Storage
During storage, RBCs undergo various metabolic, structural and morphological changes, the so-called storage lesions1. The acceptable extent of the metabolic changes forms a major part of the blood bank quality guidelines. However, the consequences of these changes for RBC survival after transfusion are mostly unknown. Strikingly, it still has not been determined which events in the blood bank are responsible for the disappearance of up to 30 percent of the transfused RBCs within the first hours after transfusion in the patient2. Also, the relationship between RBC survival after transfusion and the occurrence of transfusion-related pathologies such as iron accumulation, inflammation, and formation of anti-RBC antibodies is far from understood.
Since the changes in most metabolic parameters such as 2,3-DPG, ATP, and pH are rapidly reversible, the metabolic events underlying these parameters are not likely to contribute to most adverse transfusion effects. The storage-associated morphological changes, namely the partially reversible transition from a discoid to an echinocyte/stomatocyte, and finally to an irreversible spherocyte-like morphology1,3, suggest that alterations in membrane structure are more likely to cause a decrease in transfusion efficacy and an increase in harmful effects. The last few years have witnessed a strong increase in proteomic and biochemical data on RBC biology during blood bank storage3–11. Especially the data on storage-related exposure of removal signals such as phosphatidylserine (PS) and band 3-derived senescent cell antigens, support the theory that physiological aging-related changes in the RBC membrane are major, functionally relevant quality determinants of RBC concentrates11.
In the context of membrane alterations, in contrast to detailed analyses of the molecular constituents, relatively little attention has been devoted to the changes in deformability that accompany aging in vivo and in vitro. Here, we review the available data on aging-associated and storage-associated alterations in RBC deformability. Some relevant, preliminary data from our own laboratory will serve as the starting points for an inventory of the data on alterations in RBC deformability during storage, the putative consequences, and the proteomic and metabolomic indications for the underlying molecular mechanisms.
Deformability
The capacity of RBCs to adapt their shape to the dynamic flow conditions, both in the capillaries and - in extremis - in the spleen, is essential for a proper functioning, i.e. flow through the microcirculatory bed. RBC deformability is a major determinant of RBC survival, as deduced from the association between abnormal RBC shape, anemia, and splenic sequestration12–14. Deformability is determined by the mechanical properties of the RBC membrane, the viscosity of the cytoplasm - which is mainly determined by the mean cellular hemoglobin concentration (MCHC) -, and the surface area-to-volume ratio (S/V). Both an increase and a decrease in S/V, an increase in MCHC, and a decrease in membrane elasticity may all lead to a decrease in deformability. Thus, membrane loss by vesiculation and altered transport of ions and water across the membrane, such as occur during storage, both affect deformability. The speed and degree of relaxation, i.e. return to the normal cell shape after deformation, have been attributed to the elastic properties of the cytoskeleton15, as well as to the viscosity of the cytoplasm16. Deformability can be measured using RBC filtration, aspiration through a micropipette, and light scattering in a rheometer or a flow chamber17. A decrease in deformability, as measured by ektacytometry, occurs during physiological RBC aging in vivo18. Again, the relative contributions of the aging-associated decrease in S/V ratio and of the increase in MCHC are not clear.
Changes in the MCHC occur during storage in SAGM, as the RBC volume starts to increase in the first week of storage19. Also, proteomic and biochemical data suggest oxidation as well as breakdown of structural proteins already in the first weeks of storage6–8. These changes may be responsible for the decreased capacity to maintain membrane organization, as deduced from the increased susceptibility to osmotic stress-induced PS exposure, and to band 3 crosslinking-nduced binding of autologous IgG11,20. Since alterations in MCHC and membrane organization affect deformability, and since deformability is associated with RBC survival12,13, storage-associated changes in deformability may contribute to the fast removal of a considerable fraction of the transfused RBC2. Unraveling the details of the role of deformability in RBC removal, the underlying molecular changes, and thereby the putative mechanism(s), is the main reason for our interest in this topic.
Storage and deformability
In general, most older data suggest that cold storage induces a reduction of the deformability, as measured with various techniques17,21, and this reduction has not only been associated with morphological changes21,22, but also with the post-transfusional survival23. Interestingly, our recent data, obtained by automated ektacytometry using the Laser-assisted Optical Rotational Cell Analyzer (LORCA), did not indicate that the deformability of SAGM-stored RBCs decreases with storage time, not even after a storage period of five weeks (Figure 1A). It is possible that in the final weeks the decrease in S/V resulting from vesiculation, which would lead to a decrease in deformability, is compensated by a decrease in MCHC and an increase in deformability.
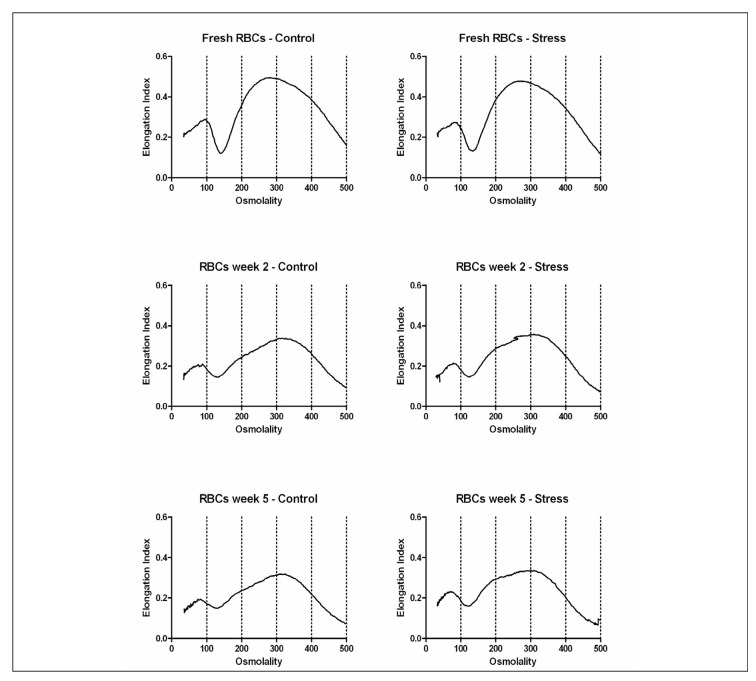
Figure 1.
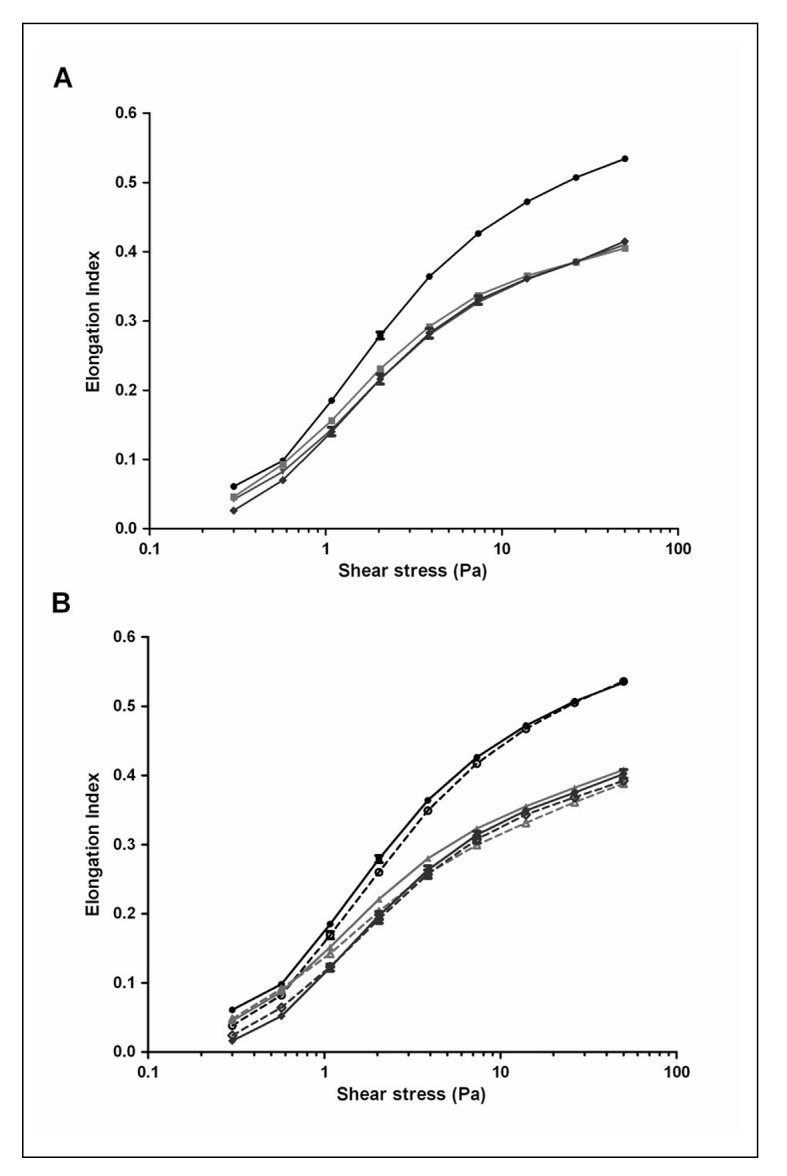
Effect of storage time and osmotic stress on deformation. RBC deformability was monitored using a laser-assisted optical rotational cell analyzer (LORCA, Mechatronics, Hoorn, the Netherlands) as previously described44. RBCs suspended in a viscous solution of polyvinylpyrrolidone are subjected to increasing shear stress. The resulting laser diffraction pattern, changing from circular (rest) to elliptical (high shear) are expressed by the Elongation Index (EI), calculated from the major (A) and minor (B) ellipse axis as (A–B)/(A+B). More deformable RBCs are more elongated and produce a higher EI.
(A) Shear stress EI curves for fresh RBCs (●) and after 1 (▼), 3 (■) and 5 weeks (♦) of storage. (B) Shear stress EI curves for fresh RBCs (●) and after 2 (▲) and 5 weeks (♦) of storage after overnight incubation in normal Ringer (–––) or hyperosmotic Ringer (⋯⋯) at 37 °C as described before20.
These data are in accordance with recent data using the same technique, suggesting that deformability of leukoreduced, SAGM-stored RBCs is not significantly reduced up to five weeks of storage24,25. In contrast, a comparison of fresh RBCs isolated directly from whole blood with RBCs in their first week in the blood bag, strongly suggests that deformability does already decrease during the three days of processing from blood to blood bag in the blood bank (Figure 1A). The same conclusion can be drawn from the LORCA data obtained from RBCs that were processed and stored in different media26. On one hand, this decrease in deformability may be caused by the same changes in membrane organisation that underly the increased PS exposure of one week-old blood bank RBCs compared with fresh RBCs20. On the other hand, hyperosmotic stress, which induces a strong increase in PS exposure especially upon prolonged storage20, does not seem to affect deformability (Figure 1B), suggesting that decreased deformability and increased PS exposure are not causally related. Thus, at this time it is not clear if increased PS exposure induces a decrease in membrane elasticity or if they share a common cause.
Neither the LORCA measurements, nor the osmotic gradient ektacytometry data indicate the occurrence of storage-associated differences in deformability, be it a decrease in the maximal elongation index (EI) in the first weeks of storage, or a shift in the hyperbolic part of the osmoscan in the last weeks of storage (Figure 2). The major changes seem to occur during the processing of the RBC in the blood bank, with no further, detectable changes after the first week. In fresh as well as in stored RBC, hyperosmotic stress does not result in any additional changes in the osmoscans (Figure 2). From a comparable study on freshly isolated RBC fractions with various cell volumes and densities, it was concluded that the shape of the hyperbolic part of the osmoscan is largely dependent on the MCHC18. Whereas in RBCs aged in vivo, the shift is towards the left, indicating an increase in MCHC that is associated with a decrease in osmotic resistance18,27, in blood bank RBCs the shift towards the right (Figure 2) suggests a decrease in MCHC and an opposite effect on osmotic resistance.
Figure 2.
Effect of storage time and stress on osmotic fragility as measured with osmotic gradient ektacytometry. Osmotic gradient ektacytometry, i.e. RBC deformability measured during a gradient of increasing osmolality (osmoscan) was performed using a the new LORCA MaxSis (see also the legend to Figure 1). Osmoscans of fresh RBCs, RBCs with a storage time of 2 weeks and RBCs with a storage time of 5 weeks were acquired after overnight incubation in normal Ringer (control) or hyperosmotic Ringer (stress) at 37 °C20,27.
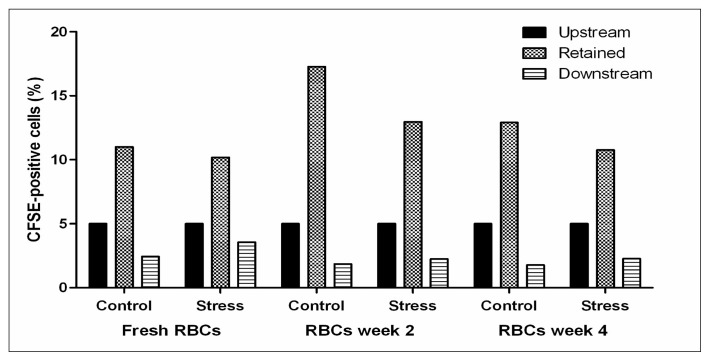
Short-term storage is already accompanied by a decrease in deformability as measured by rotational ektacytometry, and also may give rise to RBCs that are retained in the spleen more than fresh RBCs. When RBCs were passed through a recently developed spleen-mimicking device14, the percentage of RBCs that did not pass the device was increased with storage time (Figure 3). The difference between week 2 and week 4 may be explained by the loss of damaged RBCs. Curiously, these pilot data suggest that hyperosmotic stress may actually facilitate trafficking through the spleen, which would induce spleen-dependent vesiculation and/or phagocytosis28. A similar function has been postulated for the ligation of complement receptor 1-initiated increase in deformability16.
Figure 3.
Perfusion of stored RBCs through a spleen-mimicking device. RBCs were obtained from whole blood or from blood bags stored for two and four weeks. A suspension consisting of approximately 5% CFSE-labeled RBCs incubated overnight in normal Ringer (control) or hyperosmotic Ringer (stress) and 95% untreated/ unlabeled RBCs was passed through a bead-sorting device at a flow rate of 60 mL/h14. Flow cytometry was used to determine the percentage of labeled cells in the initial upstream sample, in the sample retrieved from in between the beads, and in the downstream fractions. The percentage of CFSE-labeled cells in the upstream samples was set at 5% and the retained and downstream samples were corrected accordingly.
Phosphatidylserine is widely recognized as a signal for macrophage recognition and RBC removal29. Although increased PS exposure by itself may not be sufficient for removal, it may induce adherence of RBCs to other cells, such as the blood vessel endothelium, and thereby facilitate the induction of phagocytosis. We found that an hyperosmotic stress-induced increase in PS exposure was associated with an increase in antibody-induced phagocytosis of stored RBCs by human monocyte-like THP1 cells (Table I). Thus, a stress-induced alteration in passage through the spleen may be functionally associated with increased phagocytosis.
Table I.
The effect of osmotic stress on antibody-induced phagocytosis1.
| Anti-Rh D antibody | % phagocytosis | |
|---|---|---|
| Control | Stress | |
| 0 | 0.0 | 0.0 |
| 1:25 | 3.7 | 5.9 |
| 1:5 | 11.6 | 17.2 |
| 1:1 | 21.7 | 30.5 |
RBCs (2x106) that had been incubated in iso-osmotic (control) or hyperosmotic medium (stress) were opsonized without (0) or with anti-Rhesus D antiserum in various dilutions (1:1, 1:5, 1:25), labeled with CFSE and co-incubated with THP1 cells (1x105) at 37 °C in a 5% CO2-incubator for three hours. After removal of the non-phagocytized RBCs with NH4-lysis buffer and washing at 4 °C, Fc receptors were blocked with PBS containing one percent pooled human serum. The percentage of THP1 cells that had phagocytized RBCs was determined by flow cytometry. Anti-CD235a antibody staining was used to discriminate between RBC adhesion and uptake. CFSE+CD235a− events were considered to be THP1 cells that had phagocytized RBCs.
Storage, deformability, proteomics and metabolomics
The presently available data, confirmed and extended by our own preliminary data using the spleen-mimicking device and osmoscan, suggest that the most pronounced effect of the changes RBCs undergo during blood bank storage may not only be a decreased deformability in the circulation, but also a decreased capacity to pass through the spleen. Since the spleen facilitates vesiculation and thereby possibly the removal of damaged membrane patches enriched in removal signals28,30, this may result in accelerated removal of stored RBCs. Also, a comparison of the osmoscan (Figure 2) and spleen-mimicking device data (Figure 3) indicates that the decrease in MCHC during the first weeks of storage, that by itself would result in an increase in deformability, is not a major determinant of changes in deformability of transfused RBCs in all relevant physiological conditions.
Therefore, identification of the storage-associated alterations in the interaction between the cytoskeleton and the lipid bilayer that determine membrane elasticity, may be instrumental in identifying the processes that trigger deformability-linked removal of transfused RBCs. In most recent theories on the functionally relevant changes in the membrane composition of stored RBCs, alterations in band 3 occupy a central position. Storage-associated breakdown as well as aggregation of band 3 have been observed by biochemical and immunochemical analysis4,5,7,10,31. Together with proteomic data, these analyses show that breakdown of band 3, ankyrin and spectrin, and membrane accumulation of hemoglobin occur mainly after three weeks of storage, but that the effect of these processes are already detectable within the first two weeks of storage3,4,8. The proteomic data also emphasize the importance of the “repair and destroy” proteins that protect against oxidative stress and unfolding32, and warrant a closer, experimental look at the changes in membrane-bound chaperone proteins, proteasome components, and small G proteins already observed in the first weeks of storage8.
Already during the first days of storage, signs of oxidation are observed in the cytoskeletal proteins 4.2 and 4.1, spectrin, and in band 3. These are followed by breakdown of actin, GAPDH, band 4.9 and ankyrin, as well as crosslinking of spectrin6. These data indicate that oxidative damage of membrane and membrane-associated proteins may precede proteolysis.
Thus, an oxidation-induced alteration of the linkage between membrane and cytoskeleton is a possible molecular mechanism causing the observed changes in deformability as revealed by the osmoscan and spleen-mimicking device data, that warrants further investigation. Oxidation may influence the phosphorylation status of band 3, and thereby affect the interaction between membrane and cytoskeleton33,34. Recent research on signaling in RBCs strongly suggests that a change in the interaction between integral membrane and cytoskeleton proteins, possibly caused by decreased phosphorylation, is the most likely reason for the early storage-associated susceptibility to the osmotic stress-induced decrease in deformability. Casein kinase II-catalyzed phosphorylation of beta spectrin and protein kinase C-catalyzed phosphorylation of protein 4.1 are associated with a complement receptor-mediated increase in deformability16. These data confirm the involvement of serine phosphorylation of spectrin in the mechanical properties of the RBC membrane35. Phosphorylation of band 4.1 promotes dissociation of actin from the cytoskeleton, also contributing to an increase in deformability36,37. Also, Lyn and/ or Syk-catalyzed tyrosine phosphorylation of the cytoplasmic domain of band 3 is associated with a decreased binding of the band 3 with the cytoskeleton, and with a “vesiculating” morphology38. Syk has a high preference for oxidized band 333, which may be relevant in view of the early oxidation events during storage6. Recent (phospho)proteomic-inspired data show an association between Lyn signaling and altered cytoskeleton-membrane interaction, resulting in an abnormal cell shape39.
Also, altered phosphorylation may be responsible for the metabolic changes, such as the recently described increase in glycolytic intermediates within the first two weeks of storage3,40. The rate of glycolysis is influenced by the storage-associated decrease in pH and NAD+, but we postulate that the metabolic changes are mainly due to alterations in band 3 structure and function. The cytoplasmic domain of band 3 has a high affinity for key enzymes of the glycolysis, and binding is regulated by phosphorylation41,42.
Conclusions and perspectives
The published data together with our results presented here support the theory that phosphorylation-driven changes in the RBC membrane during blood bank processing and especially in the first two weeks of storage are responsible for the accompanying changes in deformability. After 14 to 21 days of storage, functionally relevant molecular changes are likely to become irreversible, since they comprise progressive proteolysis and vesicle formation. Uncovering the triggering events and the underlying signaling pathways will be instrumental in understanding and preventing the untimely disappearance of a considerable fraction of the transfused RBCs within the first hours after transfusion.
In our perspective, the most fruitful approach to elucidate the basis for reduced RBC survival after transfusion starts with investigating the effects of signaling-based manipulation of RBCs in vitro on functionally relevant parameters. These parameters should be informative on the activation of, and recognition and removal by the immune system, on deformability in the capillaries and in the spleen, and on the susceptibility to osmotic stress, as may be critical during passage of transfused RBCs through the kidneys20,43. Proteomics and metabolomics will be instrumental in analyzing the accompanying changes in membrane structure. The main challenge is the development of biologically relevant read-out systems, and the transition of blood bank research from quality control in the blood bag to quality control in the patient.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Luten M, Roerdinkholder-Stoelwinder B, Schaap NPM, et al. Survival of red blood cells after transfusion: a comparison between red cell concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered erythrocyte concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 5.Bosman GJCGM, Klaarenbeek JM, Luten M, Bos HJ. Storage-related changes in erythrocyte band 3: not a case for the Diego blood group antigens. Cell Mol Biol. 2005;51:195–200. [PubMed] [Google Scholar]
- 6.D’Amici GM, Rinalducci S, Zolla L. Proteomic analysis of RBC membrane protein degradation during blood storage. J Proteome Res. 2007;6:3242–55. doi: 10.1021/pr070179d. [DOI] [PubMed] [Google Scholar]
- 7.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–20. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 8.Bosman GJCGM, Lasonder E, Luten M, et al. The proteome of erythrocyte membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 9.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation and signalling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 10.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 11.Bosman GJCGM, Lasonder E, Groenen-Döpp YAM, et al. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteom. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–92. [PubMed] [Google Scholar]
- 13.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411–26. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 14.Deplaine G, Safeukui I, Jeddi F, et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood. 2011;117:88–95. doi: 10.1182/blood-2010-10-312801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantaleo A, De Franceschi L, Ferru E, et al. Current knowledge about the functional role of phosphorylative changes of membrane proteins in normal and diseased red cells. J Proteom. 2010;73:445–55. doi: 10.1016/j.jprot.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Glodek AM, Mirchev R, Golan DE, et al. Ligation of complement receptor 1 increases erythrocyte membrane deformability. Blood. 2010;116:6063–71. doi: 10.1182/blood-2010-04-273904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahrstein G, Manny N, Yedgar S. Circulatory risk in the transfusion of red blood cells with impaired flow properties induced by storage. Transf Med Rev. 2011;25:24–35. doi: 10.1016/j.tmrv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Bosch FH, Werre JM, Schipper L, et al. Determinants of red blood cell deformability in relation to cell age. Eur J Haematol. 1994;52:35–41. doi: 10.1111/j.1600-0609.1994.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 19.Luten M, Roerdinkholder-Stoelwinder B, Rombout-Sestrienkova E, et al. Red cell concentrates of hemochromatosis patients comply with the storage guidelines. Transfusion. 2008;48:436–41. doi: 10.1111/j.1537-2995.2007.01547.x. [DOI] [PubMed] [Google Scholar]
- 20.Bosman GJCGM, Cluitmans JCA, Groenen YAM, et al. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during erythrocyte storage. Transfusion. 2011;51:1072–8. doi: 10.1111/j.1537-2995.2010.02929.x. [DOI] [PubMed] [Google Scholar]
- 21.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 22.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transf Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 23.Almac E, Ince C. The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol. 2007;21:195–208. doi: 10.1016/j.bpa.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Farges E, Grebe R, Baumann M. Viscoelastic and biochemical properties of erythrocytes during storage with SAG-M at +4°C. Clin Hemorheol Microcirc. 2002;27:1–11. [PubMed] [Google Scholar]
- 25.Henkelman S, Dijkstra-Tiekstra MJ, de Wildt-Eggen J, et al. Is red blood cell rheology preserved during routine blood bank storage? Transfusion. 2010;50:941–8. doi: 10.1111/j.1537-2995.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 26.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werre JM, Willekens FL, Bosch FH, et al. The red cell revisited - matters of life and death. Cell Mol Biol. 2004;50:139–45. [PubMed] [Google Scholar]
- 28.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Döpp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–51. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 29.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy-le-grand) 2004;50:147–58. [PubMed] [Google Scholar]
- 30.Willekens FL, Werre JM, Groenen-Döpp YA, et al. Erythrocyte vesiculation: a self-protective mechanism? Brit J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 31.Bosman GJCGM, Kay MMB. Erythrocyte aging: a comparison of model systems for simulating cellular aging in vitro. Blood Cells. 1988;14:19–46. [PubMed] [Google Scholar]
- 32.Goodman SR, Kurdia A, Ammann L, et al. The human red blood cell proteome and interactome. Exp Biol Med (Maywood) 2007;232:1391–408. doi: 10.3181/0706-MR-156. [DOI] [PubMed] [Google Scholar]
- 33.Pantaleo A, Ferru E, Giribaldi G, et al. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem J. 2009;418:359–67. doi: 10.1042/BJ20081557. [DOI] [PubMed] [Google Scholar]
- 34.Pantaleo A, Ferru E, Carta F, et al. Irreversible AE1 tyrosine phosphorylation leads to membrane vesiculation in G6PD deficient red cells. PLoS One. 2011;6:e15847. doi: 10.1371/journal.pone.0015847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manno S, Takakuwa Y, Nagao K, Mohandas N. Modulation of erythrocyte membrane mechanical function by beta-spectrin phosphorylation and dephosphorylation. J Biol Chem. 1995;270:5659–65. doi: 10.1074/jbc.270.10.5659. [DOI] [PubMed] [Google Scholar]
- 36.Manno S, Takakuwa Y, Nagao K, Mohandas N. Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J Biol Chem. 2005;280:7581–7. doi: 10.1074/jbc.M410650200. [DOI] [PubMed] [Google Scholar]
- 37.De Oliveira S, Silva-Herdade AS, Saldanha C. Modulation of erythrocyte deformability by PKC activity. Clin Hemorheol Microcirc. 2008;39:363–73. [PubMed] [Google Scholar]
- 38.Ferru E, Giger K, Pantaleo A, et al. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117:5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Franceschi L, Tomelleri C, Matte A, et al. Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood. 2011;118:5652–63. doi: 10.1182/blood-2011-05-355339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPDSAGM. J Proteomics. 2012 Mar 20; doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Low PS, Rathinavelu P, Harrison ML. Regulation of glycolysis via reversible enzyme binding to the membrane protein band 3. J Biol Chem. 1993;268:14627–31. [PubMed] [Google Scholar]
- 42.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci USA. 2005;102:2402–7. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang F, Lang KS, Lang PA, et al. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8:1183–92. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- 44.Hardeman MR, Goedhart PT, Dobbe JGG, Lettinga KP. Laser-assisted optical rotational cell analyzer (L.O.R.C.A.). I. A new instrument for measurement of various structural hemorheological parameters. Clin Hemorheol. 1994;14:605–18. [Google Scholar]