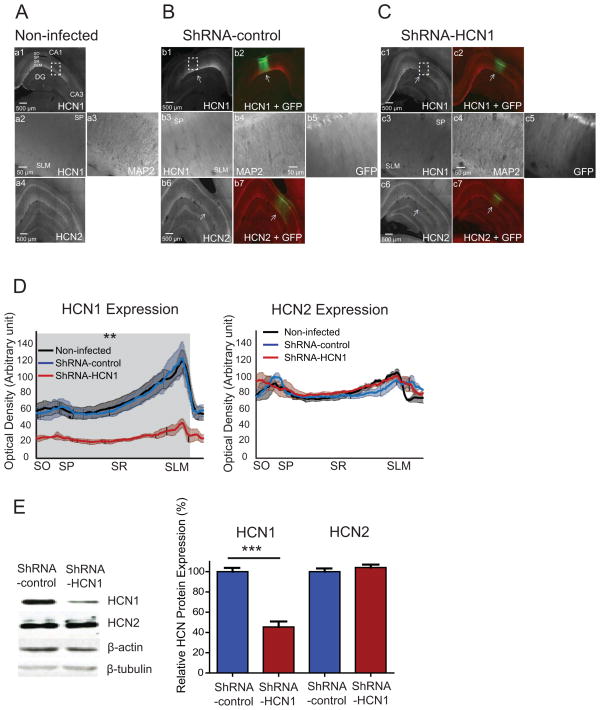
We developed a lentivirus-based silencing RNA system expressing short hairpin RNA (shRNA) against HCN1 mRNA (Figure 1A) to knockdown the expression level of HCN1 protein in the dorsal hippocampal CA1 region. To achieve higher transfection efficiency, we used the U6 promoter to drive shRNA expression. Therefore, we first confirmed that HCN1 subunits are only expressed in neurons, and not glia cells (Figure S1). Given that the brain parenchyma in young animals has more extracellular space to provide better spread of lentiviral particles (Thorne and Nicholson, 2006; Zhao et al., 2008), we used 4- to 5-week-old rats for infusion of lentivirus in this study. Lentivirus expressing shRNA-HCN1 was infused in the CA1 region of the dorsal hippocampus, which expressed on 7 days post infusion (DPI) and up to at least six-months (Figure 1B) and spread mediolaterally (about 0.7–1.0 mm) and anteroposteriorly (about 1.2–1.6 mm) (Figure 1C). We quantified the local silencing efficiency of HCN1 protein by immunohistochemistry and western blotting. The HCN1 protein expression was significantly decreased without alteration in HCN2 and MAP2 protein expression in the shRNA-HCN1-infected region as compared to non-infected or shRNA-control-infected CA1 regions (Figure 2A–D). Quantification of protein expression from isolated lentiviral shRNA-HCN1-infected dorsal CA1 region showed a 58% reduction in HCN1 protein expression without change in HCN2 and β-tubulin protein expression as compared to shRNA-control-infected region (Figure 2E), suggesting specificity for knockdown of HCN1 channels.
Figure 1. Lentivirus expressing shRNA-HCN1 in the CA1 region of dorsal hippocampus.
(A) A schematic diagram showing the lentiviral shRNA expression vector system. The 19 nucleotide shRNA is specific to the HCN1 gene. In dual promoter vector system, shRNA is under control of U6 promoter and GFP is controlled by synapsin1 promoter. (B) Expression of lentivirus over time in the dorsal CA1 region. Rats infused with lentivirus in the dorsal hippocampal CA1 region were transcardially perfused on day 7, 10, 28, and 180 after infusion (days post infusion, DPI). The ages of animals are in parentheses (postnatal day). (C) Distribution of lentivirus in the dorsal hippocampal CA1 region. A single infusion of lentivirus spread out mediolaterally (about 0.7 – 1.0 mm) and anteroposteriorly (about 1.2–1.6 mm). The arrow indicates the injection track. DH: Dorsal hippocampus: VH: Ventral hippocampus
Figure 2. Specific knockdown of HCN1 by lentiviral-shRNA-HCN1 in the CA1 region of the dorsal hippocampus.
(A) 70-μm-thick dorsal hippocampal slices from non-infected rats were immunolabeled with HCN1 (a1, a2), HCN2 (a4), and dendritic marker MAP2 (a3). a2 and a3 are enlarged from the dashed box. (B) In the shRNA-control-infected dorsal hippocampal CA1 region, protein expression of HCN1 (b1, b3), HCN2 (b6), and MAP2 (b4) were similar to non-infected group. b3, b4, and b5 are enlarged from the dashed box. The labeling of HCN1 (b1) and HCN2 protein (b6) were superimposed with GFP expression in b2 and b7. The arrows indicate lentivirus-infected CA1 region. (C) In the ShRNA-HCN1-infected dorsal hippocampal CA1 region, protein expression of HCN1 (c1, c3) was significantly reduced without affecting the expression of HCN2 (c6) and MAP2 (c4). c3, c4, and c5 are enlarged from the dashed box in c1. The labeling of HCN1 (c1) and HCN2 protein (c6) were superimposed with GFP expression in c2 and c7. (D) Quantification of HCN1 and HCN2 protein expression from the perisomatic region to the distal dendritic area in the CA1 region. The gray shade indicates significant difference in HCN1 protein expression between shRNA-HCN1-infected region (n=3) and non-infected (n=3) or shRNA-control-infected regions (n=3) (Unpaired t-test). There was no significant difference in HCN2 protein expression from the perisomatic region to the distal dendritic area in the CA1 region (Unpaired t-test). (E) Western blotting of shRNA-control-infected (n=7) and shRNA-HCN1-infected (n=7) CA1 lysates with antibodies against HCN1, HCN2, β-actin, and β-tubulin. The protein expression of HCN1 and HCN2 were quantified and normalized by β-tubulin. The right displays the summary of HCN1 and HCN2 protein expression in shRNA-control-infected and shRNA-HCN1-infected regions. Data are expressed as mean ± SEM. **p < 0.01, and ***p < 0.001 compared with non-infected or lentiviral-shRNA-control-infected group. SO: Stratum Oriens; SP: Stratum Pyramidale; SR: Stratum Radiatum; SLM: Stratum Lacunosum Moleculare.
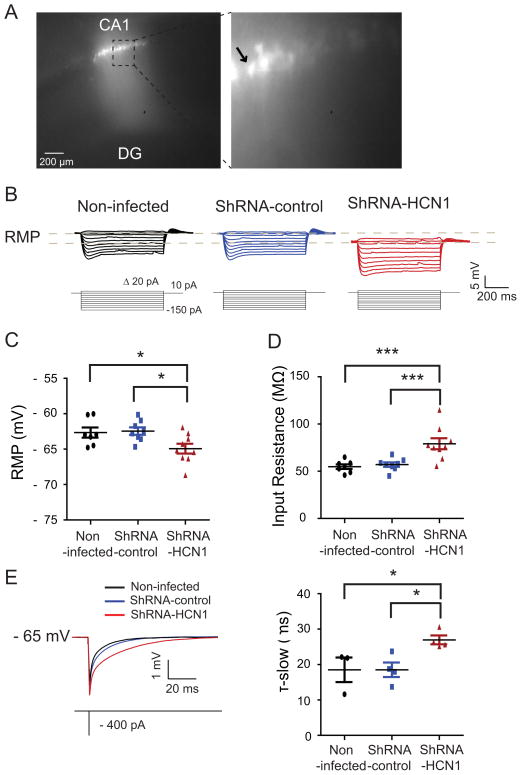
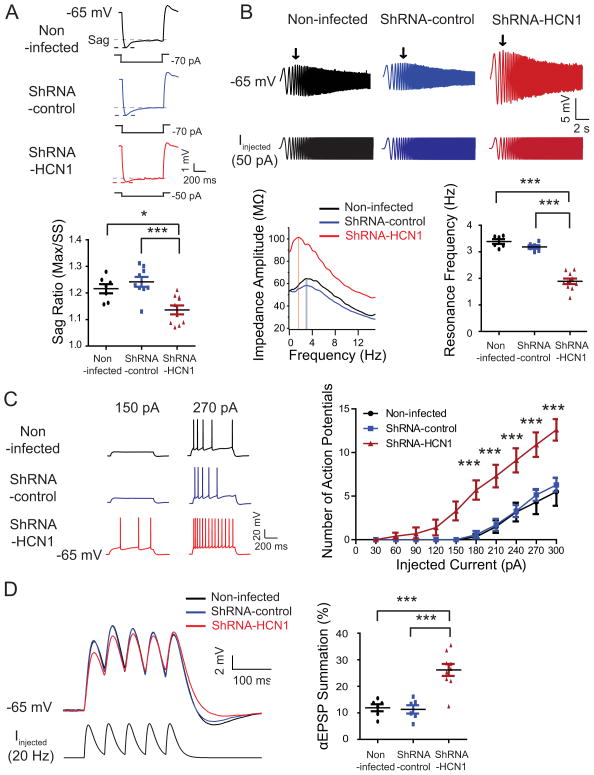
To determine whether silencing of HCN1 gene had an effect on the physiology of the dorsal CA1 pyramidal neurons, Ih-sensitive electrophysiological parameters were measured using the whole-cell current-clamp method (Narayanan and Johnston, 2007) (Figures 3 and S2). ShRNA-HCN1-infected CA1 pyramidal neurons had hyperpolarized resting membrane potentials (Figures 3C), higher steady-state input resistance (Figure 3D), and slower membrane time constant (Figure 3E) than non-infected or shRNA-control-infected CA1 pyramidal neurons. For proper comparison between groups, we held membrane potentials at −65 mV with current injection and compared electrophysiological properties (Figures 4 and S3). ShRNA-HCN1-infected CA1 pyramidal neurons had less voltage sag (Figure 4A) and lower resonance frequency (Figure 4B) compared to non-infected or shRNA-control-infected CA1 pyramidal neurons. In addition, shRNA-HCN1-infected CA1 pyramidal neurons generated more action potentials in response to depolarizing current steps (30–300 pA in 30 pA increments for 750 ms) (Figure 4C), suggesting increased cellular excitability (Shah et al., 2004). Similar results, however, were also obtained with neurons at their normal resting potentials (Figure S2). To examine subthreshold synaptic integration (αEPSP), the response to repetitive current injections similar to multiple excitatory postsynaptic currents were measured using a train of 5 alpha current injections (α=0.1, 20 Hz) (Brager and Johnston, 2007; Dembrow et al., 2010; Poolos et al., 2002). ShRNA-HCN1-infected CA1 pyramidal neurons had larger αEPSP summation than non-infected or shRNA-control-infected CA1 pyramidal neurons (Figure 4D). In agreement with our biochemical results, these data indicate that silencing of the HCN1 gene by shRNA-HCN1 produced electrophysiological changes consistent with a reduction in Ih.
Figure 3. Alteration in intrinsic membrane properties by knockdown of HCN1 in the dorsal CA1 pyramidal neurons.
(A) Photomicrograph of a representative recorded lentivirus-infected dorsal CA1 pyramidal neurons. The left displays photomicrograph of lentivirus-infected dorsal hippocampal CA1 region. The right enlarged from the dashed box. The arrow indicates the recorded pyramidal neuron. (B) Representative voltage responses with step current commands ranging from -150 pA to +10 pA (Δ=20 pA) at resting membrane potential. (C) ShRNA-HCN1-infected CA1 pyramidal neurons (n=9) displayed hyperpolarized membrane potential as compared to non-infected- (n=7) or shRNA-control-infected (n=8) pyramidal neurons. (D) Knockdown of HCN1 in the dorsal CA1 pyramidal neurons (n=9) resulted in higher input resistance than non-infected- (n=7) or shRNA-control-infected (n=8) pyramidal neurons. (E) ShRNA-HCN1-infected pyramidal neurons (n=4) displayed slower time constant compared to non-infected- (n=3) or shRNA-control-infected (n=4) pyramidal neurons. The top displays representative traces and current commands. ShRNA-HCN1-infected neurons were compared with non-infected or shRNA-control-infected CA1 pyramidal neurons. Data are expressed as mean ± SEM. *p < 0.05 and ***p < 0.001.
Figure 4. Knockdown of HCN1 induced physiological changes consistent with reduction of Ih in the dorsal CA1 pyramidal neurons.
For proper comparison between groups, CA1 pyramidal neurons were held at -65 mV. (A) ShRNA-HCN1-infected CA1 pyramidal neurons (n=10) showed less voltage sag compared to non-infected- (n=7) or shRNA-control-infected pyramidal neurons (n=9). The left are representative voltage traces and current commands. The sag is defined as the ratio of the maximum hyperpolarization (Max) to the steady-state hyperpolarization (SS). (B) ShRNA-HCN1-infected pyramidal neurons (n=10) had lower membrane resonance frequency than non-infected- (n=6) or shRNA-control-infected pyramidal neurons (n=6). The top are representative voltage traces and current commands. The arrows point to the maximum depolarization. The bottom left displays the profile of impedance amplitude. The resonance frequencies are marked by vertical lines. (C) ShRNA-HCN1-infected pyramidal neurons (n=10) had more action potentials than non-infected- (n=6) or shRNA-control-infected pyramidal neurons (n=7). The top are representative voltage traces. (D) Knockdown of HCN1 in dorsal CA1 pyramidal neurons (n=9) resulted in higher αEPSP summation as compared to non-infected- (n=6) or shRNA-control-infected pyramidal neurons (n=6). The left are representative voltage traces and current commands. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with non-infected or shRNA-control-infected CA1 pyramidal neurons.
Knockdown of HCN1 in dorsal hippocampus exerted anxiolytic-like effects
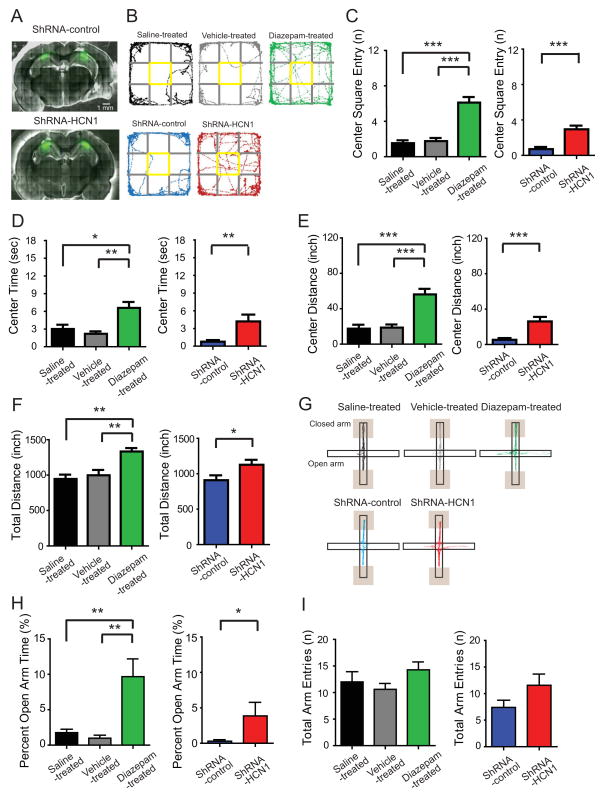
To test whether HCN1 gene silencing in the dorsal hippocampus affected locomotion and anxiety, rats infused bilaterally with lentivirus expressing either shRNA-control or shRNA-HCN1 were examined in the open field test (OFT). The OFT is a behavioral test for assessing anxiety level and exploration activity in rats (Borelli et al., 2004; Liu et al., 2010; Prut and Belzung, 2003). As a control experiment, we tested one anxiolytic drug, diazepam, and compared the results with saline- and vehicle-treated rats. Rats that did not receive lentiviral-shRNA infusions were placed into the open field arena 30 min after i.p. injection of saline, vehicle (0.5% Tween-20 in saline), or diazepam (1.0 mg/kg in vehicle solution). Diazepam-treated rats displayed anxiolytic-like behaviors as defined by increased number of center square entries (Figure 5C), duration of center square entries (Figure 5D) and distance travelled in the center square (Figure 5E) compared to saline-treated or vehicle-treated rats, confirming anxiolytic-like behaviors in this behavior paradigm (see also Figure S4). Surprisingly, rats infused with lentiviral-shRNA-HCN1 into the dorsal CA1 region displayed significantly larger number of center square entries (Figure 5C), longer duration of center square entries (Figure 5D), and longer distance travelled in the center square (Figure 5E) compared to shRNA-control-infected rats, indicating less anxiety-like behavior in shRNA-HCN1-infected animals. For exploration activity, diazepam-treated rats showed significantly increased total distance (Figures 5B and 5F), consistent with diazepam-induced hyperexploration (Ennaceur et al., 2010). Like diazepam-treated rats, shRNA-HCN1-infected rats also showed significantly longer total distance travelled than shRNA-control-infected rats (Figures 5B and 5F), indicating more exploration in the novel open field environment. To further confirm the anxiolytic-like effect of HCN1 knockdown in the dorsal CA1 region, we used an elevated plus maze (EPM) test to assess anxiety level of these animals. The EPM test is a pharmacologically validated behavioral test for evaluating anxiety responses of rodents (Pellow et al., 1985). Rats were allowed to explore on the elevated plus maze 30 min after i.p. injection of saline, vehicle (0.5% Tween-20 in saline), or diazepam (1.0 mg/kg in vehicle solution) for 6 min. Diazepam-injected rats displayed anxiolytic-like behaviors as defined by increased percentage of time spent in open arms (Figure 5H) without significant change in total arm entries (Figure 5I), confirming anxiolytic-like behaviors in this paradigm. Like diazepam-treated rats, shRNA-HCN1-infected rats displayed significant increase in the percentage of time spent in open arms (Figure 5H) without significant alteration in total arm entries (Figures 5I and S5) compared to shRNA-control-infected rats, indicating anxiolytic-like behavior. Taken together, knockdown of HCN1 in the CA1 region of the dorsal hippocampus produced anxiolytic-like effects in the open field test and the elevated plus maze test.
Figure 5. Knockdown of HCN1 by lentiviral-shRNA-HCN1 in the CA1 region of the dorsal hippocampus produced anxiolytic-like effect in the open field test and elevated plus maze test.
(A) Representative coronal sections of the brains display the areas infected by lentivirus (green). (B) Representative video tracking images during the last 5 min of open field test of age-matched individual rats treated with saline (i.p.), vehicle (i.p.), diazepam (1 mg/kg i.p.) or infected with lentiviral shRNA-control (infusion in dorsal CA1 region) or shRNA-HCN1 (infusion in dorsal CA1 region). During the open field test, the number of center square entries (C), the duration (D), and the travelled distance (E) in the center square were measured. Like diazepam-treated rats, shRNA-HCN1-infected rats showed significantly increased center square entries, center time, and center distance compared to shRNA-control-infected rats, indicating anxiolytic-like behaviors. (F) Like diazepam-induced hyperexploration in the open field test, shRNA-HCN1-infected rats also showed increased exploration activity compared to shRNA-control-infected rats for the last 5 min of the test. (G) Representative video tracking images during the 6 min of elevated plus maze test of age-matched individual rats treated with saline (i.p.), vehicle (i.p.), diazepam (i.p.) or infected with lentiviral shRNA-control or shRNA-HCN1. The traces do not include locations near the end of closed arms since it is too dim to reliably recognize the body center (Shaded boxes). During the elevated plus maze test, the percentage of open arm time (H) was measured. Similar to the diazepam-induced increase in the percentage of open arm time, shRNA-HCN1-infected rats displayed significantly higher percentage of open arm time compared to shRNA-control-infected rats. (I) There is no significant difference in total arm entries between groups. Data are expressed as mean ± SEM with significance indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
Knockdown of HCN1 in dorsal hippocampus produced antidepressant-like effects
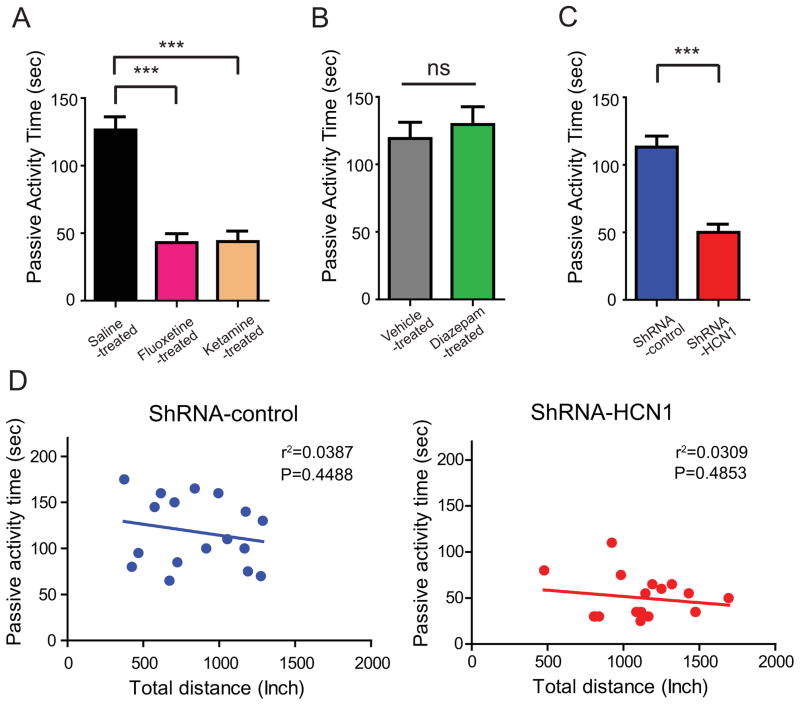
The modified forced swim test (FST) is used to screen antidepressant effects in rats and mice (Lucki, 1997). Animals subjected to an inescapable stress environment typically exhibit two different behaviors—active activity (swimming and climbing) and passive activity (immobility) (Lucki, 1997). Passive activity can thus be an indicator of behavioral despair in this paradigm (Porsolt et al., 1977). This passive activity can be reduced by acute or chronic treatment with clinical antidepressant drugs (Liu et al., 2010; Lu et al., 2006). We replicated this finding in rats treated with either a sub-anesthetic dose of ketamine (15 mg/kg i.p.) or fluoxetine (10 mg/kg i.p.). Fluoxetine and ketamine treated rats displayed significantly reduced duration of passive activity in the forced swim test (Figure 6A) compared to saline-treated rats, confirming antidepressant-like effects of these compounds in this behavioral paradigm. Additionally, diazepam did not have antidepressant-like effects in forced swim test (Figure 6B) even though it showed hyperexploration in OFT, suggesting specificity of this behavioral test (Figure S6). To determine whether knockdown of HCN1 in the dorsal CA1 region can produce antidepressant-like effects, rats were microinjected bilaterally with lentivirus expressing either shRNA-control or shRNA-HCN1. Similar to fluoxetine or ketamine treatment, knockdown of HCN1 was associated with an antidepressant-like effect compared with shRNA-control-infected animals (Figure 6C). Because shRNA-HCN1-infected rats displayed higher exploration activity (Figures 5B and 5F) in the open field test, we further analyzed whether there was a relationship between exploration activity and duration of passive activity. We found, however, that there was no correlation between passive activity in FST and exploration activity in OFT (Figure 6D), suggesting specificity of antidepressant-like effects by knockdown of HCN1 in the dorsal hippocampal CA1 region.
Figure 6. Knockdown of HCN1 by lentiviral-shRNA-HCN1 in the CA1 region of the dorsal hippocampus promoted antidepressant-like effects in the forced swim test.
(A) 10- to 12-week-old rats were treated with saline i.p. (n=17), 10 mg/kg fluoxetine i.p. (n=13), or 15 mg/kg ketamine i.p. (n=13) 30 min before the forced swim test as a positive control. Rats treated with fluoxetine or ketamine displayed significantly less passive activity time compared to rats treated with saline. (B) Diazepam-treated rats (1mg/kg i.p. n=12) had no effect on the forced swim test compared to vehicle-treated rats (n=13). (C) ShRNA-HCN1-infected rats (n=18) displayed significantly less passive activity time compared to shRNA-control-infected rats (n=17). Behavior despair as indicated by passive activity time was determined in the last 4 min of the forced swim test. (D) There was no significant correlation between the passive activity time (sec) in the forced swim test and the total distance (inch) in the open field test analyzed with linear regression. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001.
Enhancement of dorsal hippocampal activity and upregulation of BDNF-mTOR signaling in the dorsal hippocampal CA1 region by knockdown of HCN1
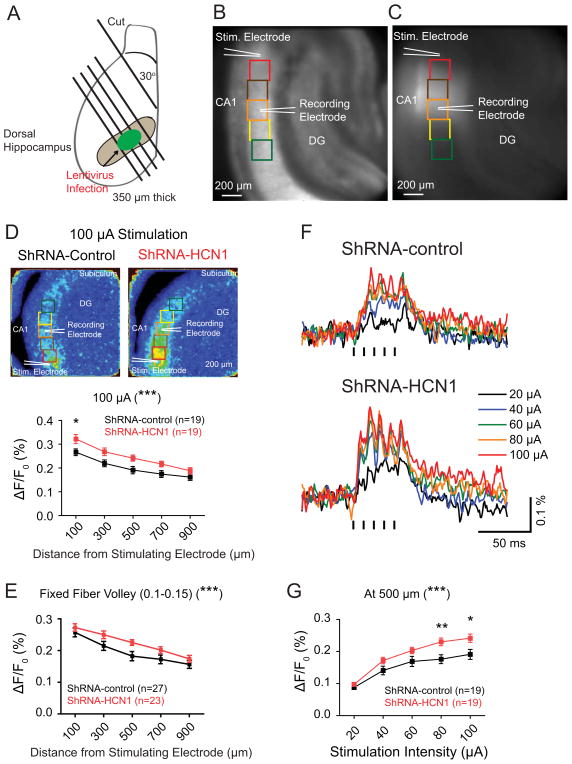
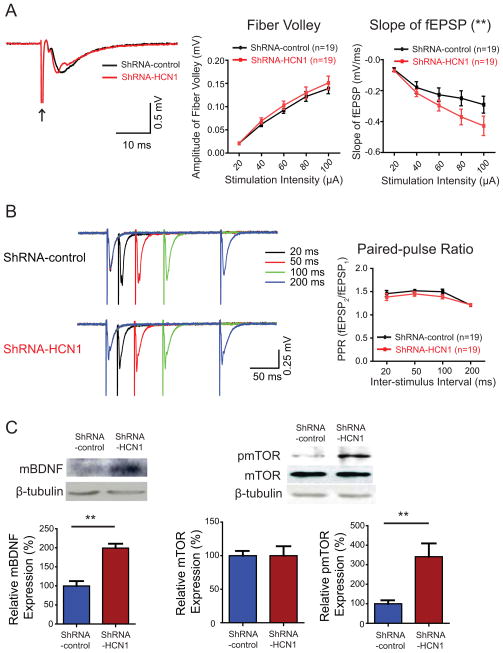
Patients with treatment-resistant depression have pathologically altered activity in the limbic-cortical area (Mayberg et al., 2005). Animal models of depression induced by chronic mild stress display an increase in the ventral CA1 activity. This could be reversed by fluoxetine, highlighting the significance of hippocampal activity in the treatment of depression (Airan et al., 2007). Because genetic silencing of HCN1 gene in a small region of the dorsal hippocampal CA1 region produced anxiolytic- and antidepressant-like effects, it is possible that chronic knockdown of HCN1 could cause changes in hippocampal activity. We used voltage sensitive dye (VSD) imaging to determine whether a localized, chronic knockdown of HCN1 can change hippocampal activity. We placed a stimulating electrode in the stratum radiatum (SR), near the border between the CA1 and CA2 regions to activate the Schaffer collaterals (Chang et al., 2007). Both VSD optical signals and extracellular field potentials were recorded in the SR of the CA1 region (Figures 7B and 7C). Knockdown of HCN1 led to enhanced VSD optical signals in response to a burst of stimuli (five 0.2 ms current pulses at 100 Hz, 100 μA) as compared to shRNA-control-infected group (Figure 7D), indicating widespread enhancement of hippocampal activity. At the area 500 μm away from the stimulating electrode, where the recording electrode was placed, the peak amplitude of VSD optical signals in shRNA-HCN1-infected slices were significantly larger than those evoked in shRNA-control-infected slices (Figures 7F and 7G). To compare VSD optical signals in response to a similar number of activated Schaffer collaterals, we grouped data with a fixed range of fiber volley amplitude (FV, 0.1–0.15 mV) and, consistently, the shRNA-HCN1-infected group showed significantly increased VSD optical signals as compared to shRNA-control-infected group (Figure 7E). It has been demonstrated that VSD optical signals reflecting membrane depolarization of postsynaptic neurons are correlated with extracellular field potentials (Tominaga et al., 2000). The widespread enhancement of VSD optical signals in the CA1 region of shRNA-HCN1-infected slices suggested that basal synaptic transmission might have been changed. Indeed, we found that there were significant differences in the slope of field potentials without change in the amplitude of presynaptic fiber volleys between shRNA-control- and shRNA-HCN1-infected groups (Figures 8A and S7), indicating enhanced synaptic transmission in the shRNA-HCN1-infected CA1 region. The paired-pulse ratio (PPR) was not significantly different between shRNA-control- and shRNA-HCN1-infected slices, suggesting no significant difference in presynaptic neurotransmitter release probability between these two groups (Figure 8B).
Figure 7. Knockdown of HCN1 enhanced voltage sensitive dye (VSD) optical signals in the dorsal hippocampal CA1 region.
(A) Schematic illustration of the dorsal hippocampal slices prepared from lentivirus-infected animals. (B) Photomicrograph of a representative hippocampal slice displays the location of the stimulating electrode, the recording electrode, and the regions of interest (ROIs). The size of ROIs is 200 × 200 μm. The Stimulating electrode was placed in the stratum radiatum (SR) close to the CA2 region and the recording electrode was placed in the SR of CA1 region, 500 μm away from the stimulating electrodes. (C) A representative image displays the lentivirus-infected dorsal CA1 region. (D) VSD optical signals were widely increased along the CA1 axis in response to a train of stimuli (five, 0.2 ms current pulses at 100 Hz, 100 μA). The left are representative maximal VSD optical signals (color scale: 0–0.6%). (E) Knockdown of HCN1 enhanced widespread VSD optical signals within a fixed range of fiber volley amplitude (0.1–0.15 mV) compared to shRNA-control-infected group. (F) Representative traces of VSD optical signals from the ROIs, 500 μm away from the stimulating electrode, in response to a train of stimuli (five 0.2 ms current pulses at 100 Hz, 20–100 μA). (G) Knockdown of HCN1 enhanced VSD optical signals at the ROI 500 μm from the stimulating electrode in response to a train of stimuli (five 0.2 ms current pulses at 100 Hz, 20–100 μA). Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with shRNA-control-infected group.
Figure 8. Enhancement of synaptic transmission and BDNF-mTOR signaling by knockdown of HCN1 in the dorsal CA1 region.
(A) Representative traces of field excitatory postsynaptic potentials (fEPSPs) recorded in the median stratum radiatum (SR) of the CA1 region in response to a single stimulation (100 μA, arrow). The slope of fEPSP as a function of stimulation intensity from 20 μA to 100 μA showed significant difference between shRNA-control- and shRNA-HCN1-infected dorsal hippocampal CA1 regions without change in the amplitude of presynaptic fiber volley. (B) Representative traces of fEPSPs evoked by paired pulses at four inter-stimulus intervals (ISI; 20 ms, 50 ms, 100 ms, and 200 ms) in shRNA-control and shRNA-HCN1-infected dorsal hippocampal CA1 region. Paired-pulse ratio was not significantly different between shRNA-control- and shRNA-HCN1-infected dorsal hippocampal CA1 regions. (C) Mature BDNF (mBDNF) protein expression was significantly increased in shRNA-HCN1-infected dorsal CA1 region (n=3) compared to shRNA-control infected group (n=3). mBDNF protein expression was normalized by β-tubulin. Knockdown of HCN1 in dorsal CA1 region (n=6) increased phosphorylation of mTOR compared to shRNA-control-infected group (n=6). Phospho-mTOR protein expression was normalized by total mTOR and β-tubulin. Data are expressed as mean ± SEM. **p < 0.01.
Recently, it has been reported that a low dose of ketamine increased BDNF protein synthesis and activated mTOR signaling pathway, leading to antidepressant-like effect (Autry et al., 2011; Li et al., 2010). In addition, ketamine is also known as an inhibitor of HCN1 channels (Chen et al., 2009). Because we observed that knockdown of HCN1 channels in the dorsal hippocampal CA1 region produced antidepressant-like effect, it is possible that this manipulation also altered BDNF-mTOR signaling pathway. Indeed, knockdown of HCN1 in the dorsal CA1 region resulted in significant increase in mature BDNF expression and phosphorylation of mTOR in dorsal hippocampus (Figure 8C), suggesting possible cellular mechanisms underlying the antidepressant-like effect. Taken together, knockdown of HCN1 in the dorsal hippocampal CA1 region resulted in widespread enhancement of VSD optical signals with an enhancement in synaptic transmission, which is likely associated with the upregulation of BDNF-mTOR signaling.
Discussion
We used a lentiviral shRNA system to locally silence HCN1 gene in the dorsal hippocampus. This resulted in altered physiological properties of individual CA1 neurons, increased synaptic transmission, higher hippocampal CA1 activity during stimulation, and upregulated BDNF-mTOR signaling at the hippocampal network level, and produced anxiolytic- and antidepressant-like behavioral effects in rats.
Lewis et al. reported that three different lines of knockout mice (HCN1, HCN2, and TRIP8b) with elimination or reduction of functional Ih displayed antidepressant-like behaviors (Lewis et al., 2011). To determine which brain regions are important for this antidepressant-like behavior, we developed a lentiviral shRNA-HCN1 system that allowed for focused silencing of the HCN1 gene in a small population of neurons. A single infusion of lentivirus expressing shRNA-HCN1 infected about one-third of the dorsal CA1 region and produced a decrease in HCN1 protein expression and physiological changes consistent with the reduction of Ih. Knockdown of HCN1 channels in the CA1 pyramidal neurons led to changes in the intrinsic membrane properties as well as an increase in cellular excitability measured as a change in the number of action potentials elicited by defined current injections.
Anxiolytic- and antidepressant-like effects by knockdown of HCN1 in the dorsal hippocampus
Anxiety- and depression-like symptoms induced by chronic mild stress can be reversed by acute or chronic administration of antidepressant drugs (Liu et al., 2010; Lu et al., 2006). In a clinical study, chronic administration of serotonin selective reuptake inhibitors (SSRIs) showed therapeutic effects in patients with general anxiety disorder (Ball et al., 2005). Our behavioral data indicated that knockdown of HCN1 in the dorsal hippocampal CA1 region promoted both anxiolytic- and antidepressant-like effects. In the open field test, shRNA-HCN1-infected rats showed significantly more center square entries, longer duration and longer travelled distance in the center square, and increased open arm time and entries in the elevated plus maze test compared to shRNA-control-infected rats. In contrast, TRIP8b and HCN1 knockout mice lacking functional Ih did not display anxiolytic-like behaviors in the elevated plus-maze test and dark/light box test (Lewis et al., 2011). One possibility is that whole brain deletion of TRIP8b or HCN1 gene might have masked the anxiolytic-like effects due to the lack of functional HCN1 protein in other brain regions that are involved in different physiological functions and behavior phenotypes (Nolan et al., 2004; Nolan et al., 2003; Wang et al., 2007). Another possibility is that absence of functional HCN1 protein during development might have evoked some compensatory mechanisms, which mask the anxiolytic-like effects of HCN1 gene deletion in the dorsal hippocampal CA1 region. The observation that knockdown of HCN1 in the dorsal hippocampal CA1 region produced antidepressant-like effects in the forced swim test was similar to that previously found from global knockout animals (Lewis et al., 2011). TRIP8b knockout mice lacking functional Ih showed antidepressant-like behavior as indicated by significantly reduced immobility time in the forced swim and tail suspension tests. This is in general agreement with antidepressant-like effects by knockdown of HCN1 in the dorsal hippocampal CA1 region. Because lentiviral shRNA-HCN1-infected rats displayed increased exploration during 5 min open field test, it is possible that increased exploration attempts might affect passive activity in the forced swim test. However, linear regression analysis from behavior test results indicated that there were no correlation between exploration activity in OFT and duration of passive activity in FST, suggesting specificity for antidepressant-like effect of HCN1 knockdown in the dorsal hippocampal CA1 region.
Widespread enhancement of hippocampal activity by knockdown of HCN1 in dorsal CA1 region
Why does knockdown of HCN1 in a small CA1 region of the dorsal hippocampus produce anxiolytic- and antidepressant-like effects? It is becoming increasingly clear that small populations of neurons in specific regions can mediate certain behaviors (Han et al., 2009; Silva et al., 2009). Moreover, it has been shown that chronic electrical stimulation in limbic-cortical region from patients with treatment-resistant depression reduced pathologically elevated metabolic activity, implying that alternation in limbic-cortical activity may be involved in this treatment of severe depression (Mayberg, 2003; Mayberg et al., 2005). In a functional neuroimaging study, patients with severe depression showed reduced posterior hippocampal volume, suggesting the posterior hippocampus, the equivalent of dorsal hippocampus in rodents, might be involved in both affective status and spatial learning in humans (Campbell and Macqueen, 2004). Indeed, depressed patients showed deficits in spatial learning and memory assessed by a virtual navigation task as compared to healthy subjects (Gould et al., 2007). In a non-clinical study, Airan et al. showed that animal models of depression induced by chronic mild stress displayed increased ventral CA1 activity using voltage sensitive dye imaging, which can be reversed by clinical antidepressant drugs delivered by i.p. injection, indicating that modulation of hippocampal activity might be required for the treatment of depression (Airan et al., 2007). In our experiments, knockdown of HCN1 in the dorsal hippocampal CA1 region resulted in a widespread increase of VSD optical signals in response to afferent stimulation, indicating an enhancement of dorsal hippocampal activity. This discrepancy might be related to brain region, dorsal hippocampus vs. ventral hippocampus, or specificity, knockdown of HCN1 vs. antidepressant drug delivered by i.p. injection. Another possibility is that we consistently placed the stimulating electrode in the middle of stratum radiatum, close to the border between CA1 and CA2 regions, to activate Shaffer collaterals. In contrast to our experimental configuration, the stratum pyramidale was targeted for stimulation in Airan et al.’s study, which might have activated significantly more inhibitory axons. Because we observed behavioral changes with the expression of shRNA-HCN1 in a small CA1 region of the dorsal hippocampus, one concern is that lentivirus might have been transported retrogradely to other brain regions. We found no evidence, however, of GFP positive cells outside of the dorsal hippocampus including the dorsal raphe containing serotonergic cell bodies and the locus coeruleus containing noradrenergic cell bodies (data not shown), suggesting that anxiolytic- and antidepressant-like behaviors are due to knockdown of HCN1 channels in the dorsal hippocampal CA1 region. This is consistent with the report that VSV-G pseudotyped lentivirus did not transport retrogradely in the brain (Louboutin et al., 2007), suggesting local expression in the primary injection area. It has been reported that hippocampal theta waves propagate along the septotemporal axis (Lubenov and Siapas, 2009). Thus, we cannot rule out the possibility that knockdown of HCN1 channels in the dorsal hippocampus influenced the initiation and propagation of theta waves to the ventral region and thereby contributing to the affective state of animals.
Chen et al. reported that ketamine, an NMDA receptor antagonist and clinical anesthetic, inhibited h-channels and reduced total Ih conductance in cortical and CA1 pyramidal neurons by acting on HCN1 subunits (Chen et al., 2009). In agreement with our results, a sub-anesthetic dose of ketamine, which would inhibit HCN1 containing h-channels, produced early onset and long lasting therapeutic antidepressant effects in both animal models of depression and in patients with treatment-resistant depression (Autry et al., 2011; Berman et al., 2000; Li et al., 2010). This ketamine-mediated antidepressant-like effect depends on BDNF protein synthesis and activation of mTOR pathway (Autry et al., 2011; Li et al., 2010). Consistent with these findings, lentiviral shRNA-HCN1-infected dorsal CA1 region showed significantly increased mature BDNF protein expression and phosphorylation of mTOR, indicating upregulation of BDNF-mTOR signaling pathway. This upregulation of BDNF-mTOR signaling may be mediating the enhancement of hippocampal activity, the increase in synaptic transmission and the alteration in behaviors.
Ketamine is known as a psychotomimetic agent producing hallucination, delirium, and confusion (Remerand et al., 2007; Webster and Walker, 2006). In addition, chronic administration of clinical antidepressant drugs increased the risk of seizures in depressed patients (Alldredge, 1999; Peck et al., 1983; Salzberg and Vajda, 2001). Thus, another alternative therapy to treat depression is invaluable. In our VSD imaging and field potential recording, we did not observe any abnormal epileptic-like activity in the shRNA-HCN1-infected CA1 region, suggesting local silencing of HCN1 expression might provide an alternative treatment to existing pharmacological interventions for treating depression and anxiety disorders.
Experimental procedures
Animals
Male Sprague Dawley rats (4- to 12-week-old) were used for these experiments. Rats were housed 2–3 per cage in a temperature (25°C) and light (12 hour light/12 hour dark cycles) controlled room. All procedures were carried out in accordance to the University of Texas at Austin Institutional Animal Care and Use Committee (IACUC).
Generation of lentivirus
Lentivirus production is described in supplemental experimental procedures.
Stereotaxic microinjection
Stereotaxic microinjection is described in supplemental experimental procedures.
Biochemical assay
Western blotting and immunohistochemistry are described in supplemental experimental procedures.
Dorsal hippocampal slice preparation and electrophysiology
4- to 5-week old rats were bilaterally microinjected with lentivirus expressing shRNA-control or shRNA-HCN1 in the dorsal hippocampal CA1 region. After behavior test, dorsal hippocampal slices (350 μm) were prepared from 10- to 12-week-old lentivirus-infected male Sprague-Dawley rats. Then slices were transferred to a holding chamber for 20–30 min at 35°C containing (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 2 MgCl2, 10 dextrose, 1.3 ascorbic acid, and 3 sodium pyruvate, bubbled with 95% O2 - 5% CO2. Whole-cell current-clamp recordings were performed on slices submerged in a recording chamber filled with aCSF heated to 32–34 °C flowing at a rate of 1 to 2 ml/min. ACSF contained (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, and 12.5 dextrose, bubbled with 95% O2 - 5% CO2. Lentivirus-infected CA1 pyramidal neurons were visualized using a microscope (Zeiss Axioskop) fitted with differential interference contrast optics and a GFP filter set (Stuart et al., 1993). Patch pipettes (4–7 MΩ) were prepared with capillary glass (external diameter.65 mm, World Precision Instruments) using Flaming/Brown micropipette puller and filled with an internal solution containing (in mM) 120 K-gluconate, 20 KCl, 10 HEPES, 4 NaCl, 7 K2-phosphocreatine, 4 Mg-ATP, 0.3 Na-GTP (pH 7.3 with KOH). Neurobiotin (Vector Labs) was used (0.1–0.2 %) for subsequent histological processing (DAB staining). Data were acquired with a Dagan BVC-700A amplifier (Dagan, Minneapolis, MN) and were filtered at 3 kHz, sampled at 10 kHz, and digitized by an ITC-18 interface (Instructech Corporation, Port Washington, NY) connected to a computer running custom software written in IGOR Pro (Wavemetrics). Data analyses were also performed with custom software written in IGOR Pro. The experiments were performed under blind conditions. No correction for liquid junction potential.
Behavioral procedures
122 rats (9- to 12-week) were assigned into seven groups as follows; Group I: saline i.p. injected (n=17), group II: vehicle (0.5 % Tween-20 in saline) i.p. injected (n=13), group III: diazepam i.p. injected (1 mg/kg, n=18), group IV: ketamine i.p. injected (15 mg/kg, n=13), group V: Fluoxetine i.p. injected (10 mg/kg, n=13), group VI: lentivirus expressing shRNA-control microinjected into the dorsal hippocampal CA1 region (n=24), group VII: lentivirus expressing shRNA-HCN1 microinjected into the dorsal hippocampal CA1 region (n=24). All behavior evaluations were done blindly. Experiment was performed in noise and light controlled behavior room. Custom written programs were used to analyze animal behaviors (http://clm.utexas.edu/djlab).
Open field test
The apparatus was consisted of opaque and matte finished black acrylic sheet (36″ × 36″ × 24″). Each rat was randomly assigned and placed into the center of the field following 30 min saline, vehicle, and diazepam i.p. injection. Behavior in the open field arena was recorded for 6 min using a CCD camera. The surface of the open field arena was cleaned with 70% EtOH in order to remove permeated odors by previous animals after each trial. To analyze behaviors for the last five minutes, the open field arena was divided into 9 equal-size squares (12″ by 12″). Basal exploration activity was measured by total travelled distance (inch) and anxiety level was assessed by the number of center square entries, the duration, and the travelled distance in the center square. A line crossing was defined as the body center crosses a line.
Elevated plus maze test
The apparatus was made of black acrylic sheet. Four arms (50-cm long and 10- cm wide) are connected and elevated to a height of 50-cm from the floor. Two arms are open (open arms) and the other two arms are enclosed within 40-cm walls. Each rat was placed into the intersection of the four arms (center area) 30 min following saline, vehicle, or diazepam i.p. injection. Behavior in elevated plus maze was recorded for 6 min using a CCD camera. The surface of the elevated plus maze was cleaned with 70% EtOH in order to remove permeated odors by previous animals after each trial. To analyze behavior test for 6 min, the number of arm entries and the percentage of open arm time (duration in open arm/total time) were assessed. Arm entry was defined as 50% of the body being positioned within the arm.
Forced swim test
The tank is made of transparent Plexiglas cylinder (80 cm tall × 30 cm in diameter) filled with water (23–24 °C) to a depth of 45 cm. Water in the tank was changed after each trial. For the first exposure, rats without drug treatment were placed in the water for 15 min (pre-test session). Twenty-four hours later, rats were placed in the water again for a 6 min session (test session) 30 min following saline, vehicle, diazepam (1 mg/kg), ketamine (15 mg/kg), or fluoxetine (10 mg/kg) i.p. injection. Forced swim test was recorded by a video cameras positioned on the top of the water tank. A passive activity was defined as floating and making only those movements necessary to keep the nose above the water. Behaviors from forced swim test was quantified by using a time sampling technique to rate the predominant behavior over a 5-sec interval as described (Lucki, 1997) and a custom written program.
Voltage sensitive dye imaging and extracellular field potential recording
4- to 5-week old rats were bilaterally microinjected with lentivirus expressing shRNA-control or shRNA-HCN1 in the dorsal hippocampal CA1 region. After behavior test, dorsal hippocampal slices (350 μm) were prepared from 10- to 12-week-old lentivirus-infected male Sprague-Dawley rats. Slices were stained with the voltage-sensitive dye RH-414 (Molecular probes) for at least 30 min (0.15 μM in aCSF). Recording pipettes (1–2 MΩ) were filled with aCSF and placed in the stratum radiatum (SR) of the CA1 region. Synaptic responses were evoked by a glass stimulating electrode placed in the stratum radiatum near the border between the CA1 and the CA2 regions. The filter set is consisted of a 510–560 nm excitation filter, a 590 nm longpass emission filter, and a 590 nm longpass dichroic mirror. Optical signals were sampled at 1 kHz with a fast CCD camera (CCD-SMQ; RedShirtImaging, GA). Custom software written in C++ was used to control the camera, the amplifier and to analyze the optical and field potential signals. All optical signals were displayed as the change in fluorescence divided by resting fluorescence (ΔF/F). Average of four trials was analyzed. The experiments were performed under blind conditions.
Data Analysis
Quantification of data from immunohistochemistry and western blotting was determined by optical density analysis using the ImageJ program. Input resistance was measured by the slope of the linear fit of the V-I plot between +10 and −150 pA current injection. Voltage sag was calculated as the ratio of the maximum voltage change to the steady-state voltage change resulting from hyperpolarizing current injections. Slow time constant was calculated from a double-exponential fit of the averaged voltage decay resulting from 100 trials of identical 1-ms, 400-pA current injections. Resonance frequency was measured as the frequency of the peak impedance using a sinusoidal current injection of constant amplitude and linearly spanning 0–15 Hz in 15 sec. Temporal summation ratio was measured as the amplitude of the fifth αEPSP relative to the first in a train of five αEPSPs at 20 Hz [(αEPSP5 −αEPSP1)/α EPSP1]. Paired-pulse ratio (PPR) was calculated as the ratio of the slope of the second fEPSP to the slope of the first fEPSP. The slope of fEPSP was measured by the initial part of fEPSP (0.5 ms). Lentivirus-infected rats were excluded from behavior results if GFP expression is not limited in the dorsal CA1 region.
Statistical Analysis
All data were expressed as mean ± SEM. The data from whole-cell current-clamp recordings were analyzed using unpaired t-test. Unpaired t-test and one-way ANOVA were used for the analysis of behavioral results followed by Tukey post hoc test. Two-way ANOVA was used for the analysis of VSD optical signals and field potentials followed by Bonferrori post hoc test. Biochemical results were analyzed using unpaired t-test. P<0.05 was considered as statistically significant.
Supplementary Material
Highlights.
Knockdown of HCN1 led to physiological changes consistent with a reduction of Ih
Knockdown of HCN1 in the dorsal CA1 region increased BDNF-mTOR signaling.
Dorsal hippocampal CA1 activity was enhanced by knockdown of HCN1.
Antidepressant- and anxiolytic-like effects were produced by knockdown of HCN1.
Acknowledgments
This work was supported by National Institutes of Health grant MH48432 (D.J.). We thank Drs. Rick Gray, Randy Chitwood, Nikolai Dembrow, Darrin Brager, Kelly Dougherty, Brian Kalmbach, and Yul Young Park for reviewing the manuscript, providing helpful comments, and giving technical support during this study. We also thank Brandy Routh, Ann Clemens, Sachin Vaidya, and Andrea Haessly Dickson for giving helpful comments on the manuscript. We indebted to the labs of Drs. Aldrich, Mauk, and Raab-Graham for their consistent support of this study, and to Dr. Dane Chetkovich for HCN1 and HCN2 antibodies and Dr.Paul Pfaffinger for the lentiviral vector.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Alldredge BK. Seizure risk associated with psychotropic drugs: clinical and pharmacokinetic considerations. Neurology. 1999;53:S 68–75. [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011 doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SG, Kuhn A, Wall D, Shekhar A, Goddard AW. Selective serotonin reuptake inhibitor treatment for generalized anxiety disorder: a double-blind, prospective comparison between paroxetine and sertraline. J Clin Psychiatry. 2005;66:94–99. doi: 10.4088/jcp.v66n0113. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Borelli KG, Nobre MJ, Brandao ML, Coimbra NC. Effects of acute and chronic fluoxetine and diazepam on freezing behavior induced by electrical stimulation of dorsolateral and lateral columns of the periaqueductal gray matter. Pharmacol Biochem Behav. 2004;77:557–566. doi: 10.1016/j.pbb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex. 2007;17:702–712. doi: 10.1093/cercor/bhk021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chang PY, Taylor PE, Jackson MB. Voltage imaging reveals the CA1 region at the CA2 border as a focus for epileptiform discharges and long-term potentiation in hippocampal slices. J Neurophysiol. 2007;98:1309–1322. doi: 10.1152/jn.00532.2007. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009;29:600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Fernandez T, Nakamura K, Gross CG. Functional differentiation along the anterior-posterior axis of the hippocampus in monkeys. J Neurophysiol. 1998;80:1002–1005. doi: 10.1152/jn.1998.80.2.1002. [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for str-ess-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. Distinguishing anxiolysis and hyperactivity in an open space behavioral test. Behav Brain Res. 2010;207:84–98. doi: 10.1016/j.bbr.2009.09.042. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, Burgess N, Manji HK, Zarate CA., Jr Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry. 2007;164:516–519. doi: 10.1176/ajp.2007.164.3.516. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi F, Wittchen HU, Holting C, Hofler M, Pfister H, Muller N, Lieb R. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS) Psychol Med. 2004;34:597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2011;72:341–348. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Vaidya SP, Blaiss CA, Liu Z, Stoub TR, Brager DH, Chen X, Bender RA, Estep CM, Popov AB, et al. Deletion of the Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Auxiliary Subunit TRIP8b Impairs Hippocampal Ih Localization and Function and Promotes Antidepressant Behavior in Mice. J Neurosci. 2011;31:7424–7440. doi: 10.1523/JNEUROSCI.0936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-Derived Neurotrophic Factor Val66Met Allele Impairs Basal and Ketamine-Stimulated Synaptogenesis in Prefrontal Cortex. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele E, Strayer DS. Strategies for CNS-directed gene delivery: in vivo gene transfer to the brain using SV40-derived vectors. Gene Ther. 2007;14:939–949. doi: 10.1038/sj.gt.3302939. [DOI] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999a;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendrites. London: Oxford University Press; 1999b. Dendritic voltage-gated ion channels; pp. 139–155. [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Morrish AC, Gorzalka BB. Local enhancement of cannabinoid CB1 receptor signalling in the dorsal hippocampus elicits an antidepressant-like effect. Behav Pharmacol. 2007;18:431–438. doi: 10.1097/FBP.0b013e3282ee7b44. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron. 2007;56:1061–1075. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarizationactivated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- Padovan CM, Guimaraes FS. Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats. Pharmacol Biochem Behav. 2004;77:15–19. doi: 10.1016/j.pbb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Peck AW, Stern WC, Watkinson C. Incidence of seizures during treatment with tricyclic antidepressant drugs and bupropion. J Clin Psychiatry. 1983;44:197–201. [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Remerand F, Couvret C, Pourrat X, Le Tendre C, Baud A, Fusciardi J. Prevention of psychedelic side effects associated with low dose continuous intravenous ketamine infusion. Therapie. 2007;62:499–505. doi: 10.2515/therapie:200802. [DOI] [PubMed] [Google Scholar]
- Salzberg MR, Vajda FJ. Epilepsy, depression and antidepressant drugs. J Clin Neurosci. 2001;8:209–215. doi: 10.1054/jocn.2000.0896. [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawalla SB, Farabaugh A, Johnson MW, Morray M, Delgado ML, Pingol MG, Rosenbaum JF, Fava M. Fluoxetine treatment of depressed patients with comorbid anxiety disorders. J Psychopharmacol. 2002;16:215–219. doi: 10.1177/026988110201600304. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Tominaga Y, Yamada H, Matsumoto G, Ichikawa M. Quantification of optical signals with electrophysiological signals in neural activities of Di-4-ANEPPS stained rat hippocampal slices. J Neurosci Methods. 2000;102:11–23. doi: 10.1016/s0165-0270(00)00270-3. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Webster LR, Walker MJ. Safety and efficacy of prolonged outpatient ketamine infusions for neuropathic pain. Am J Ther. 2006;13:300–305. doi: 10.1097/00045391-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Keating K, Dolman C, Thorpe R. Characterization of complete particles (VSV-G/SIN-GFP) and empty particles (VSV-G/EMPTY) in human immunodeficiency virus type 1-based lentiviral products for gene therapy: potential applications for improvement of product quality and safety. Hum Gene Ther. 2008;19:475–486. doi: 10.1089/hum.2007.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.