Abstract
Background
SAGM is currently the standard additive solution used in Europe, while AS-3 is the third additive solution that has been licensed in the USA, and is also the one used in part of Canada. Although AS-3 is based on a saline-adenine-glucose solution, it also contains citrate and phosphate. Storage of red blood cell concentrates in CPD-SAGM is known to lead to the accumulation of a wide series of storage lesions, including membrane protein fragmentation and vesiculation, as we could previously determine through 2-dimensional gel electrophoresis.
Materials and methods.
Through 2D-SDS-IEF-polyacrilamide gel electrophoresis we performed a time course analysis (day 0, 21 and 42 of storage) of red blood cell membranes from leukocyte-filtered concentrates either stored in CPD-SAGM or CP2D-AS-3.
Results and discussion.
From the present study it emerges that the membrane protein profile of red blood cells stored in presence of AS-3 appears to be slightly different from (better than) previous reports on SAGM-stored counterparts. However, the increase of total membrane spot number due to the presence of fragments at day 21 and the significant decrease at day 42 are suggestive of a universal phenomenon which is not efficiently tackled by either of the two additive solutions investigated in the present study.
Conclusion
To further delve into the storage lesion issue for RBCs stored in AS-3, it would be interesting in the future to assay metabolic changes over storage progression as well.
Keywords: red blood cell, storage, SAGM, AS-3, proteomics
Introduction
In a recent and comprehensive review1, Hess shed light on the history of red blood cell (RBC) storage solutions, whose early days date back to the 19001, when Rous and Turner developed the first citrate and glucose mixture for storing rabbit RBCs2 and Robertson used it in the first blood bank in France during World War I3.
The inclusion of phosphate in 1950s and adenine in 1970s paved the way for the design and diffusion of additive solutions (1980s), which allowed further extending the shelf life and improving the quality of the RBC storage1. Of note, most of the additive solutions which are routinely exploited everyday worldwide have been known for decades4.
Compositional changes over the years were mainly based on the need to improve storage safety and effectiveness. Indeed, at first mixtures of sodium citrate and dextrose caramelized when the solutions were heated, whereas solutions of sodium citrate alone could be autoclaved1. On the other hand, pH lowering to 5.8 allowed sterilization of citrate and glucose (acid citrate dextrose - ACD) solutions as well, enabling storage of RBCs for up to 21 days5.
The subsequent development in the field of additive solutions was characterized by the addition of sodium phosphate to ACD (citrate phosphate dextrose - CPD), which reduced phosphate leakage from stored RBCs by reducing the gradient in phosphate concentration between the cytosol and the supernatant. In clinical terms, storage of whole blood in CPD resulted in improved in vivo recovery at 24h from transfusion, although it did not produce any substantial improvement to the shelf life of the transfusion product6.
The introduction of plastic bags7 and adenine (CPDA-1)8 to the blood processing workflow resulted in further improvements (storage up to five weeks), the latter being related to the restoration of cell shape, ATP concentration and viability. Indeed, RBCs lose adenine and adenosine through deamination reactions over storage durations, which leads to impaired RBC recovery and osmotic fragility9.
Additive solutions came soon afterwards, as they were added to packed RBCs to provide additional volume and nutrients for longer storage and better flow4. The first additive solution was SAG, named after its constituents, saline, adenine and glucose, decreasing storage haematocrit and viscosity to approximately 55% and 10 cps, respectively10. However, high biological variability of haemolysis still hampered the extension of the shelf life of RBC concentrates over 5 weeks, at least until the introduction of mannitol (a free radical scavenger and membrane stabilizer) by Hogman11. This solution, SAGM, gained widespread distribution and is now the standard additive solution used in Europe, while AS-1 and AS-5 (widely used in the USA) are two SAGM variants which differ only modestly in their concentrations of salt, sugar and mannitol1. AS-3 is the third additive solution that has been licensed in the USA, and is also used in part of Canada1. Again, it is based on SAG but also contains citrate and phosphate (the compositional differences between AS-3 and SAGM are highlighted in Table I). Citrate and mannitol both serve the same membrane-protective function in AS-3 and SAGM, respectively, although the former also functions as an impermeable ion that balances the osmotic pressure of small ion-permeable RBCs12. Another main difference is that AS-3 additive solution depends on a version of the primary CPD anticoagulant with higher dextrose content, called CP2D (Table I).
Table I.
Composition of SAGM and AS-3 additive solution.
| CP2D + AS-3 | CPD + SAGM |
|---|---|
| CP2D | CPD |
| Each 100 mL contains: | Each 100 mL contains: |
| Citric Acid (Monohydrate), 0.327 g | Citric Acid (monohydrate), 0.327 g |
| Sodium Citrate (Dihydrate), 2.630 g | Sodium Citrate (dehydrate), 2.630 g |
| Monobasic Sodium Phosphate (Monohydrate), 0.222 g | Sodium Dihydrogen Phosphate (dihydrate), 0.251 g |
| Dextrose (Anhydrous), 4.640 g | Dextrose (monohydrate), 2.55 g |
|
| |
| AS-3 | SAGM |
| Each 100 mL contains: | Each 100 mL contains: |
| Dextrose (Anhydrous), 1.000 g | Dextrose (monohydrate), 0.900 g |
| Sodium Chloride, 0.410 g | Sodium Chloride, 0.877 g |
| Adenine, 0.030 g | Adenine, 0.0169 g |
| Citric Acid (Monohydrate), 0.042 g | Mannitol, 0.525 g |
| Sodium Citrate (Dihydrate), 0.588 g containing 15 mEq of Sodium. | |
| Monobasic Sodium Phosphate (Monohydrate), 0.276 g | |
It is reported in the literature that none of these additive solutions appear to have significant advantages over the others. Indeed, AS-3 and SAGM are both associated with 78–84% recovery and 0.4% haemolysis after 6 weeks of storage1,4. However, although liquid storage of RBCs delivers a blood-derived therapeutic which is safe and effective, concerns still arise and persist about the quality issue of units stored longer than 14 days, as it emerged from clinical retrospective studies14,15, and laboratory evidence (about morphology16,17, metabolism18,19, membrane protein profiles,20–22 and protein biomarkers23,24). Although clinical prospective studies are either not yet conclusive or still in progress25,26,27, questions arise and persist as to whether the actual guidelines for RBC collection and processing in the frame of storage for transfusion purposes might already be good, albeit not good enough28.
Laboratory studies have already provided clear hints about the necessity to pursue a better, rather than a longer storage29. Indeed, in recent years the application of proteomics technology to the field of transfusion medicine30,31 has revealed major changes in the RBC membrane proteome as storage progresses, either in leukofiltered20 and non-leukofiltered21 RBC concentrates stored in CPD-SAGM.
Through two-dimensional gel-electrophoresis (2DE), an approach which allows separating proteins on the basis of their isoelectric point and molecular weight (MW), we previously reported that, as storage progresses, the membrane proteome undergoes some major alterations including the increase from the second to the third week of storage of the overall number of protein spots and the subsequent progressive decrease until the end of storage20,21. While late decrease of overall spot number is consistent with an increased rate of vesiculation22, transitional increase of the protein spot number might be attributed to (i) migration of intact proteins to the membrane; (ii) fragmentation of higher MW proteins; (iii) protein aggregation; all these phenomena being triggered by metabolic alterations19,20 and oxidative stress20,21.
In the present study, we wanted to assess through a gel-based approach (2DE) whether the membrane protein profiles of RBCs stored in CP2D-AS-3 followed a trend that could be compared to the one we have already reported for CPD-SAGM counterparts.
Materials and methods
Sample collection
SAGM
RBC samples to be stored in SAGM were collected as previously reported20,21.
Whole blood (450 mL ± 10%) was collected from healthy volunteer donors into CPD anticoagulant (63 mL) and leukodepleted. After separation of plasma by centrifugation, RBCs were suspended in 100 mL of SAG-M (Saline, Adenine, Glucose, Mannitol) additive solution. We studied RBC units collected from 4 male donors [age 38±12.5 (mean±S.D.)], upon signing of informed consent according to the declaration of Helsinki.
AS-3
The AS-3 samples were collected from a clinical study conducted at Dartmouth-Hitchcock Medical Center with the authorization of the Committee for the Protection of Human Subjects. Written informed consent was obtained from normal, healthy subjects meeting Food and Drug Administration (FDA; 21CFR640) and AABB7 donation criteria. Subjects donated 1 unit of whole blood (500±50 mL) into a standard, licensed primary collection container with CP2D anticoagulant solution (Pall Medical, Covina, CA). Collected blood was leukoreduced by means of an integral, whole blood leukoreduction filter and processed by centrifugation into RBCs by removing plasma and adding 110 mL of AS-3 (Nutricel, Pall Medical).
RBC units were stored under standard blood bank conditions (1–6 °C) and samples were removed aseptically for the analysis at day 0, 21 and 42 of storage for subsequent membrane protein extraction and 2DE analysis.
RBC protein extraction
Extraction of human erythrocyte membrane proteins was performed at day 0, 21 and day 42, based on the conventional method as described by Olivieri et al.33. The erythrocytes were isolated by centrifuging twice at 1,000 × g for 10 min. Packed cells were washed three times in 5 mM phosphate buffer pH 8.0, containing 0.9% w/v NaCl; then, they were centrifuged at 300 × g for 10 min, at 4 °C. Erythrocytes were then processed as in D’Amici et al.21. After 15 min of incubation at room temperature, cells were pelleted and then lysed with 9 vol of cold 5 mM phosphate buffer pH 8.0 containing 1 mM EDTA, 1 mM phenylmethanesulfonyl fluoride (PMSF). Cytosol was collected after centrifugation at 17,000 × g for 20 min at 4 °C and its protein content was estimated by the DC protein assay method (Bio-Rad, Hercules, CA, USA). Membranes were washed with the same buffer until free of hemoglobin and then, in order to remove non-specifically membrane-bound cytosolic proteins, were washed three times with 0.9% w/v NaCl and collected at 17,000 × g, for 20 min at 4 °C. Protein content was estimated by the bicinchoninic acid method34 and ghosts prepared in this way were used for the following steps.
Two-dimensional electrophoresis
To remove lipids, proteins were precipitated from a desired volume of each sample with a cold mix of tri-n-butyl phosphate/acetone/methanol (1:12:1). After incubation at 4 °C for 90 min, the precipitate was pelleted by centrifugation at 2,800 g, for 20 min at 4 °C. After washing with the same solution, the pellet was air-dried and then solubilized in the focusing solution containing 7 M urea, 2 M thiourea, 2% (w/v) ASB 14, 0.8% (w/v) pH 3–10 carrier ampholyte, 40 mM Tris, 5 mM TBP, 10 mM acrylamide, 0.1 mM EDTA (pH 8.5), 2% (v/v) protease inhibitor cocktail (Sigma Aldrich, Milan, Italy), and 2 mM PMSF. Before focusing, the sample was incubated in this solution for 3 h at room temperature, under strong agitation. To prevent over-alkylation, acrylamide was destroyed by adding an equimolar amount of DTE. A total of 250 μL of the resulting protein solution was then used to rehydrate 13 cm long IPG 3–10 NL (Amersham Biosciences, Cernusco sul Naviglio, Italy) for 8 h. IEF was carried out on a Multiphor II (Amersham Biosciences) with a maximum current setting of 50 μA/strip at 20 °C. The total product time voltage applied was 40 000 Vh for each strip. For the second dimension, the IPG strips were equilibrated for 30 min in a solution containing 6 M urea, 2% (w/v) SDS, 20% (v/v) glycerol, and 375 mM Tris-HCl (pH 8.8), with gentle agitation. The IPG strips were then laid on a 5–16% T gradient SDS-PAGE gel with 0.5% (w/v) agarose in the cathode buffer (192 mM glycine, 0.1% w/v SDS and Tris to pH 8.3). The anode buffer was 375 mM Tris-HCl, pH 8.8. The electrophoretic run was performed at a constant current (10 mA for 60 min, followed by 40 mA until the run was completed). During the whole run, the temperature was set at 13 °C. Proteins were visualized by Coomassie Brilliant Blue G-250 stain35.
Image statistical analysis
Twenty-four stained gels (4 biological replicates × 3 periods − day 0, 21 and 42 – x 2 groups – SAGM and AS-3) were digitalized using an ImageScanner and LabScan software 3.01 (Bio-Rad, Hercules, CA).
Overall spot number has been calculated through ad hoc statistical software PDQuest 8.0 (Bio-Rad). Normalization and background subtraction have been automatically performed and a Master Map has been created for day 0, 21 and 42 gels for both groups (SAGM and AS-3). In Master Maps, spots have been included only if present in at least 3 out of 4 replicates. Total spot numbers have been thus calculated for each Master Map.
In-gel digestion and protein identification by MALDI-TOF TOF
In the light of our previous investigations in the field of RBC storage through membrane proteomics via gel-based approaches, we expected an increase in protein fragmentation proportional to the storage duration and inversely proportional to the extent of vesiculation events2,5. Since newly appearing spots in the low MW range (below 25 kDa) could not derive from de novo protein synthesis, since RBCs are enucleated and thus devoid of any new protein synthesis capacity, differential protein expression (changes in the photodensity of protein spots) in the low apparent MW region of the 2DE gels was taken into account and considered significant at p-values <0.05.
Protein spots were carefully excised from stained gels and subjected to in-gel trypsin digestion according to Shevchenko et al.36.
Twenty microliters of the tryptic protein digests was loaded onto activated (0.1% TFA in acetonitrile) ZipTip columns and washed three times with 10 μL of 0.1% TFA in DD-H2O. The peptides were eluted with 1 μL of matrix solution (0.7 mg/mL α-cyano-4-hydroxy-trans-cinnamic acid [Fluka, Seelze, Germany] in 85% acetonitrile, 0.1% TFA and 1 mM NH4H2PO4) and spotted directly on the MALDI-TOF target plate for automatic identifications (PAC384 pre-spotted anchor chip). Proteins were identified, as previously reported37 and per manufacturer’s specifications, through an Autoflex II MALDITOF/TOF mass spectrometer with the LIFT module (Bruker Daltonics, Bremen, Germany) was used for mass analysis of peptide mixtures. A peptide mixture (Peptide calibration standard I, Bruker Daltonics) was used for external calibration, while thinternal calibration was performed using the trypsin autolysis products. Proteins were identified by PMF using the database search program MASCOT (http://www.matrixscience.com/) upon removal of background ion peaks. Accuracy was set within 50 ppm, while the enzyme chosen was trypsin and only 1 missed cleavage was allowed; fixed carbamidomethylCys and variable Met-oxidation, was used as optional search criterion. PMF-based protein identification was confirmed by MS/MS analyses of precursor ions and repeated MASCOT based database searches. Runs were performed automatically through FlexControl setting and Biotools processing of MS data (PMF) and validation of identifications through MS/MS (LIFT analysis) on the three most intense ion peaks.
Results and discussions
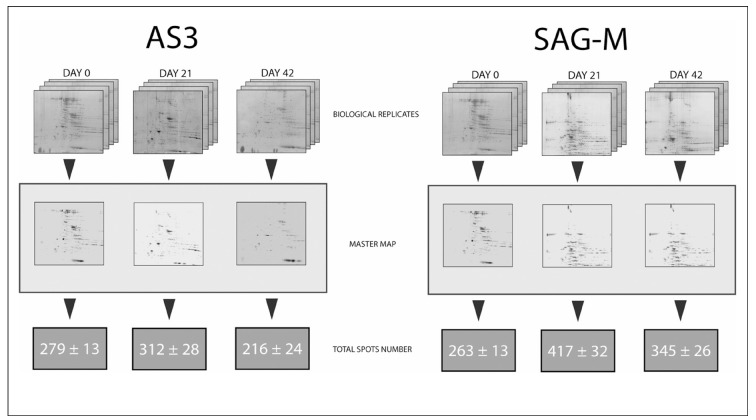
Twenty-four 2DE gels were performed to monitor changes of the RBC membrane proteome over storage under blood bank conditions in presence of AS-3 or SAGM. Figure 1 summarizes the overall number of biological replicates for each arm of the study (four each for AS-3 and SAGM) and the master maps for each storage period (day 0, 21 or 42 of storage). Below each figure, the overall number of protein spots from Coomassie staining is indicated.
Figure 1.
Total spot number at day 0, 21 and 42 of storage from 2DE analyses of RBC membranes obtained from cells stored in AS-3 (left side) or SAGM (right side). Total spot numbers were calculated on master maps obtained from 4 distinct biological replicates for each group by means of the PDQuest 8.0 software. Results are reported as means ± S.D..
Although the results are not directly comparable to our previous paper on 2DE analyses of RBC membrane proteome in CPD-SAGM21, since in that case RBCs had not been leukoreduced, it is still possible to observe that after three weeks of storage (two in D’Amici et al., 200721) the overall number of spots significantly increases, 45% in the previous (from 126.60±2.07 to 232.80±7.66) and 59% in the current investigation (from 263±13 to 417±32). It is also worthwhile to stress that, in our 2007 report21, spot number increased in the Silver Stained gels as well (from 392±15 to 487±24 spots in day 0 and 14, respectively), although inconsistencies in the SAGM initial spot number might be either due to bioinformatic improvements in the PDQuest software, different staining protocol and the nonleukodepleted nature of the RBC concentrates tested in 2007, rather than to actual technical advances in 2DE runs over the last two years.
Nevertheless, it is also worthwhile to stress that the overall spot number decreased again after the third week of storage, as to remain only slightly higher than day 0 SAGM controls by day 42 (Figure 1). This was evident both in the present study (263±13 vs 345±26 at day 0 and 42, respectively) and in the previous one21 (392±15 vs. 447±21 at day 0 and 42 in Silver staining stained gels), which we previously interpreted as fragmentation events occurring in the early weeks of storage (second to the third20,21) and vesiculation taking place soon afterwards. In a more recent investigation20, we could further confirm the latter hypothesis and delve into membrane protein profiles through 2DE analyses of RBCs extracted in presence of N-ethylmaleimed. We could indeed individuate the progressive accumulation at the membrane level at storage day 35 of vesicle-related proteins, such as alpha-soluble NSF attachment protein, alpha SNAP, 55 KDa erythrocyte membrane protein isoform 1, stomatin, ankyrin and biliverdin reductase, and 14-3-3 zeta/delta20.
Figure 1 also indicates for the first time that a similar trend could be observed as well for RBCs stored in presence of AS-3. While the overall number of protein spots at day 0 was almost comparable for SAGM and AS3 membrane protein profiles (263±13 vs. 279±13, respectively), a slight increase could be observed after three weeks of storage (312±28 total spots) and a significant decrease by day 42 (216±24, 69% of the total spot number after 21 day of storage).
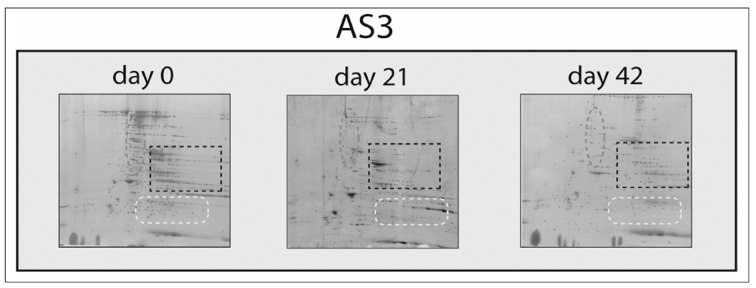
Qualitative differences among Master Maps (at day 0, 21 and 42 of RBC storage in AS3) are further highlighted in Figure 2. The overall decrease in spot number from Master Maps at day 42 in comparison to day 0 controls are particularly evident in the areas delimited by the grey ellipse (high MW region, previously reported in SAGM-stored RBCs to host structural proteins undergoing fragmentation) and the white rectangle with rounded edges. Of note, the high MW region (grey ellipse in Figure 2) appears to be depleted in protein spots yet at day 21, when new protein spots appear, especially at low MW (white rectangle with rounded edges).
Figure 2.
A detail of 2DE maps for AS-3-stored RBC membranes at day 0, 21 and 42 (from left to right). The grey ellipse, black rectangle and white rectangle with rounded edges indicate a high MW region, average MW region with medium to high pI and a low MW region, respectively. As storage progresses, fragmentation of proteins from the grey ellipsoid region increases the number of spots at low MW. A right-shift (increase in apparent pI) can be observed for most of the spots by storage day 42.
Prolonged storage (42 days) resulted in a right-shift of the pIs (spots display higher apparent pIs). This is particularly evident in the blue rectangle in Figure 2.
Although the less drastic increase in the total spot number at day 21 in comparison to day 0 controls suggested that the fragmentation phenomenon was less intense in AS-3 than in SAGM-stored RBCs, protein fragments still accumulated at the membrane level in AS-3-stored RBCs, as we could highlight through the selection of newly appearing protein spots by day 21 in the low MW range (below 25 kDa apparent MW - Figure 3). Indeed, we could find at least eight newly appearing protein spots (p-value < 0.05) from 2DE analyses of RBC membrane obtained from cells stored in AS3 for 21 days (Figure 3). Spot excision and tryptic digestion allowed MALDI-TOF/TOF-based identification of each spot as follows (Table II): (i) protein fragments of higher MW structural proteins (spots no. 121 - spectrin beta; 148 - ankyrin 2.2; 153 - heat shock cognate 71 kDa protein isoform 1; 234 - protein 4.1 isoform 4; 247 - ankyrin isoform 2; 282 - heat shock 70kDa protein 8 isoform 1; 315 - protein 4.1 isoform 4); (ii) intact proteins migrating to the membrane (spots no. 111 - alpha globin).
Figure 3.
Eight newly appearing protein spots in day 21 2DE gels of RBC membranes, in comparison to day 0 controls.
Table II.
Protein spots identified through mass spectrometry as protein fragments.
| N° spot | Mr, Da | pI | N° of peptides identified | Mascot Score | NCBI Accession Number | Protein ID [Homo sapiens] |
|---|---|---|---|---|---|---|
| 111 | 13574 | 7.98 | 1 (MS/MS) | 126 | gi|28549 | alpha globin |
| 121 | 173310 | 4.93 | 10 (MS) | 103 | gi|119601287 | spectrin, beta, erythrocytic (includes spherocytosis, clinical type I), isoform CRA_g |
| 148 | 188894 | 6.15 | 28 (MS) | 180 | gi|747710 | alt. ankyrin (variant 2.2) |
| 153 | 70854 | 5.37 | 1 (MS/MS) | 131 | gi|5729877 | heat shock cognate 71 kDa protein isoform 1 |
| 234 | 71911 | 6.19 | 10 (MS) | 131 | gi|42716291 | protein 4.1 isoform 4 |
| 247 | 188882 | 6.15 | 8 (MS) | 69 | gi|70780355 | ankyrin-1 isoform 2 |
| 282 | 70855 | 5.28 | 11 (MS) | 87 | gi|62897129 | heat shock 70kDa protein 8 isoform 1 variant |
| 315 | 71911 | 6.19 | 8 (MS) | 114 | gi|42716291 A | protein 4.1 isoform 4] |
Notably, two distinct fragments of protein 4.1 could be detected (spots no. 234 and 315), which in the 2DE map showed highly divergent apparent pIs and MWs. However, through mass spectrometry we could only distinguish them on the basis of the presence of two additional peptides in spot no. 234 in comparison to spot no. 315 (highlighted in bold red and yellow in the peptide list and protein sequence, respectively, in the supplementary Figure), which indicate a higher sequence coverage of the former, at least justifying the higher apparent MW (sequence coverage 15% and 13% for spot no. 234 and 315, respectively).
Fragmentation of structural proteins in the frame of RBC storage has long been reported20,21,22,38 and might stem from the exacerbation of oxidative stress under cold liquid storage conditions20. This in turn results from the alteration of the metabolic poise19,38, which leads to the impaired capability of RBCs to face oxidative stress as storage progresses20. Although the present study is only based upon 2DE proteomics observations, it appears that RBCs stored either in CPD-SAGM or AS-3 are affected by the same fragmentation/vesiculation phenomena, though to a different extent, underlying a universal mechanism for RBC ageing in vitro39,40 that none of the hereby tested additive solutions appears to attenuate.
Conclusion
From the present study it emerges that the membrane protein profile of RBCs stored in presence of AS-3 appears to be slightly different from previous reports on SAGM-stored RBC counterparts. This interpretation is made with some caution since the donor populations are different in the AS-3 and SAGM cohorts. However, the increase of total membrane spot number due to the presence of fragments at day 21 and the significant decrease at day 42 are suggestive of a universal phenomenon which is not efficiently tackled by none of the two additive solutions investigated in the present study. To further delve into the storage lesion issue for RBCs stored in AS-3, it would be interesting in the future to assay metabolic changes over storage progression as well, in like fashion to the recently proposed study for RBCs stored in CPD-SAGM19.
Since oxidative stress is now universally recognized as the underlying phenomenon triggering alterations of the RBC proteome and a whole series of storage lesions19,20,41–43, it is mandatory to pursue alternative storage strategies which tackle oxidative stress at its roots, either implying the use of additive solutions with substantial compositional modifications (for example, alkaline pH13) or the introduction of novel strategies for RBC storage which envisage elimination of oxygen32,44–46.
Acknowledgments
Gian Maria D’Amici, Cristiana Mirasole, Angelo D’Alessandro and Lello Zolla are supported by the Italian National Blood Center (Centro Nazionale Sangue - CNS - Istituto Superiore Sanità - Rome, Italy). AS-3 samples were prepared with a grant from NIH to TY (2R44HL088848) with special thanks to Michelle Dumas for technical work.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91(1):13–9. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 2.Rous P, Turner JW. The preservation of living red blood cells in vitro. J Exp Med. 1916;23:219–37. doi: 10.1084/jem.23.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson OH. Transfusion with preserved red blood cells. Br Med J. 1918;1:691–5. doi: 10.1136/bmj.1.2999.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore GL. Additive solutions for better blood preservation. CRC Crit Rev Clin Lab Sci. 1987;25:211–28. doi: 10.3109/10408368709105883. [DOI] [PubMed] [Google Scholar]
- 5.Loutit JF, Mollison PL. Advantages of a disodiumcitrate-glucose mixture as a blood preservative. Br Med J. 1943;2:744–5. doi: 10.1136/bmj.2.4327.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebaugh FG, Jr, Ross JF. The radioactive sodium chromate method for erythrocyte survival. Vox Sang. 1985;49:304–7. doi: 10.1111/j.1423-0410.1985.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 7.Artz CP, Howard JM, Davis JH, Scott R., Jr . Plastic bags for intravenous infusions: observations in Korea with saline, dextran and blood. In: Howard JM, editor. Battle Casualties in Korea: Studies of the Surgical Research Team. Vol. 2. Washington, DC: Army Medical Service Graduate School; 1954. pp. 219–24. [Google Scholar]
- 8.Shields CE. Effect of adenine on stored erythrocytes evaluated by autologous and homologous transfusions. Transfusion. 1969;9:115–9. doi: 10.1111/j.1537-2995.1969.tb05528.x. [DOI] [PubMed] [Google Scholar]
- 9.Simon ER, Chapman RG, Finch CA. Adenine in red cell preservation. J Clin Invest. 1962;41:351–9. doi: 10.1172/JCI104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogman CF, Hedlund K, Zetterstrom H. Clinical usefulness of red cells preserved in protein-poor media. N Engl J Med. 1978;299:1377–82. doi: 10.1056/NEJM197812212992502. [DOI] [PubMed] [Google Scholar]
- 11.Hogman CF, Hedlund K, Sahlestrom Y. Red cell preservation in protein-poor media. III. Protection against in vitro hemolysis. Vox Sang. 1981;41:274–81. doi: 10.1111/j.1423-0410.1981.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis HG, Gore DM, Briggs C, et al. Cold storage of ‘cryohydrocytosis’ red cells: the osmotic susceptibility of the cold-stored erythrocyte. Br J Haematol. 2003;122:859–68. doi: 10.1046/j.1365-2141.2003.04487.x. [DOI] [PubMed] [Google Scholar]
- 13.Högman CF, Löf H, Meryman HT. Storage of red blood cells with improved maintenance of 2,3-bisphosphoglycerate. Transfusion. 2006;46(9):1543–52. doi: 10.1111/j.1537-2995.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 14.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 15.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49(7):1384–94. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 16.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102(1):6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 17.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22(2):90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 18.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012 doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Amici GM, Rinalducci S, Zolla L. Proteomic analysis of RBC membrane protein degradation during blood storage. J Proteome Res. 2007;6(8):3242–55. doi: 10.1021/pr070179d. [DOI] [PubMed] [Google Scholar]
- 22.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48(5):827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 23.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 24.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50(2):376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett-Guerrero E, Stafford-Smith M, Waweru PM, et al. A prospective, double-blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion. 2009;49(7):1375–83. doi: 10.1111/j.1537-2995.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix J, Hébert P, Fergusson D, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;25(3):197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Steiner ME, Assmann SF, Levy JH, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43(1):107–16. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess JR. Red cell storage: when is better not good enough? Blood Transfus. 2009;7(3):172–3. doi: 10.2450/2009.0110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolla L. Blood Proteomics. Preface. J Proteomics. 2010;73(3):361–4. doi: 10.1016/j.jprot.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Liumbruno G, D’Alessandro A, Grazzini G, Zolla L. How has proteomics informed transfusion biology so far? Crit Rev Oncol Hematol. 2010;76(3):153–72. doi: 10.1016/j.critrevonc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Liumbruno G, D’Alessandro A, Grazzini G, Zolla L. Blood-related proteomics. J Proteomics. 2010;73(3):483–507. doi: 10.1016/j.jprot.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, AuBuchon JP, Tryzelaar L, et al. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92(1):22–31. doi: 10.1111/j.1423-0410.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 33.Olivieri E, Herbert B, Righetti PG. The effect of protease inhibitors on the two-dimensional electrophoresis pattern of red blood cell membranes. Electrophoresis. 2001;22(3):560–5. doi: 10.1002/1522-2683(200102)22:3<560::AID-ELPS560>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 34.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 35.Candiano G, Bruschi M, Musante L, et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25(9):1327–33. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 36.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 37.Suckau D, Resemann A, Schuerenberg M, et al. A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal Bioanal Chem. 2003;376:952–65. doi: 10.1007/s00216-003-2057-0. [DOI] [PubMed] [Google Scholar]
- 38.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40(3):353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 39.Bosman GJ, Lasonder E, Groenen-Döpp YA, et al. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73(3):396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Lion N, Crettaz D, Rubin O, Tissot JD. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73(3):374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Karon BS, Hoyer JD, Stubbs JR, Thomas DD. Changes in Band 3 oligomeric state precede cell membrane phospholipid loss during blood bank storage of red blood cells. Transfusion. 2009;49(7):1435–42. doi: 10.1111/j.1537-2995.2009.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhary R, Katharia R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. Blood Transfusion. 2011;10:59–62. doi: 10.2450/2011.0107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanias T, Acker JP. Biopreservation of red blood cells--the struggle with hemoglobin oxidation. FEBS J. 2010;277(2):343–56. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8(4):220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumont LJ, Yoshida T, AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009;49(3):458–64. doi: 10.1111/j.1537-2995.2008.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida T, AuBuchon JP, Dumont LJ, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion. 2008;48(10):2096–105. doi: 10.1111/j.1537-2995.2008.01812.x. [DOI] [PubMed] [Google Scholar]