Abstract
Introduction
Juvenile polyposis (JP) is an autosomal dominant disease that predisposes to GI malignancies. Germline mutations in the tumor suppressor gene SMAD4 account for approximately 20% of JP cases. SMAD4 is the common intracellular mediator of the TGF-β and bone morphogenetic protein (BMP) pathways. Since mutations in BMP receptor 1A also cause JP, we hypothesize that altered BMP signaling is the underlying defect in JP. We therefore set out to investigate the effect of SMAD4 mutations on BMP signaling.
Methods
SMAD4 mutations identified in JP patients were selected for analysis. These were created in SMAD4 pCMV expression vectors (EV) using a PCR-based, site-directed mutagenesis (SDM) approach. SDM clones were confirmed by direct sequencing, then co-transfected with an IdI-BMP Luciferase Responsive Element (BRE-Luc) vector and Renilla control vector into HEK-293T cells. Lysates were then collected after 48 hours, and luciferase activity was quantified using a luminometer. A pCMV empty vector was used as a negative control, and its luciferase activity was considered the baseline for cellular BMP signaling. Results obtained for each SDM clone were compared to those with the wild type (WT) vector. Statistical analysis was performed with the Student’s t-test.
Results
Eleven distinct mutations from 16 JP patients were analyzed; seven mutations were nonsense, and four were missense. Both type of mutations resulted in reduction of BMP signaling; missense mutations produced an 8–30% reduction in luciferase activity, whereas nonsense mutations led to 30–60% reduction in luciferase activity when compared to the WT clone (Figure 1). All nonsense mutations led to significantly reduced activity relative to WT (P < 0.05), while the reduction in signaling seen in missense mutations was not statistically significant.
Conclusion
SMAD4 germline mutations as seen in the JP patients appear to negatively impact downstream BMP signaling. Nonsense mutations resulted in significantly reduced luciferase activity when compared to missense mutations. These results support the hypothesis that disruption of the BMP signaling pathway is the likely etiology of JP in patients with SMAD4 mutations.
Keywords: juvenile polyposis, SMAD4, bone morphogenetic protein (BMP)
INTRODUCTION
Juvenile polyposis (JP) is an autosomal dominant disorder with a prevalence of 1 in 100,000 individuals [1]. Affected patients develop hamartomatous polyps in the gastrointestinal tract, most notably in the colorectum and stomach [2]. Although these polyps are typically benign in nature, there is a potential for malignancy, with the risk of developing colon cancer estimated to be up to 50% over a patient’s lifetime [3–5].
Two genes have previously been linked to the development of this disease: SMAD4, located on chromosome 18q21.1 [6, 7], and BMPR1A, located on 10q22–23 [8]. Mutations of these genes are responsible for approximately 40% of reported JP cases [9]. Both of these genes are in the bone morphogenetic protein (BMP) pathway, which is part of the transforming growth factor-β (TGF-β) superfamily. The BMP pathway plays a role in various cellular processes, including differentiation, development, reproduction, and apoptosis [10–12]. Since germline mutations in SMAD4 and BMPR1A cause JP, and these genes are both involved in the bone morphogenetic protein (BMP) signaling pathway, aberrant BMP signaling is implicated in the evolution of JP.
Uncovering the effect of specific JP patient mutations on BMP signaling is important to better delineate the mechanism of polyp formation and cancer predisposition. SMAD4, the intracellular mediator of BMP signaling, forms hetero-oligomers with SMADs 1,5 and/or 8 after their phosphorylation by ligand-activated BMPR1A. The aim of this study was to recreate mutations found in JP probands in an expression vector, and to evaluate their effect on BMP signaling in an in vitro model.
MATERIALS AND METHODS
Reporter Plasmids
Germline SMAD4 mutations found in JP patients [9] were recreated in a SMAD4 pCMV6-XL5 expression vectors (Origene, Rockville, MD). A PCR-based, site-directed mutagenesis (SDM) approach was used to generate the individual mutations. Primers containing specific patient mutations were designed using the QC Primer Design Software (Stratagene). PfuUltra (Stratagene) was then used to amplify mutant constructs under the following conditions: 95° for 30 s, 65° for 1 min, and then 7 min at 68° for 18 cycles. E. coli bacterial cells were then transformed with the mutant expression vectors. For each mutation, 10 colonies of the transformed E. coli cells were selected at random, and were allowed to propagate 12 h in an orbital shaker at 37° in 5 mL of Luria Burtani media supplemented with 100 ug/mL of ampicillin. Plasmid DNA was then extracted using the PureLink Quick Plasmid Miniprep Kit (Invitrogen, Carlsbad, CA). The plasmid DNA was then sequenced to verify successful creation of each mutation.
Cell Culture and Transfection
Human embryonic kidney cells (HEK-293T), obtained from American Type Culture Collection (Manassas, VA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin G-streptomycin sulfate (PS). After confluence of 90% was achieved, the cells were cotransfected with 1 ug of the BRE-Luc vector (a plasmid reporter vector with a BMP responsive element promoter cloned upstream from a luciferase gene), 1 ug of the SMAD4 expression vector (wild-type or mutants), and 200 ng of Renilla (internal control vector for transfection efficiency), using 6 uL Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). Each transfection was performed in triplicate. After 4 h, fresh media was added.
Luciferase Assays
Forty-eight hours post-transfection, 500 uL of lysis buffer was added to the cells, which were incubated at room temperature for 15 min. Twenty uL of lysate was transferred to a reading tube that contained 100 uL of the Luciferase Assay Reagent II (LARII) solution, and luciferase activity was measured at 562 nm for 10 s using a TD 20/20 luminometer (Turner BioSystems, Sunnyvale, CA). Once the initial readings were performed, 100 uL of Stop and Glo reagent (Promega, Madison, WI) was added, and Renilla luciferase activity measured at 480 nm for 10 s. The final amount of luciferase activity for each construct was determined by subtracting the background luciferase activity from the control pGL3 basic vector without construct, and then normalizing to the Renilla luciferase activity for each individual reaction. A Student’s t-test was then performed to assess the statistical significance of differences between triplicate results obtained for each mutant construct relative to the wild-type.
RESULTS
In our juvenile polyposis database of 119 probands, 22 patients with SMAD4 mutations were identified. Six of these patients shared one mutation (Exon 9: Del AGAC 1244-47, D415EfsX20), while two others shared substitutions of nucleotide 1081. After accounting for the duplication of these mutations, 14 distinct mutations were identified. One mutation was a splice site variant, and was not further evaluated. Eleven of these mutations were re-created by SDM in the SMAD4 expression vector. Seven of these mutations were non-sense: 608delC (P203HfsX38, Exon 4); 1037delC (P346LfsX38, Exon 8); 1162C > T (E388X, Exon 9); 1193G > A (W398X, Exon 9); 1244_7delACAG (D415EfsX20, Exon 9); 1343_65del22 (Q448QfsX37, Exon 10); and 1588delC (R530TfsX7, Exon 11). Four mutations were missense: 989A > G (E330G, Exon 8); 1081C > A (R361S, Exon 8); 1393G > A (V465M, Exon 10); 1525T > A (W509R, Exon 11).
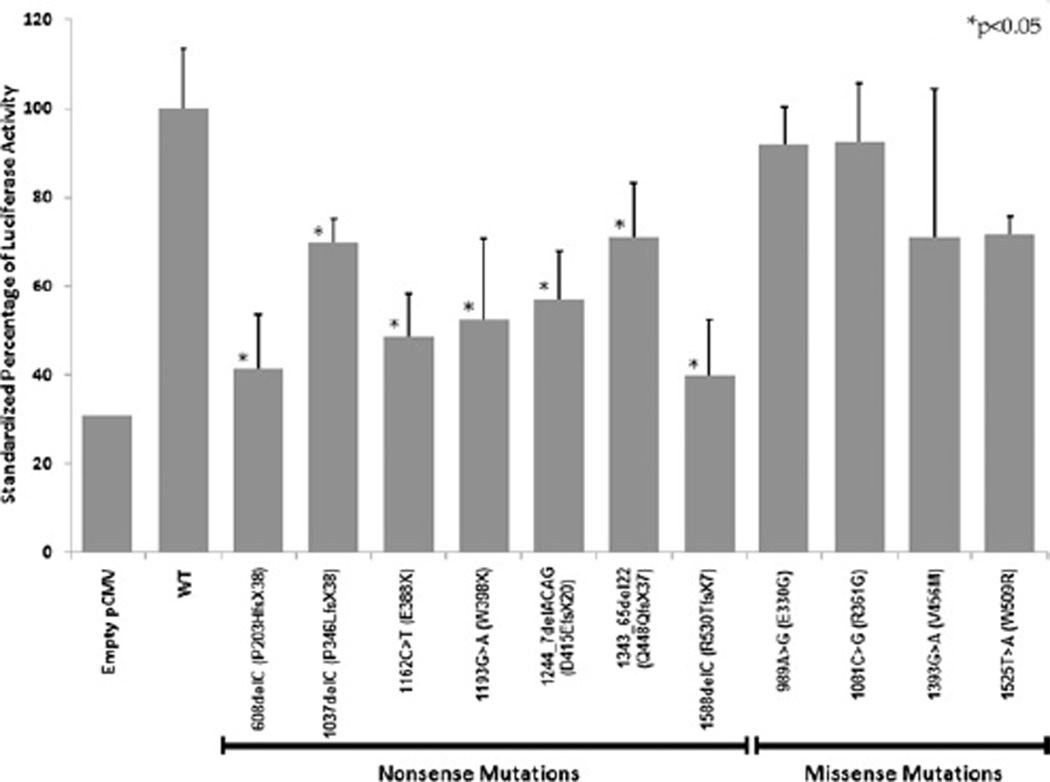
Luciferase activity from the different constructs is shown in Fig. 1. The light units produced by the wild-type (WT) expression vector (standardized to the Renilla control) were set at 100%. The negative control, which consisted of a pCMV vector without an insert, produced 31% of the wild-type light units, indicating the baseline level of BMP signaling mediated through endogenous cellular SMAD4. As a group, the non-sense mutations led to a 30%–60% reduction in luciferase activity compared with the wild-type vector. More specifically, the 608delC mutation led to a 59%reduction in luciferase activity, 1037delC was reduced 30%, 1162 C > T 52%, 1193 G > A 48%, 1244_7delACAG 33%, 1343_65del22 29%, and 1588delC 61% (P < 0.05 for all constructs).
FIG. 1.
The effect of SMAD4 mutations on BRE-Luc luciferase activity. Asterisks denote values that were statistically significantly different from wild-type (P < 0.05).
The missense mutations led to lesser reductions in BMP signaling, ranging from 8%–30%. The 989 A > G (E330G) substitution led to an 8% reduction, 1081 C > A (R361S) an 8% reduction, 1393 G > A (V465M) a 30% reduction, and 1525 T > A (W509R) a 30% reduction in luciferase. These differences were not statistically significant from that found for the wild-type vector.
DISCUSSION
Bone morphogenetic proteins (BMPs) are part of the transforming growth factor-β superfamily, and were first identified by Urist in 1965 to induce post-natal osteogenesis by an autoinduction pathway [13]. BMPs are secreted in an active form, and bind to their associated cellular receptors, the BMP type I and type II receptors. BMPs bind first to the ligand-specific type II receptor, which then recruits and phosphorylates the type I receptor. This complex of the type I and II receptor is then responsible, via its serine-threonine kinase activity, for initiation of an intracellular signaling cascade via the SMAD proteins [8].
SMAD proteins are the intracellular effectors of the BMP signaling pathway. The receptor-SMADs (R-SMADs) are phosphorylated by the receptor complex activity; R-SMADs specific to the TGF-β pathway are SMADs 2 and 3, whereas R-SMADs for the BMP pathway are SMADs 1, 5, and 8 [14]. The R-SMADs then associate with the common-SMAD (Co-SMAD), SMAD4. These proteins then translocate to the nucleus, associate with DNA binding proteins, and serve as transcription factors for gene expression via direct binding to specific SMAD binding elements (SBEs) in DNA [15].
BMPs are implicated in many cellular processes, including differentiation of cell lines. Therefore, it is plausible that changes in BMP signaling may play a role in cancer evolution. Thawani et al. reviewed BMPs and their role in cancer, which are thought to induce epithelial cells to change to a mesenchymal-like cell [16]. In embryogenesis, this epithelial-to-mesenchymal transition (EMT) causes migration of cells for formation of different organ systems. However, when this type of change occurs post-development, the cell’s invasive and metastatic potential increases [17]. For example, in non-small-cell lung cancer (NSCLC) and mammary cancer cell lines, BMP2 increased cellular migration, and BMP4 was implicated in increased invasive potential. In samples from osteosarcomas, tumors that metastasized had increased expression of BMP receptors as compared to their non-metastasizing counterparts [16].
To evaluate BMP signaling, we chose a well-described vector, containing a BMP responsive element (BRE) upstream from the luciferase gene. Korchynskyi and ten Dijke created this luciferase vector by cloning the most active SBEs in the inhibitor of differentiation (Id1) promoter, which is activated by BMP signaling [10]. They showed that this vector is activated only by BMPs and not TGF-β. Follow-up studies by Logeart-Avramoglou confirmed specificity to the BMP pathway, and demonstrated 100-fold increased sensitivity and decreased time requirements over previous alkaline phosphatase assays [18]. The usefulness of this vector has been validated in studies of the effects of BMP-6 on angiotensin II [19], the efficacy of chimeric activin-BMP receptors on signaling [20], and osteoblast differentiation [21].
In this study, SMAD4 germline mutations as seen in JP patients negatively impacted downstream BMP signaling. Both non-sense and missense mutations resulted in reduction of BMP signaling, with non-sense mutations leading to a significant decrease in luciferase activity, while the decreases in luciferase activity in the missense mutation group were not statistically significant. The decrease in luciferase activity exhibited in the non-sense mutations is likely due to the nature of the mutation. As these mutations lead to premature stop codons, these proteins become truncated, making them more susceptible to proteasomal degradation, transcripts to non-sense-mediated decay, and to deleterious effects in binding to R-SMADs or DNA. The significance of missense mutations is less clear, and depends on their location and effect on protein structure, which is more difficult to predict. Moren et al. studied four missense mutations in the amino-terminal domain of SMAD4, and found that all could bind R-SMADs, but had reduced DNA binding and protein stability. Furthermore, two did not translocate to the nucleus well and showed reduced TGF-β signaling [22]. It is possible that we did not detect difference in BMP signaling with missense mutations because the specific changes did not disrupt binding to the specific Id1 promoter SBEs in the Bre-Luc vector, or that stimulation of transfected cells with BMPs could be necessary to detect more subtle changes in BMP signaling.
Our study confirms thatSMAD4mutations lead to decreased BMP signaling. Both predisposing genes are members of the BMP pathway and, therefore, decreased BMP signaling is likely the key element in the etiology of juvenile polyposis in our population. Further study of BMP signaling alterations would be warranted in other cancers as well, as they may be an underappreciated mechanism of invasion and metastasis.
REFERENCES
- 1.Burt RW, Bishop DT, Lynch HT, et al. Risk and surveillance of individuals with heritable factors for colorectal cancer. WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68:655. [PMC free article] [PubMed] [Google Scholar]
- 2.Jass J. Pathology of polyposis syndromes with special reference to juvenile polyposis. In: Utsonomiya JL, Lynch HT, editors. Hereditary colorectal cancer. Tokyo: Springer Verlag; 1990. pp. 343–350. [Google Scholar]
- 3.Howe JR. Juvenile polyposis syndrome. In: Scriver CR, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 805–818. [Google Scholar]
- 4.Haggitt RC, Reid BJ. Hereditary gastrointestinal polyposis syndromes. Am J Surg Pathol. 1986;10:871. doi: 10.1097/00000478-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 5.van Hattem WA, Brosens LA, de Leng WW, et al. Large genomic deletions of SMAD4, BMPR1A and PTEN in juvenile polyposis. Gut. 2008;57:623. doi: 10.1136/gut.2007.142927. [DOI] [PubMed] [Google Scholar]
- 6.Howe JR, Ringold JC, Summers RW, et al. A gene for familial juvenile polyposis maps to chromosome 18q21.1. Am J Hum Genet. 1998;62:1129. doi: 10.1086/301840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe JR, Roth S, Ringold JC, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 8.Howe JR, Bair JL, Sayed MG, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- 9.Calva-Cerqueira D, Chinnathambi S, Pechman B, et al. The rate of germline mutations and large deletions of SMAD4 and BMPR1A in juvenile polyposis. Clin Genet. 2009;75:79. doi: 10.1111/j.1399-0004.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 10.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 11.Chen YG, Lui HM, Lin SL, et al. Regulation of Cell Proliferation, Apoptosis, and Carcinogenesis by Activin. Exp Biol Med (Maywood) 2002;227:75. doi: 10.1177/153537020222700201. [DOI] [PubMed] [Google Scholar]
- 12.Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 13.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 14.ten Dijke P, Korchynskyi O, Valdimarsdottir G, et al. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Blanco Calvo M, Bolos Fernandez V, Medina Villaamil V, et al. Biology of BMP signalling and cancer. Clin Transl Oncol. 2009;11:126. doi: 10.1007/s12094-009-0328-8. [DOI] [PubMed] [Google Scholar]
- 16.Thawani JP, Wang AC, Than KD, et al. Bone morphogenetic proteins and cancer: Review of the literature. Neurosurgery. 2010;66:233. doi: 10.1227/01.NEU.0000363722.42097.C2. [DOI] [PubMed] [Google Scholar]
- 17.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 18.Logeart-Avramoglou D, Bourguignon M, Oudina K, et al. An assay for the determination of biologically active bone morphogenetic proteins using cells transfected with an inhibitor of differentiation promoter-luciferase construct. Anal Biochem. 2006;349:78. doi: 10.1016/j.ab.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Otani H, Otsuka F, Inagaki K, et al. Aldosterone breakthrough caused by chronic blockage of angiotensin II type 1 receptors in human adrenocortical cells: Possible involvement of bone morphogenetic protein-6 actions. Endocrinology. 2008;149:2816. doi: 10.1210/en.2007-1476. [DOI] [PubMed] [Google Scholar]
- 20.Korupolu RV, Muenster U, Read J, et al. Activin A/bone morphogenetic protein (BMP) chimeras exhibit BMP-like activity and antagonize activin and myostatin. J Biol Chem. 2008;283:3782. doi: 10.1074/jbc.M704530200. [DOI] [PubMed] [Google Scholar]
- 21.Jang WG, Kim EJ, Lee KN, et al. AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophys Res Commun. 2011;404:1004. doi: 10.1016/j.bbrc.2010.12.099. [DOI] [PubMed] [Google Scholar]
- 22.Moren A, Itoh S, Moustakas A, et al. Functional consequences of tumorigenic missense mutations in the amino-terminal domain of Smad4. Oncogene. 2000;19:4396. doi: 10.1038/sj.onc.1203798. [DOI] [PubMed] [Google Scholar]