Abstract
Protein tyrosine kinases of the Janus kinase (JAK) family are associated with many cytokine receptors, which, on ligand binding, regulate important cellular functions such as proliferation, survival, and differentiation. In multiple myeloma, JAKs may be persistently activated due to a constant stimulation by interleukin (IL)-6, which is produced in the bone marrow environment. INCB20 is a synthetic molecule that potently inhibits all members of the JAK family with a 100- to 1,000-fold selectivity for JAKs over >70 other kinases. Treatment of multiple myeloma cell lines and patient tumor cells with INCB20 resulted in a significant and dose-dependent inhibition of spontaneous as well as IL-6-induced cell growth. Importantly, multiple myeloma cell growth was inhibited in the presence of bone marrow stromal cells. The IL-6 dependent cell line INA-6 was particularly sensitive to the drug (IC50 < 1 μmol/L). Growth suppression of INA-6 correlated with an increase in the percentage of apoptotic cells and inhibition of signal transducer and activator of transcription 3 phosphorylation. INCB20 also abrogated the protective effect of IL-6 against dexamethasone by blocking phosphorylation of SHP-2 and AKT. In contrast, AKT phosphorylation induced by insulin-like growth factor-I remained unchanged, showing selectivity of the compound. In a s.c. severe combined immunodeficient mouse model with INA-6, INCB20 significantly delayed INA-6 tumor growth. Our studies show that disruption of JAKs and downstream signaling pathways may both inhibit multiple myeloma cell growth and survival and overcome cytokine-mediated drug resistance, thereby providing the preclinical rationale for the use of JAK inhibitors as a novel therapeutic approach in multiple myeloma.
Introduction
Janus kinases (JAK) are cytoplasmic protein tyrosine kinases that are constitutively associated with several cytokine and growth hormone-like receptors that by themselves lack intrinsic tyrosine kinase activity. The family of JAKs comprises four members in the mammalian system: JAK1, JAK2, JAK3, and TYK2 (1). They are structurally unique in having a COOH-terminal kinase domain that is preceded by a pseudokinase domain. JAKs are ubiquitously expressed with the exception of JAK3, which is mainly restricted to hematopoietic cells. Activation of JAKs under normal physiologic conditions occurs by ligand binding and receptor chain oligomerization. The present view is that receptor oligomerization leads to a conformational change that brings the JAKs into apposition, which allows them to phosphorylate each other. Subsequently, they phosphorylate specific tyrosine motifs within the cytoplasmic tail of the receptor chains, which then act as recruitment sites for other signaling molecules such as signal transducer and activator of transcription (STAT) factors, Src kinases, protein tyrosine phosphatases, and several adaptor proteins (2). Thus, JAKs provide important links between cytokine receptors and downstream effector proteins, ultimately resulting in transcriptional regulation of specific genes that mediate cellular responses.
Constitutive or enhanced JAK activation has been implicated in neoplastic transformation and abnormal cell proliferation in various hematologic malignancies including lymphoid and myeloid leukemias, Hodgkin’s lymphoma, and various B-cell non-Hodgkin’s lymphomas (3, 4). Direct evidence comes from the identification of TEL-JAK2 fusion proteins as a result of chromosomal translocations in lymphoid leukemias and a case of atypical chronic myeloid leukemia (5, 6). The TEL-JAK2 chimera was able to transform Ba/F3 cells and render them factor-independent. Importantly, TEL-JAK2 transgenic mice develop T-cell leukemia with constitutive activation of STAT1 and STAT5 in leukemic tissues (7). Other mechanisms that may lead to increased JAK activation include gene amplification, phosphorylation by oncogenic tyrosine kinases, increased growth factor production, and disruption of normal negative regulation. The recent discovery of a specific JAK2 point mutation in myeloproliferative disorders has revealed a causal role for constitutive JAK activation in the pathogenesis of this disease category (8).
In multiple myeloma cells, JAKs may be persistently activated by constant stimulation with interleukin (IL)-6, which is produced primarily in the bone marrow environment and mediates multiple myeloma cell growth (9, 10). IL-6 binding to its specific receptor (gp80/CD126) leads to homodimerization of the gp130 chain and activation of JAKs, which then phosphorylate gp130 at specific tyrosine residues (11). The JAK kinases that are associated with gp130 are JAK1, JAK2, and TYK2, and their activation following stimulation with IL-6 has been shown for murine and human plasmacytoma cell lines and patient samples (12–17). The signaling pathways downstream of gp130/JAK include STAT3, the Ras/Raf/mitogen-activated protein kinase, and the phosphatidylinositol 3-kinase/AKT pathways. In multiple myeloma cells, all three pathways can be activated by IL-6 and mediate cell growth, survival, and drug resistance (10). Additional cytokines besides IL-6, which activate JAKs and may promote multiple myeloma cell growth, are mostly within the gp130 family and include oncostatin M, leukemia inhibitory factor, IL-11 (12, 18), novel neurotrophin-1/B-cell-stimulating factor-3 (19), and human herpesvirus-8 IL-6 homologue (20). IL-21 may also act as a growth and survival factor for multiple myeloma cells (21).
Altogether, JAK kinases play a critical role in the pathophysiology of multiple myeloma primarily through their association with cytokine receptors. Disruption of JAK activity and downstream signaling pathways may inhibit myeloma cell growth, survival, and overcome drug resistance. The aim of this study is to evaluate the effects of a JAK selective inhibitor, INCB20, on human multiple myeloma cells.
Materials and Methods
Reagents and Kinase Assays
INCB000020 (INCB20) is a synthetic compound, which has been extensively evaluated for its ability to inhibit JAK family members and other kinases (Incyte). The enzyme assays for human JAKs were done using recombinant catalytic domains of the respective kinases expressed in the baculovirus system, with ATP concentrations at Km. Enzyme activities were assayed by measuring the phosphorylation of the peptide biotin-EQEDEPEGDYFEWLE. The phosphorylated peptide was detected by a time-resolved fluorescence resonance energy transfer method. Reactions included the indicated amount of purified JAKs, the specified amount of ATP, and 500 nmol/L peptide in 40 μL assay buffer containing 50 mmol/L Tris-HCl (pH 7.8), 100 mmol/L NaCl, 5 mmol/L DTT, and 0.1 mg/mL bovine serum albumin. Reactions were incubated at room temperature for 1 h and then stopped on addition of 20 μL of 45 mmol/L EDTA, 300 nmol/L streptavidin-allophycocyanin, and 6 nmol/L europium-labeled anti-phosphotyrosine antibody Py20 in assay buffer (Perkin-Elmer). Streptavidin-allophycocyanin and Eu-Py20 were allowed to bind for 40 min before fluorescence was measured using a Fusion plate reader (Perkin-Elmer). Kinetic variables for JAKs were determined by measuring initial rates at several different ATP concentrations. IC50 values were determined using an ATP concentration equal to the Km for ATP ([ATP] = 90 μmol/L for JAK1, 30 μmol/L for JAK2, 3 μmol/L for JAK3, and 20 μmol/L for TYK2). INCB20 was shown to be competitive with ATP by determining the IC50 at several ATP concentrations ranging from 5 μmol/L to 1 mmol/L. The derived IC50 values were fit to IC50 = Ki (1 + [ATP]/Km), where Ki is the JAK-INCB20 dissociation constant and Km is the concentration of ATP at half-maximal reaction rate in the absence of inhibitor. Similar experiments were done with tyrphostin AG490 (Invitrogen). To determine the in vitro specificity of INCB20, the inhibition of a panel of other kinases was measured using the Kinase Profiler Service (Upstate Biotechnology). For cell culture experiments, INCB20 was dissolved in DMSO (endotoxin and cell cultured tested; Sigma); a 5 mmol/L stock solution was prepared and stored at room temperature until use. Recombinant human IL-6 was purchased from R&D Systems, insulin-like growth factor-I (IGF-I) was obtained from Research Diagnostic, and AG490 and LY294002 were from LC Laboratories.
Cells and Culture Conditions
The human IL-6-dependent cell line INA-6 was established and characterized as described (22). Cell lines MM1.S and L363 were kindly provided by Dr. S. Rosen (Northwestern University) and Dr. V. Diehl (University of Cologne). LP-1 was a kind gift from Dr. P.L. Bergsagel (Weill Medical College, Cornell University), OCI-My7 cells were kindly provided by Dr. H.A. Messner (Ontario Cancer Institute), and doxorubicin-resistant RPMI8226 cells (Dox40) were provided by Dr. W. Dalton (H. Lee Moffitt Cancer Center). U266, RPMI8226, and NCI-H929 cell lines were obtained from the American Type Culture Collection. Cells were maintained in RPMI 1640 with 10% or 20% heat-inactivated fetal bovine serum (Harlan), 2 mmol/L L-glutamine, and antibiotics. Recombinant human IL-6 (2.5 ng/mL) was added to INA-6 cells. Primary tumor cells were isolated from the bone marrow sample of a patient with multiple myeloma as described previously (23). Peripheral blood mononuclear cells were prepared from human whole blood samples using Ficoll-Hypaque separation method and T cells were obtained from the peripheral blood mononuclear cells by centrifugal elutriation. Bone marrow stromal cell (BMSC) cultures were established from patient bone marrow mononuclear cells and grown in RPMI 1640 with 20% fetal bovine serum and supplements as described above.
Cell Growth Assays
DNA synthesis was measured by [3H]thymidine uptake. Briefly, 2 × 104 to 3 × 104 cells per well were cultured in 96-well flat-bottomed plates for 48 or 72 h. INCB20 and IL-6 were added as indicated. Control cultures contained DMSO at concentrations equivalent to the highest INCB20 concentration (0.2% at maximum). Cells were pulsed with 0.5 μCi (0.0185 MBq)/well [3H]thymidine (Amersham) for 6 h (cell lines), harvested onto glass fiber filters, and counted in a β-scintillation counter. Because primary myeloma cells exert a relatively low proliferation rate, DNA synthesis of patient cells was measured during the last 16 h of culture. For evaluation of INCB20 effects on cell viability, a colorimetric, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium-based method was used (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega). T cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, and antibiotics. After washing once with PBS, cells were resuspended and plated at 6,000 per well in 96-well plates and treated with INCB20 at various concentrations. The plates were incubated at 37°C in 5% CO2 atmosphere for 3 days and the proliferation was determined by adding an equal volume of CellTiter-Glo Reagent and detecting luminescence on TopCount NXT. All experiments were done in triplicate.
Multiple Myeloma/BMSC Cocultures
In coculture experiments, stromal cells and primary tumor cells were derived from different patients (24). BMSC were trypsinized, washed, and transferred to 96-well flat-bottomed plates (0.5 × 104-1 × 104 cells per well). Multiple myeloma cells (2 × 104 INA-6 and 3 × 104 patient cells) and INCB20 were added the following day, and cell growth evaluated after 2 or 3 more days. For signaling experiments, BMSC were transferred into 6-well plates in 2 mL RPMI 1640 with 10% fetal bovine serum, and 8 × 106 IL-6-starved INA-6 cells were added the following day. Cocultures were left untreated for 24 h; INCB20 was then added for 2 h and cell lysates were prepared. DMSO concentration in all INCB20-containing cultures was adjusted to the highest drug concentration (0.02% final).
Measurement of IL-6 Levels
IL-6 levels were determined by ELISA (R&D Systems) according to the manufacturer’s protocol. Supernatants were collected from BMSC cultures left untreated or treated with 5 μmol/L INCB20 for 48 h and stored at −20°C until use.
Determination of Apoptotic Cell Numbers
After extensive washing, 5 × 105 INA-6 cells were plated into 24-well culture plates in medium supplemented with 10% fetal bovine serum and 10 ng/mL IL-6. INCB20 was added as indicated. Control cultures contained DMSO concentrations equivalent to the highest drug concentration. After 48 h, apoptotic cells were detected by measuring Apo2.7 antigen expression (Beckman Coulter). Samples were analyzed using a Cytomics FC500 flow cytometer (Coulter).
Western Blot Analysis
Multiple myeloma cells were starved and treated with INCB20 for 2 h before cytokines were added. Whole-cell lysates were prepared in buffer containing 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100, 5 mmol/L NaF, 2 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L DTT, and a protease inhibitor cocktail (Complete; Roche) and cleared by centrifugation at 14,000 rpm for 15 min. Lysates were subjected to SDS-PAGE and proteins were transferred to Hybond-C Extra nitrocellulose membranes (Amersham). Membrane staining was done with antibodies specific for phosphorylated AKT and SHP-2 (Cell Signaling); phosphorylated and total STAT1, STAT3, and extracellular signal-regulated kinase 1/2 (Santa Cruz Biotechnology); and tubulin (Sigma). Peroxidase-conjugated and human immunoglobulin preadsorbed anti-rabbit and anti-mouse IgG antibodies (Santa Cruz Biotechnology) and a chemiluminescence system (Western Lightning Plus; Perkin-Elmer Life Sciences) were used for detection.
Animal Studies
Animal studies were done under Animal Welfare Regulation Guidelines in a facility (DuPont Experimental Station) accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Six-week-old severe combined immunodeficient mice (Charles River) were injected s.c. with ~1 × 106 viable INA-6.Tu1 cells freshly harvested from a tumor bearing mouse. The INA-6.Tu1 subline was derived from a tumor of INA-6 cells grown in the peritoneum of severe combined immunodeficient mice. These cells engraft into severe combined immunodeficient mice with a reliable and reproducible rate of >90% and in a reasonable time (4–5 weeks). The cells have maintained their cytokine dependence in vitro and are responsive to IL-6. However, in contrast to the original INA-6, they respond to other cytokines of the gp130 family as well (19, 22). One week after inoculation, animals were monitored daily for signs of tumor growth. When tumors were detectable, visually or by palpation, their size was monitored twice each week by measuring tumor size in two dimensions with a caliper. Tumor volume was calculated as (length × width2)/2. When tumors were well established (>125 mm3), animals were randomly assigned into treatment groups with similar median tumor volumes (n = 6 per group for efficacy and n = 3 per group for pharmacodynamic studies). Mice were dosed twice daily by i.p. injection with vehicle or INCB20. At the end of the experiment, tumors were harvested and weighed, thus confirming our tumor measurement data. Tumors for STAT3 Western blot analysis were harvested after 2 days of dosing.
Results
INCB20 Is a Potent and Specific Pan-JAK Inhibitor
The ability of INCB20 to inhibit JAK family members was tested in an in vitro kinase assay that contained purified, recombinant JAK family kinase and a peptide substrate. The effect of various concentrations of INCB20 on JAK2 activity is shown in Fig. 1, where an IC50 of 0.5 nmol/L was obtained. Using similar measurements, the corresponding IC50 values for inhibition of the other JAK kinases were determined to be 0.9 nmol/L for JAK1, 0.5 nmol/L for JAK3, and 0.3 nmol/L for TYK2 (data not shown). AG490 was a relatively weak inhibitor with IC50 values of 10.2 μmol/L for JAK1, 11.2 μmol/L for JAK2, and 15.4 μmol/L for JAK3 (data not shown). The profiling of INCB20 against a panel of kinases revealed a 100- to 1,000-fold selectivity for JAKs over >70 other kinases tested including Abl, Aurora-A, c-Raf, CSK, fibroblast growth factor receptor 3, glycogen synthase kinase 3β, IGF-I receptor, insulin receptor, Lck, platelet-derived growth factor receptorα, PKBβ, PKCI, and Zap-70 (25). In summary, INCB20 potently inhibited all four JAK kinases and as such was used to evaluate the effect of JAK inhibition in multiple myeloma cells.
Figure 1.
INCB20 is a potent JAK inhibitor. The potency of INCB20 was assessed using recombinant kinase domains from each of the JAK family members and the biotinylated substrate EQEDEPEGDYFEWLE. Phosphor-ylated peptides were detected by time-resolved fluorescence resonance energy transfer (see Materials and Methods for details). All assays were done using the Km ATP concentration for the respective enzymes and were repeated at least three times. Representative dose-response curve for JAK2.
INCB20 Inhibits Spontaneous and IL-6-Mediated Growth of Human Multiple Myeloma Cells
A panel of human multiple myeloma cell lines was treated with INCB20 at different concentrations, and cell growth was monitored by measuring [3H]thymidine uptake after 48 h. INCB20 induced a dose-dependent decrease in DNA synthesis (Fig. 2A). The IC50 values were in the low micromolar range. Similar results were obtained in a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay, which indicates the proportion of living cells, indicating that inhibition of proliferation by the JAK inhibitor was due to induction of cell death (data not shown). Cell line INA-6 was particularly sensitive to treatment, with an IC50 of ~0.5 μmol/L and complete inhibition of cell proliferation at a 1 μmol/L concentration. INA-6 cells are strictly dependent on exogenous IL-6, which activates STAT3 in these cells (20, 22). Considering the dependency of primary multiple myeloma cells on cytokines and the human bone marrow environment (26), the INA-6 cell line may therefore be more likely to represent a disease-relevant phenotype. Other cell lines not reliant on exogenous IL-6 are still cytokine responsive.
Figure 2.
INCB20 inhibits growth of human myeloma cells. A, effect of INCB20 on multiple myeloma cell lines. Cell growth was assayed by measuring [3H]thymidine uptake during the last 6 h of a 48 h culture time. Results were compared with untreated cultures. Experiments were done in triplicate. INA-6 cells were cultured in the presence of 10 ng/mL IL-6. Cell growth was not significantly affected in vehicle control cultures containing DMSO at concentrations equivalent to the highest drug concentration (data not shown). B, effect of INCB20 on patient tumor cells. Multiple myeloma cells enriched from patient bone marrow sample were cultured in medium alone or in the presence of 50 ng/mL IL-6 for 72 h. [3H]thymidine uptake was measured during the following 16 h. Bars, 1 SD of triplicate cultures. C, INCB20 has only limited effects on viability of normal T cells and peripheral blood mononuclear cells as evaluated by luminescence and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay.
A marked inhibition of cell growth by INCB20 was also seen with enriched primary patient tumor cells (Fig. 2B). In these cells, the addition of IL-6 induced a significant proliferative response and a shift in IC50 toward higher concentrations but did not protect from INCB20 inhibition. INCB20 therefore inhibits spontaneous as well as IL-6-mediated growth of multiple myeloma cells. The proliferation and viability of human embryonic kidney cells, umbilical cord vein endothelial cells, and Bcr-Abl-transformed cells was not affected at concentrations ≥25 μmol/L, nor was the viability of resting T cells or peripheral blood mononuclear cells (Fig. 2C; data not shown). This observation indicates that the drug has less cytotoxic effect in either quiescent hematopoietic cells or those whose viability is driven by other cytokines.
INCB20 Inhibits Multiple Myeloma Cell Growth in the Presence of BMSC
We next examined the ability of INCB20 to block growth of multiple myeloma cells adherent to BMSC. Growth of INA-6 cells, as well as primary patient cells, was markedly enhanced in the presence of allogeneic BMSC (Fig. 3A and B). Importantly, BMSC-induced growth of multiple myeloma cells could be inhibited by INCB20 in a dose-dependent manner, with IC50 values again in the low micromolar range. In contrast, BMSC viability was not affected at INCB20 concentrations that were 10 times that required to completely block multiple myeloma growth (Fig. 3C). Moreover, INCB20 had no measurable effect on the function of the nonproliferating BMSC. For instance, we measured IL-6 levels in the supernatants of BMSC cultures, because IL-6 was the only cytokine that promoted growth and survival of INA-6 cells, among several cytokines tested (22). IL-6 levels were not significantly different in INCB20-treated cultures (5.4 ng/mL) compared with controls (5.9 ng/mL), with concentrations that are sufficient for optimal cell growth (20). These results suggest that growth inhibition of INA-6 cells in the presence of BMSC was not due to lack of growth factor support.
Figure 3.
INCB20 inhibits multiple myeloma cell growth in the presence of BMSC. INA-6 (A) or patient tumor cells (B) were cultured alone or in the presence of BMSC and treated with different concentrations of INCB20. Cell growth was assayed by measuring [3H]thymidine uptake after 48 h (INA-6) and 72 h (patient cells). [3H]thymidine uptake of BMSC alone was not significant (118 ± 32 and 561 ± 200 counts/min, respectively). Mean of triplicate cultures; bars, 1 SD. DMSO at concentrations equivalent to the highest INCB20 concentration had no effect (data not shown). C, INCB20 had no significant effect on BMSC cell viability. BMSC (1 × 104) were cultured overnight and INCB20 at indicated concentrations was added the following day. Control cultures contained DMSO at concentrations equivalent to the highest INCB20 concentration. After 48 h, a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay was done. The number of viable cells is expressed as the percentage of absorbance values achieved in untreated cultures. Mean of triplicate cultures; bars, 1 SD.
INCB20 Induces Apoptosis and Blocks STAT3 Phosphorylation
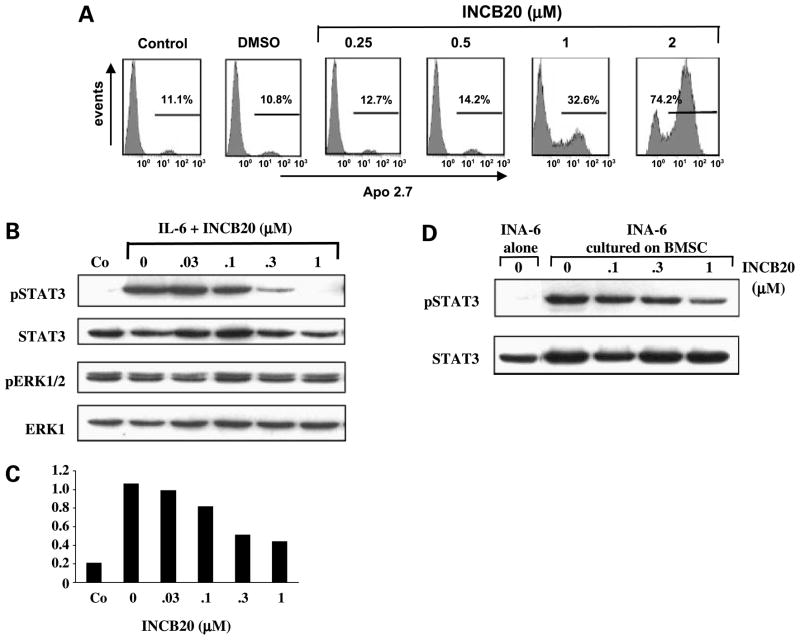
Because IL-6 plays a role in multiple myeloma and activates JAK kinases, the IL-6-dependent INA-6 cell line was chosen as an in vitro model to evaluate mechanisms of growth inhibition by INCB20. As shown before, INA-6 cell growth is promoted by either IL-6 or BMSC and this was inhibited by INCB20. Loss of viability of INA-6 cells is due to apoptosis as evidenced by a dose-dependent increase in the number of APO2.7 antigen-expressing cells (Fig. 4A). For example, a 6.9-fold increase in APO2.7-positive cells was observed at 48 h in INCB20-treated cultures versus vehicle control at a drug concentration of 2 μmol/L.
Figure 4.
INCB20 induces apoptosis and blocks STAT3 phosphorylation in INA-6 cells. A, INA-6 cells were cultured for 48 h with different drug concentrations of INCB20 in the presence of 10 ng/mL IL-6 and subjected to flow cytometry.The percentage of Apo2.7-positive cells is indicated. Control cultures contained DMSO at concentrations equivalent to the highest drug concentration. B, INA-6 cells were IL-6 and serum starved for 4 h, and INCB20 was added 2 h before cells were stimulated with 10 ng/mL IL-6 for 15 min or left untreated (Co). Whole-cell lysates were prepared immediately after IL-6 stimulation and subjected to SDS-PAGE, and membranes were stained with antibodies for phosphorylated and total STAT3 and extracellular signal-regulated kinase. DMSO concentration was equal in all drug-treated samples and equivalent to the highest INCB concentration (0.04% final). C, levels of phosphorylated STAT3 shown in B was analyzed with the Quantity One software (Bio-Rad Laboratories) and expressed as relative value compared with total STAT3. D, INCB20 inhibits STAT3 phosphorylation in the presence of BMSC. INA-6 cells were cultured alone or on a layer of BMSC for 24 h and then treated with INCB20 for 2 h or left untreated. Whole-cell lysates of nonadherent (INA-6) cells were prepared and subjected to SDS-PAGE. Membranes were stained with antibodies for phosphorylated and total STAT3.
When INA-6 cells were pretreated with INCB20, IL-6-induced STAT3 tyrosine phosphorylation was inhibited in a dose-dependent manner (Fig. 4B and C). At 1 μmol/L INCB20, STAT3 phosphorylation was completely blocked, correlating with the magnitude of growth inhibition at that drug concentration. In contrast, extracellular signal-regulated kinase 1/2 phosphorylation, which is constitutive in INA-6 cells due to an activating mutation in the N-ras gene (22), was not affected by INCB20 (Fig. 4B), providing further evidence for specific inhibition of the JAK/STAT pathway. In an attempt to mimic the in vivo situation more accurately, INA-6 cells were cultured on BMSC for 24 h and then treated with INCB20 for 2 h. In this setting, a significant decrease of STAT3 phosphorylation levels was observed in INCB20-treated cultures (Fig. 4D). Inhibition of IL-6 and BMSC-induced growth of INA-6 cells by INCB20 therefore correlates with induction of apoptosis and decreased STAT3 phosphorylation.
In vivo Effect of JAK Inhibition on Myeloma Tumor Growth and Survival
To determine how JAK inhibition affects the growth and/or survival of multiple myeloma cells in vivo, we established a s.c. xenograft tumor model using a previously described tumorigenic subclone of INA-6 (22). In culture, INA-6.Tu1 cells respond to INCB20 similarly to the parental cells; importantly, growth of these cells in vivo does not select for cytokine independence. Using these cells, we inoculated a cohort of mice s.c. and monitored tumor growth over time. INA-6 cells produce only small amounts of κ light chains when cultured in vitro, whereas heavy chain secretion is negligible. In the s.c. model used in the present study, the tumor size could be easily monitored by direct measurement without the need to follow a surrogate marker. When tumors were well established, mice were randomly grouped into four cohorts with similar mean tumor volumes and subsequently treated with vehicle or INCB20 at increasing doses for 6 consecutive days. Inhibition of JAK kinases dramatically affected tumor growth in a dose-responsive manner (Fig. 5). At these concentrations, INCB20 had no significant effect on growth of Bcr-Abl-driven K562 tumor xenografts (data not shown). Analysis of H&E-stained tissue sections showed a preponderance of pyknotic nuclei, consistent with an apoptotic response (data not shown).
Figure 5.
Effect of INCB20 on INA-6 tumors in severe combined immunodeficient mice. INA-6 tumor-bearing mice were treated with vehicle or INCB20 at indicated concentrations twice a day for 6 consecutive days. For STAT3 phosphorylation, a separate cohort of mice was treated in a similar manner. Tumor samples were harvested after four doses. Whole-cell lysates were prepared and subjected to Western blotting. Equal amounts of protein were analyzed.
To investigate the relationship between JAK inhibition and tumor growth, a separate cohort of mice was treated in a similar manner with INCB20 using the same doses administered in the efficacy study. Tumor cells were harvested from the mice after 2 days of dosing and cellular proteins were extracted and normalized for protein concentration to analyze the levels of phosphorylated STAT3. A correlation was observed between dose levels of INCB20 that were sufficient to inhibit STAT3 phosphorylation and tumor growth. Whereas modest inhibition of STAT3 phosphorylation resulted in modest tumor growth inhibition, complete inhibition of STAT3 phosphorylation resulted in tumor regression (Fig. 5). Similar data were observed in tumors harvested as early as 2 h after a single dose of JAK inhibitor, suggesting that inhibition of STAT3 phosphorylation precedes tumor cell death, similar to what is observed in tissue culture studies (Fig. 4B).4 Importantly, explanted tumors were confirmed to be still IL-6-dependent and responsive to INCB20 (data not shown).
INCB20 Abrogates IL-6-Mediated Protection against Dexamethasone-Induced Apoptosis
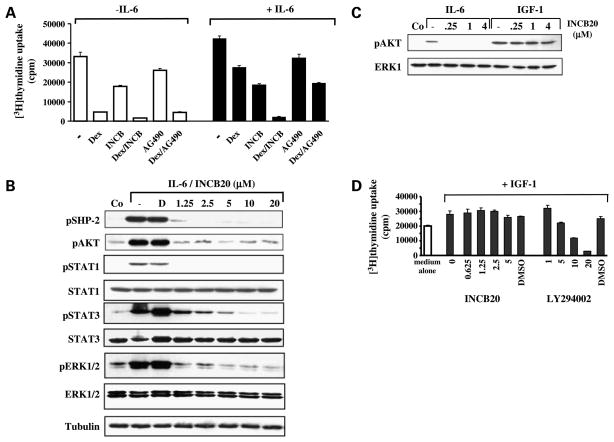
Dexamethasone is the most commonly used drug in the primary treatment for multiple myeloma. Consistent with our earlier studies, growth inhibition of sensitive MM1.S cells by dexamethasone was abrogated in the presence of exogenous IL-6. This protective effect is completely abolished when INCB20 is added at a concentration that by itself has only a marginal effect on cell growth (Fig. 6A). Our prior studies have shown that IL-6-mediated resistance to dexamethasone is associated with activation of the phosphatases SHP-2 and AKT (27, 28). Conversely, treatment of MM1.S cells with INCB20 blocked IL-6-induced phosphorylation of both SHP-2 and AKT (Fig. 6B and C). Phosphorylation of STAT1, STAT3, and extracellular signal-regulated kinase 1/2 was also inhibited in these cells, consistent with the central role of JAKs in gp130 downstream signaling pathways (Fig. 6B). INCB20 had no effect on AKT phosphorylation or cell growth induced by IGF-I, which, on the other hand, can be blocked with a phosphatidylinositol 3-kinase inhibitor (Fig. 6C and D). These results are consistent with the kinase profile of INCB20 as well as the observation that the kinase activity of the IGF-I receptor lies within the receptor β subunit itself and does not involve JAK kinases (29).
Figure 6.
INCB20 overcomes cytokine-mediated protection against dexamethasone by inhibiting IL-6-mediated but not IGF-I-mediated signaling pathways. A, INCB20 inhibits IL-6-mediated protection against dexamethasone in MM1.S myeloma cells. MM1.S cells were cultured for 72 h in the presence or absence of IL-6 (50 ng/mL), dexamethasone (1 μmol/L), INCB20 (5 μmol/L), AG490 (10 μmol/L), or a combination of the drugs. Mean [3H]thymidine uptake (counts/min) of triplicate cultures; bars, 1 SD. B, inhibition of IL-6-mediated signaling pathways. Serum-starved MM1.S cells were left untreated or treated with INCB20 for 2 h and then stimulated with 50 ng/mL IL-6 for 15 min. Control cultures did not contain cytokines or drug. Whole-cell lysates were subjected to SDS-PAGE, and membranes were stained with antibodies for phosphorylated and total proteins as indicated. D, DMSO. C, inhibition of IL-6-induced but not IGF-I-induced AKT phosphorylation. Serum-starved MM1.S cells were left untreated or treated with increasing concentrations of INCB20 for 2 h and then stimulated with 50 ng/mL IL-6 or 200 ng/mL IGF-I for 15 min. Control cultures did not contain cytokines or drug. D, effect of INCB20 and Ly294002 on IGF-I-induced proliferation. MM1.S cells were cultured in medium alone or in the presence of 200 ng/mL IGF-I with and without inhibitors. After 72 h, [3H]thymidine uptake during the last 6 h was measured. Vehicle control cultures contained DMSO at concentrations equivalent to the highest drug concentration. Mean [3H]thymidine uptake (counts/min) of triplicate cultures; bars, 1 SD.
Discussion
In multiple myeloma, the tumor cells mainly reside in the bone marrow, where they bind to stromal cells and induce the secretion of cytokines. Among them, IL-6 is one of the most important factors mediating multiple myeloma cell growth, survival, and drug resistance (9, 10). The gp130 signal transducer chains of the IL-6R complex are constitutively associated with JAK kinases, which play a critical role in IL-6-mediated cellular response (11). Evidence for JAK activation in multiple myeloma cells in vivo comes from studies showing constitutive activation of STAT3 in tumor samples, whereas little or no activated STATs were detected in bone marrow from normal individuals or patients with no evidence of bone marrow metastases (30–32). Here, we show that the selective JAK inhibitor INCB20 induces cytotoxicity in multiple myeloma cells by blocking IL-6 signaling pathways.
Inhibition of cell growth by INCB20 was achieved in nine multiple myeloma cell lines; sensitivity to INCB20 treatment, however, varied depending on the cell line tested. The shift in potency of INCB20 in the cellular assays compared with the enzyme assays can be explained by the higher ATP concentration in a cell compared with the concentrations used in the cell-free kinase assays (10- to 100-fold). As such, there is much more ATP for the inhibitor to compete with. Furthermore, INCB20 is active only as a free (unbound) compound and, like most other kinase inhibitors, has a high tendency to bind to serum proteins. The fact that most multiple myeloma cell lines are factor independent may explain why IL-6-dependent INA-6 cells are particularly susceptible to growth inhibition by INCB20.
Remarkably, growth of INA-6 cells and, even more important, of patient multiple myeloma cells was inhibited not only in the presence of IL-6 but also when cultured on BMSC with similar IC50 concentrations. This setting may more accurately reflect the in vivo situation (26). Our data therefore suggest that signaling pathways involving JAKs are critical for multiple myeloma cell growth and that additional growth factors in the bone marrow microenvironment may not be able to overcome the inhibitory effect of a JAK inhibitor. Although the results of the primary patient sample were consistent with the observations in INA-6 cells, the effect of INCB20 may not be reproduced in every single patient sample but shows the potential and proof-of-concept of JAK inhibition in multiple myeloma. Our hypothesis will require further examinations but is supported by a recent work with another pan-JAK inhibitor that significantly decreased cell viability in the majority of primary myeloma samples (33) and by our own results using an independent JAK inhibitor (25).5 Death of INA-6 cells may be the direct result of STAT3 inactivation by INCB20, also providing evidence for the efficiency of this compound to block activation of an immediate JAK target. Notably, by blocking JAK/STAT3 activation in multiple myeloma cells, an important survival pathway is interrupted: STAT3 increases the transcription of multiple Bcl-2 family proteins (30, 34). STAT3 may also directly contribute to the malignant progression of multiple myeloma by allowing accumulation of long-lived plasma cells (35, 36). For INA-6 cells, STAT3 is obligatory for survival confirmed by transfecting the cells with mutated gp130 chimeras that lack STAT3-binding sites (37). We cannot completely exclude that inhibition of cytokine-mediated AKT activity, or inhibition of another enzyme required for myeloma cell proliferation and survival, may have also contributed to the observed effects.
Many multiple myeloma cell lines, which are no longer dependent on exogenous cytokines, are still cytokine responsive. In these cells, inhibition of JAK may not induce spontaneous apoptosis but rather sensitize them to chemo-therapeutic agents. For example, abrogation of STAT3 activation and subsequent down-regulation of Bcl-xL expression sensitizes U266 cells to Fas- or drug-induced cell death (30, 38). In IL-6-responsive MM1.S, INCB20 inhibited all major IL-6 signaling pathways including STAT3, extracellular signal-regulated kinase 1/2, and AKT. This result is consistent with the role for JAKs as central and upstream kinases. Specifically, inhibition of AKT and SHP-2 activation may account for the abrogation of IL-6-mediated dexamethasone resistance (27, 28, 39). In contrast, IGF-I-induced effects were not blocked by INCB20, confirming selectivity of the compound.
Treatment strategies targeting IL-6 in multiple myeloma have already been examined in vitro as well as clinically, including monoclonal antibodies to IL-6 or the IL-6R, as well as IL-6R superantagonists (40–43). One advantage of using a JAK inhibitor versus antibodies is the potential to inhibit the effects of more than one cytokine at the same time. This would not only affect directly multiple myeloma cell growth but also may diminish tumor-associated complications such as bone resorption (44). In addition, antibody therapies may have limited activity due to stochiometric issues including the extremely high levels of IL-6 present in the myeloma bone marrow milieu (45).
Although we have not assessed the effects of INCB20 on normal lymphocyte activation and function, pharmacological inhibition of JAK activity may likely have side effects due to the central role of JAKs in hematopoiesis, lymphocyte development, and immunoregulation (1). JAK1 is important in IFN signaling; conversely, its inhibition might lead to increased susceptibility to viral and bacterial pathogens. TYK2 mediates the biological response to IL-12 and lipopolysaccharide. TYK2 deficiency might result in increased pathogen susceptibility as well; however, only subtle defects were observed in TYK2 knockout mice. Inhibition of JAK2 may cause anemia, leukopenia, or thrombocytopenia due its role in hematopoietic cytokine signaling (46). Significant reductions in T-cell and natural killer cell numbers have been observed in nonhuman primates on extended dosing with a selective JAK3 inhibitor (47). Some effect on B-cell development and function will also have to be expected; however, not all mitogenic and survival pathways of B cells are driven by JAKs and therefore will not be affected. Moreover, patients with myeloma already have an impaired immunoglobulin and antibody-specific synthesis; nevertheless, therapy with the anti-CD20 monoclonal antibody rituximab is well tolerated (48). That being said, it is difficult to faithfully predict the potential side effects of periodic (or even constant) inhibition of select JAK family members. Similar hematologic toxicities result from various cytotoxic chemo-therapeutics and can be dealt with clinically with proper medical support. In our animal studies, the compound was generally well tolerated and did not produce gross toxicity. Furthermore, clinical data on the moderately JAK3 selective inhibitor CP-690,550 in patients with psoriasis or rheumatoid arthritis have been reported in which systemic JAK inhibition appears to be safe and well tolerated (49). However, more formal toxicity studies will be needed to clearly define the potential liabilities of JAK inhibitors.
JAKs represent an attractive target for the development of novel targeted therapies in various clinical settings including hematologic malignancies, autoimmune disease, and organ transplantation. Our studies show that INCB20 abrogates JAK activity and thereby blocks downstream signaling pathways mediating multiple myeloma cell growth and survival. These preclinical studies provide the rationale for clinical protocols of JAK1/2 inhibitors, alone or combined with novel therapies, to improve patient outcome in multiple myeloma.
Acknowledgments
We thank Min Wei for excellent technical assistance.
Grant support: NIH grants RO-1 50947, PO-1 78378, and P50 CA100707; The Multiple Myeloma Research Foundation, The Fund to Cure Myeloma, and Doris Duke Distinguished Clinical Research Scientist Award.
Footnotes
J.S. Fridman, unpublished results.
J.S. Fridman and K. Vaddi, unpublished data.
Disclosure of Potential Conflicts of Interest
E. Caulder, C.L. Neilan, K. Vaddi, J. Li, and J.S. Fridman: employees with ownership interest in Incyte Corp. No other potential conflicts of interest were disclosed.
References
- 1.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 2.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 3.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95:19–29. [PubMed] [Google Scholar]
- 4.Verma A, Kambhampati S, Parmar S, Platanias LC. Jak family of kinases in cancer. Cancer Metastasis Rev. 2003;22:423–34. doi: 10.1023/a:1023805715476. [DOI] [PubMed] [Google Scholar]
- 5.Lacronique V, Boureux A, Valle VD, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–12. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 6.Peeters P, Raynaud SD, Cools J, et al. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–40. [PubMed] [Google Scholar]
- 7.Carron C, Cormier F, Janin A, et al. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood. 2000;95:3891–9. [PubMed] [Google Scholar]
- 8.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–72. [PubMed] [Google Scholar]
- 10.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–18. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger LC, Hawley TS, Lust JA, Goldman SJ, Hawley RG. Tyrosine phosphorylation of JAK-TYK kinases in malignant plasma cell lines growth-stimulated by interleukins 6 and 11. Biochem Biophys Res Commun. 1994;202:596–605. doi: 10.1006/bbrc.1994.1970. [DOI] [PubMed] [Google Scholar]
- 13.Stahl N, Boulton TG, Farruggella T, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6β receptor components. Science. 1994;263:92–5. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 14.Ogata A, Chauhan D, Teoh G, et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–21. [PubMed] [Google Scholar]
- 15.Hideshima T, Chauhan D, Teoh G, et al. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clin Cancer Res. 2000;6:1180–9. [PubMed] [Google Scholar]
- 16.Rawat R, Rainey GJ, Thompson CD, Frazier-Jessen MR, Brown RT, Nordan RP. Constitutive activation of STAT3 is associated with the acquisition of an interleukin 6-independent phenotype by murine plasma-cytomas and hybridomas. Blood. 2000;96:3514–21. [PubMed] [Google Scholar]
- 17.De Vos J, Jourdan M, Tarte K, Jasmin C, Klein B. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells. Br J Haematol. 2000;109:823–8. doi: 10.1046/j.1365-2141.2000.02127.x. [DOI] [PubMed] [Google Scholar]
- 18.Tani Y, Nishimoto N, Ogata A, Shima Y, Yoshizaki K, Kishimoto T. Gp130 in human myeloma/plasmacytoma. Curr Top Microbiol Immunol. 1995;194:229–33. doi: 10.1007/978-3-642-79275-5_27. [DOI] [PubMed] [Google Scholar]
- 19.Burger R, Bakker F, Guenther A, et al. Functional significance of novel neurotrophin-1/B cell-stimulating factor-3 (cardiotrophin-like cytokine) for human myeloma cell growth and survival. Br J Haematol. 2003;123:869–78. doi: 10.1046/j.1365-2141.2003.04686.x. [DOI] [PubMed] [Google Scholar]
- 20.Burger R, Neipel F, Fleckenstein B, et al. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–63. [PubMed] [Google Scholar]
- 21.Brenne AT, Ro TB, Waage A, Sundan A, Borset M, Hjorth-Hansen H. Interleukin-21 is a growth and survival factor for human myeloma cells. Blood. 2002;99:3756–62. doi: 10.1182/blood.v99.10.3756. [DOI] [PubMed] [Google Scholar]
- 22.Burger R, Guenther A, Bakker F, et al. Gp130 and ras mediated signaling in human plasma cell line INA-6: a cytokine-regulated tumor model for plasmacytoma. Hematol J. 2001;2:42–53. doi: 10.1038/sj.thj.6200075. [DOI] [PubMed] [Google Scholar]
- 23.Tai YT, Teoh G, Shima Y, et al. Isolation and characterization of human multiple myeloma cell enriched populations. J Immunol Methods. 2000;235:11–9. doi: 10.1016/s0022-1759(99)00199-4. [DOI] [PubMed] [Google Scholar]
- 24.Hönemann D, Chatterjee M, Savino R, et al. The IL-6 receptor antagonist SANT-7 overcomes bone marrow stromal cell-mediated drug resistance of multiple myeloma cells. Int J Cancer. 2001;93:674–80. doi: 10.1002/ijc.1388. [DOI] [PubMed] [Google Scholar]
- 25.Fridman S, Li J, Liu P, et al. Discovery and preclinical development of selective JAK inhibitors for the treatment of haematological malignancies. Haematologica. 2007;92:117. [Google Scholar]
- 26.Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–13. [PubMed] [Google Scholar]
- 27.Chauhan D, Pandey P, Hideshima T, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–50. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 28.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 29.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–37. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 30.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 31.Quintanilla-Martinez L, Kremer M, Specht K, et al. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol. 2003;162:1449–61. doi: 10.1016/S0002-9440(10)64278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-κB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 33.Pedranzini L, Dechow T, Berishaj M, et al. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66:9714–21. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- 34.Puthier D, Bataille R, Amiot M. IL-6 up-regulates mcl-1 in human myeloma cells through JAK/STAT rather than ras/MAP kinase pathway. Eur J Immunol. 1999;29:3945–50. doi: 10.1002/(SICI)1521-4141(199912)29:12<3945::AID-IMMU3945>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 36.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 37.Brocke-Heidrich K, Kretzschmar AK, Pfeifer G, et al. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood. 2004;103:242–51. doi: 10.1182/blood-2003-04-1048. [DOI] [PubMed] [Google Scholar]
- 38.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–26. [PubMed] [Google Scholar]
- 39.Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60:6763–70. [PubMed] [Google Scholar]
- 40.Bataille R, Barlogie B, Lu ZY, et al. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood. 1995;86:685–91. [PubMed] [Google Scholar]
- 41.van Zaanen HC, Lokhorst HM, Aarden LA, et al. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998;102:783–90. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsunenari T, Koishihara Y, Nakamura A, et al. New xenograft model of multiple myeloma and efficacy of a humanized antibody against human interleukin-6 receptor. Blood. 1997;90:2437–44. [PubMed] [Google Scholar]
- 43.Sporeno E, Savino R, Ciapponi L, et al. Human interleukin-6 receptor super-antagonists with high potency and wide spectrum on multiple myeloma cells. Blood. 1996;87:4510–9. [PubMed] [Google Scholar]
- 44.Silvestris F, Lombardi L, De Matteo M, Bruno A, Dammacco F. Myeloma bone disease: pathogenetic mechanisms and clinical assessment. Leuk Res. 2007;31:129–38. doi: 10.1016/j.leukres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Lu ZY, Brailly H, Wijdenes J, Bataille R, Rossi JF, Klein B. Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments. Blood. 1995;86:3123–31. [PubMed] [Google Scholar]
- 46.O’Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov. 2004;3:555–64. doi: 10.1038/nrd1441. [DOI] [PubMed] [Google Scholar]
- 47.Paniagua R, Si MS, Flores MG, et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation. 2005;80:1283–92. doi: 10.1097/01.tp.0000177643.05739.cd. [DOI] [PubMed] [Google Scholar]
- 48.Treon SP, Pilarski LM, Belch AR, et al. CD20-directed serotherapy in patients with multiple myeloma: biologic considerations and therapeutic applications. J Immunother. 2002;25:72–81. doi: 10.1097/00002371-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson B, Gaweco A, Changelian P, et al. Improvement in psoriatic lesions during a 14-day trial of CP-690,550 (CP), an orally active inhibitor of Janus kinase 3 (JAK3) Ann Rheum Dis. 2007;66:155. [Google Scholar]