SUMMARY
Humans are metacognitive: they monitor and control their cognition. Our hypothesis was that neuronal correlates of metacognition reside in the same brain areas responsible for cognition, including frontal cortex. Recent work demonstrated that non-human primates are capable of metacognition, so we recorded from single neurons in the frontal eye field, dorsolateral prefrontal cortex, and supplementary eye field of monkeys (Macaca mulatta) that performed a metacognitive visual-oculomotor task. The animals made a decision and reported it with a saccade, but received no immediate reward or feedback. Instead, they had to monitor their decision and bet whether it was correct. Activity was correlated with decisions and bets in all three brain areas, but putative metacognitive activity that linked decisions to appropriate bets occurred exclusively in the SEF. Our results offer a survey of neuronal correlates of metacognition and implicate the SEF in linking cognitive functions over short periods of time.
INTRODUCTION
We not only perform cognitive functions, we also evaluate and alter them. For example, after creating a lecture, we may reflect on how we organized its content. If the lecture is not ready yet, we may think about how to improve its logical structure. Monitoring and controlling cognitive processes is called metacognition (Flavell, 1976).
Researchers have incorporated metacognition into psychological frameworks (Nelson and Narens, 1990) and attempted to localize its neuronal basis in the human brain. Metacognitive skills are impaired in patients with lesions of medial and lateral frontal cortex (Pannu et al., 2005; Schnyer et al., 2004) and in subjects who experience transcranial magnetic stimulation over dorsolateral prefrontal cortex (Rounis et al., 2010). Functional magnetic resonance imaging has implicated multiple brain regions involved in metacognition, including dorsolateral prefrontal cortex (Kikyo et al., 2002) medial prefrontal cortex (Chua et al., 2006) and cingulate cortices (Chua et al., 2006; Kikyo et al., 2002).
Little is known about how the brain encodes metacognitive processes at the single neuron level. An animal model would facilitate such research, and recent behavioral studies have provided evidence for some degree of metacognition in rats (Foote and Crystal, 2007), dolphins (Smith et al., 1995), rhesus monkeys (Hampton, 2001; Smith et al., 1998), and orangutans (Suda-King, 2008). When offered the chance to take a test or decline it, these animals may opt-out on relatively difficult trials, ensuring a small reward rather than risking no reward if they take the test and fail it. Gorillas, chimpanzees, bonobos, orangutans (Call, 2010), and rhesus monkeys (Hampton et al., 2004) seek information to improve future decisions, an example of metacognitive control, and rhesus monkeys can be trained to bet whether a past decision was correct or incorrect, an example of metacognitive monitoring (Kornell et al., 2007). We recently designed a streamlined version of such a betting task that involves visual stimuli and saccadic eye movement reports, and we reported evidence that monkeys can monitor their own decisions (Middlebrooks and Sommer, 2011).
Here we recorded from single neurons in macaque frontal cortex during the betting task to search for neuronal activity related to metacognition, which we hypothesized may co-localize with neuronal activity related to cognition. Only two studies previously recorded single neuron activity related to possible metacognitive processing. Kiani and Shadlen (2009), using an opt-out task, reported that neuronal activity in monkey lateral intraparietal cortex correlated with choices to abort a task. Kepecs et al. (2008), using a delayed reward task, showed that activity in rat orbitofrontal cortex predicted whether an animal would opt out of waiting for reward after an incorrect decision. Our task is fundamentally different from the opt-out tasks used in both prior studies. A monkey had to make a decision and then place a bet on the correctness of that decision (Figure 1a). Appropriate wagers required retrospective monitoring, a metacognitive process. Every trial contained the same sequence of task events, and every trial required the monitoring of decisions, allowing us to directly compare activity between trials to identify neuronal correlates of decision-making, wagering, and monitoring.
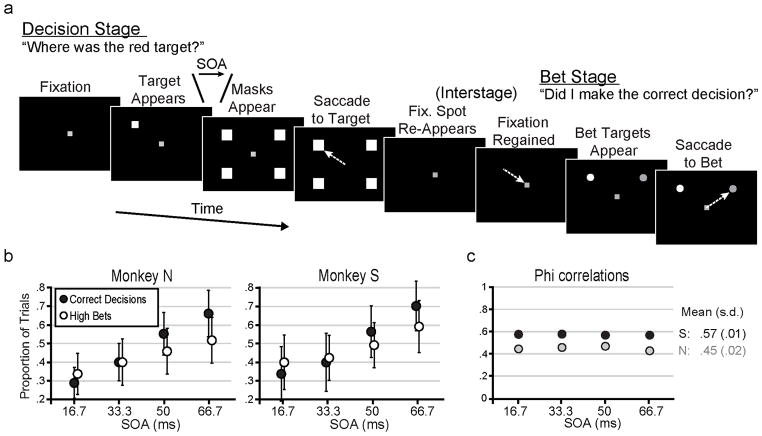
Figure 1.
Task and behavior. (a) Each trial consisted of a Decision Stage and a Bet Stage, separated by an interstage period. In the Decision Stage, monkeys foveated a fixation spot, a target appeared at one of four locations, and after a variable stimulus onset asynchrony (SOA), masks appeared at the four locations. A correct decision (shown) was made if a saccade (arrow) went to the target location. A saccade to any other location was an incorrect decision (not shown). During the interstage period, the fixation spot re-appeared and monkeys foveated it to initiate the Bet Stage. Two bet targets appeared and monkeys placed their bet by making a saccade to one of them. They received the outcome of the bet (reward or timeout) to end the trial. (b) Overall proportion of correct decisions (black circles) and high bets (white circles) made by each monkey as a function of SOA. Error bars represent standard deviations (s.d.). (c) Overall phi correlations (Kornell et al., 2007) for Monkeys N (grey) and S (black) as a function of SOA. Mean and s.d. across SOAs are shown to the right. See also Figure S1.
We recorded from three frontal areas: the frontal eye field (FEF), dorsolateral prefrontal cortex (PFC), and supplementary eye field (SEF). Each area has neuronal activity related to vision, saccades, and reward (Boch and Goldberg, 1989; Bruce and Goldberg, 1985; Ding and Hikosaka, 2006; Funahashi et al., 1991; Kim et al., 2008; Mohler et al., 1973; Roesch and Olson, 2003; Russo and Bruce, 1996; Stuphorn et al., 2000; Watanabe, 1996). FEF and PFC contain neurons involved in decision making (Kim and Shadlen, 1999), target selection (Schall et al., 1995), attention (Iba and Sawaguchi, 2003; Thompson and Bichot, 2005), and maintaining information during a delay (Funahashi et al., 1989; Kim et al., 2008; Sommer and Wurtz, 2001). FEF neurons in particular predict upcoming decisions in a reverse-masking task (Thompson and Schall, 1999) that inspired the decision-making portion of our task. PFC neurons have been implicated in a range of high-level cognitive processes including executive function (Miller and Cohen, 2001), abstract rule encoding (Wallis and Miller, 2003), and behavioral context (Johnston and Everling, 2006), suggesting they collectively function to guide behavior for a desired outcome (Tanji and Hoshi, 2008). SEF neurons have been implicated in performance monitoring by signaling error, conflict, and reward (Nakamura et al., 2005; Stuphorn et al., 2000). Given these different characteristics, we predicted that FEF neurons would be more “low-level” in encoding the decision alone, while PFC and SEF would be more “high-level” in linking the decision to the appropriate bet.
We analyzed neuronal activity from FEF, PFC, and SEF with respect to three main functions of the task: making decisions, placing bets, and linking decisions to appropriate bets. Activity in all three areas correlated with decisions and likewise with bets, but only activity in the SEF correlated with monitoring decisions to guide bets. Of the three areas, the SEF seems the most involved in metacognition.
RESULTS
Behavior
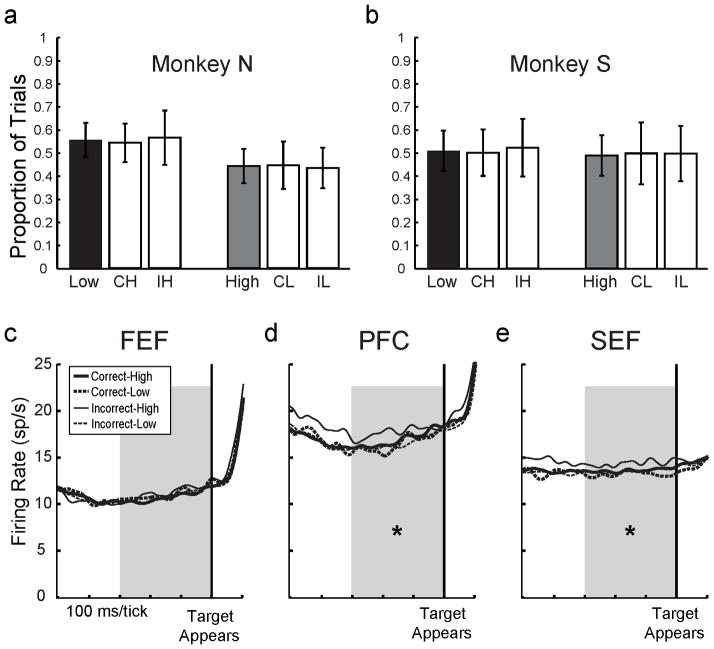
We previously provided a detailed analysis on the monkeys’ behaviors during sessions prior to neuronal recordings (Middlebrooks and Sommer, 2011). Here we analyze behavioral data collected during the recording sessions of the present study (150 sessions for Monkey N, 182 for Monkey S). On average, each monkey made more correct decisions in the Decision Stage, and placed more high bets in the Bet Stage, as a function of longer SOA (Figure 1b, one-way ANOVAs, each p < .001). In principle, SOA alone could have provided information to guide betting; monkeys could have ignored their trial-by-trial decisions and just bet high more often if the masks appeared later or the task seemed easier. We analyzed the data from each SOA separately to address this potential confound. Trial-by-trial analyses revealed that for each monkey, within each SOA, bets were correlated appropriately with decisions (χ2 test, p < .001 for each SOA and each monkey; details in Middlebrooks and Sommer, 2011). We quantified performance across SOAs using two phi correlation methods (Kornell et al., 2007; Zar, 1999). Phi correlation values could range from 0 (random betting) to 1 (perfect association between decisions and bets). Both monkeys’ phi correlations, assessed with either method (Figures 1c and S1), were significant at each SOA and constant across SOAs (one-way ANOVA, p > .05).
Another potential confound is the use of motor-related cues. Monkeys could possibly detect their saccade latencies during the Decision Stage and use this information to help place bets. This explanation is feasible if latency distributions differ between Correct-High vs. Correct-Low trials and between Incorrect-High and Incorrect-Low trials, but they did not (Table S1). All of these results replicate our prior findings (Middlebrooks and Sommer, 2011) and indicate that, within each trial during neuronal recordings, monkeys maintained information about their decision to guide their bet, a metacognitive strategy.
Single neuron recordings
We studied 87 neurons in the FEF (Monkey N: 35, Monkey S: 52), 112 in the PFC (N: 54, S: 58), and 133 in the SEF (N: 61, S: 72). As expected, neurons in all three areas were highly modulated during the task (Figure S2). The monkeys’ betting behavior did not vary significantly between recording sessions in the three cortical areas (phi correlations for Monkey N: FEF, .51; PFC, .49; SEF, .47; for Monkey S: FEF, .59; PFC, .54; SEF, .54; no differences between areas by ANOVAs, p > .05, for both monkeys). Because the monkeys were well trained, the neuronal recording data included more Correct-High and Incorrect-Low trials (the appropriate decision-bet pairings) than Correct-Low and Incorrect-High trials (Table S2 shows the breakdown of trial outcomes).
Decision-related neuronal activity
To test whether neurons encoded the decision, we compared all correct with all incorrect trials, regardless of subsequent bets (i.e. high and low bet trials pooled).
Sensory-related activity comparison
First we focused on neuronal activity related to the visual target. Using a similar masked target task, Thompson and Schall (1999) demonstrated that signals predictive of a monkey’s decision occur in the early visual responses of FEF neurons, prior to the start of motor-related processes (reviewed by Schall and Thompson, 1999; Schall, 2001; see also Schall et al., 1995; Sato and Schall, 2003). We analyzed trials in which the target appeared in the hemifield contralateral to the neuron’s location in the brain, because for FEF, visual receptive fields are typically contralateralized (Bruce and Goldberg, 1985). Contralateral biases are common in SEF and PFC, too (Funahashi et al., 1989, 1990, 1991; Russo and Bruce, 1996), and we wanted to analyze data from all three areas in the same way for a fair comparison.
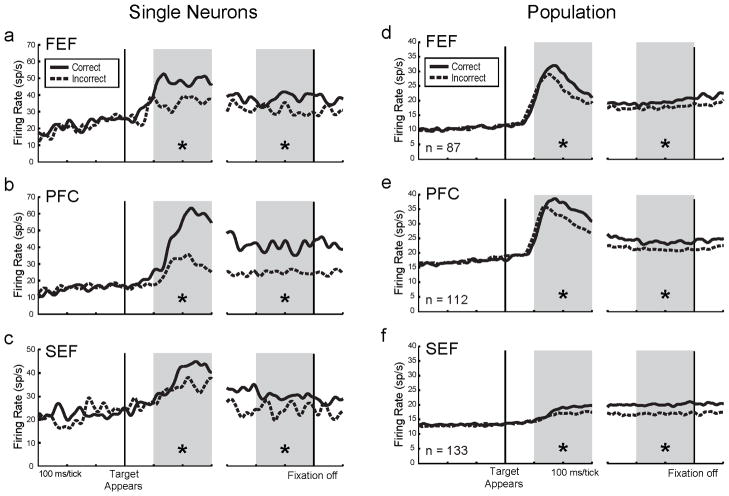
Single neuron examples are shown for FEF, PFC, and SEF (Figure 2a-c). Each neuron was active during the early visual response (visual-1) and delay epochs (grey shadings), and each was more active on correct than incorrect trials in both epochs (t-test, p < .05). At the population level, all three frontal areas showed this effect (Figure 2d-f and Table 1). We repeated these analyses using only those neurons that were significantly active within each epoch, and this yielded the same results (Table S3). These findings extend the results of Thompson and Schall (1999) to show that visual and delay activity correlate with decisions in a masked target task in the SEF and PFC as well as in FEF.
Figure 2.
Decision-related neuronal activity. Within each panel, neuronal firing rates for all correct trials (solid lines) and incorrect trials (dashed lines) are aligned to Decision Stage target onset (left) and fixation spot offset (right), and grey shadings indicate visual-1 (left) and delay epochs (right). Asterisks indicate a significant difference in activity (p < .05) within the epoch. (a–c) Single neuron examples. Each neuron was more active during correct than incorrect trials in both epochs. (d–f) Population activity. In all three areas, activity was greater for correct than for incorrect trials in both epochs (Table 1 shows corresponding numerical data). Population spike density functions are the average of all individual neuron spike density functions from each area. See also Figure S2.
Table 1.
Decision-related Activity: Population
| FEF | Baseline | Visual-1 | Delay | Presaccadic-1 | Postsaccade |
|---|---|---|---|---|---|
|
| |||||
| Correct | 10.4 (.9) | 26.8 (2.0) | 19.5 (1.6) | 29.3 (2.6) | 20.2 (2.4) |
| Incorrect | 10.2 (.9) | 23.8 (1.7) | 17.6 (1.4) | 30.5 (2.7) | 20.4 (2.5) |
| p | .33 | < .001* | .017* | .16 | .75 |
|
| |||||
| PFC | |||||
|
| |||||
| Correct | 16.0 (1.3) | 33.9 (2.6) | 23.5 (1.7) | 25.7 (2.2) | 25.9 (2.4) |
| Incorrect | 16.4 (1.3) | 30.7 (2.4) | 20.7 (1.5) | 25.0 (2.0) | 28.0 (2.3) |
| p | .47 | < .001* | < .001* | .39 | .86 |
|
| |||||
| SEF | |||||
|
| |||||
| Correct | 13.4 (1.1) | 18.5 (1.4) | 20.1 (1.5) | 22.2 (1.5) | 22.7 (1.5) |
| Incorrect | 13.1 (1.1) | 17.0 (1.3) | 17.02 (1.2) | 20.9 (1.4) | 20.5 (1.3) |
| p | .20 | < .001* | < .001* | .024* | < .001* |
Population decision-related firing rates during Decision Stage epochs. For each cortical region, all correct vs. all incorrect firing rates (spikes/s) are shown with standard errors in parentheses and paired t-test p-values underneath. Asterisks and bold fonts represent significant differences (p < .05) between correct and incorrect trials.
Motor-related activity comparison
To analyze activity related to decision saccades, we compared the correct and incorrect trials for which a saccade was made into the contralateral field. We analyzed activity just before and after the saccade (presaccadic-1 and postsaccade epochs, respectively). Only the SEF population had activity in these epochs that differentiated correct from incorrect decisions (Table 1). Repeating this analysis on the subsets of neurons active within each epoch (i.e. only neurons with significant pre- or postsaccadic activity), SEF neurons were more active during correct than incorrect decisions within the postsaccade epoch (Table S3) but not the presaccadic-1 epoch. FEF and PFC showed no effect in either epoch.
Relationship to bet-related activity
We expected bet-related activity to resemble decision-related activity, given the high trial-by-trial correlations between decisions and bets: correct decisions were mostly followed by high bets and incorrect decisions by low bets (Table S2). To analyze bet-related activity explicitly, we compared high bet with low bet trials regardless of preceding decisions (i.e. pooled correct and incorrect trials). The results, as expected, were similar to those from the decision-related activity analysis, and are summarized in the Supplemental Information (Bet-related activity section of Supplemental Results; Tables S4 and S5).
Metacognition-related neuronal activity
To test whether neuronal activity correlated with metacognitive monitoring, we compared trials when the monkey made the same decision but different bets. Our rationale was that metacognition is the process that links a decision to a bet, allowing purposeful instead of random wagering. Signals related to metacognition should differ between trials when a decision is followed by an appropriate vs. inappropriate bet.
We first compared neuronal activity between Correct-High (CH) and Correct-Low (CL) trials. This was a straightforward analysis because visual stimuli and saccade directions were equivalent in CH and CL trials throughout the Decision Stage. We included only those trials in which the targets were located in, and the saccades were directed into, the contralateral field. The critical time period was the interstage epoch: the time span after the decision was reported but before the bet targets appeared.
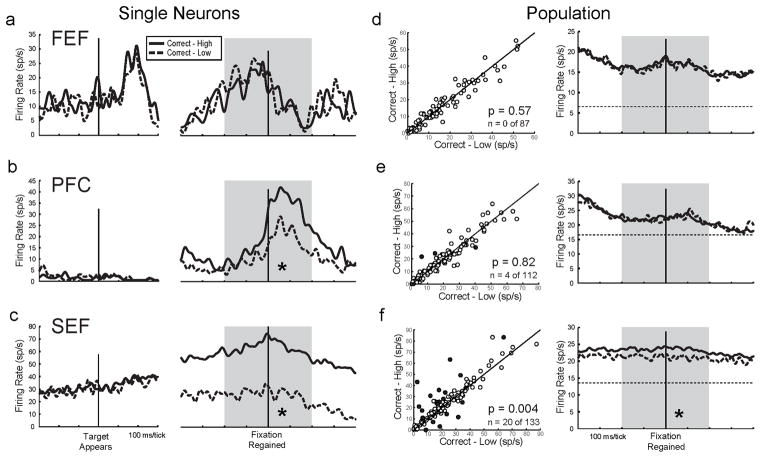
In the FEF, neuronal activity was no different in CH vs. CL trials during the interstage epoch. A single neuron example (Figure 3a) was equally active for CH and CL trials during the interstage epoch (grey shading), and the same negative result was found for the FEF population (Figure 3d, left). FEF population activity profiles overlapped for CH and CL trials (Figure 3d, right). In the FEF, visual receptive fields and movement fields are often much smaller than a hemifield, so for a more careful test of FEF activity, we then limited our analyses to directions associated with the visual receptive field and/or movement field for each neuron; the results were still negative, however (Figure S3a,d).
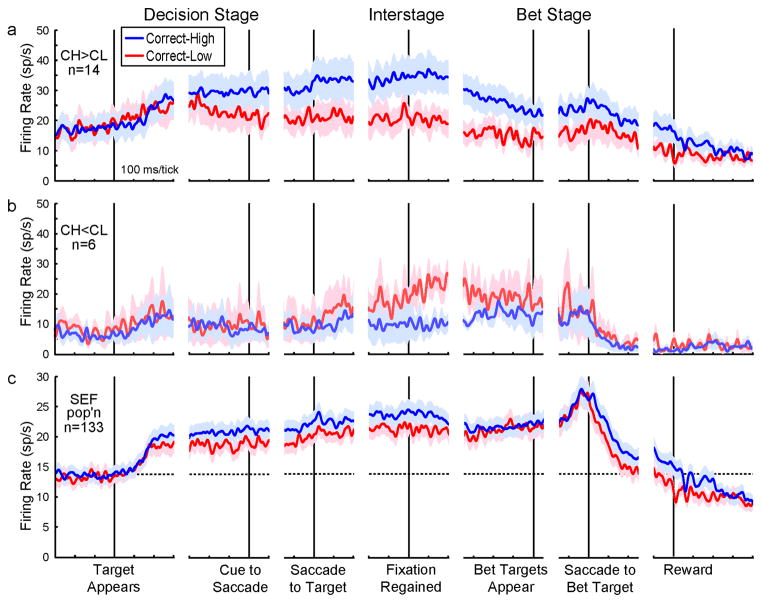
Figure 3.
Metacognition-related activity. For the single neuron examples (a–c), firing rates for all Correct-High (CH) trials (solid lines) and Correct-Low (CL) trials (dashed lines) are aligned to Decision Stage target onset (left) and on regaining fixation to begin the Bet Stage (right). Grey shading indicates the interstage epoch. For the population data (d–f), scatterplots (left) show CH vs. CL firing rates for each neuron during the interstage epoch, p values of t-tests of population CH vs. CL activity, and numbers (n) of individual neurons with significant CH vs. CL activity (each denoted with a filled dot). Activity profiles (right) show population spike density functions, with baseline activity levels (dashed horizontal lines) provided for reference. Asterisks indicate significant differences within the interstage epoch (p < .05 for single neurons and < .025 for population data). The FEF neuron (a) was not significantly different between trial types (post-ANOVA t-test, p > .05), but the PFC neuron (b) and SEF neuron (c) had significantly greater activity in CH than in CL trials (both p < .001). (d) In the FEF, single neurons (left) and population profiles (right) showed no significant differences in activity between CH and CL trials. (e) In the PFC, a few single neurons showed CH vs. CL differences (left), but this was not significant at the population level, and population profiles overlapped (right). (f) In the SEF, many individual neurons showed CH vs. CL differences (left), this was significant at the population level, and population profiles were higher for CH than CL trials throughout the interstage epoch (right). Table 2, Interstage column, shows corresponding numerical data. See also Figure S3.
PFC neuron activity was marginally better at distinguishing CH from CL trials. An example neuron (Figure 3b) was more active for CH trials than CL trials during the interstage epoch. In the PFC population, however (Figure 3e, left), there was no average activity difference between CH and CL trials, the incidence of individually significant neurons was not greater than expected by chance (4/112 neurons compared with 5/112 expected false positives at the p < .05 criterion for individual neurons; Fisher Exact Test, p = .999), and average activity profiles for CH and CL trials overlapped (Figure 3e, right). Results were similarly negative for analyses restricted to visual and movement fields (Figure S3b,e).
The SEF seemed to be the major player in sustaining a metacognitive signal. The SEF neuron in Figure 3c, for example, was 2.5 times more active during the interstage epoch for CH than CL trials. Overall, 15% (20/133) of individual SEF neurons had significantly different activity in CH vs. CL trials (Figure 3f, left, filled circles), a proportion significantly greater than expected by chance (6/133 neurons false positives were expected at p < .05; 20/133 neurons is significantly greater; Fisher Exact Test, p = .0063). CH activity exceeded CL activity for 70% (14/20) of the individually significant neurons and at the population level (Figure 3f, right). The SEF results were similarly positive for analyses restricted to visual and movement fields (Figure S3c,f).
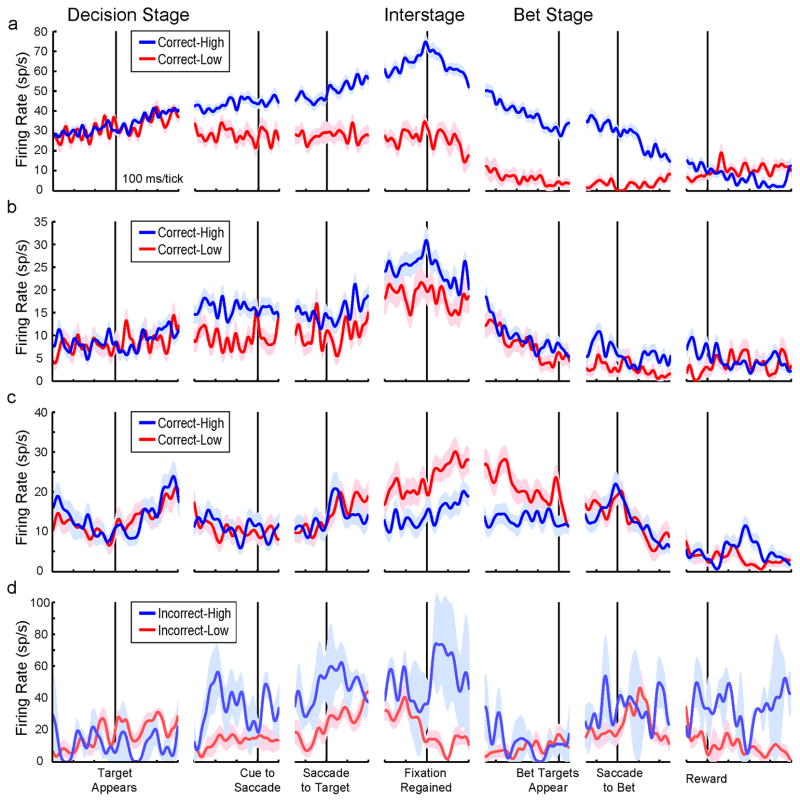
In the SEF, differential CH-CL activity could emerge long before the interstage epoch. Individual neurons showed a variety of time courses. Figure 4a and b show example CH > CL neurons, and Figure 4c shows an example CH < CL neuron. Pooling together the subset of 14 neurons for which CH activity significantly exceeded CL activity during the interstage epoch, it can be seen that the average effect started well before the interstage epoch and had a long time course (Figure 5a). CH and CL activity separated before the cue to make a saccade, i.e., during the time when monkeys may have made their decision but before they reported it. The subset of 6 neurons with the reverse effect (CH < CL) had a more transient average time course (Figure 5b). Overall activity was higher for the CH > CL subset than for the CH < CL subset, including during the baseline period (first 300 ms of time courses), hinting that the former subset may include more inhibitory interneurons than the latter subset (e.g. Connors and Gutnick, 1990; Constantinidis and Goldman-Rakic, 2002). We cannot provide further support for this possibility, however, because we did not store action potential waveforms (e.g. Mitchell et al., 2007), and we found no significant differences in spiking statistics between the subsets (see Spike Burstiness section in Supplemental Results; Anderson et al., 2011).
Figure 4.
Time courses of example SEF neurons. Activity profiles depict means (lines) and SEMs (shading) and are aligned to events indicated at bottom. (a and b) Two example neurons for which Correct-High (CH) activity significantly exceeded Correct-Low (CL) activity during the interstage epoch. (b) Example neuron for which CL activity significantly exceeded CH activity during the interstage epoch. (d) Example neuron for which Incorrect-High (IH) activity significantly exceeded Incorrect-Low (IL) activity during the interstage epoch. Its noisy IH activity was typical, due to small numbers of trials (IH was the least likely trial outcome).
Figure 5.
Time courses of SEF population activity. Conventions as in Figure 4. Average activity profiles are shown for (a) the 14 SEF neurons for which CH activity significantly exceeded CL activity during the interstage epoch, (b) the 6 SEF neurons for which CL activity significantly exceeded CH activity during the interstage epoch, and (c) the entire population of SEF neurons. In the population, SEF activity distinguished CH from CL trials ~200ms after target onset (“Target Appears”), and this differential activity was maintained through the interstage period (after “Fixation Regained” and before “Bet Targets Appear”). Table 2, bottom row, shows results of statistical analyses for this time range (Baseline through Interstage epochs). Population differential activity re-emerged after the saccade to the bet target. Only contralateral data are included here. Population baseline firing rate is shown with a horizontal dashed line. See also Figure S4.
In the entire population of SEF neurons (Figure 5c), population differential activity emerged early in the Decision Stage and then was maintained, steadily and significantly, through the interstage epoch. The numerical data corresponding to this sustained effect are listed in Table 2, bottom row. The SEF population results were the same when we extended the analysis beyond contralateral space to all directions (summarized in Figure S4a, top). When we considered only the subset of SEF neurons significantly active within each epoch, we found a similar pattern differentiating CH versus CL activity (Figure S4a, middle and bottom). Finally, the population-level CH > CL effect during the interstage epoch was significant for each monkey individually (Table S6).
Table 2.
Metacognition-related Activity: Population
| FEF | Baseline | Visual-1 | Delay | Presaccadic-1 | Postsaccade | Interstage |
|---|---|---|---|---|---|---|
|
| ||||||
| CH | 10.4 (0.9) | 27.2 (2.0) | 19.9 (1.7) | 27.8 (2.3) | 20.5 (2.2) | 16.4 (1.5) |
| CL | 10.6 (1.0) | 27.3 (2.1) | 19.3 (1.7) | 26.9 (2.2) | 21.0 (2.1) | 16.1 (1.5) |
| p | .64 | .82 | .40 | .37 | .59 | .57 |
|
| ||||||
| PFC | ||||||
|
| ||||||
| CH | 15.8 (1.3) | 33.9 (2.6) | 23.3 (1.7) | 25.4 (2.1) | 27.0 (2.4) | 21.9 (2.0) |
| CL | 15.9 (1.3) | 32.7 (2.4) | 23.1 (1.7) | 24.3 (1.9) | 26.3 (2.4) | 22.0 (2.0) |
| p | .79 | .23 | .82 | .23 | .35 | .82 |
|
| ||||||
| SEF | ||||||
|
| ||||||
| CH | 13.6 (1.1) | 18.5 (1.4) | 20.1 (1.6) | 22.2 (1.5) | 22.9 (1.5) | 23.6 (1.6) |
| CL | 13.1 (1.2) | 17.7 (1.5) | 18.6 (1.4) | 20.1 (1.4) | 21.0 (1.4) | 21.3 (1.5) |
| p | .25 | .10 | < .001* | .017* | < .001* | < .001* |
Population metacognition-related firing rates linking decisions to bets. For each cortical region, all Correct-High (CH) vs. all Correct-Low (CL) firing rates (spikes/s) are shown with standard errors in parentheses and paired t-test p-values underneath. Asterisks and bold fonts represent significant differences between CH and CL trials (p < .025 criterion, see Experimental Procedures).
The complementary approach to testing whether neuronal activity correlates with metacognitive behavior is to compare Incorrect-High (IH) vs. Incorrect-Low (IL) trials. Analyses of IH and IL trials are complicated by two issues, however. First, the target location is not coincident with the saccade destination, by definition of an incorrect trial. Incorrect saccades may go to the other target location in the same hemifield or to either target location in the other hemifield. Thus different directions had to be analyzed as a function of epoch (see IH versus IL section in Supplemental Results). Second, IH trials were the rarest outcome (only 10% of all trials; Table S2), resulting in few trials to analyze for many neurons. Nevertheless, we performed the IH vs. IL analyses and found, as with the CH vs. CL analyses, significant effects during the interstage epoch in the SEF population (p = .005), but not in the PFC or FEF populations (Figure S3g–i). For most of the individually significant SEF neurons (9/10), IH activity exceeded IL activity. These neurons showed a variety of time courses (one is shown in Figure 4d), but on average they distinguished between IH and IL trial outcomes continuously from the end of the Decision Stage to the end of the trial (Figure S4b). We directly compare IH to CH activity, and IL to CL activity, below in the Reward anticipation section.
For the FEF and PFC populations, CH vs. CL differences failed to reach significance not only during the interstage epoch, as described above, but also in every epoch (Table 2, top and middle rows). IH and IL activity differences were similarly insignificant across epochs (except for one epoch in the FEF; Supplemental Results, IH versus IL section). Finally, no CH-CL or IH-IL differences were significant in any epoch for the subsets of FEF and PFC neurons that were significantly active in each epoch (not shown).
Effects of SOA
We varied the SOA in the task to elicit large numbers of correct and incorrect trials (and their associated bets) to analyze. This raises two questions. Did varying SOAs contribute to differences in trial durations between trial outcomes (e.g. CH vs. CL) that could have influenced our neuronal results? And, more to the point, did metacognition-related signals in SEF vary with SOA? Regarding the first question, we did not expect SOA distributions (and thus trial lengths) to vary appreciably between trial outcomes, given that metacognitive behavior was stable across SOAs (e.g. Figure 1c). Betting depended on trial-by-trial decisions, not SOAs. The only exception might be if a monkey “guessed” during the more difficult, shorter SOA trials; it might choose a target randomly and then bet low to be safe. If its choice was correct, the outcome would be a CL trial. Hence short SOAs might be slightly more prevalent in CL trials than in CH trials. Consistent with this expectation, we found that average SOAs were 48.3 ms (SD 17.9 ms) in CH trials and 45.1 ms (SD 18.2 ms) in CL trials, a slight but significant difference (Mann-Whitney U test, p < .001). This 3.2 ms difference in mean trial duration was negligible compared to the overall trial duration of ~2 s, so it is unlikely to have influenced our neuronal data. Regarding the second question, we analyzed whether our main indicators of metacognitive signals, CH firing rates, CL firing rates, and differential CH-CL activity, varied across SOAs. We analyzed each of these three data sets for all six epochs of Table 2 (baseline through interstage), for contralateral directions and all directions. Firing rates did not vary significantly as a function of SOA for any of the 36 tests (ANOVAs, p > .05 for all). In sum, variations in SOA were critical for the task design but had no significant influence on the neuronal effects that we found, just as they had no influence on metacognitive behavior (e.g. Figure 1c).
Bet Stage-related activity
We also analyzed CH vs. CL differences for time periods after the interstage epoch, through the Bet Stage of the task. Briefly, at the population levels, none of the three cortical areas had activity correlated with metacognition after the interstage epoch and before the bet. In the SEF, CH and CL signals clearly became different again after the bet, through delivery of the reward (Figure 5c and Supplemental Results, Bet Stage-related activity section; Tables S7 and S8). In the SEF population, this disappearance and resurgence of CH > CL activity might be explained by opposing dynamics of CH > CL and CH < CL neurons. The individually significant CH > CL sustained their signal through the entire Bet Stage (Figure 5a), but the CH < CL neurons were transiently active in the late interstage and early Bet Stage (Figure 5b), so they may have effectively nullified the CH > CL signal during that time at the population level.
Reward anticipation
Many neurons in the SEF encode reward anticipation (Roesch and Olson, 2003; So and Stuphorn, 2010). In our experimental design, reward amounts were determined entirely by behavior: the decision and the bet. We could not know what reward amounts the monkeys expected on given trials, but it is likely that they placed high bets in anticipation of high reward and low bets in anticipation of low reward. If our SEF neurons represented reward anticipation, this might explain the higher firing rates in CH vs. CL trials and IH vs. IL trials. Quantitatively, the reward anticipation hypothesis predicts that activity should be equal for all trials in which the same bet was made after different decisions: firing rates should be indistinguishable between CH and IH trials and between CL and IL trials. We found that, to the contrary, SEF activity strongly differentiated between CH and IH trials and between CL and IL trials through the Decision Stage and into the Bet Stage. As with our usual analyses, we considered trials for which targets were located within, and saccades were directed into, the contralateral field. During the Decision Stage (Table S9), the CH-IH difference in population activity began in the visual-1 epoch and lasted through the interstage epoch. In the subsets of neurons with significant activity in each epoch, the same pattern of results was observed with the exception of the presaccadic-1 epoch. SEF activity also was different in the Decision Stage between CL and IL trials. As a population, the difference was significant during the delay and interstage periods. For the subsets, CL-IL firing rates were different from the visual-1 epoch through the interstage epoch, except in the presaccadic-1 epoch. Thus, although we would not rule out effects of reward anticipation during the Decision Stage, we found little evidence for it.
During the Bet Stage (Table S10), SEF population activity became more similar between CH and IH trials and between CL and IL trials; differences in activity between these trial outcomes diminished and eventually ceased. This implies that neuronal correlates of reward anticipation may have contributed more to SEF population activity near the end of the trial. On a related note, SEF neurons are known to modulate with reward delivery (Stuphorn et al., 2000). We also observed reward modulation, in that SEF neurons had higher firing rates for the worst reward outcome (IH, resulting in time-out and no reward) than any of the other trial outcomes (IH: 16.1 ± 1.6 sp/s, CH: 12.0 ± 1.4 sp/s, p < .001; CL: 10.3 ± 1.2 sp/s, p < .001; IL: 11.0 ± 1.3 sp/s, p < .001; see also Table S10).
Influence of past trial outcomes
Given the elevated activity in the SEF at the end of IH trials, we asked whether inter-trial effects may have influenced our data. In strategic decision making tasks, choices and neuronal activity can be affected by the outcomes of previous trials (Barraclough et al., 2004; Seo and Lee, 2009), suggesting that carryover of neuronal activity from one trial to the next could guide choices (Sutton and Barto, 1998). First, using our behavioral data and considering all directions of target locations and saccades, we analyzed the rates at which monkeys switched their bets from trial to trial; that is, the rates of making low bets after CH or IH trials or high bets after CL or IL trials. If a bet is influenced by previous trial outcome, monkeys should switch bets with relatively low likelihood after CH and IL trials (“win-stay” strategy) but with high likelihood after IH and CL trials (“lose-switch” strategy). We found no such inter-trial effects: the rates of placing low bets after high bet trials (i.e., switching after CH or IH trials) were no different from the average rate of placing low bets (Figure 6a and b, left data). The same was true for rates of placing high bets after low bet trials (Figure 6a and b, right data; t-tests, all p > .05).
Figure 6.
Inter-trial effects. (a and b) Rate of placing bets as a function of previous trial outcome for each monkey. In each graph, the average low bet rate (black bar) is plotted next to low rates after previous CH and IH trials (white bars), and the average high bet rate (grey bar) is plotted next to high rates after previous CL and IL trials (white bars). Error bars are standard deviations. None of the “switch” rates were different from the respective average bet rates (paired t-tests, p > .05). (c–e) Neuronal activity during baseline period (shaded) as a function of previous trial outcome for the populations of FEF, PFC, and SEF neurons. Asterisks indicate whether IH activity was greater than all three of the other trial outcomes by paired t-tests (p < .05).
At the neuronal level, we found carryover of previous trial information that differed between brain areas. Data were pooled over all directions. In the SEF population, baseline firing rates were higher after IH trials than after other trial outcomes (Figure 6e; paired t-tests, all p < .05). The effect was individually significant for 13% (17/133) of the SEF neurons. The effect disappeared as soon as the Decision Stage began (target appearance) and did not return throughout the course of the trial; no other epochs in SEF distinguished between previous trial outcomes (paired t-tests, all p > .05).
In contrast, neurons in both PFC and FEF carried information about previous IH trials into various Decision Stage epochs of the next trial. PFC carried substantial previous-trial information, as seen previously (Barraclough et al., 2004). Like in the SEF, baseline firing rates in the PFC were higher after IH trials than after other trial outcomes (Figure 6d, paired t-tests, all p < .05). The effect was individually significant for 11%, 12/112, of the PFC neurons. This IH-related signal was sustained through the next two (visual-1 and delay) epochs (not shown). In the FEF, previous IH trials had no effect on baseline activity but led to significantly higher firing rates during the postsaccade period (paired t-tests, all p < .05, not shown).
In sum, IH trials seemed to affect neuronal activity in the next trial. In the SEF, this influence ended after the baseline period, matching the monkeys’ behavior in that previous-trial information did not account for current trial performance. In PFC and FEF, previous-trial information persisted into the current trial to affect neuronal activity in various epochs.
Discussion
We recorded from single neurons in the FEF, PFC, and SEF while monkeys performed a visual oculomotor task in which they monitored their own decisions. Neuronal activity correlated with decisions and bets was found in all three areas, but joint activity that linked decisions to appropriate bets was found exclusively in the SEF. This putative metacognitive activity began swiftly in the SEF during the Decision Stage and continued into the Bet Stage. Monkey behavior was independent of previous trial outcome, as was SEF activity (but not PFC or FEF activity).
We had predicted that both the SEF and PFC would participate in metacognitive monitoring, but our data supported a role only for the SEF. The putative metacognitive activity in SEF arose early in trials (Figure 5a and c), beginning soon after the start of the decision-related signal (Figure 2f) and before the monkey reported its decision with a saccade. The time course suggests that monitoring a decision occurs in near simultaneity with making the decision. This seems analogous to the time course of monitoring motor operations (“corollary discharge”); when motor areas finalize a movement command, upstream areas monitor it within milliseconds (Sommer and Wurtz, 2004). It should be noted that most (15/20) of our individual SEF neurons with a metacognitive signal also exhibited a decision-related signal. This close relationship between metacognitive and decision-related signals may be no coincidence: in the SEF, decision-related signals may evolve into metacognitive signals. A decision-related signal that outlasts the decisive act (the saccade to the target) provides information that could be monitored for later behavior (the bet). Although decision-related signals occurred in all three areas, our data suggest differences in how the signals are used. In SEF, the prolonged decision-related signal seems to be maintained for internal use (e.g., determining the bet to make). In PFC and FEF, the briefer signal may guide only immediate acts (e.g., planning the decision saccade).
Metacognition-related activity in SEF had not been reported previously. No fMRI studies reported human SEF signals during metacognition tasks, although many fMRI results have implicated regions interconnected with SEF, such as anterior cingulate and medial prefrontal regions (Chua et al., 2006; Kikyo et al., 2002). Our recording strategy was to study every neuron encountered, so our population data may be considered a representative sample of SEF neurons (leaving aside issues of sampling biases related to neuron size, e.g. Sommer and Wurtz 2000). The signals that we found in individually significant neurons were prominent, but the gross signal in the entire SEF population was small (Figure 5c and Table 2), suggesting that it may not be distinguishable with fMRI. In future work we will concentrate our recording efforts on only those SEF neurons that show metacognition-related activity (differential CH vs. CL and IH vs. IL signals) to investigate them in more detail.
Prior recording studies of monkey SEF reported neurons signaling reward, errors, conflict, and/or inhibition of planned saccades, collectively referred to as performance monitoring (Nakamura et al., 2005; Stuphorn et al., 2000). We found two lines of evidence for reward signals in the SEF: elevated firing rates during the reward epoch of CH vs. CL trials, and information about worst-outcome, IH trials, in the reward period that carried over to the next trial (a “lack of reward” signal). Neither signal can explain our putative metacognitive activity in SEF because both start after the bet on one trial and end before the next trial’s decision. Regarding error signals (Stuphorn et al., 2000), an “error” in our task is not straightforward. An error could be a trial that earned no reward (IH), but we did not observe increased or decreased firing rates on IH trials until around the time of reward, as mentioned. A subtler interpretation is that an error occurred when less reward was earned than potentially available (CL trials). Yet we did not see SEF activity greater on CL than CH trials in any epoch, nor transient decreases in activity on CL trials. Finally, a transient error signal might occur after any incorrect decision (e.g., during the postsaccade and/or interstage epochs), since incorrect decisions were always less advantageous than correct decisions. We did not observe SEF neurons with that sort of signal either. In short, we saw little or no evidence of error signals in our SEF data.
We found, as well, that reward anticipation (Roesch and Olson, 2003; So and Stuphorn, 2010) was not a plausible explanation for the metacognitive signals. Our experiment did not explicitly vary reward anticipation, but it could be argued that “bet anticipation” is the same thing, as long as the animals expected all high bets to yield high reward and all low bets to yield low reward. We found little evidence for bet or reward anticipation. The activity of our SEF neurons differentiated between trials that culminated in identical bet selection (CH vs. IH, and CL vs. IL trials). This differential activity occurred throughout the Decision Stage and interstage periods, when putative metacognitive signals dominated. Signals related to identical bet selection became less distinguishable in the Bet Stage, suggesting that reward anticipation signals “took over” in the betting phase of the task. Our results cannot resolve the extent to which metacognition and reward anticipation signals are conveyed by separate SEF neurons or multiplexed in single neurons. In a recent study (So and Stuphorn, 2012), monkeys performed a gambling task in which a delay was imposed between when the monkey made a choice and when reward was delivered. SEF neurons recorded during the task carried multiple signals; some activity patterns varied with expected reward, some with experienced reward, and others with the difference between expected and experienced reward. Similar signals in SEF were reported during a token-based gambling task (Seo and Lee, 2009), in which reward was delivered after earning a sufficient number of tokens across trials. These reports complement our conclusion that SEF signals correlate with metacognitive monitoring only within a trial, not across trials. This comparison between studies highlights a key difference between our task and most other gambling tasks. Our monkeys gained no advantage by adjusting their bets based on previous trial outcomes; the reward yielded by a bet depended only on the decision made by the monkey earlier in the same trial. Our reward probabilities depended critically on the ability to monitor decisions (details in Middlebrooks and Sommer, 2011). In probabilistic gambling tasks, on each trial the reward probabilities are set by computer according to some distribution, and thus monkeys learn to keep track of those expected probabilities in addition to, or instead of, their own behavior. A salient goal of future work would be to design experiments that manipulate both reward expectation and metacognitive monitoring in systematic ways, to reconcile the extent that both signals may be carried by SEF neurons.
It was also possible that the neurons may have been coding the actual (as opposed to expected) upcoming reward. We found, however, that neuronal firing rates across trial outcomes did not parallel relative reward values, so actual rewards were not predicted by firing rates. Lastly, riskiness (McCoy and Platt, 2005) could be proposed as an alternative account of our data. If the neurons were signaling levels of risk, we would expect high firing rates for all high bets and low firing rates for low bets, but we did not observe this pattern (for more on these issues, see Supplemental Discussion).
Neither the FEF nor the PFC showed much evidence of metacognition-related activity. Instead, activity in both areas was correlated with the initial stage of the task: making the decision. This supported our initial prediction about the FEF, which was based on similar results from Thompson and Schall (1999). As discussed in that prior study and related work from the Schall laboratory, differences in FEF visual responses correlate with making decisions but are not trivially explained by other factors (e.g. saccade preparation; see Supplemental Discussion). In the PFC, we expected to find prominent metacognitive signals because it has been implicated previously in human metacognition (Rounis et al., 2010). The PFC is a large, functionally heterogeneous region (e.g. Romanski, 2004), and our posterior sampling of it (Figure S2a) may have missed metacognition-related areas. However, the neurons we recorded featured all of the familiar hallmarks of dorsolateral PFC (Funahashi et al., 1989, 1990, 1991): visual responses, strong delay activity, and postsaccadic activity (Figure S2c). In the context of visual-saccadic tasks, the neurons seemed typical. It could be that metacognitive processing in PFC (and/or FEF) occurs in specific, yet rare, neurons. FEF and PFC activity also may be more dependent on spatial parameters of the task than SEF. FEF neurons can have quite spatially restricted visual receptive and movement fields (Bruce and Goldberg, 1985), but even when we analyzed target locations confined to those fields, we found no metacognition-related effects.
Our results complement a recent report that LIP activity correlated with monkeys’ tendency to opt-out of making a decision (Kiani and Shadlen, 2009), suggesting that the activity signals confidence. Both the fundamental task design and the visual stimuli used in the LIP study differed from those used here. Moving-dots stimuli (Kiani and Shadlen, 2009) require evidence accumulation over time, but the Decision Stage of our task requires detection of a single brief stimulus. A possible advantage of our task is that its brief stimulus presentation demands a more immediate monitoring of the decision to guide the eventual metacognitive judgment. Given the short latency at which the metacognitive signals separated and the long duration of the separation, SEF neuronal activity seems to transcend general confidence and correspond more to monitoring of the monkeys’ percept. Another possible advantage of our task is that we were able to establish that the metacognition-related signals in SEF represented processes beyond reward anticipation, which was less clear in LIP using the opt-out task (Kiani and Shadlen, 2009) or in OFC using a delayed-reward task (Kepecs et al., 2008).
Studies of metacognition naturally lead to questions about broader implications. One interpretation is that metacognition is associated with conscious awareness (Nelson, 1996), but we favor a more conservative view that self-monitoring does not presuppose self-awareness (Reder and Schunn, 1996). As we argued previously (Middlebrooks and Sommer, 2011), metacognition may be to cognition as corollary discharge is to action; both describe the ability of the brain to internally monitor its operations. Just as it appears that all animals that move have internal circuits for monitoring their movements (Crapse and Sommer, 2008), all animals with even rudimentary cognitive abilities may monitor those abilities. This monitoring ability, however, does not necessarily imply states of self-awareness anywhere near the levels experienced by humans.
EXPERIMENTAL PROCEDURES
Surgery
Two male rhesus monkeys (labeled N: 6.6 kg, and S: 6.0 kg) were surgically prepared for neuronal recordings and eye position measurements. Using aseptic procedures, ceramic screws and an acrylic implant were affixed to the skull. Recording chambers and a head-restraint socket (Crist Instruments, Hagerstown, MD) were embedded in the implant. Chambers were positioned over FEF/PFC (1 chamber with access to both regions) and SEF using stereotaxic coordinates (FEF/PFC: A25, L20; SEF: A25, midline). In the same surgery, we implanted scleral search coils. Animals recovered for 1–2 wk before training resumed. Procedures were approved by and conducted under the auspices of the University of Pittsburgh Institutional Animal Care and Use Committee and were in compliance with the guidelines set forth in the United States Public Health Service Guide for the Care and Use of Laboratory Animals.
Tasks
Receptive field mapping tasks
To determine appropriate target locations for the metacognition task (described below), we initially characterized the receptive field of each neuron using simple visual oculomotor tasks (see Sommer and Wurtz, 2004). First, the monkey made visually guided saccades to targets in eight directions (cardinal directions and diagonals). After the neuron’s preferred direction was established, the monkey performed visually guided saccades of varying amplitudes in that direction. If necessary, directions and amplitudes were adjusted and the tasks repeated to refine the assessment of the field. Once the receptive field center was located, we had the monkey make memory-guided saccades to that location, to distinguish visual-, delay-, and saccade-related activity (Mays and Sparks, 1980). We accepted neurons with any combination of these signals.
Metacognition task
The task was described previously in detail (Middlebrooks and Sommer, 2011). Each trial consisted of a Decision Stage and a Bet Stage, separated by an interstage period (Figure 1a). In the Decision Stage the animal was required to detect and report the location of a masked visual target (Thompson and Schall, 1999), and in the Bet Stage to report, via a wager, whether a correct or incorrect decision had been made in the Decision Stage (Shields et al., 2005). Appropriate betting, thus optimal reward delivery, required the animal to maintain a representation of its decision. It is the maintenance of that decision signal, and its use for betting, that we refer to as metacognition. To obtain reward on any trial, completion of both the Decision and Bet Stages was required.
Decision Stage
The monkey fixated a spot for 500–800 ms (randomized; Figure 1a, left). Then a dim target appeared in one of four possible locations (also randomized). The locations were constant in a session but could vary between sessions; eccentricities ranged from 5–25 deg. and directions, relative to horizontal, ranged from 0–60 deg. For each neuron, these parameters were chosen so that, when possible, at least one target location was in the receptive field center. The locations were mirror symmetric across the vertical meridian. After the target appeared, identical mask stimuli (white squares) appeared at all four locations. The interval between target appearance and masks appearance, the stimulus onset asynchrony (SOA), was randomized by trial (16.7, 33, 50, or 66.7 ms) to vary task difficulty. After the masks appeared, a random delay of 500–1000 ms ensued, during which the monkey maintained fixation while the masks remained visible. Then the fixation spot was extinguished, cueing the monkey to report its decision by making a saccade to the perceived target location within 1000 ms. The monkey received no performance feedback until after the Bet Stage, but the computer tracked whether the decision was correct (saccade landed in an electronic window around the target location) or incorrect (saccade landed anywhere else). If at any time during the Decision Stage the monkey broke fixation, made a saccade before cued to go, or failed to make a saccade, the trial was aborted (and repeated later) and the next trial immediately began.
Bet Stage
A new fixation spot appeared 350 ms after the decision saccade that concluded the Decision Stage (Figure 1a, right). The monkey foveated the spot and, 500–800 ms later, two bet targets appeared: a red “high-bet” target and a green “low-bet” target (for Monkey N; color assignments reversed for Monkey S). In a session the two locations were constant, but the appearance of high-bet or low-bet targets varied randomly between the locations. One location was in the center of the receptive field and the other was at the mirror symmetric location in the other hemifield. A monkey reported its bet by making a saccade to one of the targets, then received reward or timeout as described below, and the trial ended. A monkey optimized its reward if it bet high after a correct decision and low after an incorrect decision. If, during the Bet Stage, the monkey broke fixation or made a saccade to a non-bet-target location, the trial was aborted and a brief timeout ensued before a new trial began.
Reward
The amount of reward delivered after each trial was based on how appropriate the bets were relative to the decisions. If the monkey made a correct decision and bet high, it earned maximum reward: 5 drops of water. If the monkey made an incorrect decision and bet high, it received no reward and a 5-second timeout. Betting low earned a sure but minimal reward: 3 drops after a correct decision and 2 after an incorrect decision. The reward schedule was based on previous studies (e.g. Kornell et al., 2007) and fine-tuned to elicit best performance.
Neuronal recordings
A single tungsten electrode (.3–1 MΩ impedance at 1 kHz; FHC, Bowdoinham, ME) was lowered through a 23 g. guide tube using a custom microdrive system (ftp://lsr-ftp.nei.nih.gov/lsr/StepperDrive/). A plastic grid with 1×1 mm hole spacing (Crist Instruments, Hagerstown, MD) was attached inside the recording chamber. The FEF was confirmed with microstimulation by evoking saccades at low current threshold (< 50 μA; Bruce and Goldberg, 1985). The PFC was recorded from the same chamber as FEF. PFC recordings included locations a few mm anterior to identified FEF, in areas ventral, dorsal, and within the principal sulcus (identified by the isolation of neurons at lower depths than locations dorsal or ventral). The SEF was identified by moderate-current microstimulation (typically 50–100 μA) that evoked or delayed saccades (Russo and Bruce, 1996; Schlag and Schlag-Rey, 1987). Standard extracellular recording techniques were used to isolate action potentials of single neurons (Sommer and Wurtz, 2004). All data were collected using the REX real-time system (Hays et al., 1982) and analyzed using MATLAB (R20010a, The MathWorks, Inc.).
Analyses
We defined multiple epochs throughout the metacognition task, and measured and analyzed the average firing rates within these epochs. Baseline was 300 ms before Decision Stage target onset. During the Decision Stage, we analyzed a visual-1 epoch 100–300 ms after target onset. The visual-1 epoch was selected to start after the masks appeared in every trial, to end before the onset of our epoch for delay activity, and to capture a broad duration of visual-related activity. Also in the Decision Stage, we analyzed a delay epoch 200 ms before fixation offset, a presaccadic-1 epoch 50 ms before saccade onset, and a postsaccadic epoch 100–300 ms after saccade onset. After the Decision Stage, we defined an interstage epoch as the 400 ms surrounding the time the animal regained fixation to initiate the Bet Stage, from 200 ms before until 200 ms after that time. In the Bet Stage, we analyzed a visual-2 epoch 50–150 ms after bet target onset. The start of this epoch was sooner than that of the visual-1 epoch because there were no masks and we could simply capture the visual response starting at the earliest latencies in the areas under study (generally ~50 ms in the FEF; Pouget et al., 2005). We truncated this epoch at 150 ms after bet target onset to minimize inadvertent measurement of saccade-related activity, given that there was no imposed delay before the bet saccade. Also in the Bet Stage, we analyzed a presaccadic-2 epoch 50 ms before bet saccade onset, a reward anticipation epoch 250 ms before reward delivery, and a reward epoch 50–250 ms after reward.
We performed two types of population analyses. First we included the entire population of recorded neurons. Then we focused on only the subsets of neurons that were significantly modulated within particular epochs. A neuron was deemed significantly active in a given epoch if its average firing rate in the epoch on all correct trials (high and low bets pooled) was above its baseline firing rate as determined by paired t-tests (p < .05 criterion). Modulations below baseline were rare and such neurons were excluded from the second analysis.
To analyze decision-related activity, the average firing rate in each epoch was compared between correct trials and incorrect trials (regardless of bets). For single neuron analyses, comparisons were made using two-sample t-tests (p < .05 criterion). For population analyses, comparisons were made using paired t-tests (p < .05 criterion). Analysis of Bet-related activity was analogous, except we compared average firing rates between all high-bet and all low-bet trials (regardless of decisions).
To analyze metacognition-related activity, the aim was to compare trials in which decisions were identical, but bets (our observables of the monkey’s internal state) were different. We compared average firing rates in each epoch between Correct-High trials (correct decisions followed by high bets) and Correct-Low trials (correct decisions-low bets), or between Incorrect-High trials (incorrect decisions-high bets) and Incorrect-Low trials (incorrect decisions-low bets). For single neuron analyses, one-way ANOVAs were first calculated between all four trial conditions. If significant at p < .05, multiple comparisons (Tukey-Kramer tests) were performed between individual conditions (p < .05 criterion). For population analyses, paired t-tests were calculated between trial outcomes at p < .025, Bonferroni corrected from .05 because we used the same data to analyze reward expectation as well (see Results). Finally, to focus on activity related to targets in a neuron’s visual receptive field, or to saccades made into its movement field, we analyzed memory guided saccade data to ascertain the direction that yielded the strongest visual and presaccadic discharges. We used an epoch 50–150 ms after target onset for the visual response, and an epoch 50 ms before saccade onset for the presaccadic activity. The receptive field and/or movement field was defined as the direction that elicited the maximum firing rate within the relevant epoch. In addition, the firing rate was required to be greater than the neuron’s baseline firing rate (200 ms before target onset), assessed by t-test. We used that direction for our analyses of metacognition task activity that were restricted to the best visual target direction and best saccade direction.
Supplementary Material
Highlights.
Monkeys made decisions and wagered on their performance in a metacognitive task.
Single neurons were recorded in three frontal cortical regions.
Only supplementary eye field (SEF) neuronal activity correlated with metacognition.
The SEF metacognitive signal provided a temporal “bridge” between decision and bet.
Acknowledgments
This research was supported by the NIMH (Kirschstein NRSA F31 MH087094 to P.G.M.) and the NEI (EY017592 to M.A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson EB, Mitchell JF, Reynolds JH. Attentional modulation of firing rate varies with burstiness across putative pyramidal neurons in macaque visual area V4. J Neurosci. 2011;31:10983–10992. doi: 10.1523/JNEUROSCI.0027-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- Boch RA, Goldberg ME. Participation of prefrontal neurons in the preparation of visually guided eye movements in the rhesus monkey. J Neurophysiol. 1989;61:1064–1084. doi: 10.1152/jn.1989.61.5.1064. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Call J. Do apes know that they could be wrong? Anim Cogn. 2010;13:689–700. doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol. 2002;88:3487–3497. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. J Neurosci. 2006;26:6695–6703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH. Metacognitive aspects of problem solving. In: Resnick LB, editor. The nature of intelligence. Hillsdale, NJ: Erlbaum; 1976. pp. 231–236. [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Curr Biol. 2007;17:551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1991;65:1464–1483. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proc Natl Acad Sci U S A. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Anim Cogn. 2004;7:239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican L. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc; 1982. pp. 1–10. [Google Scholar]
- Iba M, Sawaguchi T. Involvement of the dorsolateral prefrontal cortex of monkeys in visuospatial target selection. J Neurophysiol. 2003;89:587–599. doi: 10.1152/jn.00148.2002. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cogn Neurosci. 2006;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Zariwala HA, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455:227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324:759–764. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36:177–186. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron. 2008;59:161–172. doi: 10.1016/j.neuron.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psychol Sci. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Mays LE, Sparks DL. Saccades are spatially, not retinocentrically, coded. Science. 1980;208:1163–1165. doi: 10.1126/science.6769161. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Platt ML. Expectations and outcomes: decision-making in the primate brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:201–211. doi: 10.1007/s00359-004-0565-9. [DOI] [PubMed] [Google Scholar]
- Middlebrooks PG, Sommer MA. Metacognition in monkeys during an oculomotor task. J Exp Psychol Learn Mem Cogn. 2011;37:325–337. doi: 10.1037/a0021611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mohler CW, Goldberg ME, Wurtz RH. Visual receptive fields of frontal eye field neurons. Brain Res. 1973;61:385–389. doi: 10.1016/0006-8993(73)90543-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- Nelson TO. Consciousness and metacognition. Am Psychol. 1996;51:102–116. [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. Psychol Learn Motiv. 1990;26:125–141. [Google Scholar]
- Pannu JK, Kaszniak AW, Rapcsak SZ. Metamemory for faces following frontal lobe damage. J Int Neuropsych Soc. 2005;11:668–676. doi: 10.1017/S1355617705050873. [DOI] [PubMed] [Google Scholar]
- Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol. 2005;94:2086–2092. doi: 10.1152/jn.01097.2004. [DOI] [PubMed] [Google Scholar]
- Reder LM, Schunn CD. Metacognition does not imply awareness: Strategy choice is governed by implicit learning and memory. In: Reder LM, editor. Implicit memory and metacognition. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 45–77. [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Romanski LM. Domain specificity in the primate prefrontal cortex. Cogn Affect Behav Neurosci. 2004;4:421–429. doi: 10.3758/cabn.4.4.421. [DOI] [PubMed] [Google Scholar]
- Rounis E, Maniscalco B, Rothwell JC, Passingham RE, Lau H. Theta-burst transcranial magnetic stimulation to the prefrontal cortex impairs metacognitive visual awareness. Cogn Neurosci. 2010;1:165–175. doi: 10.1080/17588921003632529. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Neurons in the supplementary eye field of rhesus monkeys code visual targets and saccadic eye movements in an oculocentric coordinate system. J Neurophysiol. 1996;76:825–848. doi: 10.1152/jn.1996.76.2.825. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural basis of deciding, choosing and acting. Nat Rev Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for right medial prefontal cortex in accurate feeling-of-knowing judgements: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42:957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Seo H, Lee D. Behavioral and neural changes after gains and losses of conditioned reinforcers. J Neurosci. 2009;29:3627–3641. doi: 10.1523/JNEUROSCI.4726-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields WE, Smith JD, Guttmannova K, Washburn DA. Confidence judgments by humans and rhesus monkeys. J Gen Psychol. 2005;132:165–186. [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) J Exp Psychol Gen. 1995;124:391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Allendoerfer KR, Washburn DA. Memory monitoring by animals and humans. J Exp Psychol Gen. 1998;127:227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- So NY, Stuphorn V. Supplementary eye field encodes option and action value for saccades with variable reward. J Neurophysiol. 2010;104:2634–2653. doi: 10.1152/jn.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So NY, Stuphorn V. Supplementary eye field encodes reward prediction error. J Neurosci. 2012;32:2950–2963. doi: 10.1523/JNEUROSCI.4419-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol. 2000;83:1979–2001. doi: 10.1152/jn.2000.83.4.1979. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. J Neurophysiol. 2001;85:1673–1685. doi: 10.1152/jn.2001.85.4.1673. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Suda-King C. Do orangutans (Pongo pygmaeus) know when they do not remember? Anim Cogn. 2008;11:21–42. doi: 10.1007/s10071-007-0082-7. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning. Vol. 9. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Schall JD. The detection of visual signals by macaque frontal eye field during masking. Nat Neurosci. 1999;2:283–288. doi: 10.1038/6398. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. New Jersey: Prentice Hall; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.