Abstract
Green leafy volatiles (GLV), six-carbon aldehydes, alcohols, and esters commonly emitted by plants in response to mechanical damage or herbivory, induced intact undamaged corn seedlings to rapidly produce jasmonic acid (JA) and emit sesquiterpenes. More importantly, corn seedlings previously exposed to GLV from neighboring plants produced significantly more JA and volatile sesquiterpenes when mechanically damaged and induced with caterpillar regurgitant than seedlings not exposed to GLV. The use of pure synthetic chemicals revealed that (Z)-3-hexenal, (Z)-3-hexen-1-ol, and (Z)-3-hexenyl acetate have nearly identical priming activity. Caterpillar-induced nocturnal volatiles, which are enriched in GLV, also exhibited a strong priming effect, inducing production of larger amounts of JA and release of greater quantities of volatile organic compounds after caterpillar regurgitant application. In contrast, GLV priming did not affect JA production induced by mechanical wounding alone. Thus, GLV specifically prime neighboring plants against impending herbivory by enhancing inducible chemical defense responses triggered during attack and may play a key role in plant-plant signaling and plant-insect interactions.
Plant defenses against herbivorous insects include both chemical and physical mechanisms that directly affect the performance of the herbivores (1). Additionally, a countermeasure against damaging insect herbivores that may be even more effective is the release of volatile organic compounds (VOC), consisting mainly of products of the shikimic acid-pathway, fatty acid-derived products and terpenes, which attract parasitoids and predators, natural enemies of the actively feeding arthropods (2, 3). Herbivore-induced VOC have also been shown to decrease oviposition rates and increase egg predation on the emitting plant in nature (4, 5). Although insect-induced VOC can serve direct and indirect defense functions, neighboring (or receiver) plants may also perceive and respond to these signals. Pathogen infection of plants often leads to the release of methyl salicylate (6), which serves as a potential signal for the induction of defense-related genes in neighboring plants. Also, after wounding and herbivore damage some plants emit methyl jasmonate (MeJA), which has been shown to effectively turn on defense genes (7). However, not all plants release MeJA, calling into question its role as a general volatile defense signal. Green leafy volatiles (GLV) consist mainly of degradation products derived from C18 fatty acids (linolenic and linoleic acid), which, after being transformed to a hydroperoxide by a lipoxygenase, are cleaved into C12 and C6 components by hydroperoxide lyase (HPL). Depending on the C18-substrate, HPL produces either (Z)-3-hexenal [(Z)-3-HAL)] or hexanal (8). Further processing by alcohol dehydrogenase, acetylation, and isomerization leads to the production of the remaining C6-components, like (Z)-3-hexenol [(Z)-3-HOL)], (Z)-3-hexenyl acetate [(Z)-3-HAC], and the respective E-isomers. The C12-component is processed to traumatin, which has long been hypothesized to play an important role in the wound response of plants (9). GLV are typically released locally by plants immediately after wounding or herbivore damage (8) but can also be induced and released systemically (10). Previous studies indicated that GLV induce certain defense-related genes (11-13). However, although plants treated with (E)-2-hexenal released significantly greater quantities of VOC than control plants, they released significantly less than plants treated with MeJA or damaged by insect herbivores (13). Also, in other studies, treatment of plants with six-carbon aldehydes induced less than the complete set of defense-related genes and resulted in a moderate plant response relative to MeJA at both the physiological and molecular levels (11, 12). Two important questions arose from these findings: Which signaling pathways are involved, and is the immediate but rather moderate activation of plant defense responses the main function of this signaling? To answer these questions, we have started a comprehensive investigation of the effects of naturally released GLV on neighboring plants. We used GLV from wounded plant tissue, caterpillar-induced night-time volatiles enriched in GLV, and pure C6 compounds to examine their effects on intact corn plants. We discovered not only that GLV stimulate transient jasmonic acid (JA) biosynthesis and VOC release in corn, but also that exposure to GLV primed corn plant defenses to respond more strongly against subsequent attack by herbivorous insects by increasing JA biosynthesis and VOC release.
Materials and Methods
Plant and Insect Material. Corn (Zea mays cv. Delprim) was grown as reported (14). Beet armyworm (BAW, Spodoptera exigua) eggs were obtained from W. J. Lewis (Insect Biology and Population Management Research Laboratory, U.S. Department of Agriculture, Agricultural Research Service, Tifton, GA) and reared on an artificial diet based on pinto beans (15). Late first and early second instar larvae were selected for the induction of nocturnal volatiles.
Preparation of Crude Regurgitant Elicitor (CRE) from Larvae of BAW. BAW were transferred to feed on corn seedlings at least 48 h before collection of regurgitant. Regurgitation was induced by holding fourth instar BAW caterpillars with forceps and gently pinching behind the head with a second pair. The regurgitant from 40-50 caterpillars was collected, boiled for 5 min to inactivate degrading enzymes (16), centrifuged to remove cell debris and denatured proteins, and the supernatant diluted 1:1 in buffer (50 mM sodium phosphate, pH 8) before use (referred to as CRE).
Chemicals. (Z)-3-HAL (50% in triacetin), (Z)-3-HOL (98% pure), cis jasmone (85% pure), methyl salicylate, and MeJA were purchased from Sigma-Aldrich. (Z)-3-HAC was made from (Z)-3-HOL by acetylation with acetyl chloride and purity estimated by GC and GC/MS analysis (95% pure). Dihydro MeJA (Bedoukian Research, Danbury, CT) was converted to dihydro JA (DhJA) by alkaline hydrolysis. [2H6]salicylic acid (SA) was purchased from CDN Isotopes (Pointe-Claire, QC, Canada). All solvents used were analytical grade.
Plant Treatments. Two different sets of experiments were conducted, one to measure the direct effect of GLV on JA, SA, and VOC production in plants, and the second to determine the effect of GLV treatment on subsequent plant response to wounding and treatment with caterpillar regurgitant. To measure the short-term production of JA and SA, intact corn plants (receiver plants) were exposed to various volatiles in 6-l Plexiglas cylinders. After 0, 30, 60, and 180 min, the intact receiver plants were removed from the chamber and the leaves frozen in liquid nitrogen for further analysis. For short-term exposure to GLV vapors, 2 g of cut-leaf material from 2- to 3-wk-old corn plants was placed on a small dish and added to the chamber with the receiver corn plant. Control plants were held in the chambers for equal periods of time in ambient air. For induction with synthetic compounds, 20 μg of (Z)-3-HAL, (Z)-3-HOL, (Z)-3-HAC, or cis jasmone (17) (dissolved in dichloromethane, 1 μg/μl), was pipetted onto a cotton ball in the Plexiglas cylinder. Controls consisted of a plant in a chamber with 20 μl of pure dichloromethane or 20 μg of triacetin in dichloromethane [for treatments with (Z)-3-HAL)] on a cotton ball. For short-term exposure to caterpillar-induced volatiles (CIV) emitted from neighboring infested plants, 2-wk-old intact corn plants held in 200-ml glass tubes were infested with 20-25 BAW caterpillars (late first to early second instar) in the morning. After 5 h, each glass tube with a caterpillar-infested corn plant (source plant) was connected by Teflon tubing (i.d. 5 mm) to a 6-liter Plexiglas cylinder containing an intact receiver plant. A vacuum was attached to each Plexiglas cylinder, and fresh charcoal-purified air was pulled at ≈200 ml/min over the infested plant and then through the Plexiglas cylinder to allow the entrained nocturnal volatiles to flow over the intact plants. As a control, uninfested corn seedlings were used as source plants.
A second control was performed by using CRE-induced corn plants as a source for VOC. Plants were induced through application of 5 μl of CRE on one wounded site on each leaf (three leaves total) and then transferred to the 200-ml (vol) glass tubes. After 5 h, the tubes were connected to the 6-l Plexiglas cylinders with the receiver plants as described above for CIV.
For overnight exposure to GLV, cut corn leaf material was added to the 200-ml glass cylinder, and GLV was drawn over the receiver plants in 6-liter cylinders at 200 ml/min, as described above. In the controls, the 200-ml cylinders were empty. For induction with synthetic compounds, 20 μg of (Z)-3-HAL, (Z)-3-HOL, or (Z)-3-HAC (dissolved in dichloromethane, 1 μg/μl) was pipetted onto a cotton ball in the Plexiglas cylinder. For controls, 20 μl of dichloromethane was added to the cotton ball. Corn plants were exposed to CIV overnight (15 h) in the same manner described for short-term exposure to CIV (see above).
For induction with CRE, intact corn plants were exposed to GLV, CIV, or the respective synthetic chemical overnight, as described above. After 15 h, plants were removed from Plexiglas cylinders. An area of ≈2 × 10 mm on the third leaf of each plant was scratched with a razor blade and 5 μl of CRE from BAW immediately added to the wounded site. For controls, buffer only was added to the wounded site. Plants were harvested 0, 30, 60, and 180 min after application of CRE, and JA and SA quantified (see below). In a separate experiment, VOC were collected from plants exposed to GLV, CIV, and synthetic compounds overnight and then treated with CRE (see below).
To estimate the amount of each compound in the volatiles to which the corn plants were exposed (Table 1), a Super Q filter-trap (Alltech Associates) was connected to the downstream side of the Plexiglas cylinders containing the treated plants, and air was drawn through the trap at 200 ml/min for various periods of time, depending on the experiment. VOC were eluted from the trap with dichloromethane, nonyl acetate added as an internal standard, and samples were analyzed by GC and GC/MS (3). The identity of each compound was confirmed by comparison of retention times and mass spectra with those of authentic chemicals, and quantities were determined by comparison of peak areas with peak area of internal standard. The average amounts of GLV released from the cut-leaf material and after caterpillar damage are shown in Table 1.
Table 1. Amount of GLV (in ng) released by source plants upstream from receiver plants during periods of treatment.
| CIV | GLV | Control | ||
|---|---|---|---|---|
| 30 min | Z-3-HAL | 356 ± 95 | 4,872 ± 633 | ND |
| Z-3-HOL | 108 ± 53 | 2,736 ± 546 | ND | |
| Z-3-HAC | 595 ± 569 | 3,720 ± 1,052 | ND | |
| Overnight | Z-3-HAL | 2,177 ± 470 | 653 ± 339 | 44 ± 37 |
| Z-3-HOL | 1,210 ± 128 | 3,137 ± 245 | 44 ± 30 | |
| Z-3-HAC | 3,540 ± 833 | 4,102 ± 663 | 207 ± 184 |
Data are from caterpillar-infested corn plants (CIV), cut leaf material (GLV), and control plants during the 30-min and overnight incubation period. Data represent mean ± SD (n = 4). ND, not detected.
Quantification of JA and SA. Extraction and quantification were performed as described (18, 19). In brief, plant tissues were frozen in liquid N2, and ≈100 mg of each sample was transferred to 2-ml screw-cap FastPrep tubes (Qbiogene, Carlsbad, CA) containing 1 g of Zirmil beads (1.1 mm; SEPR Ceramic Beads and Powders, Mountainside, NJ). Dihydro JA and [2H6]SA (100 ng) were added to the 2-ml tubes before sample addition. The samples were mixed with 300 μl of 1-propanol/H2O/HCl (2:1:0.002) and shaken for 30 s in a FastPrep FP 120 tissue homogenizer (Qbiogene). Dichloromethane (1 ml) was added to each sample, reshaken for 10 s in the homogenizer, and centrifuged at 11,300 × g for 30 s. The bottom dichloromethane/1-propanol layer was then transferred to a 4-ml glass screw-cap vial, with care taken to avoid transfer of the upper aqueous layer. The organic phase was evaporated by a constant airstream and 100 μl of diethyl ether/methanol (9:1, vol/vol) added. Carboxylic acids were converted into methyl esters by the addition of 2 μl of a 2.0 M solution of trimethylsilyldiazomethane in hexane. The vials were then capped, vortexed, and allowed to sit at room temperature for 30 min. Excess trimethylsilyldiazomethane was then destroyed by adding an equivalent molar amount of acetic acid to each sample.
Volatile metabolites were separated from the complex mixture by vapor-phase extraction as described in ref. 19. The trapped volatiles were then eluted with 150 μl of dichloromethane and analyzed by chemical ionization-GC/MS (19).
Analysis of Released VOC. Plants exposed to GLV, CIV, and pure synthetic compounds overnight (15 h) were transferred to 200-ml glass cylinders and VOC collected for 1 h without further treatment. In a second experiment, intact corn plants were exposed overnight as described above. After 15 h, plants were removed from the Plexiglas incubation chamber and induced with CRE as described above. Plants were subsequently transferred to the 200-ml glass cylinder, and VOC were collected for 1-h periods beginning 30 min after induction. Between sequential volatile collections from the same plant, there was a 30-min delay, during which the plant was watered. Volatiles were collected by pulling purified air at 200 ml/min over the plants and through Super Q filter traps. Analysis of the trapped VOC was performed as described in ref. 3. The amounts of linalool, 4,8-dimethylnona-1,3,7-triene, β-caryophyllene, bergamotene, and β-farnesene emitted by the plants during the volatile collection period were measured and summed to obtain an estimate of total volatiles.
Statistical Analysis. At least three replicates of all experiments were conducted. Data were analyzed for significance with t test (P < 0.05). Treatments were compared to appropriate controls.
Results
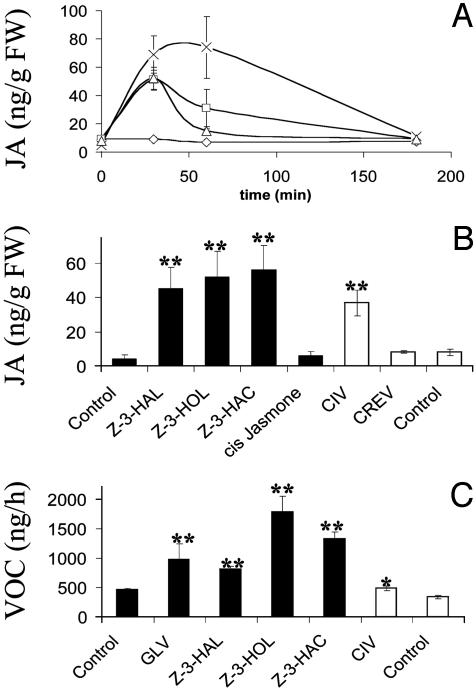
GLV Induce JA Production and Volatile Emission. When we exposed intact hydroponically grown corn seedlings to wound-induced GLV by adding cut-leaf material to the incubation chamber, JA was induced transiently, reaching a maximum [52 ng/g fresh weight (FW)] 30 min after exposure (Fig. 1A). This initial burst of JA declined rapidly and reached the baseline levels of untreated control plants after 2-3 h (9 ng/g FW; control 8 ng/g FW). The analysis of the released GLV revealed that predominantly the (Z)-3-isomers were released after wounding and caterpillar infestation. When individual seedlings in incubation chambers were exposed to the vapors of (Z)-3-HAL, (Z)-3-HOL, (Z)-3-HAC, or cis jasmone, evaporated from cotton balls in concentrations comparable to those released by cut-leaf material, all C6 compounds tested induced JA in comparable amounts (45-56 ng/g FW JA, compared to 4 ng/g FW in the control plants; Fig. 1B). Higher concentrations of synthetic compounds did not elevate the amount of induced JA significantly above this level but maintained it over a longer period (Fig. 1A). Synthetic compounds were still active at concentrations as low as 3 nM in the gas phase, inducing 15-20 ng/g FW of JA compared to 5 ng/g FW in control plants. Levels of endogenous JA did not change in control plants and plants treated with cis jasmone (17) (Fig. 1B). SA was not affected by treatment with GLV.
Fig. 1.
Effects of GLV, CIV, and pure C6 compounds on JA production and the release of volatiles in intact corn plants. Error bars represent SD (n = 4). Data were analyzed for significance with t test (*, P ≤ 0.05; **, P ≤ 0.01). (A) Induction of JA in corn seedlings by GLV and 20 μg or 1 mg of (Z)-3-HAC in a 6-liter Plexiglas container. Corn plants were incubated for 0, 30, 60, and 180 min. Controls were treated the same way, except that no volatile compounds were added to the incubation chambers. ⋄, control; ▵, GLV-treated; □, ≤20 μg of (Z)-3-HAC; ×, 1 mg of (Z)-3-HAC. Twenty micrograms of (Z)-3-HAC corresponds to 30 nM maximum concentration in the gas phase, and 1 mg corresponds to 1.66 μM maximum concentration in the gas phase. Data points are connected by smoothed lines. (B) Black bars indicate levels of JA in intact corn plants after 30-min exposure to volatiles from synthetic compounds (Z)-3-HAL, (Z)-3-HOL, (Z)-3-HAC, or cis jasmone (20 μg each). White bars indicate JA levels in corn plants after 30-min exposure to CIV, crude regurgitant elicitor-induced volatiles (CREV), and control. Intact uninfested corn plants were used as a source of volatiles in the controls. (C) Induction of VOC after overnight exposure to GLV, synthetic C6 compounds, CIV, or controls, as described above. The amounts of linalool, 4,8-dimethylnona-1,3,7-triene, β-caryophyllene, bergamotene, and β-farnesene emitted by the plants during the volatile collection period were measured and summed to obtain total volatiles.
In a second experiment, nocturnal VOCs from plants infested with BAW, consisting predominantly of (Z)-3-HAL, (Z)-3-HOL, and (Z)-3-HAC, were used as a source of naturally released GLV. Importantly, plants exposed to these CIV also exhibited an increase of JA after 30 min (38 ng/g FW; Fig. 1B), although these plants received less GLV compared to those plants exposed to cut-leaf material (Table 1). SA was again not affected by this treatment, even with longer treatments of 8 h (data not shown). To examine the relevance of GLV further, CRE-induced volatiles (CREV), which contain only trace amounts of GLV, were used to induce plants. CREV failed to induce JA in receiver plants (Fig. 1B).
After overnight treatment of corn plants with either GLV or pure C6 compounds, we observed the induction of small but significant amounts of VOC compared to control plants (Fig. 1C). Overnight exposure of corn plants to CIV also resulted in a slight stimulation of volatile release by receiver plants (Fig. 1C).
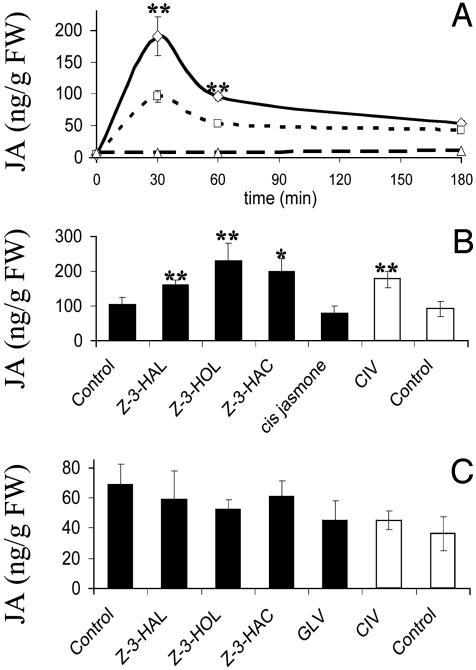
GLV Pretreatment Enhances JA Production and Volatile Emission in Response to Treatment with Caterpillar Regurgitant. To test the priming hypothesis, corn plants were pretreated with either wound-induced GLV, CIV, or synthetic C6 compounds overnight (15 h), as described above. Then, CRE was applied to the plants the next day as a mimic of actual caterpillar feeding. We measured CRE-induced JA and the release of induced VOC as indicators of the induction of defense responses. After overnight exposure to GLV, CIV, and synthetic C6 compounds, the resting levels of JA in these plants were the same as in the control plants (8 ng/g FW). Thirty minutes after induction with CRE, the level of endogenous JA rose in control plants to 96 ng/g FW, whereas in GLV-pretreated plants, 190 ng/g FW were found (Fig. 2 A and B). The level of JA in GLV-pretreated plants remained higher over a period of 3 h but followed the same trend as JA in CRE-induced control plants (Fig. 2A). The same effect was observed for pretreatment with pure C6 compounds and CIV (Fig. 2B). Surprisingly, wound-induced JA was not affected by previous exposure of corn plants to GLV (Fig. 2C).
Fig. 2.
Effects of pretreatment with GLV and pure chemicals on subsequent CRE- or wound-induced JA. Error bars represent SD (n = 4). Data for induction of JA by application of CRE (with or without previous exposure to GLV) were analyzed for significance with t test (*, P ≤ 0.05; **, P ≤ 0.01). (A) Induction of JA after application of CRE to GLV-pretreated intact corn plants. ⋄, GLV plus CRE; □, control plus CRE; ▵, control. Data points are connected by smoothed lines. (B) Effects of overnight exposure to synthetic chemicals on CRE-induced JA in intact corn plants. JA was quantified from leaf tissue 30 min after application of CRE. (C) Effects of GLV, CIV, and pure C6 compounds on wound-induced JA. Corn plants were exposed to synthetic C6 compounds, GLV, CIV, and the respective control for 15 h. One leaf then was wounded with a razor blade, and JA was quantified after 30 min.
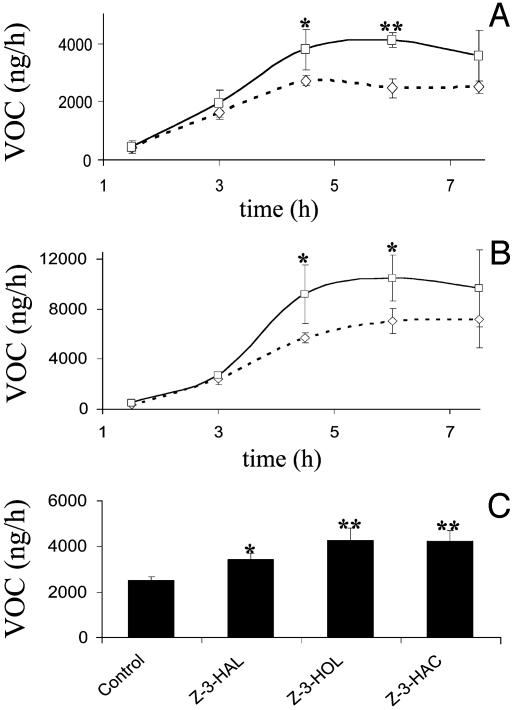
The consequences of GLV pretreatment were further demonstrated by their effect on the release of VOC induced by application of CRE. Corn plants exposed overnight to GLV, CIV, and pure C6 compounds released VOC without further treatment. However, this effect was significantly enhanced by induction with CRE. The GLV-pretreated plants released ≈4 μg of total VOC 4-6 h postinduction compared to 2.5 μg from nonprimed control plants (Fig. 3A). The same effect was observed for pretreatment with CIV (Fig. 3B) and pure C6 compounds (Fig. 3C). GLV-pretreated plants reached the maximum release rate of unprimed plants earlier and exhibited a higher absolute release rate
Fig. 3.
Effects of pretreatment with GLV, pure chemicals, and CIV on subsequent CRE-induced volatiles. Error bars represent SD (n = 4). Data were analyzed for significance with t test (*, P ≤ 0.05; **, P ≤ 0.01). After induction with CRE, volatiles were collected during the period of maximum release rate (4-5 h after induction). Note that experiments were done with different batches of plants and CRE and at different times of the year. (A) Induction of volatiles by CRE in corn seedlings after overnight exposure to GLV. □, GLV plus CRE; ⋄, control plus CRE. Data points are connected by smoothed lines. (B) Induction of volatiles by CRE in corn seedlings after overnight exposure to CIV. □, CIV plus CRE; ⋄, control plus CRE. Data points are connected by smoothed lines. (C) Induction of volatiles by CRE in corn seedlings after overnight exposure to pure C6 compounds.
Discussion
Our results clearly demonstrate a specific function for GLV in priming the defenses of corn plants against herbivorous insects. Both JA production, which is important to direct and indirect defenses, and VOC release in response to simulated herbivore attack were significantly enhanced in plants previously exposed to GLV. Furthermore, only elicitor-induced JA was affected, whereas wound-induced production of JA remained unchanged.
GLV are important components of the VOC blends released by plants as a defense against attacking insect herbivores (2, 3). Moreover, unlike the induced emission of VOC like terpenes, indole, and methyl salicylate, which may not be released for hours after the beginning of herbivore damage, GLV are released almost immediately after wounding and the onset of herbivory and are considered typical wound signals (8).
GLV have been described as inducers of defense-related processes in various plant species; however, the signaling mechanisms involved were not clearly defined (11-13, 20). Furthermore, gene expression analysis indicated that a subset of defense-related genes was induced after exposure to GLV, all of which were related to JA action. JA, also first considered to be a typical wound signal (7), plays an important role in the activation of defensive functions in plants (7, 21, 22). The specific attribute of exogenous GLV to induce JA in corn plants, as shown herein, was the first indication of a possible mechanism linking this highly volatile wound signal to defense responses. The induction of subsets of defense-related genes (11, 12) might reflect the relatively low and transient induction of JA after exposure to GLV (compare Figs. 1A and 2A).
C6 compounds are known to induce the release of VOC in tomato plants exposed to physiological concentrations (13), but the response of these plants was lower compared to those exposed to MeJA or actual insect herbivory. This coincides well with our own findings. Corn plants exposed to GLV, CIV, or pure C6 compounds also released significant amounts of VOC compared to control plants. Comparing the total amounts of released VOC after exposure to GLV with that induced by application of CRE revealed that application of CRE induced four to six times more VOC, although only one leaf was damaged.
GLV may also mediate direct defense against insect pests. Aphids feeding on potato plants depleted in HPL activity (23) exhibited a 2-fold increase in fecundity compared to those feeding on wild-type plants. Interestingly, in this example, the plants did not show any phenotypic differences compared to wild-type plants with regard to wound-induced gene expression, further indicating a specific function of GLV in defense-related processes rather than in the wound response. Together with our own results, these examples demonstrate the capability of GLV to affect plant defense responses against various threats in different plant species. However, it is obvious that all these responses are moderate compared to those after actual insect herbivore damage and would provide only a very low level of protection against attack by herbivorous insects. This led to the question of whether the capability of GLV to induce JA and thus JA-responsive defense-related genes is the primary function of these compounds, or whether the rather modest induction of defense-related processes primes the receiver plants against pending attack.
Cost/benefit analyses demonstrate that maintaining a high level of defense without being actually threatened negatively affects plant performance (24, 25) and would make such a mechanism beneficial only under conditions with a high probability of attack. However, priming by GLV provides a different way of responding to the threat of insect herbivory. Defense-related processes are turned on but incompletely compared to actual herbivore damage (11, 12). More importantly, the plant prepares, by a yet-unknown mechanism, to respond more intensely when it is subsequently attacked, as demonstrated herein for the induction of JA and release of VOC. In this way, the plant avoids great biochemical investments, which would affect the general physiology significantly unless actually attacked (24, 25).
A further, equally important aspect of priming is the specificity of the signaling process. As mentioned previously, GLV induce a subset of defense-related genes (11, 12) or cause the release of VOC (13), all processes related to JA. However, in addition to the induction of defense responses, JA is involved in various developmental processes and responses to other environmental factors in plants (21, 22). This raises the question of which JA signaling processes are induced by GLV. In corn, there are at least three different ways of inducing JA, all related to wounding but different in their regulation. GLV induces JA transiently; wounding also results in JA production; application of CRE on wounded sites combines the wound response and an elicitor response, resulting in higher amounts of induced JA. Priming with GLV specifically promoted only the CRE-induced JA production, whereas wound-induced JA or JA induced by a second application of GLV after 15 h (data not shown) was not affected. Furthermore, SA was never affected by any of the treatments with GLV, giving further proof for the specificity of the GLV signal in insect herbivore defense response.
The induction and release of VOC by plants as a response to insect herbivore damage have been demonstrated to be an effective defense strategy. Recruiting parasites and predators of the herbivore (2, 3) as well as repelling female moths, thereby avoiding egg deposition (4), helps the plant to reduce damage. Additionally, parasitization of attacking insect herbivores increased seed production in mature corn plants (26), demonstrating clearly the fitness benefits of this defense measure. It is obvious that the effectiveness of this strategy strongly depends on the timely release of a strong VOC signal. Priming corn plants with GLV results in a faster and more intense release of these VOC when induced with CRE and could give them a competitive advantage over nonprimed plants.
Our results demonstrate that GLV induce defense responses in neighboring plants via induction of JA followed by the release of low levels of typical herbivore-induced VOC. Furthermore, a specific priming of corn plants against subsequent insect herbivore attack is initialized, allowing them to respond more rapidly by enhanced JA production and an increased release of VOC (26, 27). The principle of priming plants against pathogen infections by chemicals that mimic endogenous defense-related signaling compounds is well established (28). A comparable mechanism has not been shown to date for priming against insect herbivore attack.
A further indication for the specificity of this signaling is the inactivity of other VOC in this system. However, other classes of VOC have been demonstrated to be interplant defense signals in other plant species (11, 29-31). The mechanism of priming may benefit receiver plants by reducing investment in defenses until the onset of actual herbivory. Thus, the effect of GLV is far-reaching and influences both directly and indirectly the entire tritrophic complex of plants, insect herbivores, and natural enemies of the herbivores. Future research is now directed toward the underlying molecular mechanism of this process in plants.
Acknowledgments
We thank the two reviewers for helpful advice that significantly improved the manuscript. This work was supported in part by a grant from the Defense Advanced Research Project Agency.
Abbreviations: JA, jasmonic acid; SA, salicylic acid; GLV, green leafy volatiles; CIV, caterpillar-induced volatiles; VOC, volatile organic compounds; (Z)-3-HAL, (Z)-3-hexenal; (Z)-3-HOL, (Z)-3-hexenol; (Z)-3-HAC, (Z)-3-hexenyl acetate; MeJA, methyl jasmonate; CRE, crude regurgitant elicitor; FW, fresh weight.
References
- 1.Karban, R. & Baldwin I. T. (1997) Induced Responses to Herbivory (Univ. of Chicago Press, Chicago).
- 2.Takabayashi, J. & Dicke, M. (1996) Trends Plant Sci. 109-113.
- 3.Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T. & Doolittle, R. E. J. (1991) Chem. Ecol. 17, 2235-2251. [DOI] [PubMed] [Google Scholar]
- 4.DeMoraes, C. M., Mescher, M. C. & Tumlinson, J. H. (2001) Nature 410, 577-580. [DOI] [PubMed] [Google Scholar]
- 5.Kessler, A. & Baldwin, I. T. (2001) Science 291, 2141-2144. [DOI] [PubMed] [Google Scholar]
- 6.Shulaev, V., Silverman, P. & Raskin, I. (1997) Nature 386, 718-721. [Google Scholar]
- 7.Creelman, R. A. & Mullet, J. E. (1997) Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 355-381. [DOI] [PubMed] [Google Scholar]
- 8.Hatanaka, A. (1993) Phytochemistry 34, 1201-1218. [Google Scholar]
- 9.Vick, B. A. (1993) in Lipid Metabolism in Plants, ed. Moore, T. S., Jr. (CRC Press, Boca Raton, FL), pp. 167-191.
- 10.Paré, P. W. & Tumlinson, J. H. (1997) Plant Physiol. 114, 1161-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bate, N. J. & Rothstein, S. J. (1998) Plant J. 16, 561-569. [DOI] [PubMed] [Google Scholar]
- 12.Arimura, G., Ozawa, R., Nishioka, T., Boland, W., Koch, T., Kühnemann, F. & Takabayashi, J. (2002) Plant J. 29, 87-98. [DOI] [PubMed] [Google Scholar]
- 13.Farag, M. A. & Pare, P. W. (2002) Phytochemistry 61, 545-554. [DOI] [PubMed] [Google Scholar]
- 14.Schmelz, E. A., Alborn, H. T. & Tumlinson, J. H. (2001) Planta 214, 171-179. [DOI] [PubMed] [Google Scholar]
- 15.King, E. G. & Leppla, N. C. (1984) Advances and Challenges in Insect Rearing (U.S. Govt. Printing Office, Washington, DC).
- 16.Mori, N., Alborn, H. T., Teal, P. E. & Tumlinson, J. H. (2001) J. Insect Physiol. 47, 749-757. [DOI] [PubMed] [Google Scholar]
- 17.Birkett, M. A., Campbell, C. A. M., Chamberlain, K., Guerrieri, E., Hick, A. J., Martin, J. L., Matthes, M., Napier, J. A., Pettersson, J., Picket, J. A., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 9329-9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmelz, E. A., Engelberth, J., Alborn, H. T., O′Donnel, P., Sammons, M., Toshima, H. & Tumlinson, J. H. (2003) Proc. Natl. Acad. Sci. USA 100, 10552-10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelberth, J., Schmelz, E. A., Alborn, H. T., Cardoza, Y. J., Huang, J. & Tumlinson, J. H. (2003) Anal. Biochem. 312, 242-250. [DOI] [PubMed] [Google Scholar]
- 20.Sivasankar, S., Sheldrick, B. & Rothstein, S. J. (2000) Plant Physiol. 122, 1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creelman, R. A. & Mullet, J. E. (1995) Proc. Natl. Acad. Sci. USA 92, 4114-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beale, M. H. & Ward, J. L. (1998) Nat. Prod. Rep. 15, 533-548. [DOI] [PubMed] [Google Scholar]
- 23.Vancanneyt, G., Sanz, C., Farmaki, T., Paneque, M., Ortego, F., Castanera, P. & Sanchez-Serrano, J. J. (2001) Proc. Natl. Acad. Sci. USA 98, 8139-8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heil, M. & Baldwin, I. T. (2002) Trends Plant Sci. 7, 61-67. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin, I. T. (1998) Proc. Natl. Acad. Sci. USA 95, 8113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritzsche Hoballah, M. E. & Turlings, T. C. J. (2001) Evol. Ecol. Res. 3, 553-565. [Google Scholar]
- 27.Fritzsche Hobalah, M. E., Tamo, C. & Turlings, T. C. J. (2002) J. Chem. Ecol. 28, 951-968. [DOI] [PubMed] [Google Scholar]
- 28.Conrath, U., Pieterse, C. M. J. & Mauch-Mani, B. (2002) Trends Plant Sci. 7, 210-216. [DOI] [PubMed] [Google Scholar]
- 29.Arimura, G., Ozawa, R., Shimoda, T., Nishioka, T., Boland, W. & Takabayashi, J. (2000) Nature 406, 512-515. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin, I. T. & Schultz, J. C. (1983) Science 221, 277-279. [DOI] [PubMed] [Google Scholar]
- 31.Tscharnke, T., Thiessen, S., Dolch, R. & Boland, W. (2001) Biochem. Syst. Ecol. 29, 1025-1047. [Google Scholar]