Abstract
A triptycene-based bis(benzoxazole) diacid ligand H2L2Ph4 bearing sterically encumbering groups was synthesized. Treatment of H2L2Ph4 with Fe(OTf)3 afforded a C2-symmetric trinuclear iron(III) complex, [NaFe3(L2Ph4)2(μ3-O)(μ-O2CCPh3)2(H2O)3](OTf)2 (8). The triiron core of this complex adopts the well known “basic iron acetate” structure where the heteroleptic carboxylates, comprising two dianionic ligands (L2Ph4)2− and two Ph3CCO2−, donate the six carboxylate bridges. The (L2Ph4)2− ligand undergoes only minor conformational changes upon formation of the complex.
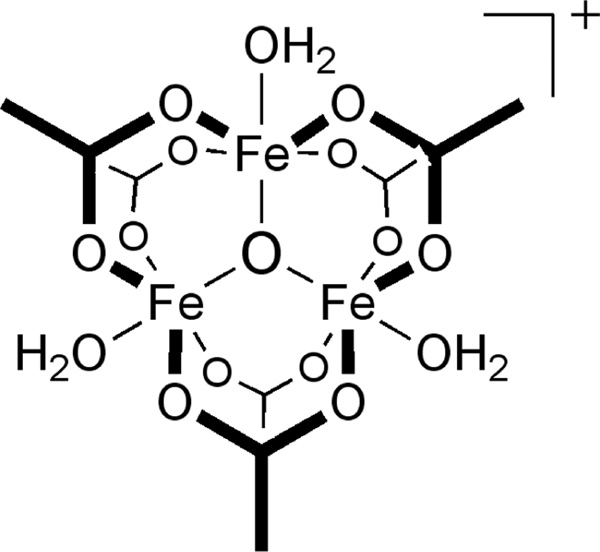
The chemical and physical properties of oxo-bridged triiron carboxylates, also known as “basic iron acetates” (Figure 1), were studied decades ago, and the trinuclear μ3-oxo structural motif is recognized as a ubiquitous feature of iron carboxylate chemistry.1 The widespread occurrence of the {Fe3O(O2CR)6}+ motif is most likely a consequence of its thermodynamic stability, and the unit has been employed as a useful building block of metal-organic frameworks (MOFs) and other solid state materials.2–5 In addition, this family of complexes displays interesting catalytic properties in olefin epoxidation6 and the oxidation of C–H bonds.7,8 Aside from its distinctive {Fe3O(O2CR)6}+/0 core, several other features are characteristic of basic iron carboxylates. Most such complexes are bridged by ligands derived from six monocarboxylate ligands (Figure 1). The use of chelating diacetate ligands in the core has only recently been reported.8 Moreover, to our knowledge, basic iron acetates are all derived from a homoleptic set of carboxylate bridges. No {Fe3O(O2CR)6}+ cores bearing a mixture of different carboxylate bridges are known. Here, we describe a novel chiral C2-symmetric complex having a triiron(III) basic carboxylate structure that extends the type of known compounds in this class. This cluster contains a chelating dicarboxylate ligand with a triptycene backbone. We report the synthesis, structure, and characterization of the ligand, as well as the triiron(III) complex.
Figure 1.
Basic iron acetate
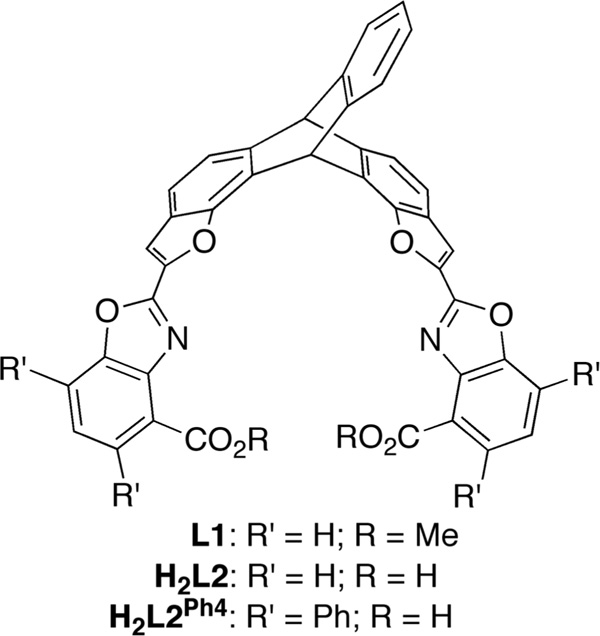
In line with the long-term goal of our laboratory to prepare biomimetic models of the diiron active site of soluble methane monooxygenase hydroxylase (sMMOH) and related bacterial monooxygenases (BMMs),9–12 we previously synthesized a triptycene-based diester ligand L1 and studied its iron complexes (Figure 2).13 To better match the active sites of the BMMs, it is desirable to replace the ester ligands of L1 with more biologically relevant carboxylate groups, such as those present in the related ligand H2L2. Although H2L2 can be prepared by hydrolysis of L1, subsequent attempts to access iron complexes of this ligand afforded only intractable materials. We hypothesize that the bridging coordination mode of carboxylate ligands14 may facilitate the formation of insoluble coordination polymers in the case of H2L2. Inspired by the success imparted by sterically encumbering carboxylate ligands to prevent such polymerization reactions and produce the desired diiron cores,10,15 we designed a modified version of the ligand, H2L2Ph4, with bulky phenyl groups incorporated at the ortho positions of each carboxylic acid in H2L2. For synthetic convenience, the para positions of the carboxylate arenes were also similarly functionalized.
Figure 2.
ligand L1 and proposed diacid ligands
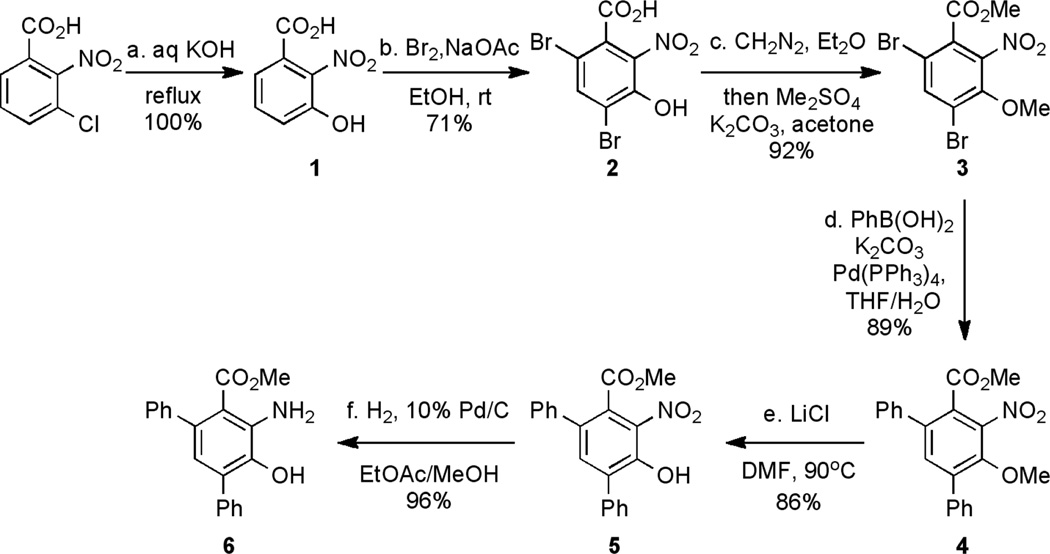
Our proposed synthetic pathway to H2L2Ph4 used the diphenyl substituted methyl (3-hydroxy)anthranilate analog 6 as the starting material. The synthesis of 6 commenced with 3-chloro-2-nitrobenzolic acid (Scheme 1). Hydroxylation of this electron deficient chlorobenzene gave 3-hydroxy-2-nitrobenzoic acid (1) in quantitative yield. Although dibromination of 1 using Br2/HOAc has been reported,16 we found the conditions to be too harsh, for they produced many side products. An improved reaction condition (Br2/NaOAc in EtOH) was devised that was sufficiently mild, affording 4,6-dibromo-3-hydroxy-2-nitrobenzoic acid (2) in 71% yield. An X-ray crystal structure of 2 confirmed the desired positions of the two bromine atoms (Figure S2). A two-step, one-pot protection reaction of both the carboxylic acid and the phenol of 2 using diazomethane and dimethylsulfate/K2CO3, respectively, gave methyl 4,6-dibromo-3-methoxy-2-nitrobenzoate (3) in 92% overall yield. Suzuki coupling reaction of 3 with phenyl boronic acid successfully introduced two phenyl groups on both the ortho and para positions of the arene in 3, giving 4 in 89% yield. The selective deprotection of the methyl phenyl ether in the presence of the methyl ester in 4 proved challenging. Ester groups are more vulnerable than ethers to deprotection under most commonly used conditions. A literature search revealed that deprotection of nitro-substituted methyl phenol ethers can be accomplished using LiCl as a mild reagent in refluxing DMF.17 Using this condition, however, resulted in demethylation of the ester moiety. We hypothesized that the high temperature (160 °C) employed might lead to the undesired hydrolysis of the ester group. Therefore, we carried out the reaction with 4 using a lower temperature (90 °C), and only the methyl phenyl ether moiety of 4 was deprotected by LiCl, leaving the ester group unmodified. After purification, phenol 5 was obtained in 86% yield. Finally, methyl anthranilate 6 was achieved in 96% yield after hydrogenation of 5.
Scheme 1.
Synthesis of methyl anthranilate analog 6.
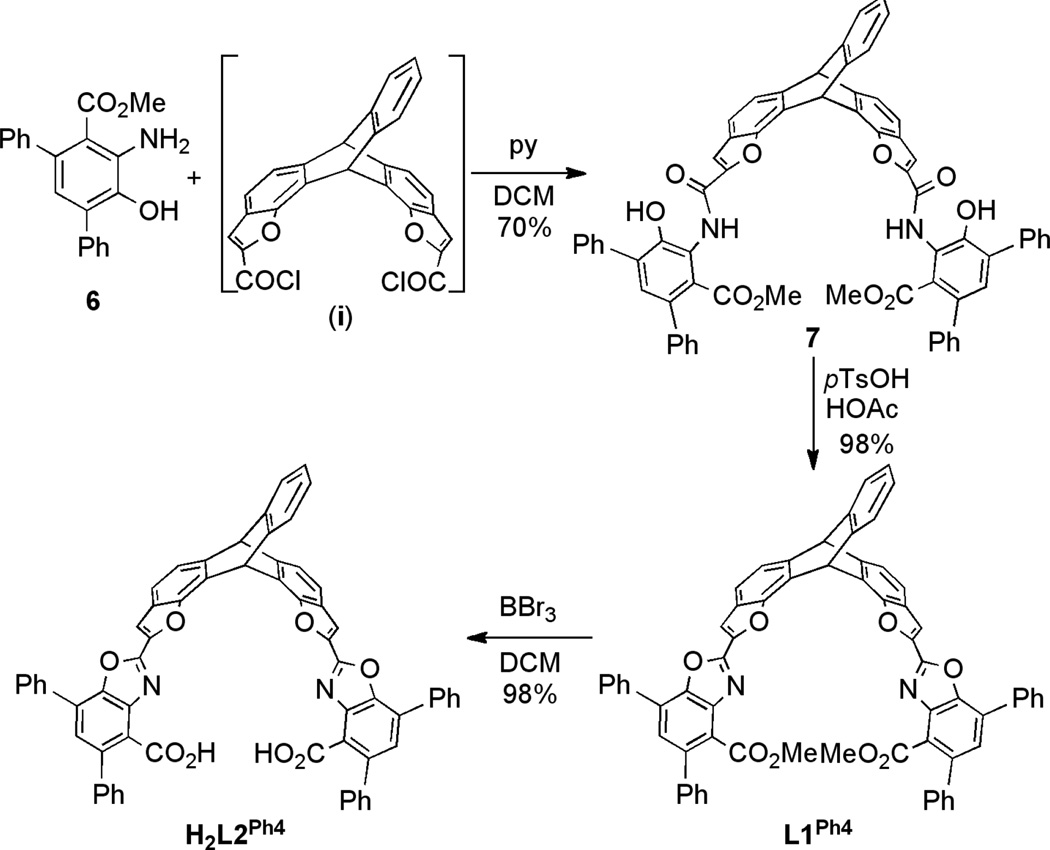
With our desired methyl anthranilate 6 in hand, we moved forward with the assembly of the triptycene backbone. As shown in Scheme 2, amide coupling of 6 with in situ generated benzoyl chloride i gave diamide 7 in 70% yield.13 Next, compound 7 was cyclized using p-TsOH·H2O in HOAc to give L1Ph4 in 98% yield. Finally, the methyl esters in L1Ph4 were successfully hydrolyzed by BBr3, giving H2L2Ph4 in 98% yield. The overall yield of this 9-step synthesis is 32%.
Scheme 2.
Synthesis of H2L2Ph4.
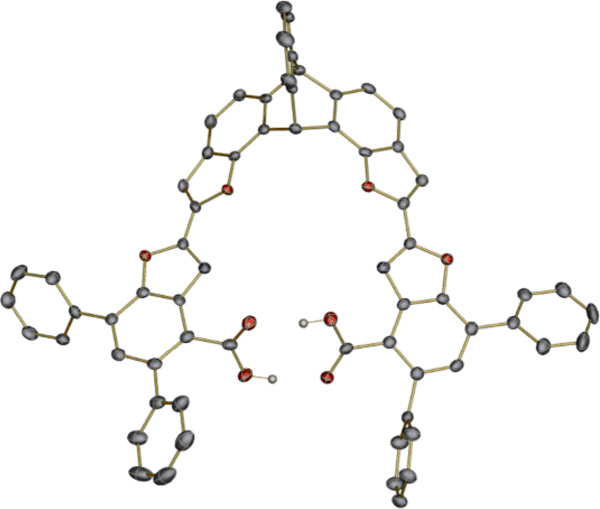
X-ray diffraction quality crystals of H2L2Ph4 were obtained by slow evaporation of a CD2Cl2 solution in an NMR tube. As shown in Figure 3, the two carboxylic acids in the crystal structure of H2L2Ph4 are oriented toward one another. The distances between two pairs of oxygen atoms are 2.676(3) Å and 2.734(3) Å, which are within hydrogen bonding distance. Furthermore, the two hydrogen atoms could be located on the difference Fourier map. The hydrogen bonds orient all of the potential donor atoms toward the center of the ligand. The distance between two nitrogen atoms is 6.013 Å and that between two furan oxygen atoms on the triptycene backbone is 4.780 Å, indicating that the ligand framework will provide a suitable binding pocket for two metal atoms. In addition, the phenyl groups of H2L2Ph4 significantly enhance the solubility of this diacid relative to the underivatized analog H2L2 in various non-polar organic solvents; the additional phenyl groups will efficiently increase the hydrophobicity of the ligand.
Figure 3.
Thermal ellipsoid plot (50% probability) of X-ray structure of ligand H2L2Ph4. Hydrogens on carbon are omitted for clarity. Carbon, gray; oxygen, red; nitrogen, blue; hydrogen, wheat.
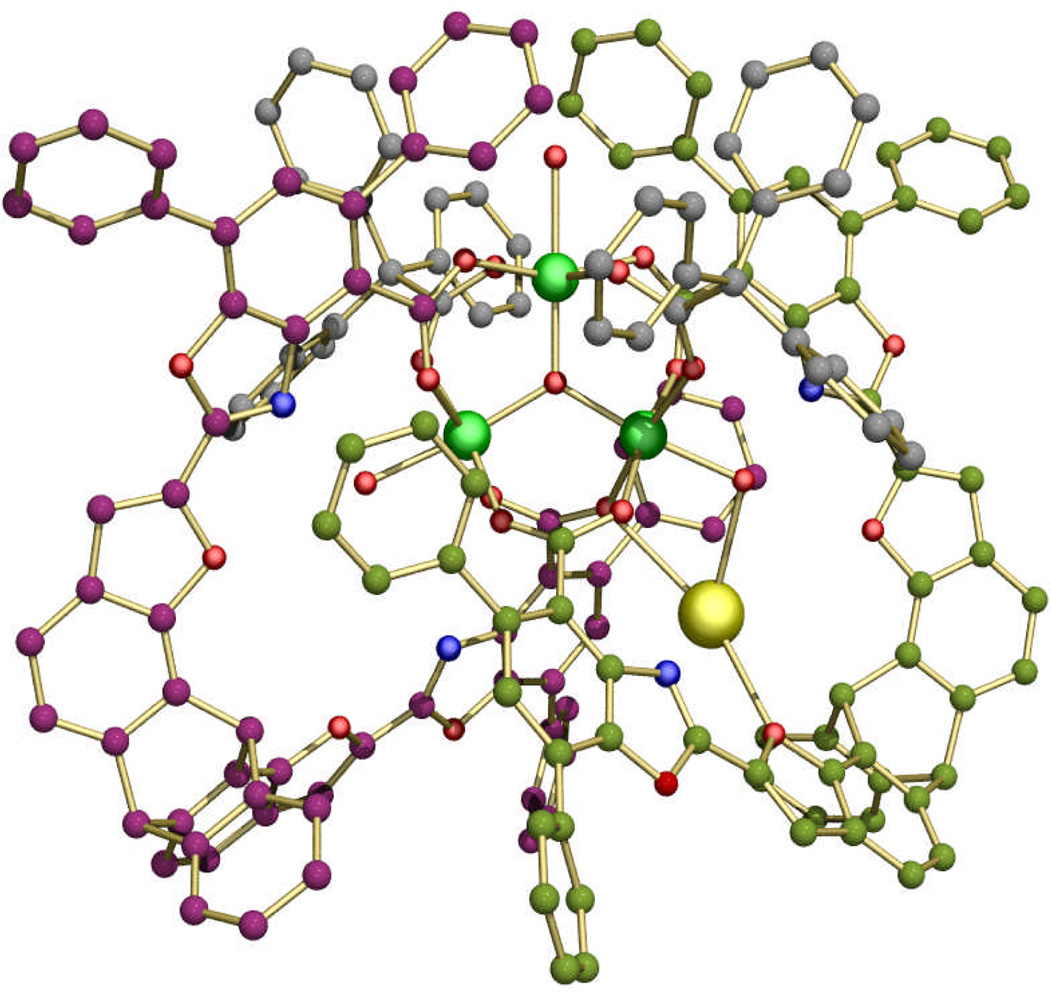
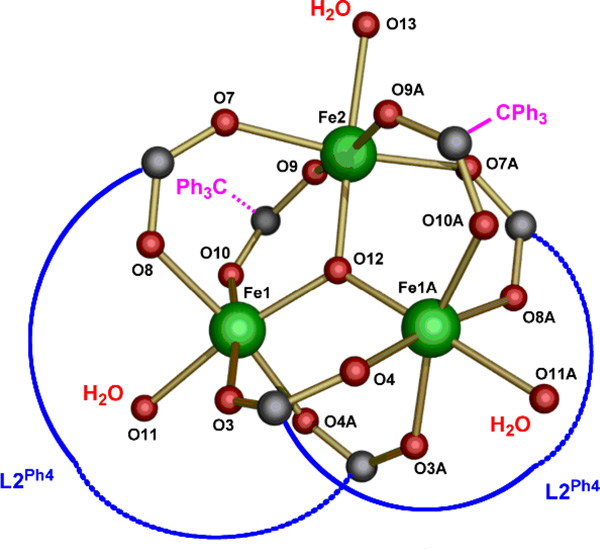
Treatment of H2L2Ph4 with Fe(OTf)3 and triethylamine in the presence of sodium 2,2,2-triphenylacetate (Ph3CCO2Na) afforded red crystalline blocks after vapor diffusion of pentane into a benzene solution of the reaction mixture. Although these crystals diffracted only to 1 Å resolution, the structure solution and refinement determined unambiguously the geometry of the complex. The complex is best formulated as [NaFe3(L2Ph4)2(μ3-O)(μ-O2CPh3)2(H2O)3](OTf)2 (8) (Figure 4), and the core is depicted in Figure 5. This latter features a triiron center, bridged internally by a μ3-oxo atom and externally by the carboxylates of (L2Ph4)2− and by Ph3CCO2−. Complex 8 has a crystallographically required C2-axis passing through the μ3-oxo ion (O12), Fe2 atom, and a coordinated water molecule (O13) (Figure 5). A sodium ion (yellow, Figure 4) forms an intimate ion-pair complex through coordination to carboxylate, water, and benzofuran oxygen atoms of the core structure. The sodium ion is spatially disordered, with 50% occupancy, between the site in the asymmetric unit (Figure 4) and its symmetry generated crystallographic partner (not shown).
Figure 4.
Ball-and-stick representation of the X-ray crystal structure of [NaFe3(L2Ph4)2(μ3-O)(μ-O2CPh3)2(H2O)3](OTf)2 (8). Hydrogen atoms, solvent molecules, and counter anions are omitted for clarity. The carbons of two (L2Ph4)2− are colored pink and bright green. Iron, dark green; oxygen, red; nitrogen, blue; sodium, yellow. The depicted Na+ ion occurs at half-occupancy; the other ion, generated by a C2 axis, is not shown.
Figure 5.
Core structure of complex 8.
The core structure of 8 is shown in Figure 5 (see Figure S5 for detailed information). Among the six carboxylate bridges, four are derived from two (L2Ph4)2− anions and the other two are Ph3CCO2−. Although a number of triiron basic carboxylate structures are known, the structure of 8 is unique for several reasons. The use of chelating dicarboxylate ligands for the assembly of this {Fe3O}7+ core is rare. Furthermore, to the best of our knowledge, all currently known complexes with such a {Fe3O}7+ core are bridged by a homoleptic set of carboxylate ligands. Here, two chemically different carboxylates, (L2Ph4)2− and Ph3CCO2−, bridge the iron atoms. Lastly, the large chelate span of (L2Ph4)2− gives rise to a chiral structure. Because the complex crystallized in a centrosymmetric space group, both enantiomers are present. Another noteworthy feature of this structure is the apparent lack of coordination of the benzoxazole nitrogen atoms. We hypothesize that the low basicity of benzoxazole renders such coordination unfavorable.
Mössbauer spectroscopy was performed to verify the oxidation state assignment of the Fe atoms. The zero-field 57Fe Mössbauer spectrum at 77 K, together with the fit, are shown in Figure S1 and Table 1, respectively. The data were best fit with a two-site model. The approximate ratio of the two iron sites was 2:1, consistent with the presence of two chemically inequivalent Fe sites observed in the X-ray structure. The major site has an isomer shift of (δ) 0.472 mm/s and quadrupole splitting of (ΔEq) 0.604 mm/s. The minor site has an isomer shift of (δ) 0.684 mm/s and quadrupole splitting of (ΔEq) 0.600 mm/s. These values are consistent with those of other triiron(III) centers having a basic carboxylate structural motif.8,18
Table 1.
Mössbauer fits of two sites in complex 8
| δ [mm/s] | ΔEq [mm/s] |
Γ | Area [%] |
|
|---|---|---|---|---|
| Site 1 | 0.472±0.02 | 0.604±0.02 | 0.384 | 71 |
| Site 2 | 0.684±0.02 | 0.600±0.02 | 0.252 | 29 |
Conclusions
In summary, we describe the synthesis of a preorganized triptycene-based bis(benzoxazole) ligand, H2L2Ph4. To prevent undesired formation of high nuclearity clusters and polymers, sterically protecting phenyl groups were installed ortho to the carboxylic acid units in the H2L2Ph4 ligand. A triiron(III) complex [NaFe3(L2Ph4)2(μ3-O)(μ-O2CCPh3)2(H2O)3](OTf)2 (8), was obtained having a “basic iron acetate” core in the structure. Among this class of compounds, the structure of 8 is novel because it utilizes a chelating dicarboxylate ligand, is constructed from two different types of carboxylate bridges, and is chiral. The observation that the benzoxazole nitrogen fails to coordinate to iron suggests that a more basic N-donor is needed to successfully model the diiron site of sMMOH. The design and synthesis of more basic analogs of H2L2Ph4 is the focus of our ongoing work.
Supplementary Material
Acknowledgments
This work was support by a grant from the National Institute of General Medical Sciences. Dr. Apfel thanks the Alexander von Humboldt Foundation a Feodor Lynen research fellowship.
Footnotes
Electronic Supplementary Information (ESI) available: Detailed experimental procedures, compound characterization, and the results of 1H and 13C NMR spectroscopic and single crystal X-ray diffraction studies. The CSD numbers for compounds 2, H2L2Ph4 and complex 8 are CCDC 881380, CCDC 881378, and CCDC 881379, respectively. See DOI: 10.1039/b000000x/
Notes and references
- 1.Cannon RD, White RP. Prog. Inorg. Chem. 1988;36:195–298. [Google Scholar]
- 2.Serre C, Mellot-Draznieks C, Surblé S, Audebrand N, Filinchuk Y, Férey G. Science. 2007;315:1828–1831. doi: 10.1126/science.1137975. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y-Z, Tong M-L, Xue W, Zhang W-X, Chen X-M, Grandjean F, Long GJ. Angew. Chem. Int. Ed. 2007;46:6076–6080. doi: 10.1002/anie.200701954. [DOI] [PubMed] [Google Scholar]
- 4.Millange F, Serre C, Guillou N, Férey G, Walton RI. Angew. Chem. Int. Ed. 2008;47:4100–4105. doi: 10.1002/anie.200705607. [DOI] [PubMed] [Google Scholar]
- 5.Millange F, Guillou N, Walton RI, Grenèche J-M, Margiolaki I, Férey G. Chem. Commun. 2008:4732–4734. doi: 10.1039/b809419e. [DOI] [PubMed] [Google Scholar]
- 6.Ito S, Inoue K, Mastumoto M. J. Am. Chem. Soc. 1982;104:6450–6452. [Google Scholar]
- 7.Barton DHR, Gastiger MJ, Motherwell WB. J. Chem. Soc., Chem. Commun. 1983:731–733. [Google Scholar]
- 8.Rabe V, Frey W, Baro A, Laschat S, Bauer M, Bertagnolli H, Rajagopalan S, Asthalter T, Roduner E, Dilger H, Glaser T, Schnieders D. Eur. J. Inorg. Chem. 2009:4660–4674. [Google Scholar]
- 9.Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Angew. Chem., Int. Ed. 2001;40:2782–2807. doi: 10.1002/1521-3773(20010803)40:15<2782::AID-ANIE2782>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Tshuva EY, Lippard SJ. Chem. Rev. 2004;104:987–1011. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]
- 11.Friedle S, Reisner E, Lippard SJ. Chem. Soc. Rev. 2010;39:2768–2779. doi: 10.1039/c003079c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do LH, Lippard SJ. J. Inorg. Biochem. 2011;105:1774–1785. doi: 10.1016/j.jinorgbio.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Cao R, Lippard SJ. Org. Lett. 2011;13:5052–5055. doi: 10.1021/ol201882v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rardin RL, Tolman WB, Lippard SJ. New J. Chem. 1991;15:417–430. [Google Scholar]
- 15.Tolman WB, Que L., Jr J. Chem. Soc., Dalton Trans. 2002:653–660. [Google Scholar]
- 16.Linderberg M, Hellberg S, Björk S, Gotthammar B, Högberg T, Persson K, Schwarcz R, Luthman J, Johansson R. Eur. J. Med. Chem. 1999;34:729–744. [Google Scholar]
- 17.Bernard AM, Ghiani MR, Piras PP, Rivoldini A. Synthesis. 1989:287–289. [Google Scholar]
- 18.Boudalis AK, Sanakis Y, Raptopoulou CP, Terzis A, Tuchagues J-P, Perlepes SP. Polyhedron. 2005;24:1540–1548. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.