Abstract
The pathology caused by traumatic brain injury (TBI) is exacerbated by the inflammatory response of the injured brain. Two pro-inflammatory cytokines that contribute to inflammation after TBI are tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). In previous studies using the parasagittal fluid-percussion brain injury model, we reported that the anti-inflammatory drug rolipram, a phosphodiesterase 4 inhibitor, reduced TNF-α and IL-1β levels and improved histopathological outcome when administered 30 min prior to injury. We now report that treatment with (±)-rolipram given 30 min after injury significantly reduced TNF-α levels in the cortex and hippocampus. However, post-injury administration of (±)-rolipram significantly increased cortical contusion volume and increased atrophy of the cortex as compared to vehicle-treated animals at 10 days post-injury. Thus, despite the reduction in pro-inflammatory cytokine levels, histopathological outcome was worsened with post-TBI (±)-rolipram treatment. Further histological analysis of (±)-rolipram-treated TBI animals revealed significant hemorrhage in the contused brain. Given the well known role of (±)-rolipram to increase vasodilation, it is likely that (±)-rolipram worsened outcome after fluid-percussion brain injury by causing increased bleeding.
Keywords: blood-brain barrier, cAMP, cerebral blood flow, cytokine, phosphodiesterase
Introduction
An estimated 1.7 million traumatic brain injuries (TBIs) occur each year in the United States, and more than 3 million people are coping with disabilities from TBI (Faul et al. 2010; Zaloshnja et al. 2008). TBI is characterized by several pathological changes in the brain that include hemorrhage, neuronal apoptosis, and diffuse axonal tract damage and all of these pathologies are exacerbated by the inflammatory signaling initiated after injury (Bramlett and Dietrich 2002; Keane et al. 2001; Zhang et al. 2005). These pathologies continue from hours to days after the initial trauma, rendering them therapeutically accessible.
After TBI, activated microglia migrate rapidly to the injury site, and release neurotoxic pro-inflammatory cytokines that initiate neuronal death. Activated microglia initiate a positive-feedback mechanism by upregulating the expression and secretion of pro-inflammatory cytokines in nearby astrocytes as well (Merrill 1991). Two prominent pro-inflammatory cytokines released after TBI by microglia and astrocytes are tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Numerous studies have documented rapid increases in TNF-α and IL-1β mRNA and protein levels within 1 hr of trauma (Kinoshita et al. 2002; Shohami et al. 1994; Taupin et al. 1993; Vitarbo et al. 2004; Yan et al. 1992). Clinical studies have also reported increases in TNF-α in the cerebrospinal fluid of TBI survivors (Goodman et al. 1990; Ross et al. 1994).
The phosphodiesterase (PDE) 4 isoforms B2 and D2 are found in inflammatory cells such as microglia, and inhibition of PDE 4 with a selective inhibitor, rolipram, decreases inflammatory signaling in microglia (Ariga et al. 2004; Jin et al. 2005; Reyes-Irisarri et al. 2007; Zhang et al. 2002). PDE 4 inhibitors have been widely-utilized by the pharmaceutical industry as anti-inflammatory drugs, and in particular, for the treatment of asthma and chronic obstructive pulmonary disease (Torphy 1998). In a previous study using the fluid-percussion brain injury (FPI) model, we found that (±)-rolipram significantly reduced levels of the pro-inflammatory cytokines TNF-α and IL-1β when given prior to the TBI (Atkins et al. 2007).
In several CNS injury models, several studies have found that elevation of cyclic AMP (cAMP) with rolipram decreases inflammation and improves neuronal survival and axonal regeneration. In spinal cord injury, rolipram in doses ranging from 0.5-3 mg/kg given continuously after injury increased the number of spared axons and oligodendrocytes and resulted in motor improvements (Beaumont et al. 2009; Koopmans et al. 2009; Nikulina et al. 2004; Pearse et al. 2004; Whitaker et al. 2008). In transient global ischemia, which typifies the ischemic damage seen after cardiac arrest, rolipram at 0.3 mg/kg given daily improved hippocampal cell survival whereas rolipram at 3 mg/kg given daily for 7 days improved hippocampal-dependent learning (Block et al. 2001; Block et al. 1997; Imanishi et al. 1997; Kato et al. 1995; Lee et al. 2004; Li et al. 2011). We have found that (±)-rolipram, when given prior to parasagittal FPI, increases neuronal survival in the cerebral cortex and hippocampus, and decreases inflammation (Atkins et al. 2007). However, those experiments were only proof-of-concept and it is important to assess the effects of (±)-rolipram in a post-injury treatment paradigm for TBI. In this study, we tested the hypothesis that post-injury treatment with (±)-rolipram would improve histopathology and decrease markers of inflammation after TBI.
Materials and Methods
Fluid-percussion brain injury model
All surgeries were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Society for Neuroscience Guidelines for the Use of Animals in Neuroscience Research, and approved by the University of Miami Institutional Animal Care and Use Committee. Adult male Sprague Dawley rats (n=73, 280-300 gm, Charles Rivers Laboratories, Wilmington, MA) were anesthetized with 3% isoflurane, 70% N2O, and 30% O2 and received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) over the right parietal cortex. Twenty-four hr after the craniotomy, the animals were re-anesthetized (3% isoflurane, 70% N2O, and 30% O2), immobilized with pancuronium bromide (1.0 mg/kg), and mechanically ventilated with 1% isoflurane, 70% N2O, and 30% O2. When physiological measurements had stabilized, the animals received a fluid-percussion pulse (1.8-2.2 atmospheres) or sham injury. Blood gases, blood pH and mean arterial blood pressure were monitored for 30 min prior to the FPI or sham surgery and up to 3 hr post-injury to maintain normal levels.
Drugs were dissolved in 100% ethanol at 10 mg/ml, and then diluted in saline (final ethanol concentration 5%) to be administered at 6 ml/kg. (±)-Rolipram was obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). Ro 20-1724, (R)-(−)-Rolipram, and (S)-(+)-Rolipram were obtained from Tocris Bioscience (Ellisville, MO). For these studies, (±)-rolipram doses ranging from 0.5 to 6 mg/kg were tested since previous studies with other CNS injury models have reported efficacy with doses typically ranging from 0.3-3 mg/kg given either immediately or 6 hr post-injury, and then either daily or continuously until analysis (Block et al. 1997; Imanishi et al. 1997; Kato et al. 1995; Li et al. 2011; Pearse et al. 2004). Additionally, in previous experiments with FPI, pre-treatment with (±)-rolipram improved histopathology in doses ranging from 0.3 mg/kg to 3 mg/kg (Atkins et al. 2007). For chronic administration of (±)-rolipram (Fig. 3), animals were treated with vehicle (5% ethanol in saline) or (±)-rolipram (3 mg/kg, intravenously) at 30 min post-surgery, and then subcutaneously (3 mg/kg/day) using Alzet infusion pumps (DURECT Corporation, Cupertino, CA) until perfusion at day 10 post-surgery (Table I).
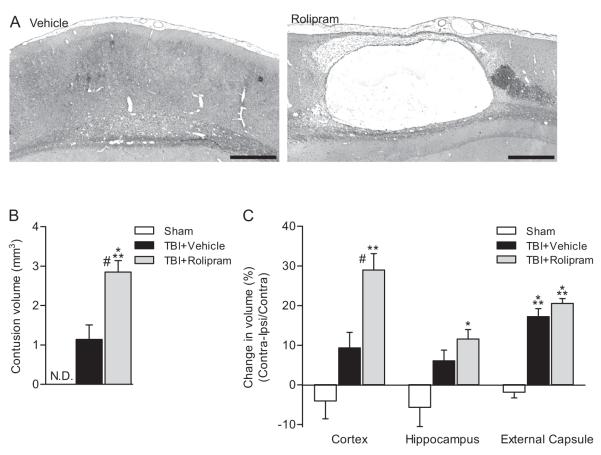
Fig. 3.
Chronic consequences of post-injury (±)-rolipram treatment. Post-injury administration of rolipram increased atrophy and cavitation of the ipsilateral parietal cortex (A). Animals received vehicle (5% ethanol) or (±)-rolipram (3 mg/kg, i.v.) 30 min after moderate parasagittal FPI or sham surgery, then continuously until perfusion (subcutaneously via osmotic miniature pumps). Animals were perfused 10 days post-surgery. Shown are H&E and Luxol fast blue stained sections at bregma level −5.3 mm. Scale bars 500 μm. Contusion volumes (B) were significantly greater in (±)-rolipram-treated TBI animals as compared to vehicle-treated TBI animals at 10 days post-injury (***P < 0.001 for sham versus (±)-rolipram-treated TBI animals, #P < 0.01 for vehicle-treated versus (±)-rolipram-treated TBI animals). TBI-induced atrophy of the parietal cortex (C) was increased in (±)-rolipram-treated animals (**P < 0.01 and ***P < 0.001 for sham versus TBI animals, #P < 0.01 for vehicle-treated versus (±)-rolipram-treated TBI animals). Mean ± SEM, n = 3 vehicle-treated sham animals, n = 5 vehicle-treated TBI animals, n = 4 (±)-rolipram-treated TBI animals.
Table I.
Experimental design: outcome measurements, treatment schedule, doses and endpoint times
| Outcome | Treatment time | Dose | Endpoint | N value |
|---|---|---|---|---|
| ELISA | 30′ post | 0.5-6 mg/kg | 3 hr post | 3-4 |
| Contusion volume | 30′ post, then daily | 0.1 or 3 mg/kg | 3 days post | 4-7 |
| Atrophy | 30′ post, then continuously | 3 mg/kg | 10 days post | 3-5 |
| BBB breakdown | 30′ post, then daily | 3 mg/kg | 3 days post | 6-7 |
| Cerebral blood flow | 30′ post | 3 mg/kg | 3 hr post | 4 |
Cytokine ELISAs
Tissue was briefly sonicated on ice (14 s, setting 2, Branson sonifier 450, Danbury, CT) in 10 volumes/weight of lysis buffer supplemented with 0.1% Igepal CA-630. Total protein was measured using the Coomassie Plus assay kit (Bio-Rad Laboratories, Hercules, CA). Cytokine levels were measured using an Il-1β or TNF-α ELISA kit (R&D Systems, Minneapolis, MN).
Histopathology analysis
Animals were anesthetized and perfused transcardially with saline (75 mL) and 4% paraformaldehyde (350 mL) in 0.1M phosphate buffer (PB), pH 7.4. The brains were embedded in paraffin and sectioned 10 μm thick. Serial sections were stained with hematoxylin and eosin (H&E) for contusion volume analysis or stained with H&E plus Luxol fast blue for atrophy measurements. Contusion volumes were assessed by an investigator blinded to the treatment groups by tracing H&E stained sections 150 μm apart over the entire extent of the contusion using Neurolucida 7.50.1 software (MicroBrightField, Inc., Williston, VT) and an Axiophot 200M microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) with a 10X objective. Atrophy was determined by tracing H&E and Luxol fast blue stained sections 300 μm apart between bregma levels −3.3 to −6.8 mm using the Neurolucida 7.50.1 software program (MicroBrightField, Inc.) by an investigator blinded to the treatment groups. As a measure of atrophy, both the ipsilateral and contralateral structures were contoured to calculate the difference in volume between the ipsilateral and contralateral structure normalized to the contralateral structure volume. Images were taken with a 20X objective and montaged using the virtual slice module in the Neurolucida 7.50.1 software program (MicroBrightField, Inc.).
Immunohistochemistry
Paraffin sections were antigen-retrieved with citric acid (10 mM, pH 6.0, 80°C, 30 min), then blocked for 1 hr at room temperature (RT) in DAKO protein block serum free solution (Dako, Carpinteria, CA). Sections were incubated with biotinylated anti-rat IgG (1:100, Vector Laboratories, Burlingame, CA) or anti-rat albumin (1:1000, MP Biomedicals, Aurora, OH). Albumin immunostaining was developed with biotinylated secondary antibodies (1:200, Vector Laboratories), and then all sections received ABC Elite and DAB (Vector Laboratories). Sections without primary antibody were run in parallel.
Images of albumin and endogenous IgG immunostaining were taken with a 20X objective on an Olympus BX51 microscope (Olympus America, Inc., Center Valley, PA) using identical settings for all images and quantified after inversion on MetaMorph 6.3r5 (Molecular Devices, LLC, Sunnyvale, CA). Integrated intensity was quantified by averaging 6 boxes randomly placed in the parietal cortex by an investigator blinded to the treatment groups.
Cerebral blood flow measurements
Animals received (±)-rolipram (3 mg/kg intravenously) or vehicle (5% ethanol in saline) at 30 min post-FPI. At 3 hr post-FPI and while still anesthetized with 1% isoflurane, 70% N2O, and 30% O2, 65 μM/Ci/kg 14C-iodantipyrine (Perkin Elmer, Waltham, MA) dissolved in 1 ml of saline was infused intravenously at a constant rate over 45 sec via a Harvard infusion pump (Dietrich et al. 1996; Wei et al. 1980). Arterial blood samples were collected at 2 sec intervals from a femoral artery catheter in pre-calibrated 20 μl heparinized capillary pipettes (Radiometer Medical, Carlsbad, CA). Animals were immediately decapitated after the infusion, and the brain was dissected and frozen by gradual immersion in liquid nitrogen. Brains were sectioned on a cryostat for coronal sections 20 μm thick. Sections were dried on slides, and exposed to Kodak Hyperfilm beta-max film (BioMax MR, Eastman Kodak Company, New Haven, CT) for 10 days. Standards used were poly(14C) methyl methacrylate (GE Healthcare, Piscataway, NJ) previously calibrated in our laboratory. Arterial blood samples were dried on filter paper and counted for 14C activity using a LS6500 liquid scintillation counter (Beckman Coulter, Inc., Indianapolis IN) in Bio-Safe II scintillation cocktail (Research Products International Corp., Mount Prospect, IL) and the arterial concentration curve for iodoantipyrine was calculated as previously described (Sakurada et al. 1978). Sections were analyzed by scanning in sections using the MCID Elite 6.0 program (Imaging Research Inc., Linton, Cambridge, England), and densitometry of the ipsilateral and contralateral parietal cortex was conducted using region of interest measurements and analyzed using MCID Elite 6.0 software. Images were averaged for each group to show the average cerebral blood flow (CBF) at bregma levels +0.7, +0.2, −0.8, −1.8, −3.8, −4.8, −5.8, and −7.3 mm.
Statistical analyses
Data represent mean ± SEM and were analyzed using one-way ANOVAs with post hoc Tukey t-tests. Significance was set at P < 0.05.
Results
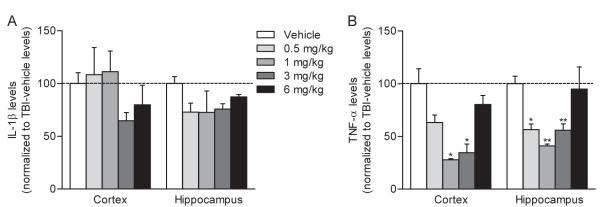
To assess the effects of post-injury treatment with rolipram on inflammatory signaling after TBI, (±)-rolipram was administered 30 min post-injury and pro-inflammatory cytokines were measured 3 hr post-injury, a time point when IL-1β and TNF-α levels are significantly increased (Kinoshita et al. 2002; Vitarbo et al. 2004). Based on the previous literature, a range of doses from 0.5 mg/kg to 6 mg/kg were tested to determine the most effective dose of (±)-rolipram that reduced IL-1β and TNF-α levels (Atkins et al. 2007; Block et al. 1997; Imanishi et al. 1997; Kato et al. 1995; Li et al. 2011; Pearse et al. 2004). Post-injury treatment with (±)-rolipram reduced TNF-α levels in both the injured parietal cortex and hippocampus at doses of 1 and 3 mg/kg (Fig. 1). IL-1β levels were nonsignificantly reduced at doses of 3 and 6 mg/kg.
Fig. 1.
Post-injury (±)-rolipram reduced cytokine levels in the injured parietal cortex. IL-1β (A) and TNF-α (B) levels were assayed by ELISA at 3 hr after moderate parasagittal FPI. Animals received vehicle or (±)-rolipram intravenously (i.v.) 30 min after trauma. (±)-Rolipram treatment nonsignificantly reduced IL-1β levels and significantly reduced TNF-α levels (*P < 0.05 for rolipram-treated versus vehicle-treated animals) in a concentration-dependent manner as compared to vehicle treatment of TBI animals (dotted line). Data represent mean ± SEM, n = 3 vehicle-treated TBI animals, n = 3 0.5 mg/kg (±)-rolipram-treated TBI animals, n = 4 1 mg/kg (±)-rolipram-treated TBI animals, n = 3 3 mg/kg (±)-rolipram-treated TBI animals, n = 4 6 mg/kg (±)-rolipram-treated TBI animals.
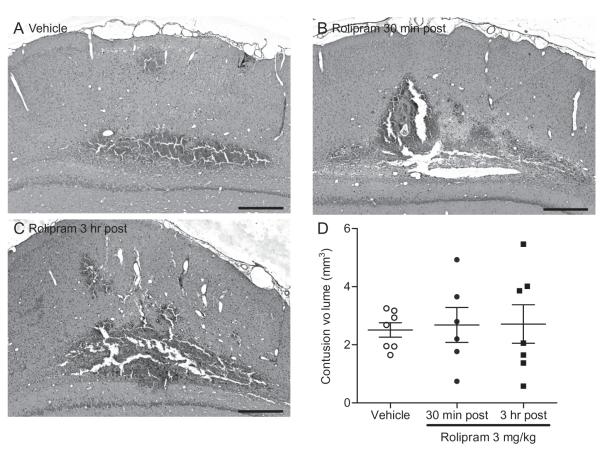
To determine if the dose of (±)-rolipram that was most effective in reducing TNF-α levels also improved histopathology, animals were treated with 3 mg/kg rolipram 30 min or 3 hr post-injury. These two time points were chosen as an initial determination of the therapeutic time window of rolipram treatment for TBI due to the rapid changes in inflammatory signaling and hemodynamics that occur in the minutes to hours after TBI (Ziebell and Morganti-Kossmann 2010). Cortical contusion volumes were assessed at 3 days post-injury, a time point when cortical contusion volumes are easily and reliably quantified (Atkins et al. 2007). (±)-Rolipram treatment had no significant effect on cortical contusion volume (Fig. 2).
Fig. 2.
Early measurements of cortical contusion size were not affected by post-injury (±)-rolipram treatment. Animals received (A) vehicle (5% ethanol) or (±)-rolipram (3 mg/kg, i.v.) at (B) 30 min or (C) 3 hr after moderate parasagittal FPI, then once per day for 3 days (intraperitoneally, i.p.). Brains were analyzed at 3 days post-injury and sections were stained with H&E. Shown is bregma level −5.8 mm. Scale bars 500 μm. Scatter plot and mean ± SEM of the cortical contusion volumes (D). n = 7 vehicle-treated TBI animals, n = 6 (±)-rolipram-treated 30 min post-injury animals, n = 7 (±)-rolipram-treated 3 hr post-injury animals.
An analysis at a longer survival time point, 10 days post-injury, indicated that continuous (±)-rolipram treatment (3 mg/kg) for 10 days post-injury caused a pronounced cavitation of the cortex (Fig. 3). There was also a significant increase in contusion volume and atrophy of the ipsilateral cortex at this longer survival time point. These results indicate that (±)-rolipram at longer survival time points worsened histopathology after TBI.
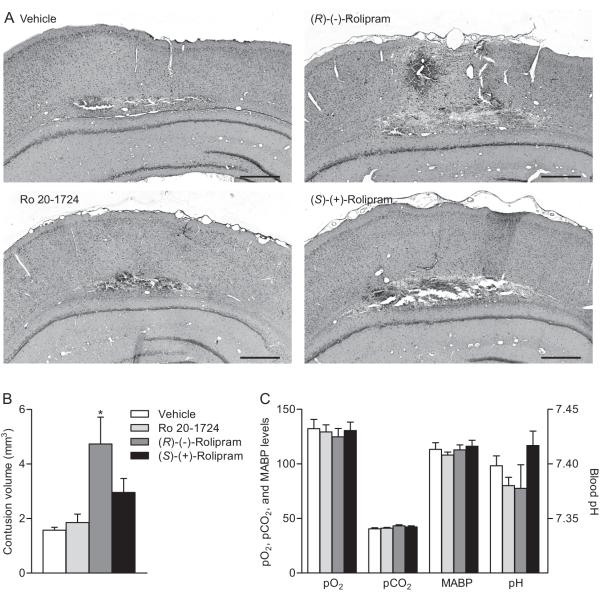
Rolipram has two PDE4 binding states, a low affinity binding state where it inhibits cAMP hydrolysis, and a high affinity binding state that does not affect cAMP hydrolysis (Jacobitz et al. 1996; Souness and Rao 1997). We hypothesized that the effects of (±)-rolipram used in the previous experiments were due to effects on cAMP hydrolysis. To test this hypothesis, cortical contusion volumes at 3 days post-FPI were assessed in animals treated with R-(−)-rolipram or S-(+)-rolipram. R-(−)-rolipram exhibits an order of magnitude higher binding specificity to the low-affinity binding state as compared to S-(+)-rolipram (Barnette et al. 1996; Schneider et al. 1986). For comparison, a structurally distinct PDE 4 inhibitor, Ro 20-1724 which binds both the high and low-affinity binding sites was tested (Zhao et al. 2003). A low dose of the R-(−)-rolipram enantiomer (0.1 mg/kg), but not S-(+)-rolipram or Ro 20-1724, given daily for 3 days beginning at 30 min post-injury, increased hemorrhage and significantly increased cortical contusion size in TBI-treated animals at 3 days post-injury (Fig. 3). There were no measureable differences on effects of blood gases, blood pressure, or blood pH.
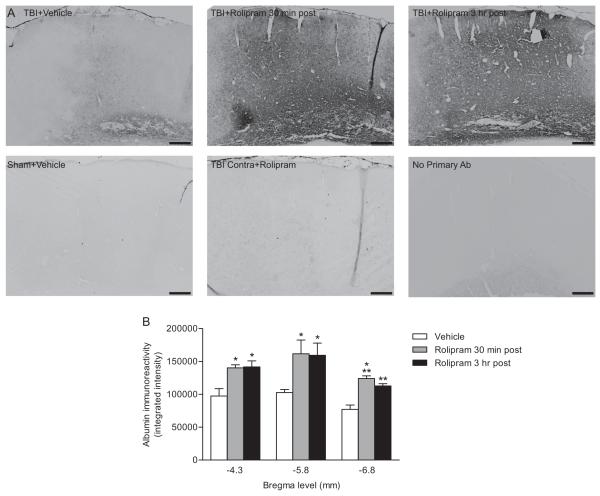
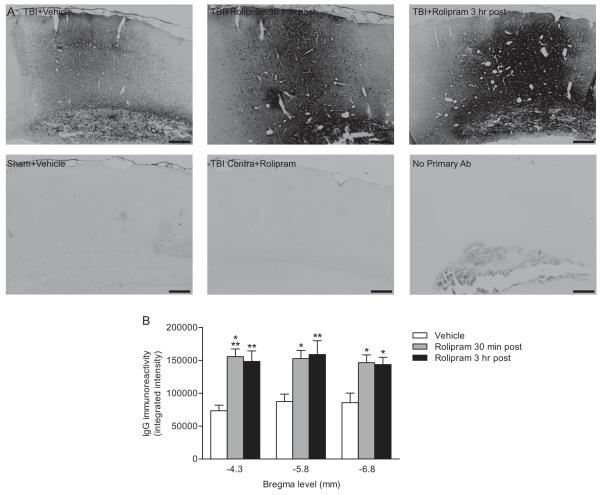
Both endogenous albumin (Fig. 5) and IgG (Fig. 6) immunostaining at 3 days post-injury was significantly increased in (±)-rolipram-treated animals (3 mg/kg daily, beginning at 30 min post-injury) as compared to vehicle-treated animals. An analysis of the contralateral cortex indicated that the increase in albumin and IgG immunostaining with (±)-rolipram treatment was restricted to the ipsilateral cortex.
Fig. 5.
Endogenous albumin immunoreactivity increased with post-injury administration of (±)-rolipram. Animals received vehicle (5% ethanol) or (±)-rolipram (3 mg/kg, i.v.) 30 min or 3 hr after moderate parasagittal FPI, then once per day (i.p.) until perfusion. Animals were perfused at 3 days post-injury and paraffin-embedded sections were immunostained for anti-rat albumin (A). Images shown are at bregma level −4.3 mm. Scale bars 250 μm. Quantification of immunostaining (B). Endogenous albumin immunoreactivity significantly increased in (±)-rolipram-treated animals treated at either 30 min or 3 hr post-injury as compared to vehicle-treated animals. Mean ± SEM, n = 7 vehicle-treated animals, n = 5 (±)-rolipram-treated animals at 30 min post-injury, n = 5 (±)-rolipram-treated animals at 3 hr post-injury, *P < 0.05, **P < 0.01, ***P < 0.001 for TBI+vehicle versus TBI+rolipram.
Fig. 6.
Post-injury administration of (±)-rolipram increased IgG immunoreactivity in the ipsilateral parietal cortex. Animals received vehicle (5% ethanol) or (±)-rolipram (3 mg/kg, i.v.) 30 min or 3 hr after TBI, and then once per day (i.p.) for 3 days. Sections were immunostained for endogenous IgG levels (A). Images were taken at bregma level −4.3 mm. Scale bars 250 μm. The intensity of IgG immunostaining was quantified in the parietal cortex (B). Endogenous IgG levels were significantly increased in (±)-rolipram-treated animals as compared to vehicle-treated animals. Mean ± SEM, n = 7 vehicle-treated animals, n = 5 (±)-rolipram-treated 30 min post-injury animals, n = 5 (±)-rolipram-treated 3 hr post-injury animals, *P < 0.05, **P < 0.01, ***P < 0.001 for TBI+vehicle versus TBI+rolipram.
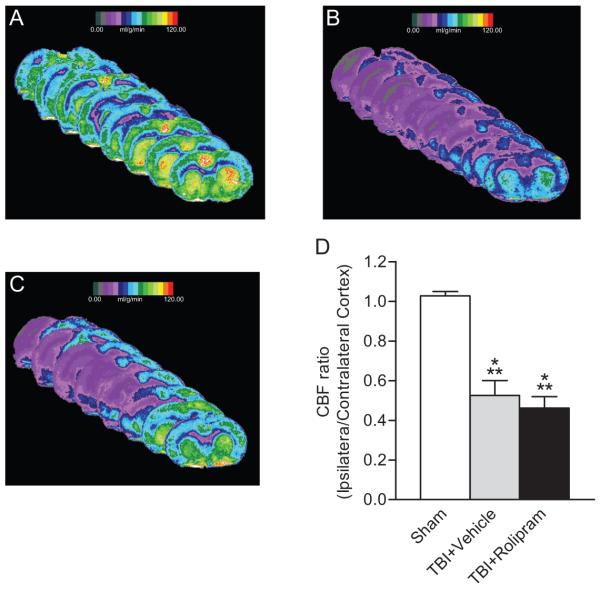
CBF using 14C-iodantipyrine autoradiography was assessed in animals treated with (±)-rolipram (Fig. 7). In accordance with previous results, there was a significant reduction in CBF in the injured parietal cortex at 3 hr post-TBI (Dietrich et al. 1996; Dietrich et al. 1998; Wei et al. 1980). However, unlike studies with non-injured animals, (±)-rolipram treatment did not increase CBF in TBI-treated animals (Rutten et al. 2009).
Fig. 7.
Measurements of CBF were unaffected by (±)-rolipram treatment in TBI animals. CBF in the ipsilateral cortex at 3 hr post-injury was quantified using 14C-iodantipyrine as the tracer and autoradiography. Images from bregma level +0.7 mm to −7.3 mm are shown for (A) sham, (B) vehicle-treated TBI animals, and (C) (±)-rolipram-treated TBI animals (3 mg/kg, i.v.). Images represent that average CBF at each bregma level for each treatment group (n = 4 animals per group). A significant decrease in CBF (D) was observed for both vehicle and (±)-rolipram-treated TBI animals as compared to sham animals. Mean ± SEM, n = 4 animals/group, ***P < 0.001 for sham versus TBI animals.
Discussion
In this study we found that post-injury treatment with (±)-rolipram, a known anti-inflammatory and vasodilatory drug, reduced pro-inflammatory cytokine levels of TNF-α, but increased hemorrhage and blood-brain barrier breakdown. The changes in IL-1β levels were inconclusive and further experiments may clarify whether (±)-rolipram in a post-treatment paradigm could also reduce levels of this or other pro-inflammatory cytokines after brain trauma. In addition, although rolipram is known to increase CBF in non-injured animals, we found that (±)-rolipram did not rescue the decrease in CBF observed in TBI animals (Rutten et al. 2009).
These results are not in accordance with our earlier study demonstrating that (±)-rolipram was highly effective in reducing histopathological changes when given 30 min prior to brain trauma (Atkins et al. 2007). Pre-treatment with (±)-rolipram during FPI decreased cortical contusion volume and levels of the pro-inflammatory cytokines, IL-1β and TNF-α. This result was supported by multiple studies reporting that rolipram works in a variety of CNS injury paradigms. Using similar doses of rolipram between 0.3-3 mg/kg, rolipram given 6 hr after cerebral ischemia and then daily until assessment improved hippocampal neuronal survival and behavioral recovery as assessed by water maze performance (Block et al. 1997; Li et al. 2011; Nagakura et al. 2002). In spinal cord injury studies, continuous post-injury treatment with rolipram in doses between 0.5-3 mg/kg improved oligodendrocyte survival and locomotor recovery (Beaumont et al. 2009; Koopmans et al. 2009; Nikulina et al. 2004; Pearse et al. 2004; Whitaker et al. 2008). Since the doses and treatment schedule in this study were similar to what was observed in previous studies demonstrating efficacy in spinal cord injury and cerebral ischemia, we speculate that the differential outcome observed with rolipram treatment with FPI may be due to differences in model-specific, pathological and hemodynamic changes.
Although (±)-rolipram did work as an anti-inflammatory drug to reduce pro-inflammatory cytokine levels in the FPI model, it increased bleeding in the injured brain. Both endothelial and smooth muscle cells contain PDEs, including several isoforms of PDE 4, the target of rolipram (Birk et al. 2004; Santos-Silva et al. 2008; Zhu et al. 2004). Application of rolipram or other PDE 4 inhibitors mediate smooth muscle relaxation and blood vessel dilation (Mehats et al. 2003; Miwa et al. 2009; Willette et al. 1997). However, vasoconstriction is typically observed after TBI and vasodilators have been shown to improve outcome after TBI (Ahn et al. 2004; Hayward et al. 2011; Lin et al. 2001; Maeda et al. 2005; Park et al. 2009; Ueda et al. 2004). In the brain at doses 100-fold lower than in our studies, rolipram increases local CBF in select regions of the brain including the temperoparietal cortex (Rutten et al. 2009). We speculate that vascular reactivity to rolipram may be altered after TBI, such that although the decrease in CBF cannot be rescued with rolipram, vulnerable areas of the brain may be prone to vasodilation and consequently increased bleeding with rolipram. Further studies with more detailed vessel staining and electron microscopy are needed to clarify why (±)-rolipram aggravated hemorrhage around the vulnerable cortical blood vessels, but did not increase CBF.
There are indications in the literature that rolipram can result in drug-induced vascular injury (Losco et al. 2004; Mecklenburg et al. 2006; Zhang et al. 2008). In one study of hypoxia-ischemia, rolipram increased lesions in the periventricular white matter and deep cortex suggesting that rolipram could have deleterious effects in some injury paradigms (Chang et al. 2008). In another study, rolipram was found to have no improvement in locomotor recovery after transient ischemia until cessation of rolipram treatment (Hatinen et al. 2008). This was due to acute side effects of rolipram causing decreased locomotion which has been reported in several studies (Barad et al. 1998; Hu et al. 2011; Silvestre et al. 1999).
Numerous studies have reported that rolipram blocks the expression and secretion of TNF-α and IL-1β from activated microglia, decreasing the inflammatory response potentially by increasing cAMP levels and activating PKA to suppress transcription of TNF-α and IL-1β (Foey et al. 2003; Prabhakar et al. 1994; Si et al. 1998; Suzumura et al. 1999; Verghese et al. 1995; Yoshikawa et al. 1999; Zhang et al. 2002). Our results that (±)-rolipram reduced TNF-α are in agreement with these previous studies that rolipram can reduce inflammation after TBI. (±)-Rolipram has two binding states to PDE 4, a low affinity binding state that inhibits cAMP hydrolysis, and a high affinity binding state that limits other non-cAMP actions of PDE4, including the prevention of apoptosis or neutrophil enzyme secretion (Houslay and Adams 2003; Zhu et al. 1998). To determine if the worsening hemorrhage and breakdown of the blood brain barrier were due to effects of cAMP or other non-cAMP actions on PDE 4, we tested whether R-(−)-rolipram versus S-(+)-rolipram would worsen cortical contusions since R-(−)-rolipram has a higher binding specificity to the cAMP catalytic site as compared to S-(+)-rolipram. In addition, we assessed a structurally distinct inhibitor, R0 20-1724 which binds both the low and high affinity binding state (Zhao et al. 2003). Since R-(−)-rolipram, but not S-(+)-rolipram or Ro 20-1724 increased cortical contusion volume, these results suggest that the worsening of histopathology and hemorrhage into the injured brain were due to the actions of decreasing cAMP hydrolysis.
Rolipram is also known to cause a rebound effect and induce increased PDE activity. Systemic administration of rolipram has been found to induce PDE 4D protein levels and subsequently decrease cAMP levels in the cortex and hippocampus below non-injured levels (Dlaboga et al. 2006; Giorgi et al. 2004). Indeed, in vascular smooth muscle cells PDE 3 and 4 activities and levels are regulated by cAMP, suggesting that daily administration of rolipram may have resulted in the opposite intended effect (Campos-Toimil et al. 2008; Tilley and Maurice 2002). Additionally, in non-injured animals, rolipram exhibits an inverted U-shaped dose-response curve such that at higher doses rolipram is not as efficacious as lower doses in improving hippocampal-dependent learning in non-injured animals (Hosseini-Sharifabad et al. 2012). Thus, careful consideration of rolipram dose is important and may have contributed to the lack of efficacy we observed using doses ranging from 0.1 to 3 mg/kg with the FPI model.
In summary, we report that (±)-rolipram, when given in a post-injury treatment paradigm for the FPI model, exacerbated neurological damage, worsened breakdown of the blood-brain barrier, and impaired behavioral recovery. These results suggest that caution is required in extrapolating the use of PDE inhibitors to different injury models.
Fig. 4.
Administration of the (R)-(−)-rolipram enantiomer 30 min post-injury worsened pathology when assessed 3 days after TBI. Representative sections from animals treated with PDE 4 inhibitors (A). Sections were stained with H&E. Shown is bregma level −5.8 mm. Scale bars 500 μm. Animals received vehicle (5% ethanol) or PDE 4 inhibitors (0.1 mg/kg, i.v.) 30 min after moderate parasagittal FPI, then once per day for 3 days (i.p.). Contusion volume analysis (B). Physiological parameters (C) of animals 15 min after the first intravenous treatment with vehicle or PDE 4 inhibitors. Mean ± SEM, n = 3 vehicle-treated animals, n = 4 Ro 20-1724-treated animals, n = 4 (R)-(−)-rolipram-treated animals, n = 4 (S)-(+)-rolipram-treated animals, *P < 0.05 for vehicle-treated versus (R)-(−)-rolipram-treated FPI animals.
Acknowledgements
Contract grant sponsor: National Institute of Neurological Disorders and Stroke; Contract grant numbers: NS056072 and NS069721. We thank Drs. Ross Bullock, Mousumi Ghosh, and Damien Pearse for helpful discussions, and Dr. Beata Frydel, Meghan O’Connell, and Olivia Nwankwo for technical assistance.
References
- Ahn MJ, Sherwood ER, Prough DS, Lin CY, DeWitt DS. The effects of traumatic brain injury on cerebral blood flow and brain tissue nitric oxide levels and cytokine expression. J Neurotrauma. 2004;21(10):1431–1442. doi: 10.1089/neu.2004.21.1431. [DOI] [PubMed] [Google Scholar]
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, Jin SL, Conti M. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173(12):7531–7538. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr., Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208(1):145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95(25):15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnette MS, Bartus JO, Burman M, Christensen SB, Cieslinski LB, Esser KM, Prabhakar US, Rush JA, Torphy TJ. Association of the anti-inflammatory activity of phosphodiesterase 4 (PDE4) inhibitors with either inhibition of PDE4 catalytic activity or competition for [3H]rolipram binding. Biochem Pharmacol. 1996;51(7):949–956. doi: 10.1016/0006-2952(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Whitaker CM, Burke DA, Hetman M, Onifer SM. Effects of rolipram on adult rat oligodendrocytes and functional recovery after contusive cervical spinal cord injury. Neuroscience. 2009;163(4):985–990. doi: 10.1016/j.neuroscience.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk S, Edvinsson L, Olesen J, Kruuse C. Analysis of the effects of phosphodiesterase type 3 and 4 inhibitors in cerebral arteries. Eur J Pharmacol. 2004;489(1-2):93–100. doi: 10.1016/j.ejphar.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Block F, Schmidt W, Nolden-Koch M, Schwarz M. Rolipram reduces excitotoxic neuronal damage. Neuroreport. 2001;12(7):1507–1511. doi: 10.1097/00001756-200105250-00041. [DOI] [PubMed] [Google Scholar]
- Block F, Tondar A, Schmidt W, Schwarz M. Delayed treatment with rolipram protects against neuronal damage following global ischemia in rats. Neuroreport. 1997;8(17):3829–3832. doi: 10.1097/00001756-199712010-00033. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol (Berl) 2002;103(6):607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154(1):82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Huang CC, Hung PL, Huang HM. Rolipram, a phosphodiesterase type IV inhibitor, exacerbates periventricular white matter lesions in rat pups. Pediatr Res. 2008;64(3):234–239. doi: 10.1203/PDR.0b013e31817cfc87. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Dewanjee S, Dewanjee MK, Ginsberg MD. Widespread hemodynamic depression and focal platelet accumulation after fluid percussion brain injury: A double-label autoradiographic study in rats. J Cereb Blood Flow Metab. 1996;16(3):481–489. doi: 10.1097/00004647-199605000-00015. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Zhao W, Dewanjee MK, Ginsberg MD. Posttraumatic cerebral ischemia after fluid percussion brain injury: An autoradiographic and histopathological study in rats. Neurosurgery. 1998;43(3):585–593. doi: 10.1097/00006123-199809000-00105. [DOI] [PubMed] [Google Scholar]
- Dlaboga D, Hajjhussein H, O’Donnell JM. Regulation of phosphodiesterase-4 (PDE4) expression in mouse brain by repeated antidepressant treatment: comparison with rolipram. Brain Res. 2006;1096(1):104–112. doi: 10.1016/j.brainres.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- Foey AD, Field S, Ahmed S, Jain A, Feldmann M, Brennan FM, Williams R. Impact of VIP and cAMP on the regulation of TNF-α and IL-10 production: Implications for rheumatoid arthritis. Arthritis Res Ther. 2003;5(6):R317–328. doi: 10.1186/ar999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154(1):99–106. doi: 10.1016/j.bbr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30(2-3):213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Hatinen S, Sairanen M, Sirvio J, Jolkkonen J. Improved sensorimotor function by rolipram following focal cerebral ischemia in rats. Restorative neurology and neuroscience. 2008;26(6):493–499. [PubMed] [Google Scholar]
- Hayward NM, Tuunanen PI, Immonen R, Ndode-Ekane XE, Pitkanen A, Grohn O. Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J Cereb Blood Flow Metab. 2011;31(1):166–177. doi: 10.1038/jcbfm.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Sharifabad A, Ghahremani MH, Sabzevari O, Naghdi N, Abdollahi M, Beyer C, Bollen E, Prickaerts J, Roghani A, Sharifzadeh M. Effects of protein kinase A and G inhibitors on hippocampal cholinergic markers expressions in rolipram- and sildenafil-induced spatial memory improvement. Pharmacol Biochem Behav. 2012;101(3):311–319. doi: 10.1016/j.pbb.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: Modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370(Pt 1):1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 2011;218(2):331–339. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi T, Sawa A, Ichimaru Y, Miyashiro M, Kato S, Yamamoto T, Ueki S. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol. 1997;321(3):273–278. doi: 10.1016/s0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- Jacobitz S, McLaughlin MM, Livi GP, Burman M, Torphy TJ. Mapping the functional domains of human recombinant phosphodiesterase 4A: structural requirements for catalytic activity and rolipram binding. Mol Pharmacol. 1996;50(4):891–899. [PubMed] [Google Scholar]
- Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175(3):1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- Kato H, Araki T, Itoyama Y, Kogure K. Rolipram, a cyclic AMP-selective phosphodiesterase inhibitor, reduces neuronal damage following cerebral ischemia in the gerbil. Eur J Pharmacol. 1995;272(1):107–110. doi: 10.1016/0014-2999(94)00694-3. [DOI] [PubMed] [Google Scholar]
- Keane RW, Kraydieh S, Lotocki G, Alonso OF, Aldana P, Dietrich WD. Apoptotic and antiapoptotic mechanisms after traumatic brain injury. J Cereb Blood Flow Metab. 2001;21(10):1189–1198. doi: 10.1097/00004647-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1β messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: Importance of injury severity and brain temperature. Neurosurgery. 2002;51(1):195–203. doi: 10.1097/00006123-200207000-00027. [DOI] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Buss A, Geoghegan L, Myint AM, Honig WH, Kern N, Joosten EA, Noth J, Brook GA. Acute rolipram/thalidomide treatment improves tissue sparing and locomotion after experimental spinal cord injury. Exp Neurol. 2009;216(2):490–498. doi: 10.1016/j.expneurol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Lee HT, Chang YC, Wang LY, Wang ST, Huang CC, Ho CJ. cAMP response element-binding protein activation in ligation preconditioning in neonatal brain. Ann Neurol. 2004;56(5):611–623. doi: 10.1002/ana.20259. [DOI] [PubMed] [Google Scholar]
- Li LX, Cheng YF, Lin HB, Wang C, Xu JP, Zhang HT. Prevention of cerebral ischemia-induced memory deficits by inhibition of phosphodiesterase-4 in rats. Metab Brain Dis. 2011;26(1):37–47. doi: 10.1007/s11011-011-9235-0. [DOI] [PubMed] [Google Scholar]
- Lin B, Ginsberg MD, Zhao W, Alonso OF, Belayev L, Busto R. Quantitative analysis of microvascular alterations in traumatic brain injury by endothelial barrier antigen immunohistochemistry. J Neurotrauma. 2001;18(4):389–397. doi: 10.1089/089771501750170958. [DOI] [PubMed] [Google Scholar]
- Losco PE, Evans EW, Barat SA, Blackshear PE, Reyderman L, Fine JS, Bober LA, Anthes JC, Mirro EJ, Cuss FM. The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeys. Toxicol Pathol. 2004;32(3):295–308. doi: 10.1080/01926230490431493. [DOI] [PubMed] [Google Scholar]
- Maeda T, Lee SM, Hovda DA. Restoration of cerebral vasoreactivity by an L-type calcium channel blocker following fluid percussion brain injury. J Neurotrauma. 2005;22(7):763–771. doi: 10.1089/neu.2005.22.763. [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Heuser A, Juengling T, Kohler M, Foell R, Ockert D, Tuch K, Bode G. Mesenteritis precedes vasculitis in the rat mesentery after subacute administration of a phosphodiesterase type 4 inhibitor. Toxicol Lett. 2006;163(1):54–64. doi: 10.1016/j.toxlet.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Mehats C, Jin SL, Wahlstrom J, Law E, Umetsu DT, Conti M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003;17(13):1831–1841. doi: 10.1096/fj.03-0274com. [DOI] [PubMed] [Google Scholar]
- Merrill JE. Effects of interleukin-1 and tumor necrosis factor-α on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13(3):130–137. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- Miwa T, Mori A, Nakahara T, Ishii K. Intravenously administered phosphodiesterase 4 inhibitors dilate retinal blood vessels in rats. Eur J Pharmacol. 2009;602(1):112–116. doi: 10.1016/j.ejphar.2008.10.060. [DOI] [PubMed] [Google Scholar]
- Nagakura A, Niimura M, Takeo S. Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br J Pharmacol. 2002;135(7):1783–1793. doi: 10.1038/sj.bjp.0704629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101(23):8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Bell JD, Siddiq IP, Baker AJ. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J Cereb Blood Flow Metab. 2009;29(3):575–584. doi: 10.1038/jcbfm.2008.151. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Lipshutz D, Bartus JO, Slivjak MJ, Smith EF, 3rd, Lee JC, Esser KM. Characterization of cAMP-dependent inhibition of LPS-induced TNF α production by rolipram, a specific phosphodiesterase IV (PDE IV) inhibitor. Int J Immunopharmacol. 1994;16(10):805–816. doi: 10.1016/0192-0561(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Reyes-Irisarri E, Sanchez AJ, Garcia-Merino JA, Mengod G. Selective induction of cAMP phosphodiesterase PDE4B2 expression in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2007;66(10):923–931. doi: 10.1097/nen.0b013e3181567c31. [DOI] [PubMed] [Google Scholar]
- Ross SA, Halliday MI, Campbell GC, Byrnes DP, Rowlands BJ. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br J Neurosurg. 1994;8(4):419–425. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- Rutten K, Van Donkelaar EL, Ferrington L, Blokland A, Bollen E, Steinbusch HW, Kelly PA, Prickaerts JH. Phosphodiesterase inhibitors enhance object memory independent of cerebral blood flow and glucose utilization in rats. Neuropsychopharmacology. 2009;34(8):1914–1925. doi: 10.1038/npp.2009.24. [DOI] [PubMed] [Google Scholar]
- Sakurada O, Kennedy C, Jehle J, Brown JD, Carbin GL, Sokoloff L. Measurement of local cerebral blood flow with iodo [14C] antipyrine. Am J Physiol. 1978;234(1):H59–66. doi: 10.1152/ajpheart.1978.234.1.H59. [DOI] [PubMed] [Google Scholar]
- Santos-Silva AJ, Cairrao E, Morgado M, Alvarez E, Verde I. PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. Eur J Pharmacol. 2008;582(1-3):102–109. doi: 10.1016/j.ejphar.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Schneider HH, Schmiechen R, Brezinski M, Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol. 1986;127(1-2):105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF α and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14(4):615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Si Q, Nakamura Y, Ogata T, Kataoka K, Schubert P. Differential regulation of microglial activation by propentofylline via cAMP signaling. Brain Res. 1998;812(1-2):97–104. doi: 10.1016/s0006-8993(98)00954-8. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Fernandez AG, Palacios JM. Effects of rolipram on the elevated plus-maze test in rats: a preliminary study. J Psychopharmacol. 1999;13(3):274–277. doi: 10.1177/026988119901300309. [DOI] [PubMed] [Google Scholar]
- Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9(3-4):227–236. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFα production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837(1-2):203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42(2):177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Tilley DG, Maurice DH. Vascular smooth muscle cell phosphodiesterase (PDE) 3 and PDE4 activities and levels are regulated by cyclic AMP in vivo. Mol Pharmacol. 2002;62(3):497–506. doi: 10.1124/mol.62.3.497. [DOI] [PubMed] [Google Scholar]
- Torphy TJ. Phosphodiesterase isozymes: Molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998;157(2):351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Suehiro E, Wei EP, Kontos HA, Povlishock JT. Uncomplicated rapid posthypothermic rewarming alters cerebrovascular responsiveness. Stroke. 2004;35(2):601–606. doi: 10.1161/01.STR.0000113693.56783.73. [DOI] [PubMed] [Google Scholar]
- Verghese MW, McConnell RT, Strickland AB, Gooding RC, Stimpson SA, Yarnall DP, Taylor JD, Furdon PJ. Differential regulation of human monocyte-derived TNF α and IL-1 β by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J Pharmacol Exp Ther. 1995;272(3):1313–1320. [PubMed] [Google Scholar]
- Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor α expression and protein levels after fluid percussion injury in rats: The effect of injury severity and brain temperature. Neurosurgery. 2004;55(2):416–424. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- Wei EP, Dietrich WD, Povlishock JT, Navari RM, Kontos HA. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ Res. 1980;46(1):37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]
- Whitaker CM, Beaumont E, Wells MJ, Magnuson DS, Hetman M, Onifer SM. Rolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injury. Neurosci Lett. 2008;438(2):200–204. doi: 10.1016/j.neulet.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Shiloh AO, Sauermelch CF, Sulpizio A, Michell MP, Cieslinski LB, Torphy TJ, Ohlstein EH. Identification, characterization, and functional role of phosphodiesterase type IV in cerebral vessels: effects of selective phosphodiesterase inhibitors. J Cereb Blood Flow Metab. 1997;17(2):210–219. doi: 10.1097/00004647-199702000-00011. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Banos MA, Herregodts P, Hooghe R, Hooghe-Peters EL. Expression of interleukin (IL)-1 β, IL-6 and their respective receptors in the normal rat brain and after injury. Eur J Immunol. 1992;22(11):2963–2971. doi: 10.1002/eji.1830221131. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler. 1999;5(2):126–133. doi: 10.1177/135245859900500210. [DOI] [PubMed] [Google Scholar]
- Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yang L, Konishi Y, Maeda N, Sakanaka M, Tanaka J. Suppressive effects of phosphodiesterase type IV inhibitors on rat cultured microglial cells: Comparison with other types of cAMP-elevating agents. Neuropharmacology. 2002;42(2):262–269. doi: 10.1016/s0028-3908(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Snyder RD, Herman EH, Knapton A, Honchel R, Miller T, Espandiari P, Goodsaid FM, Rosenblum IY, Hanig JP, Sistare FD, Weaver JL. Histopathology of vascular injury in Sprague-Dawley rats treated with phosphodiesterase IV inhibitor SCH 351591 or SCH 534385. Toxicol Pathol. 2008;36(6):827–839. doi: 10.1177/0192623308322308. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Jenkins LW, Kochanek PM, Clark RS. Bench-to-bedside review: Apoptosis/programmed cell death triggered by traumatic brain injury. Crit Care. 2005;9(1):66–75. doi: 10.1186/cc2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhang HT, O’Donnell JM. Inhibitor binding to type 4 phosphodiesterase (PDE4) assessed using [3H]piclamilast and [3H]rolipram. J Pharmacol Exp Ther. 2003;305(2):565–572. doi: 10.1124/jpet.102.47407. [DOI] [PubMed] [Google Scholar]
- Zhu B, Kelly J, Vemavarapu L, Thompson WJ, Strada SJ. Activation and induction of cyclic AMP phosphodiesterase (PDE4) in rat pulmonary microvascular endothelial cells. Biochem Pharmacol. 2004;68(3):479–491. doi: 10.1016/j.bcp.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Majluf-Cruz A, Omburo GA. Cyclic AMP-specific phosphodiesterase inhibitor rolipram and RO-20-1724 promoted apoptosis in HL60 promyelocytic leukemic cells via cyclic AMP-independent mechanism. Life Sci. 1998;63(4):265–274. doi: 10.1016/s0024-3205(98)00270-7. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7(1):22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]